Controllable Formation and Real-Time Characterization of Single Microdroplets Using Optical Tweezers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Optical Tweezers
2.2. Method
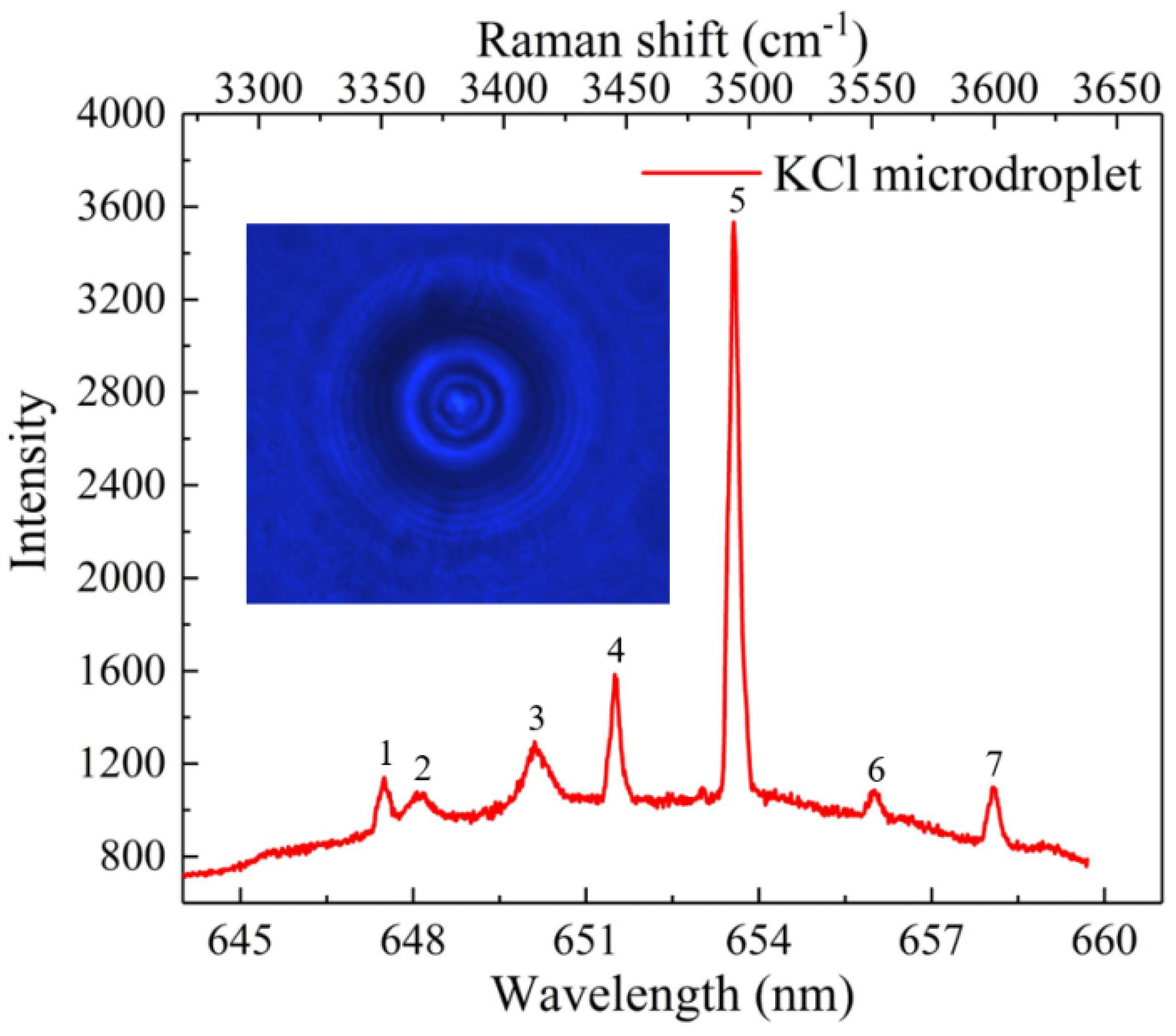
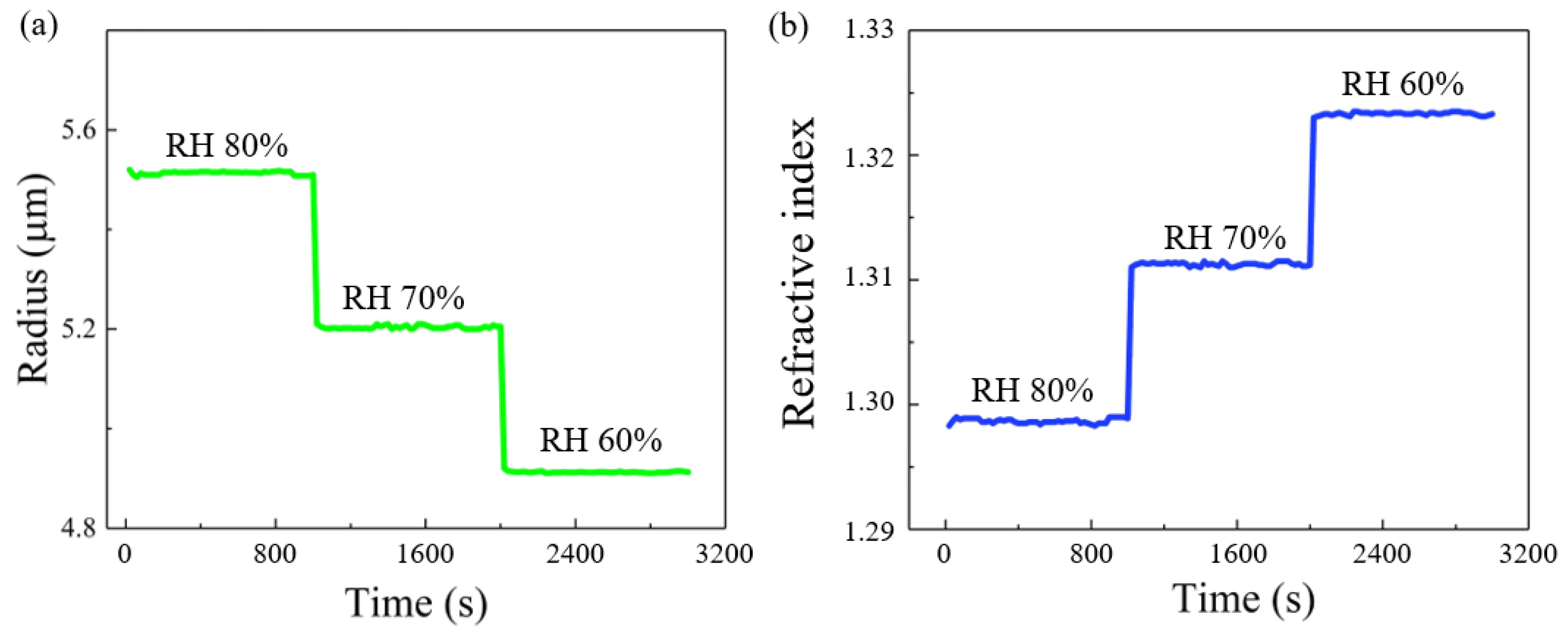
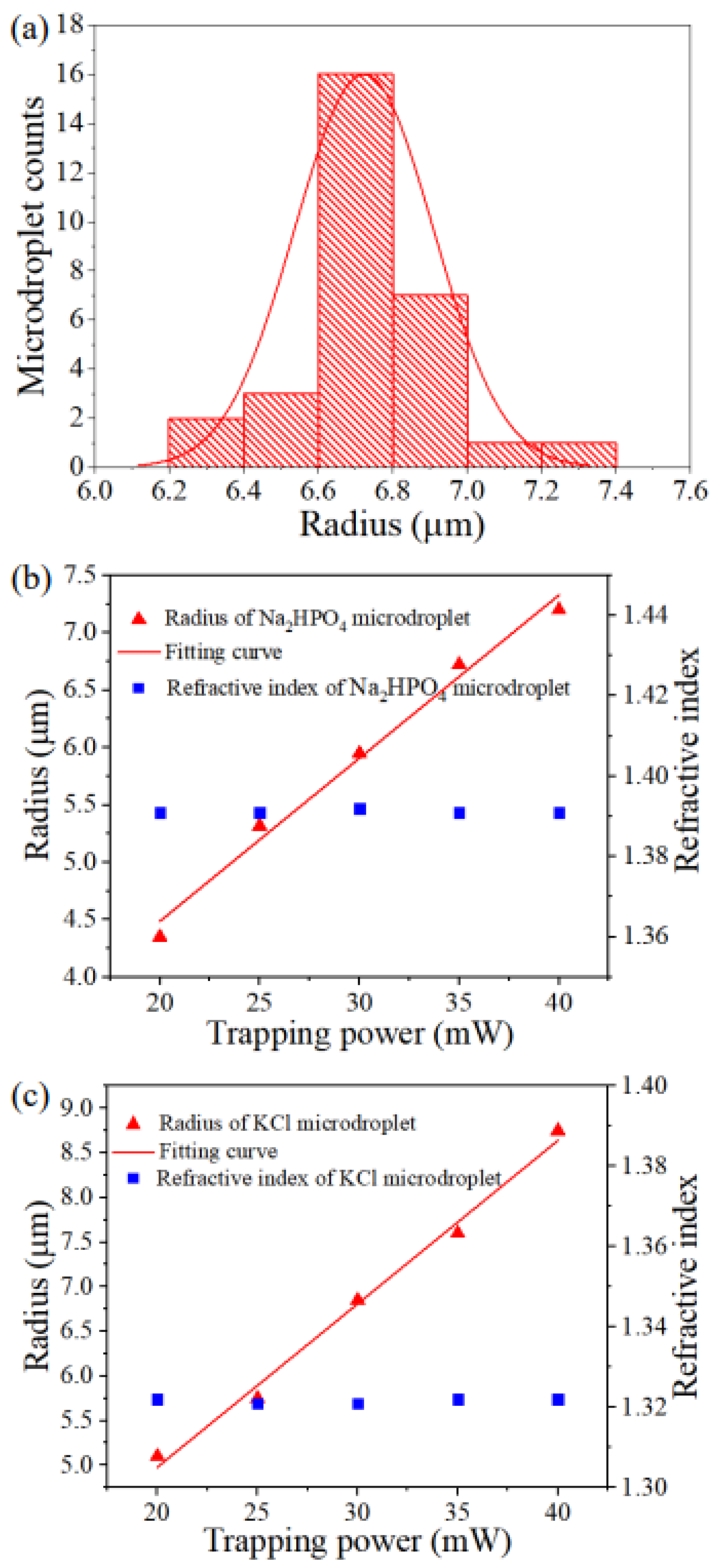
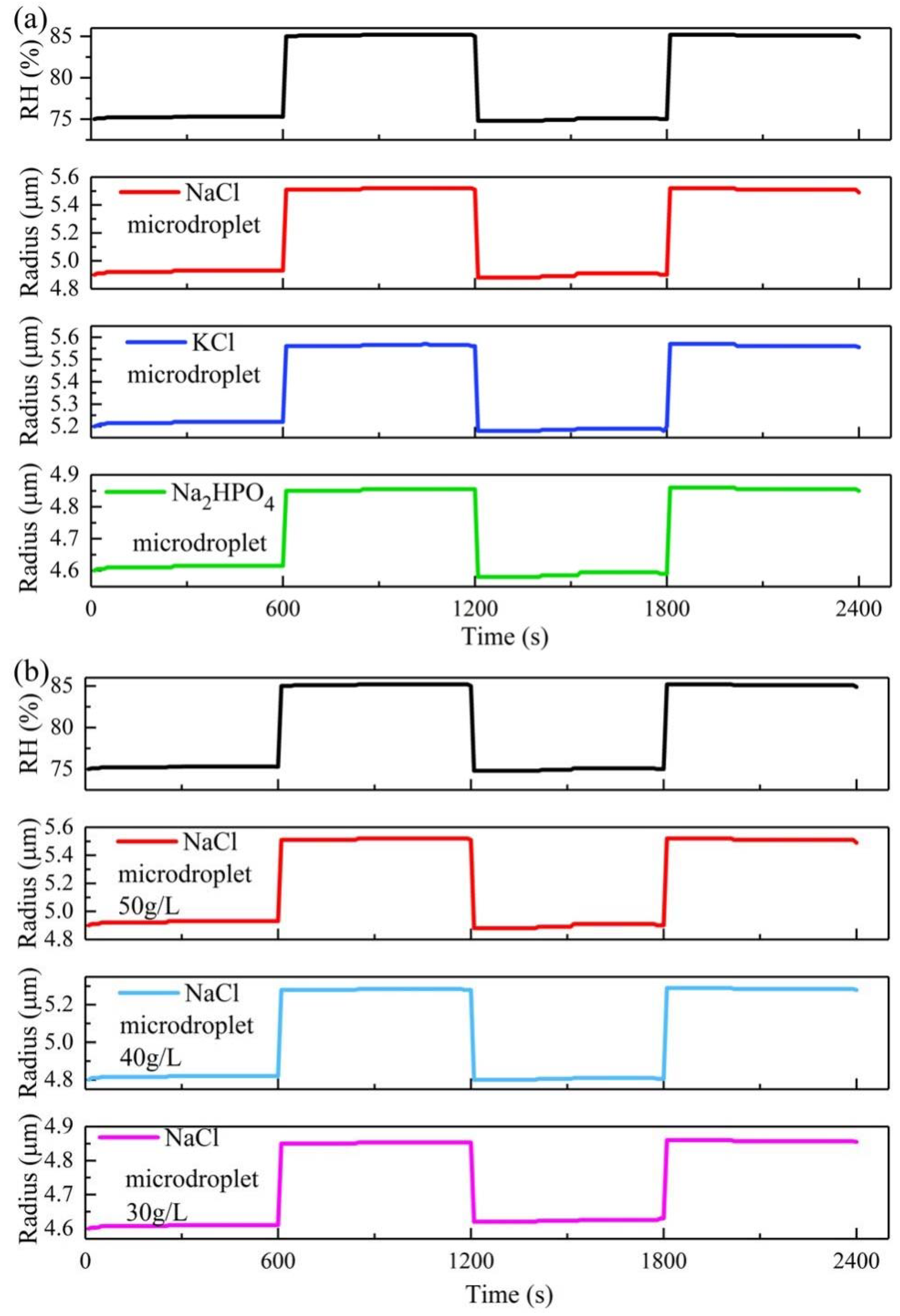
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, S.; Jeon, C.S.; Choi, N.; Moon, J.-I.; Lee, K.M.; Pyun, S.H.; Kang, T.; Choo, J. Sensitive and reproducible detection of SARS-CoV-2 using SERS-based microdroplet sensor. Chem. Eng. J. 2022, 446, 137085. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, H.; Zhao, L.; Liu, Y.; Liu, S.; Yang, J. Tapered optical fiber waveguide coupling to whispering gallery modes of liquid crystal microdroplet for thermal sensing application. Opt. Express 2017, 25, 918–926. [Google Scholar] [CrossRef]
- Humar, M.; Muševič, I. Surfactant sensing based on whispering-gallery-mode lasing in liquid-crystal microdroplets. Opt. Express 2011, 19, 19836–19844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Z.; Mi, Y.; Wang, M.; Liu, X.; Zhang, X.; Gao, K.; Shi, L.; Mugisha, E.; Chen, H.; Yan, W. Hydrophobic-substrate based water-microdroplet manipulation through the long-range photovoltaic interaction from a distant LiNbO3:Fe crystal. Opt. Express 2021, 29, 3808–3824. [Google Scholar] [CrossRef]
- Yan, X.; Bain, R.; Cooks, R.G. Organic Reactions in Microdroplets: Reaction Acceleration Revealed by Mass Spectrometry. Angew. Chem. Int. Ed. 2016, 55, 12960–12972. [Google Scholar] [CrossRef] [PubMed]
- Awerkamp, P.A.; Fish, D.; King, M.; Hill, D.; Nordin, G.P.; Camacho, R.M. 3D printed mounts for microdroplet resonators. Opt. Express 2022, 30, 1599. [Google Scholar] [CrossRef] [PubMed]
- Teshima, M.; Seki, T.; Kawano, R.; Takeuchi, S.; Yoshioka, S.; Takeoka, Y. Preparation of structurally colored, monodisperse spherical assemblies composed of black and white colloidal particles using a micro-flow-focusing device. J. Mater. Chem. C 2014, 3, 769–777. [Google Scholar] [CrossRef]
- Castillo-Orozco, E.; Kumar, R.; Kar, A. Laser-induced subwavelength structures by microdroplet superlens. Opt. Express 2019, 27, 8130–8142. [Google Scholar] [CrossRef]
- Chen, X.; Wu, T.; Gong, Z.; Guo, J.; Liu, X.; Zhang, Y.; Li, Y.; Ferraro, P.; Li, B. Lipid droplets as endogenous intracellular microlenses. Light. Sci. Appl. 2021, 10, 1–11. [Google Scholar] [CrossRef]
- Hao, H.; Leven, I.; Head-Gordon, T. Can electric fields drive chemistry for an aqueous microdroplet? Nat. Commun. 2022, 13, 280. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; He, X.; Zhu, J. Preparation and assembly of concave polymer microparticles. RSC Adv. 2015, 5, 36680–36686. [Google Scholar] [CrossRef]
- Lea-Banks, H.; Hynynen, K. Sub-millimetre precision of drug delivery in the brain from ultrasound-triggered nanodroplets. J. Control. Release 2021, 338, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.H.; Omi, S. Mechanism of formation of monodisperse polystyrene hollow particles prepared by membrane emulsi-fication technique. Effect of hexadecane amount on the formation of hollow particles. Macromol. Symp. 2002, 179, 223–240. [Google Scholar] [CrossRef]
- Pechenkin, M.A.; Möhwald, H.; Volodkin, D.V. pH- and salt-mediated response of layer-by-layer assembled PSS/PAH microcapsules: Fusion and polymer exchange. Soft Matter 2012, 8, 8659–8665. [Google Scholar] [CrossRef]
- Freger, V. Nanoscale Heterogeneity of Polyamide Membranes Formed by Interfacial Polymerization. Langmuir 2003, 19, 4791–4797. [Google Scholar] [CrossRef]
- Zhu, H.; Szymczyk, A.; Balannec, B. On the salt rejection properties of nanofiltration polyamide membranes formed by interfacial polymerization. J. Membr. Sci. 2011, 379, 215–223. [Google Scholar] [CrossRef]
- Patel, M.; Radhakrishnan, A.N.P.; Bescher, L.; Hunter-Sellars, E.; Schmidt-Hansberg, B.; Amstad, E.; Ibsen, S.; Guldin, S. Temperature-induced liquid crystal microdroplet formation in a partially miscible liquid mixture. Soft Matter 2021, 17, 947–954. [Google Scholar] [CrossRef]
- Li, S.; Hu, C.; Gao, X.; Ma, G.; Li, H.; Hu, X.; Hu, X. Optical tweezers assisted controllable formation and precise manipulation of microdroplet. Appl. Phys. Express 2019, 12, 117001. [Google Scholar] [CrossRef]
- Zhao, H.; Ibrahimova, V.; Garanger, E.; Lecommandoux, S. Dynamic Spatial Formation and Distribution of Intrinsically Disordered Protein Droplets in Macromolecularly Crowded Protocells. Angew. Chem. 2020, 132, 11121–11129. [Google Scholar] [CrossRef]
- Yan, Q.; Xuan, S.; Ruan, X.; Wu, J.; Gong, X. Magnetically controllable generation of ferrofluid droplets. Microfluid. Nanofluidics 2015, 19, 1377–1384. [Google Scholar] [CrossRef]
- Lee, C.-P.; Lan, T.-S.; Lai, M.-F. Fabrication of two-dimensional ferrofluid microdroplet lattices in a microfluidic channel. J. Appl. Phys. 2014, 115, 17B527. [Google Scholar] [CrossRef]
- Zhen, Z.; Li, Z.C.; Zhao, X.L.; Zhong, Y.J.; Zhang, L.; Chen, Q.; Yang, T.T.; Zhu, H.W. Formation of uniform water micro-droplets on wrinkled graphene for ultrafast humidity sensing. Small 2018, 14, 1703848. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Ma, L.; Chen, Y.; Deng, J.; Luo, G. A comprehensive study of droplet formation in a capillary embedded step T-junction: From squeezing to jetting. Chem. Eng. J. 2022, 427, 132067. [Google Scholar] [CrossRef]
- Ding, Y.; Howes, P.D.; Demello, A.J. Recent Advances in Droplet Microfluidics. Anal. Chem. 2020, 92, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Du, L.; Wang, Y.; Wang, K. Recent developments in microfluidic device-based preparation, functionalization, and manipulation of nano- and micro-materials. Particuology 2019, 45, 1–19. [Google Scholar] [CrossRef]
- Liu, W.-W.; Zhu, Y. “Development and application of analytical detection techniques for droplet-based microfluidics”—A review. Anal. Chim. Acta 2020, 1113, 66–84. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, L. Passive and active droplet generation with microfluidics: A review. Lab. Chip. 2017, 17, 34–75. [Google Scholar] [CrossRef]
- Vereschaka, A.; Milovich, F.; Andreev, N.; Sotova, C.; Alexandrov, I.; Muranov, A.; Mikhailov, M.; Tatarkanov, A. Investigation of the structure and phase composition of the microdroplets formed during the deposition of PVD coatings. Surf. Coatings Technol. 2022, 441, 128574. [Google Scholar] [CrossRef]
- Gateman, S.M.; Gharbi, O.; Turmine, M.; Vivier, V. Measuring changes in wettability and surface area during micro droplet corrosion measurements. Electrochimica Acta 2021, 399, 139402. [Google Scholar] [CrossRef]
- Doménech-Carbó, M.T.; Mai-Cerovaz, C.; Doménech-Carbó, A. Application of focused ion beam-field emission scanning electron microscopy-X-ray microanalysis in the study of the surface alterations of archaeological tin-glazed ceramics. Ceram. Int. 2022, 48, 14067–14075. [Google Scholar] [CrossRef]
- Xie, M.; Mills, J.K.; Wang, Y.; Mahmoodi, M.; Sun, D. Automated Translational and Rotational Control of Biological Cells with a Robot-Aided Optical Tweezers Manipulation System. IEEE Trans. Autom. Sci. Eng. 2015, 13, 543–551. [Google Scholar] [CrossRef]
- Xie, M.; Shakoor, A.; Wu, C. Manipulation of Biological Cells Using a Robot-Aided Optical Tweezers System. Micromachines 2018, 9, 245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Schonbrun, E.; Steinvurzel, P.; Crozier, K.B. Trapping and rotating nanoparticles using a plasmonic nano-tweezer with an integrated heat sink. Nat. Commun. 2011, 2, 469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaman, M.A.; Padhy, P.; Hesselink, L. Near-field optical trapping in a non-conservative force field. Sci. Rep. 2019, 9, 649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.-Y.; Kalim, S.; Callahan, C.; Teitell, M.A.; Chiou, E.P.Y. A light-induced dielectrophoretic droplet manipulation platform. Lab Chip 2009, 9, 3228–3235. [Google Scholar] [CrossRef]
- Zaman, M.A.; Padhy, P.; Ren, W.; Wu, M.; Hesselink, L. Microparticle transport along a planar electrode array using moving dielectrophoresis. J. Appl. Phys. 2021, 130, 034902. [Google Scholar] [CrossRef]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles, 1st ed.; Wiley-VCH: Weinheim, Germany, 1998; pp. 286–324. ISBN 978-0-47-129340-8. [Google Scholar]
- Petr, P.; Aleš, D. Moisture absorption and dimensional stability of poplar wood impregnated with sucrose and sodium chloride. Maderas-Cienc Tecnol. 2014, 16, 299–311. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Zhang, H.; Li, W.; Zhang, Y.; Gao, X.; Liu, H.; Li, N.; Hu, H. Controllable Formation and Real-Time Characterization of Single Microdroplets Using Optical Tweezers. Micromachines 2022, 13, 1693. https://doi.org/10.3390/mi13101693
Li S, Zhang H, Li W, Zhang Y, Gao X, Liu H, Li N, Hu H. Controllable Formation and Real-Time Characterization of Single Microdroplets Using Optical Tweezers. Micromachines. 2022; 13(10):1693. https://doi.org/10.3390/mi13101693
Chicago/Turabian StyleLi, Shuai, Hanlin Zhang, Wenqiang Li, Yizhou Zhang, Xiaowen Gao, Haiqing Liu, Nan Li, and Huizhu Hu. 2022. "Controllable Formation and Real-Time Characterization of Single Microdroplets Using Optical Tweezers" Micromachines 13, no. 10: 1693. https://doi.org/10.3390/mi13101693
APA StyleLi, S., Zhang, H., Li, W., Zhang, Y., Gao, X., Liu, H., Li, N., & Hu, H. (2022). Controllable Formation and Real-Time Characterization of Single Microdroplets Using Optical Tweezers. Micromachines, 13(10), 1693. https://doi.org/10.3390/mi13101693








