Rapid, Simple and Inexpensive Fabrication of Paper-Based Analytical Devices by Parafilm® Hot Pressing
Abstract
:1. Introduction
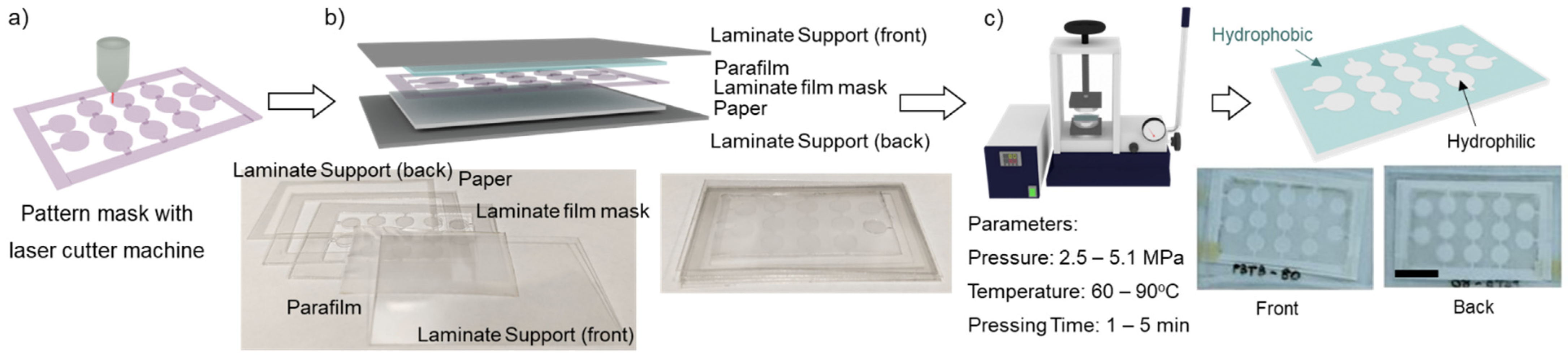
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Fabrication Parameter Study
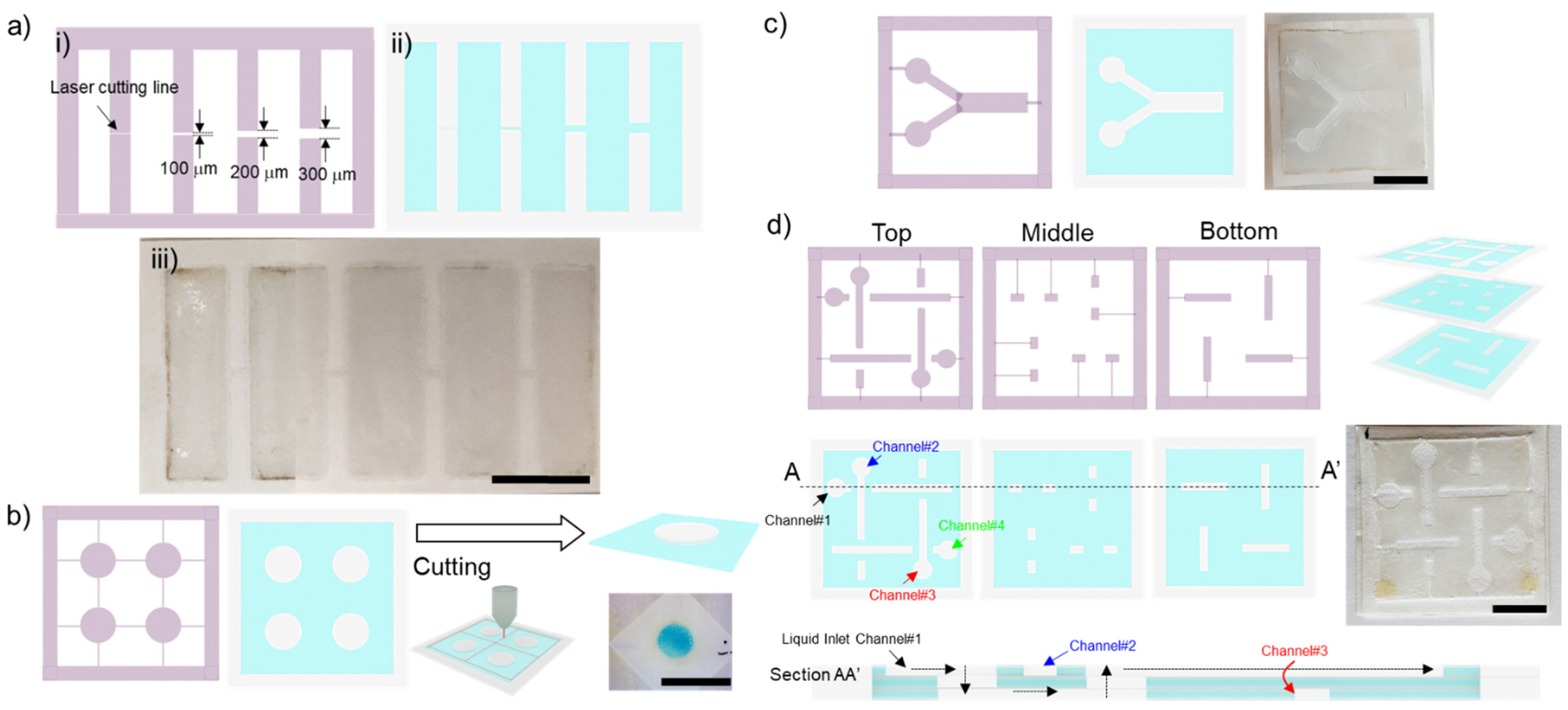
2.3. Hydrophobic Barrier Resolution
2.4. Physical and Biochemical Properties of the Paper
2.4.1. Flow Characteristic
2.4.2. Colorimetric Sandwich Immunological Assay
2.5. 2D and 3D Paperfluidic Devices
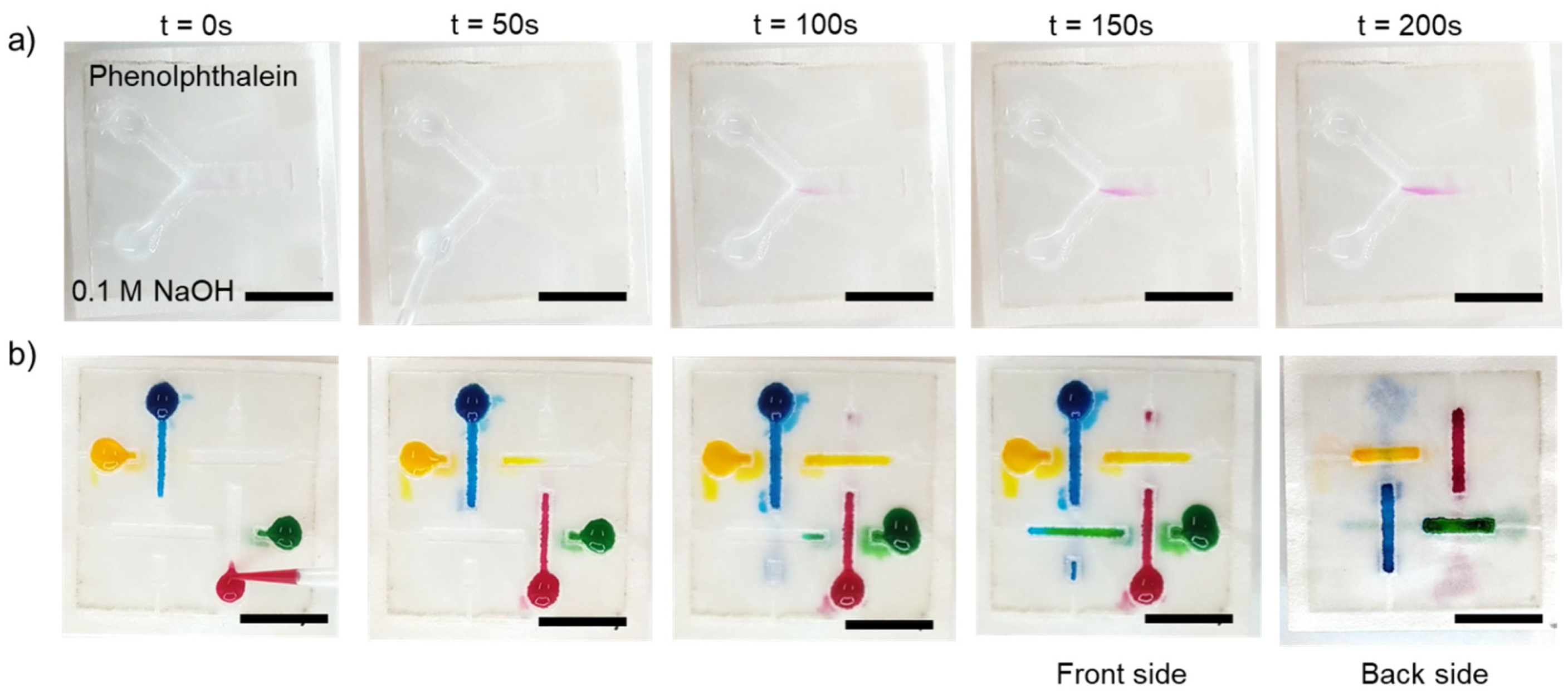
2.5.1. Diffusive Mixing in the Two-Dimensional Paperfluidic Device
2.5.2. Flow in a Three-Dimensional Paperfluidic Device
2.6. Data Acquisition and Quantification
3. Result and Discussion
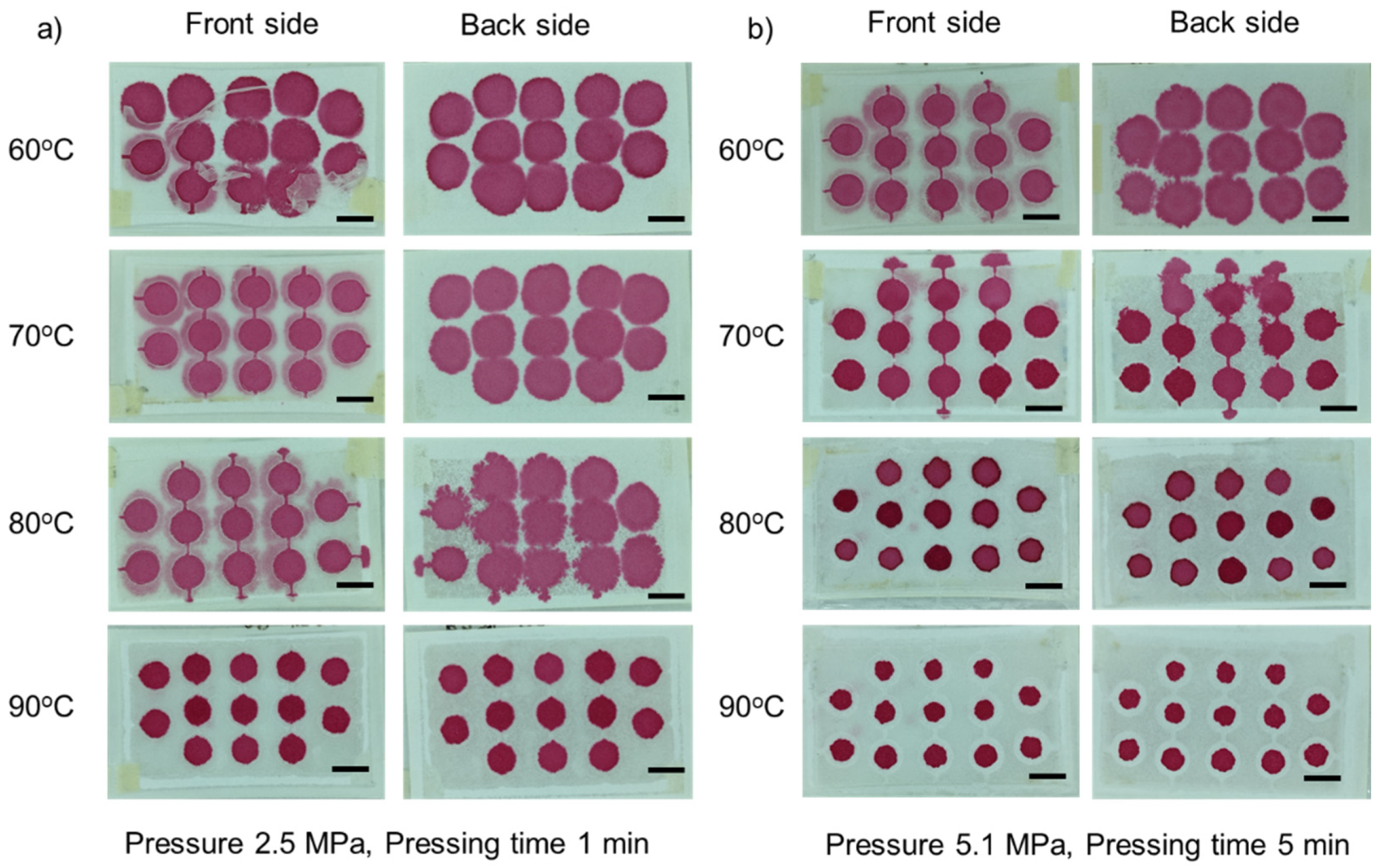
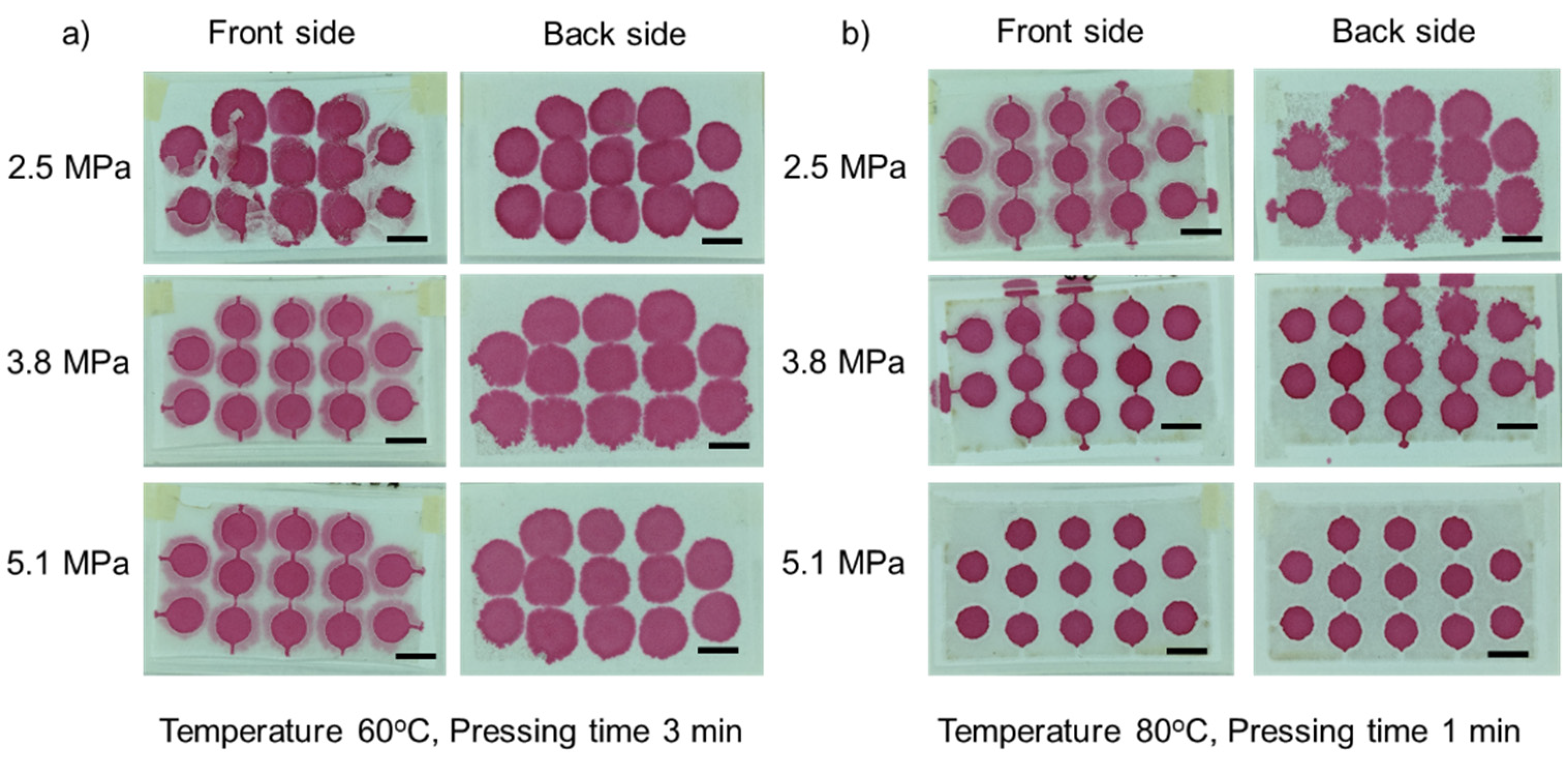
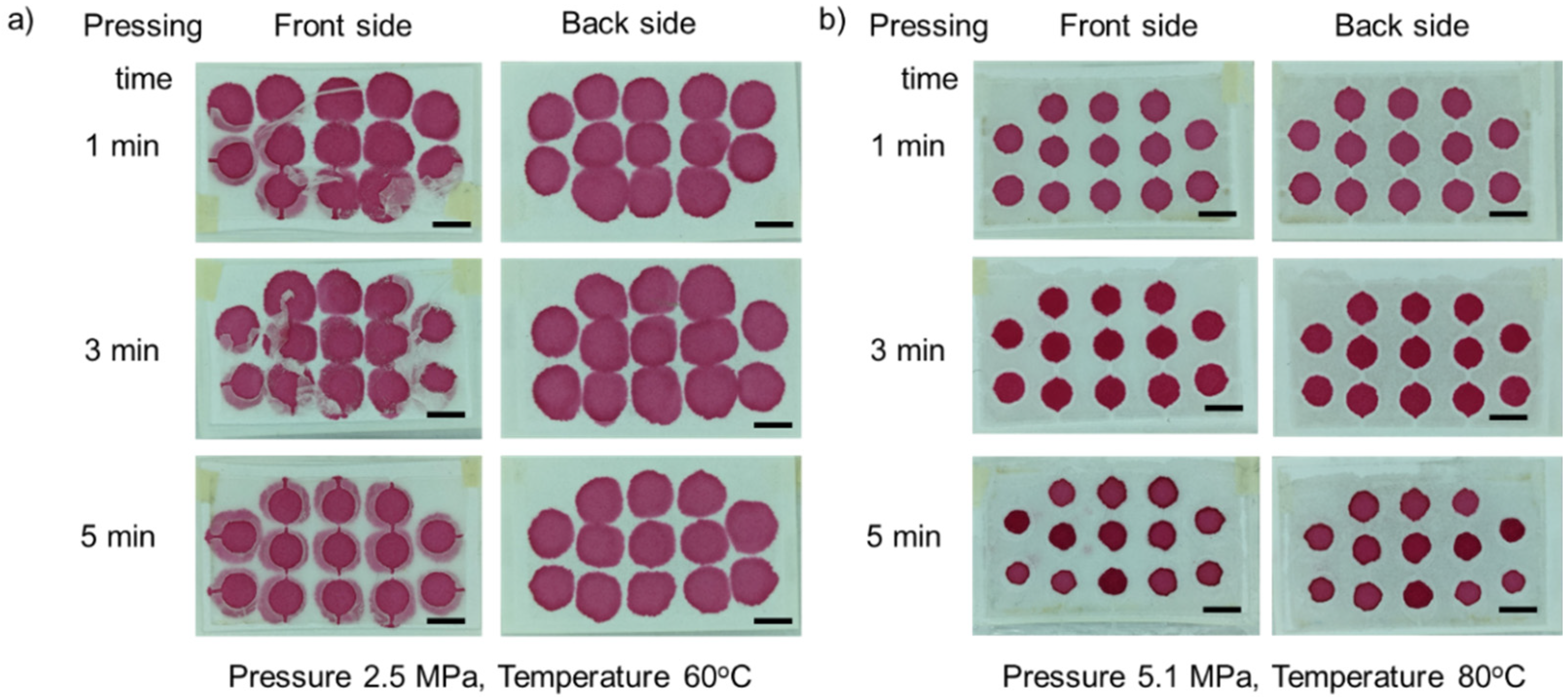
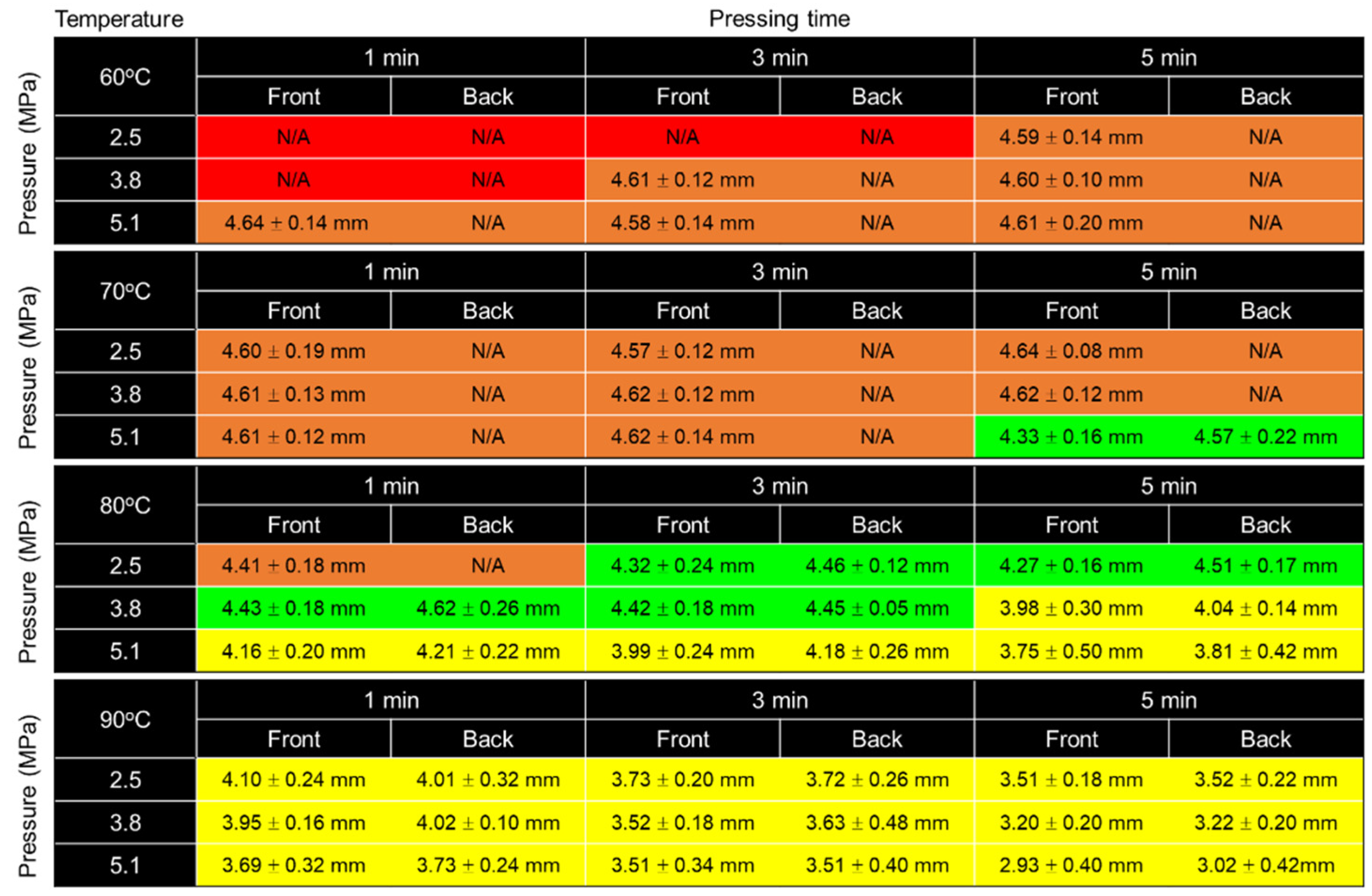
3.1. Optimization of Fabrication Parameters
3.1.1. Effect of Temperature
3.1.2. Effect of Pressure
3.1.3. Effect of Pressing Time
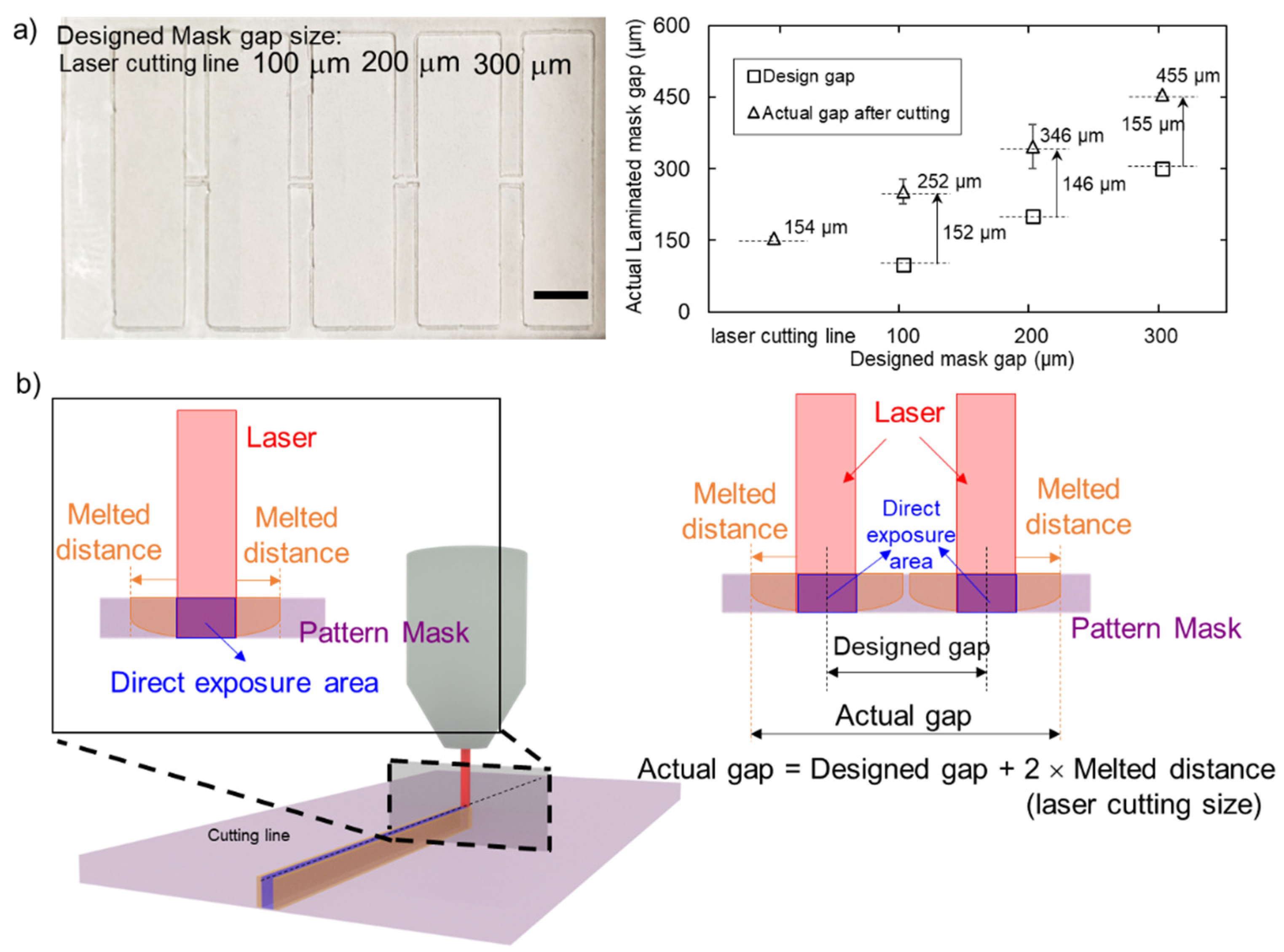
3.2. Hydrophobic Barrier Resolution
3.3. Physical and Biochemical Properties of the Paper
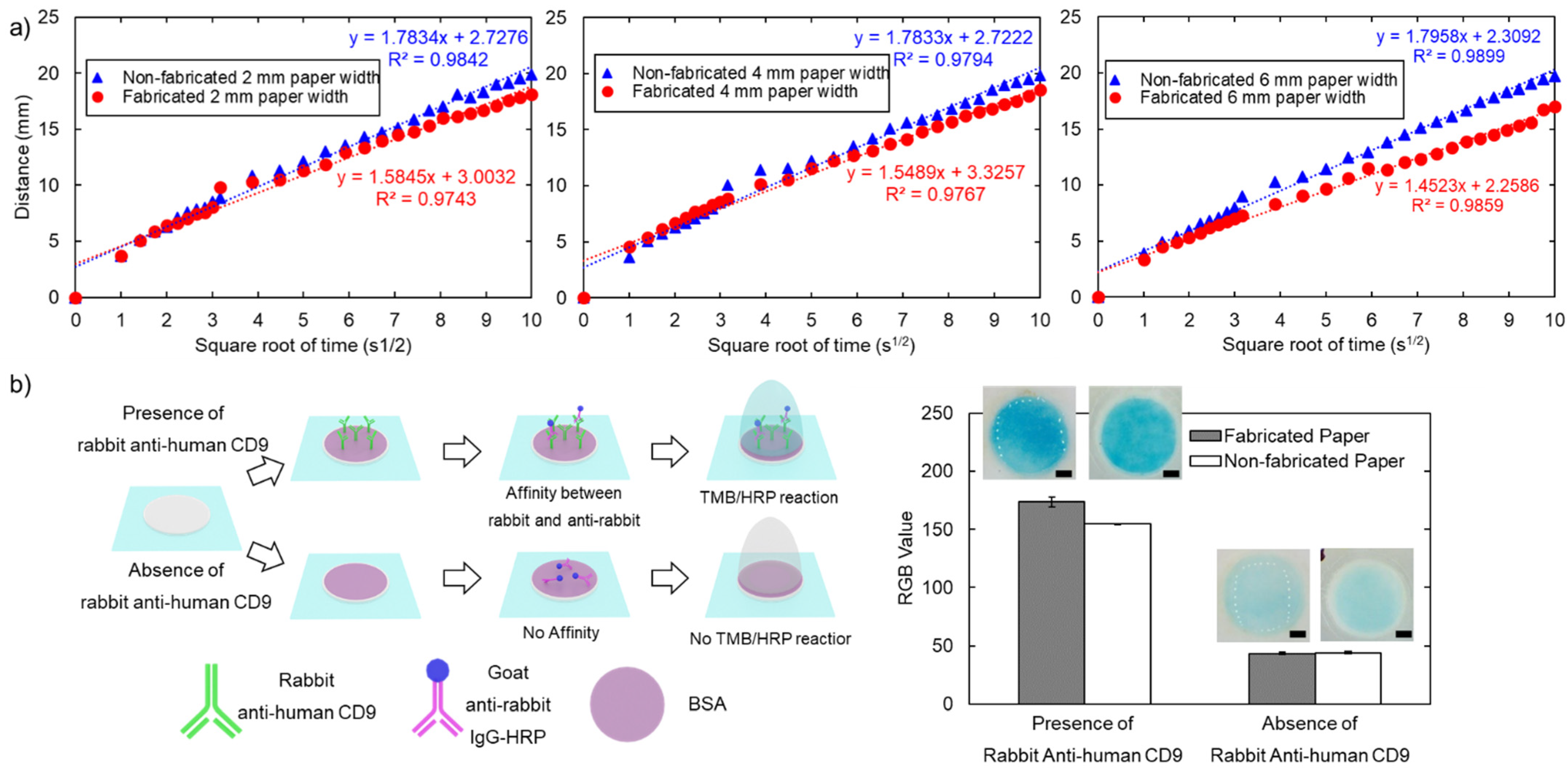
3.3.1. Flowing Characteristics
3.3.2. Colorimetric Sandwich Immunological Assay
3.4. 2D and 3D Paperfluidic Applications
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M.; Carrilho, E. Diagnostics for the Developing World: Microfluidic Paper-Based Analytical Devices. Anal. Chem. 2010, 82, 3–10. [Google Scholar] [CrossRef]
- Cate, D.M.; Adkins, J.A.; Mettakoonpitak, J.; Henry, C.S. Recent Developments in Paper-Based Microfluidic Devices. Anal. Chem. 2015, 87, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Yetisen, A.K.; Akram, M.S.; Lowe, C.R. Paper-based microfluidic point-of-care diagnostic devices. Lab Chip 2013, 13, 2210–2251. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M. Three-dimensional microfluidic devices fabricated in layered paper and tape. Proc. Natl. Acad. Sci. USA 2008, 105, 19606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akyazi, T.; Basabe-Desmonts, L.; Benito-Lopez, F. Review on microfluidic paper-based analytical devices towards commercialisation. Anal. Chim. Acta 2018, 1001, 1–17. [Google Scholar] [CrossRef]
- Lisowski, P.; Zarzycki, P.K. Microfluidic Paper-Based Analytical Devices (μPADs) and Micro Total Analysis Systems (μTAS): Development, Applications and Future Trends. Chromatographia 2013, 76, 1201–1214. [Google Scholar] [CrossRef] [Green Version]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned Paper as a Platform for Inexpensive, Low-Volume, Portable Bioassays. Angew. Chem. Int. Ed. 2007, 46, 1318–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, A.W.; Phillips, S.T.; Wiley, B.J.; Gupta, M.; Whitesides, G.M. FLASH: A rapid method for prototyping paper-based microfluidic devices. Lab Chip 2008, 8, 2146–2150. [Google Scholar] [CrossRef] [PubMed]
- Bruzewicz, D.A.; Reches, M.; Whitesides, G.M. Low-Cost Printing of Poly(dimethylsiloxane) Barriers To Define Microchannels in Paper. Anal. Chem. 2008, 80, 3387–3392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Shi, W.; Jiang, L.; Qin, J.; Lin, B. Rapid prototyping of paper-based microfluidics with wax for low-cost, portable bioassay. Electrophoresis 2009, 30, 1497–1500. [Google Scholar] [CrossRef]
- Li, X.; Tian, J.; Garnier, G.; Shen, W. Fabrication of paper-based microfluidic sensors by printing. Colloids Surf. B Biointerfaces 2010, 76, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Olkkonen, J.; Lehtinen, K.; Erho, T. Flexographically Printed Fluidic Structures in Paper. Anal. Chem. 2010, 82, 10246–10250. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-Y.; Cheng, C.-M.; Liao, Y.-C. Screen Printed Paper-based Diagnostic Devices with Polymeric Inks. Anal. Sci. 2015, 31, 145–151. [Google Scholar] [CrossRef] [Green Version]
- Songjaroen, T.; Dungchai, W.; Chailapakul, O.; Laiwattanapaisal, W. Novel, simple and low-cost alternative method for fabrication of paper-based microfluidics by wax dipping. Talanta 2011, 85, 2587–2593. [Google Scholar] [CrossRef] [PubMed]
- Juang, Y.-J.; Wang, Y.; Hsu, S.-K. One-Step Hot Microembossing for Fabrication of Paper-Based Microfluidic Chips in 10 Seconds. Polymers 2020, 12, 2493. [Google Scholar] [CrossRef] [PubMed]
- Sitanurak, J.; Fukana, N.; Wongpakdee, T.; Thepchuay, Y.; Ratanawimarnwong, N.; Amornsakchai, T.; Nacapricha, D. T-shirt ink for one-step screen-printing of hydrophobic barriers for 2D- and 3D-microfluidic paper-based analytical devices. Talanta 2019, 205, 120113. [Google Scholar] [CrossRef]
- Sousa, L.R.; Duarte, L.C.; Coltro, W.K.T. Instrument-free fabrication of microfluidic paper-based analytical devices through 3D pen drawing. Sens. Actuators B Chem. 2020, 312, 128018. [Google Scholar] [CrossRef]
- Abe, K.; Suzuki, K.; Citterio, D. Inkjet-Printed Microfluidic Multianalyte Chemical Sensing Paper. Anal. Chem. 2008, 80, 6928–6934. [Google Scholar] [CrossRef]
- Li, X.; Tian, J.; Nguyen, T.; Shen, W. Paper-Based Microfluidic Devices by Plasma Treatment. Anal. Chem. 2008, 80, 9131–9134. [Google Scholar] [CrossRef]
- Cai, L.; Xu, C.; Lin, S.; Luo, J.; Wu, M.; Yang, F. A simple paper-based sensor fabricated by selective wet etching of silanized filter paper using a paper mask. Biomicrofluidics 2014, 8, 056504. [Google Scholar] [CrossRef]
- Chitnis, G.; Ding, Z.; Chang, C.-L.; Savran, C.A.; Ziaie, B. Laser-treated hydrophobic paper: An inexpensive microfluidic platform. Lab Chip 2011, 11, 1161–1165. [Google Scholar] [CrossRef]
- Kim, Y.S.; Yang, Y.; Henry, C.S. Laminated and infused Parafilm®-paper for paper-based analytical devices. Sens. Actuators B Chem. 2018, 255, 3654–3661. [Google Scholar] [CrossRef]
- Xia, Y.; Si, J.; Li, Z. Fabrication techniques for microfluidic paper-based analytical devices and their applications for biological testing: A review. Biosens. Bioelectron. 2016, 77, 774–789. [Google Scholar] [CrossRef]
- Lim, H.; Jafry, A.T.; Lee, J. Fabrication, Flow Control, and Applications of Microfluidic Paper-Based Analytical Devices. Molecules 2019, 24, 2869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishat, S.; Jafry, A.T.; Martinez, A.W.; Awan, F.R. Paper-based microfluidics: Simplified fabrication and assay methods. Sens. Actuators B Chem. 2021, 336, 129681. [Google Scholar] [CrossRef]
- Dunfield, E.M.; Wu, Y.Y.; Remcho, T.P.; Koesdjojo, M.T.; Remcho, V.T. Simple and Rapid Fabrication of Paper Microfluidic Devices Utilizing Parafilm®. Chips Tips. 2012. Available online: https://blogs.rsc.org/chipsandtips/2012/04/10/simple-and-rapid-fabrication-of-paper-microfluidic-devices-utilizing-parafilm%C2%AE/?doing_wp_cron=1635550211.3899741172790527343750 (accessed on 1 December 2021).
- Yu, L.; Shi, Z.Z. Microfluidic paper-based analytical devices fabricated by low-cost photolithography and embossing of Parafilm®. Lab Chip 2015, 15, 1642–1645. [Google Scholar] [CrossRef] [PubMed]
- Kasetsirikul, S.; Shiddiky, M.J.A.; Nguyen, N.-T. Wicking in Paper Strips under Consideration of Liquid Absorption Capacity. Chemosensors 2020, 8, 65. [Google Scholar] [CrossRef]
- Kasetsirikul, S.; Umer, M.; Soda, N.; Sreejith, K.R.; Shiddiky, M.J.A.; Nguyen, N.-T. Detection of the SARS-CoV-2 humanized antibody with paper-based ELISA. Analyst 2020, 145, 7680–7686. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Gomez, F.A. Experimental Analysis of Fabrication Parameters in the Development of Microfluidic Paper-Based Analytical Devices (µPADs). Micromachines 2017, 8, 99. [Google Scholar] [CrossRef] [Green Version]
- Washburn, E.W. The Dynamics of Capillary Flow. Phys. Rev. 1921, 17, 273–283. [Google Scholar] [CrossRef]
- Shin, J.H.; Park, J.; Kim, S.H.; Park, J.-K. Programmed sample delivery on a pressurized paper. Biomicrofluidics 2014, 8, 054121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Shin, J.H.; Park, J.-K. Pressed Paper-Based Dipstick for Detection of Foodborne Pathogens with Multistep Reactions. Anal. Chem. 2016, 88, 3781–3788. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Ma, Y.; Zhang, A.; Zhang, L.; Zeng, L.; Liu, G. Disposable Nucleic Acid Biosensors Based on Gold Nanoparticle Probes and Lateral Flow Strip. Anal. Chem. 2009, 81, 1660–1668. [Google Scholar] [CrossRef]
- Fenton, E.M.; Mascarenas, M.R.; López, G.P.; Sibbett, S.S. Multiplex Lateral-Flow Test Strips Fabricated by Two-Dimensional Shaping. ACS Appl. Mater. Interfaces 2009, 1, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Henares, T.G.; Suzuki, K.; Citterio, D. Paper-Based Inkjet-Printed Microfluidic Analytical Devices. Angew. Chem. Int. Ed. 2015, 54, 5294–5310. [Google Scholar] [CrossRef]
- Xu, C.; Cai, L.; Zhong, M.; Zheng, S. Low-cost and rapid prototyping of microfluidic paper-based analytical devices by inkjet printing of permanent marker ink. RSC Adv. 2015, 5, 4770–4773. [Google Scholar] [CrossRef]
- Songjaroen, T.; Dungchai, W.; Chailapakul, O.; Henry, C.S.; Laiwattanapaisal, W. Blood separation on microfluidic paper-based analytical devices. Lab Chip 2012, 12, 3392–3398. [Google Scholar] [CrossRef]
- Dungchai, W.; Chailapakul, O.; Henry, C.S. A low-cost, simple, and rapid fabrication method for paper-based microfluidics using wax screen-printing. Analyst 2011, 136, 77–82. [Google Scholar] [CrossRef]
- Haller, P.D.; Flowers, C.A.; Gupta, M. Three-dimensional patterning of porous materials using vapor phase polymerization. Soft Matter 2011, 7, 2428–2432. [Google Scholar] [CrossRef]
- Kwong, P.; Gupta, M. Vapor Phase Deposition of Functional Polymers onto Paper-Based Microfluidic Devices for Advanced Unit Operations. Anal. Chem. 2012, 84, 10129–10135. [Google Scholar] [CrossRef]
- Curto, V.F.; Lopez-Ruiz, N.; Capitan-Vallvey, L.F.; Palma, A.J.; Benito-Lopez, F.; Diamond, D. Fast prototyping of paper-based microfluidic devices by contact stamping using indelible ink. RSC Adv. 2013, 3, 18811–18816. [Google Scholar] [CrossRef]
- de Tarso Garcia, P.; Garcia Cardoso, T.M.; Garcia, C.D.; Carrilho, E.; Tomazelli Coltro, W.K. A handheld stamping process to fabricate microfluidic paper-based analytical devices with chemically modified surface for clinical assays. RSC Adv. 2014, 4, 37637–37644. [Google Scholar] [CrossRef]
- He, Y.; Gao, Q.; Wu, W.-B.; Nie, J.; Fu, J.-Z. 3D Printed Paper-Based Microfluidic Analytical Devices. Micromachines 2016, 7, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Wu, W.-B.; Fu, J.-Z. Rapid fabrication of paper-based microfluidic analytical devices with desktop stereolithography 3D printer. RSC Adv. 2015, 5, 2694–2701. [Google Scholar] [CrossRef]
- Cardoso, T.M.G.; de Souza, F.R.; Garcia, P.T.; Rabelo, D.; Henry, C.S.; Coltro, W.K.T. Versatile fabrication of paper-based microfluidic devices with high chemical resistance using scholar glue and magnetic masks. Anal. Chim. Acta 2017, 974, 63–68. [Google Scholar] [CrossRef]
- Liu, N.; Xu, J.; An, H.-J.; Phan, D.-T.; Hashimoto, M.; Lew, W.S. Direct spraying method for fabrication of paper-based microfluidic devices. J. Micromech. Microeng. 2017, 27, 104001. [Google Scholar] [CrossRef] [Green Version]

| Fabrication Process | Operation Time | Hydrophobic Barrier | Advantages | Disadvantages | Ref. |
| Photolithography | 40 min | ~250 μm | Variety of patterns | Exposed to polymers and solvent, Expensive equipment and reagents | [4,7] |
| Plotting | 1 h | ~250 μm | Low-cost consumables, not exposed to harsh chemicals | Need customized plotter, Inconsistent control of hydrophobic barrier formation | [5,9] |
| Cutting | 1–3 min | 700 μm | Not exposed to harsh chemicals | Need hydrophobic substrates or cases to operate | [35] |
| Plasma Etching | 1 h | <1500 μm | Low-cost consumables | Exposed to polymers and solvent, single-use mask | [19] |
| Wet etching | ~3 h | <1000 μm | Low-cost consumables | Exposed to harsh chemicals | [20] |
| Laser etching | 2 m/s (depending on the pattern) | 600 μm | Selective modification | Require strong hydrophobic reagents | [21] |
| Inkjet etching | ~2 h 30 m | >150 μm | Precise controlled location | Exposed to polymers and solvents, require customized printers | [18] |
| Inkjet printing | 5–15 min | 300–550 μm | Low-cost thermal inkjet printers | Require formulated ink and customized printers | [11,36] |
| Flexography printing | 5–10 s | 1000 μm | Well suited for mass production | Require expensive and modified equipment | [12] |
| Wax printing | 5–10 min | 100 μm | Rapid, simple process, mass production | Require customized printer, extra heating steps, and rough channel edge. | [11,37] |
| Wax dipping | <1 min | Depend on iron mold | Low-cost and simple process | Batch-to-batch variation | [14,38] |
| Wax screen printing | <5 min | 500–1300 μm | Low-cost and simple process (0.3 USD/100 cm2) | Require patterning mesh, low resolution | [13,39] |
| Vapor deposition | ~1 h 30 m | 2500–3500 μm | Complex patterns | Require expensive equipment | [40,41] |
| Stamping | <1 min | >950 μm (Depend on stamp) | Low-cost and simple process | Batch-to-batch variation, resolution depends on the stamp | [42,43] |
| 3D printing | ~2 h | 400–500 μm | Variety of patterns | Resolution on 3D printers and printing materials | [44,45] |
| Spraying | <5 min | <1000 μm (Depend on masks) | Rapid and simple process | Non-uniformity on spraying | [46,47] |
| Lithography and embossing | ~40 m | ~150 μm | High resolution | Unsuitable for mass production | [10] |
| Parafilm Hot pressing | <5 min | >800 μm (Depend on masks) | Low cost (0.3 USD/100 cm2 paper), rapid, and simple process | Low resolution depending on the mask resolution |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasetsirikul, S.; Clack, K.; Shiddiky, M.J.A.; Nguyen, N.-T. Rapid, Simple and Inexpensive Fabrication of Paper-Based Analytical Devices by Parafilm® Hot Pressing. Micromachines 2022, 13, 48. https://doi.org/10.3390/mi13010048
Kasetsirikul S, Clack K, Shiddiky MJA, Nguyen N-T. Rapid, Simple and Inexpensive Fabrication of Paper-Based Analytical Devices by Parafilm® Hot Pressing. Micromachines. 2022; 13(1):48. https://doi.org/10.3390/mi13010048
Chicago/Turabian StyleKasetsirikul, Surasak, Kimberley Clack, Muhammad J. A. Shiddiky, and Nam-Trung Nguyen. 2022. "Rapid, Simple and Inexpensive Fabrication of Paper-Based Analytical Devices by Parafilm® Hot Pressing" Micromachines 13, no. 1: 48. https://doi.org/10.3390/mi13010048
APA StyleKasetsirikul, S., Clack, K., Shiddiky, M. J. A., & Nguyen, N.-T. (2022). Rapid, Simple and Inexpensive Fabrication of Paper-Based Analytical Devices by Parafilm® Hot Pressing. Micromachines, 13(1), 48. https://doi.org/10.3390/mi13010048









