Efficient Decellularization by Application of Moderate High Hydrostatic Pressure with Supercooling Pretreatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. NB1RGB Cell Culture
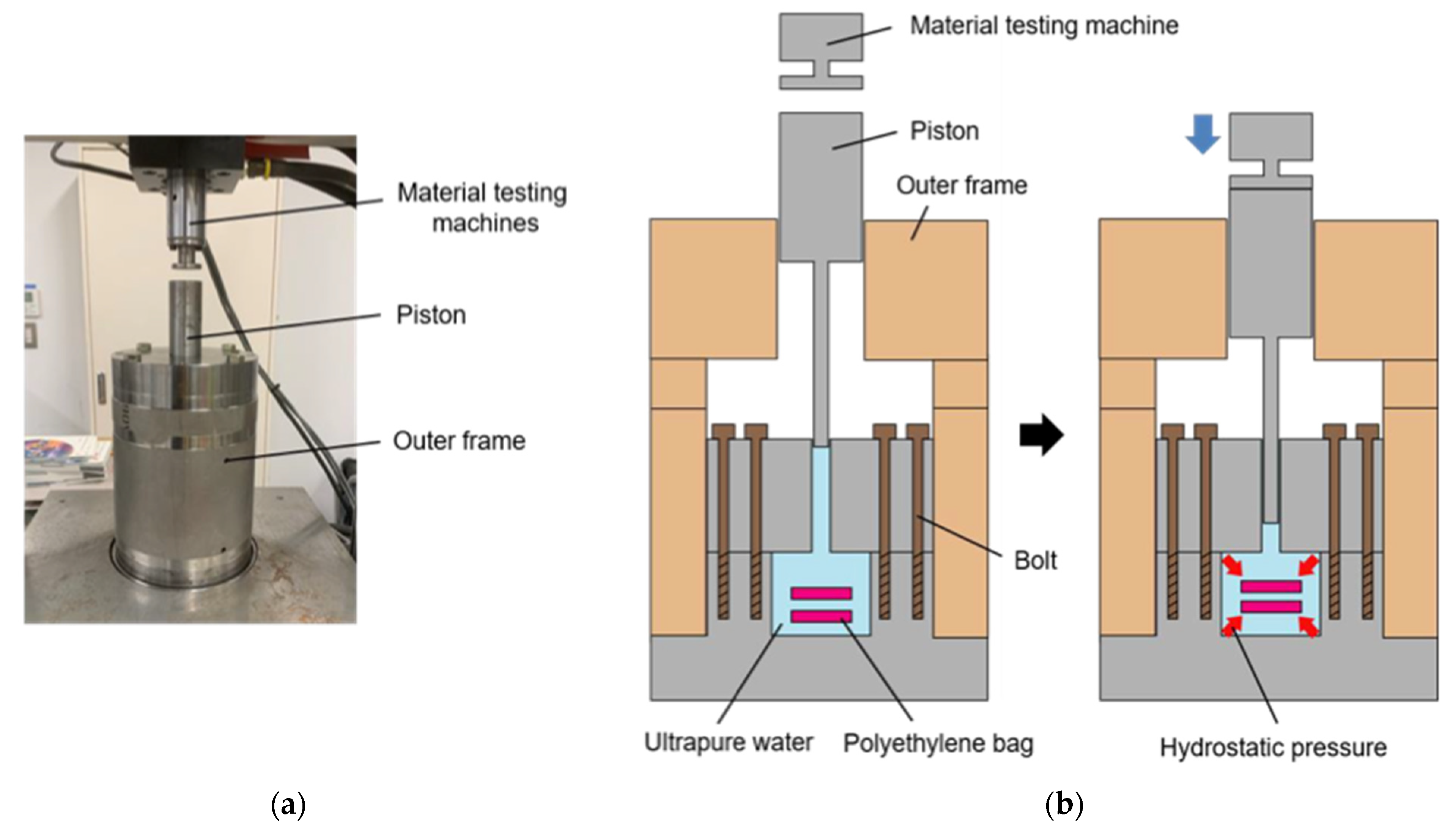
2.2. Supercooling Device for Pretreatment of Cell Suspension
2.3. High Hydrostatic Pressure Application with Supercooling Pretreatment to NB1RGB Cell Suspension
2.4. High Hydrostatic Pressure Application with Supercooling Pretreatment to Collagen Gel Scaffold
2.5. Statistical Analysis
3. Results
3.1. Effect of Single HHP Exposure with Supercooling Pretreatment on Cell Suspension
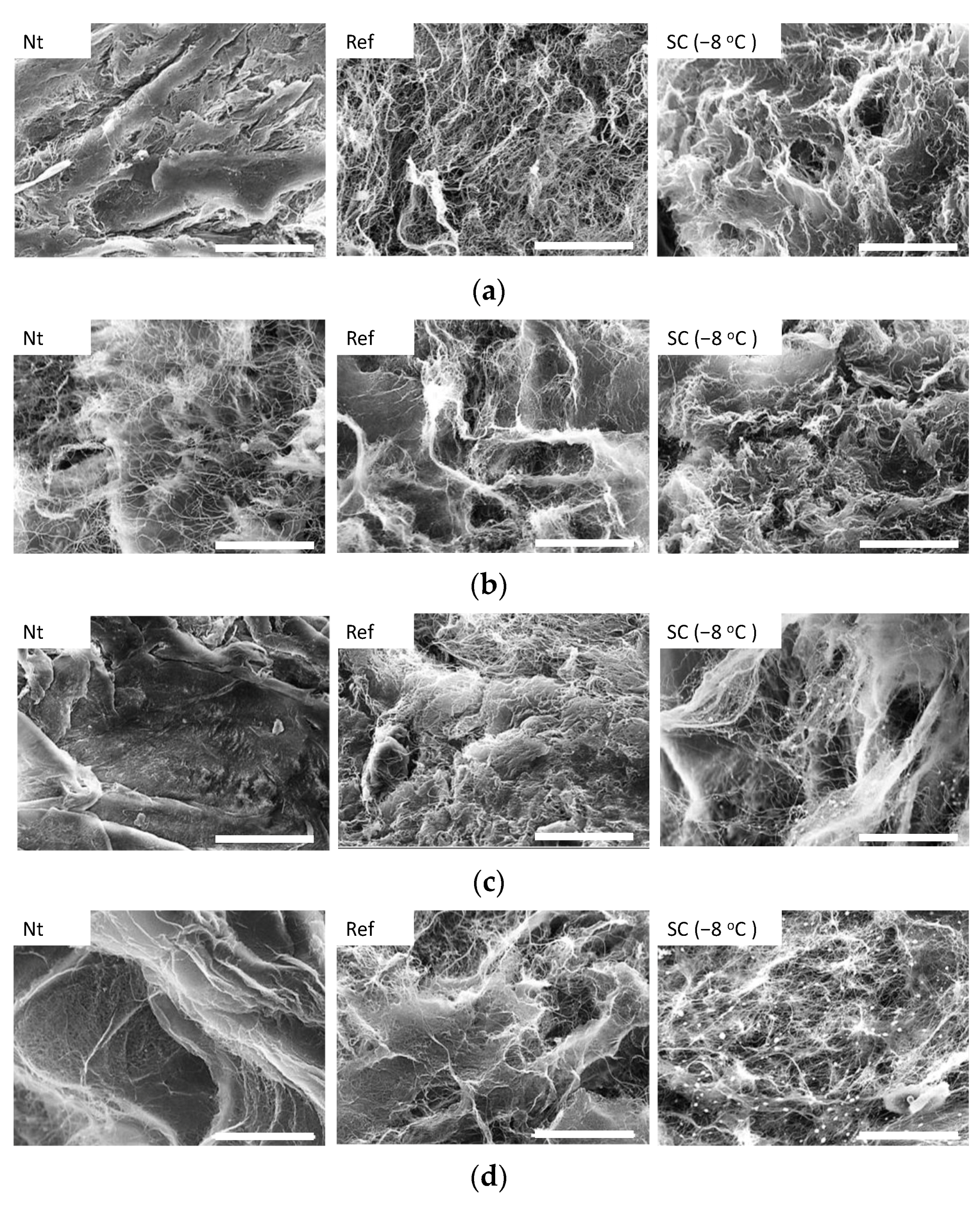
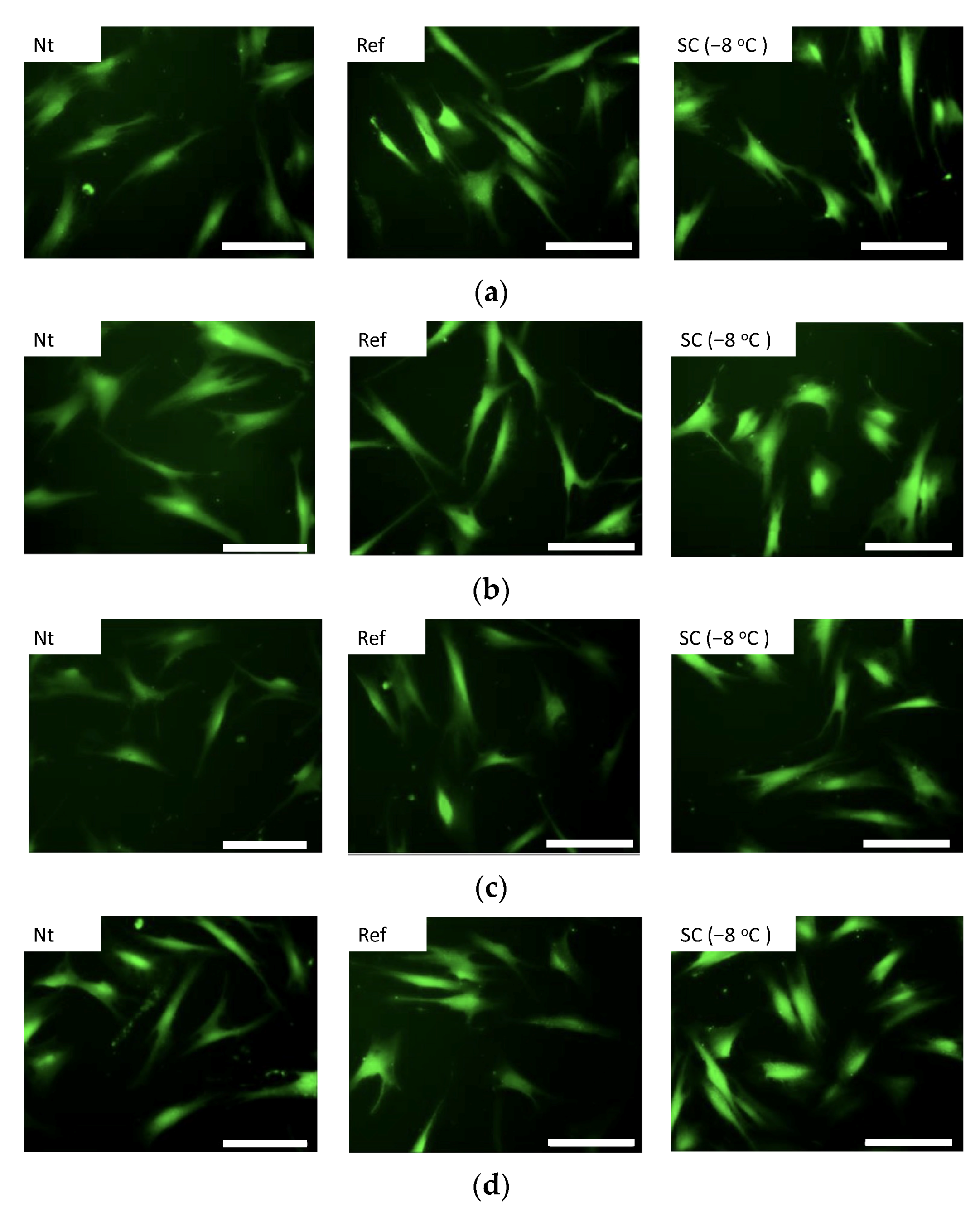
3.2. Effect of Static HHP Exposure with Supercooling Pretreatment on Micro Structure of Collagen Gel
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ikada, Y. Challenges in tissue engineering. J. R Soc. Interface 2006, 3, 589–601. [Google Scholar] [CrossRef]
- Wolff, P.; Heimann, L.; Liebsch, G.; Meier, R.J.; Gutbrod, M.; van Griensven, M.; Balmayor, E.R. Oxygen-distribution within 3-D collagen I hydrogels for bone tissue engineering. Mater. Sci. Eng. C 2019, 95, 422–427. [Google Scholar] [CrossRef]
- Liu, L.; Yang, B.; Wang, L.Q.; Huang, J.P.; Chen, W.Y.; Ban, Q.; Zhang, Y.; You, R.; Yin, L.; Guan, Y.Q. Biomimetic bone tissue engineering hydrogel scaffolds constructed using ordered CNTs and HA induce the proliferation and differentiation of BMSCs. J. Mater. Chem. B 2020, 8, 558–567. [Google Scholar] [CrossRef]
- Gao, X.; Gao, L.; Groth, T.; Liu, T.; He, D.; Wang, M.; Gong, F.; Chu, J.; Zhao, M. Fabrication and properties of an injectable sodium alginate/PRP composite hydrogel as a potential cell carrier for cartilage repair. J. Biomed. Mater. Res.-Part A 2019, 107, 2076–2087. [Google Scholar] [CrossRef]
- Haghjooy Javanmard, S.; Anari, J.; Zargar Kharazi, A.; Vatankhah, E. In vitro hemocompatibility and cytocompatibility of a three-layered vascular scaffold fabricated by sequential electrospinning of PCL, collagen, and PLLA nanofibers. J. Biomater. Appl. 2016, 31, 438–449. [Google Scholar] [CrossRef]
- Bahcecioglu, G.; Hasirci, N.; Hasirci, V. Cell behavior on the alginate-coated PLLA/PLGA scaffolds. Int. J. Biol. Macromol. 2019, 124, 444–450. [Google Scholar] [CrossRef]
- Wang, L.; Dormer, N.H.; Bonewald, L.F.; Detamore, M.S. Osteogenic differentiation of human umbilical cord mesenchymal stromal cells in polyglycolic acid scaffolds. Tissue Eng.-Part A 2010, 16, 1937–1948. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Huang, D.; Yan, Z.; Wang, C. Heparin/collagen encapsulating nerve growth factor multilayers coated aligned PLLA nanofibrous scaffolds for nerve tissue engineering. J. Biomed. Mater. Res.-Part A 2017, 105, 1900–1910. [Google Scholar] [CrossRef]
- Park, K.S.; Kim, B.J.; Lih, E.; Park, W.; Lee, S.H.; Joung, Y.K.; Han, D.K. Versatile effects of magnesium hydroxide nanoparticles in PLGA scaffold–mediated chondrogenesis. Acta Biomater. 2018, 73, 204–216. [Google Scholar] [CrossRef]
- Nasr, S.M.; Rabiee, N.; Hajebi, S.; Ahmadi, S.; Fatahi, Y.; Hosseini, M.; Bagherzadeh, M.; Ghadiri, A.M.; Rabiee, M.; Jajarmi, V.; et al. Biodegradable nanopolymers in cardiac tissue engineering: From concept towards nanomedicine. Int. J. Nanomed. 2020, 15, 4205–4224. [Google Scholar] [CrossRef]
- Porzionato, A.; Stocco, E.; Barbon, S.; Grandi, F.; Macchi, V.; De Caro, R. Tissue-engineered grafts from human decellularized extracellular matrices: A systematic review and future perspectives. Int. J. Mol. Sci. 2018, 19, 4117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Du, A.; Liu, S.; Lv, M.; Chen, S. Research progress in decellularized extracellular matrix-derived hydrogels. Regen. Ther. 2021, 18, 88–96. [Google Scholar] [CrossRef]
- Hoganson, D.M.; O’Doherty, E.M.; Owens, G.E.; Harilal, D.O.; Goldman, S.M.; Bowley, C.M.; Neville, C.M.; Kronengold, R.T.; Vacanti, J.P. The retention of extracellular matrix proteins and angiogenic and mitogenic cytokines in a decellularized porcine dermis. Biomaterials 2010, 31, 6730–6737. [Google Scholar] [CrossRef]
- Wüthrich, T.; Lese, I.; Haberthür, D.; Zubler, C.; Hlushchuk, R.; Hewer, E.; Maistriaux, L.; Gianello, P.; Lengelé, B.; Rieben, R.; et al. Development of vascularized nerve scaffold using perfusion-decellularization and recellularization. Mater. Sci. Eng. C 2020, 117, 111311. [Google Scholar] [CrossRef]
- Milan, P.B.; Amini, N.; Joghataei, M.T.; Ebrahimi, L.; Amoupour, M.; Sarveazad, A.; Kargozar, S.; Mozafari, M. Decellularized human amniotic membrane: From animal models to clinical trials. Methods 2020, 171, 11–19. [Google Scholar] [CrossRef]
- Rossi, E.A.; Quintanilha, L.F.; Nonaka, C.K.V.; De Freitas Souza, B.S. Advances in hepatic tissue bioengineering with decellularized liver bioscaffold. Stem Cells Int. 2019, 2019, 2693189. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Majid, M.; Melchiorri, A.J.; Mikos, A.G. Applications of decellularized extracellular matrix in bone and cartilage tissue engineering. Bioeng. Transl. Med. 2019, 4, 83–95. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.; Berning, K.; Tang, S.W.; Lam, Y.W. Rapid and detergent-free decellularization of cartilage. Tissue Eng.-Part C Methods 2020, 26, 201–206. [Google Scholar] [CrossRef]
- Ventura, R.D.; Padalhin, A.R.; Park, C.M.; Lee, B.T. Enhanced decellularization technique of porcine dermal ECM for tissue engineering applications. Mater. Sci. Eng. C 2019, 104, 109841. [Google Scholar] [CrossRef]
- Gilpin, A.; Yang, Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. Biomed. Res. Int. 2017, 2017, 9831534. [Google Scholar] [CrossRef] [Green Version]
- Burk, J.; Erbe, I.; Berner, D.; Kacza, J.; Kasper, C.; Pfeiffer, B.; Winter, K.; Brehm, W. Freeze-Thaw cycles enhance decellularization of large tendons. Tissue Eng.-Part C Methods 2014, 20, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Waxman, S.; Wang, C.; Jensen, A.; Loewen, R.T.; Bilonick, R.A.; Loewen, N.A. Freeze-thaw decellularization of the trabecular meshwork in an ex vivo eye perfusion model. PeerJ 2017, 5, e3629. [Google Scholar] [CrossRef] [Green Version]
- Roth, S.P.; Glauche, S.M.; Plenge, A.; Erbe, I.; Heller, S.; Burk, J. Automated freeze-thaw cycles for decellularization of tendon tissue—A pilot study. BMC Biotechnol. 2017, 17, 13. [Google Scholar] [CrossRef] [Green Version]
- Zager, Y.; Kain, D.; Landa, N.; Leor, J.; Maor, E. Optimization of irreversible electroporation protocols for in-vivo myocardial decellularization. PLoS ONE 2016, 11, e0165475. [Google Scholar] [CrossRef] [Green Version]
- Baah-Dwomoh, A.; Rolong, A.; Gatenholm, P.; Davalos, R.V. The feasibility of using irreversible electroporation to introduce pores in bacterial cellulose scaffolds for tissue engineering. Appl. Microbiol. Biotechnol. 2015, 99, 4785–4794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golberg, A.; Bruinsma, B.G.; Jaramillo, M.; Yarmush, M.L.; Uygun, B.E. Rat liver regeneration following ablation with irreversible electroporation. PeerJ 2016, 4, e1571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negishi, J.; Funamoto, S.; Kimura, T.; Nam, K.; Higami, T.; Kishida, A. Porcine radial artery decellularization by high hydrostatic pressure. J. Tissue Eng. Regen. Med. 2015, 9, E144–E151. [Google Scholar] [CrossRef]
- Funamoto, S.; Nam, K.; Kimura, T.; Murakoshi, A.; Hashimoto, Y.; Niwaya, K.; Kitamura, S.; Fujisato, T.; Kishida, A. The use of high-hydrostatic pressure treatment to decellularize blood vessels. Biomaterials 2010, 31, 3590–3595. [Google Scholar] [CrossRef]
- Le, T.M.; Morimoto, N.; Ly, N.T.M.; Mitsui, T.; Notodihardjo, S.C.; Munisso, M.C.; Kakudo, N.; Moriyama, H.; Yamaoka, T.; Kusumoto, K. Hydrostatic pressure can induce apoptosis of the skin. Sci. Rep. 2020, 10, 17594. [Google Scholar] [CrossRef]
- Potekhin, S.A.; Senin, A.A.; Abdurakhmanov, N.N.; Tiktopulo, E.I. High pressure stabilization of collagen structure. Biochim. Biophys. Acta-Proteins Proteom. 2009, 1794, 1151–1158. [Google Scholar] [CrossRef]
- Zemmyo, D.; Yamamoto, M.; Miyata, S. Fundamental study of decellularization method using cyclic application of high hydrostatic pressure. Micromachines 2020, 11, 1008. [Google Scholar] [CrossRef] [PubMed]
- Zemmyo, D.; Sudo, K.; Miyata, S. Supercool preservation of cell suspension under cryoprotectant-free culture medium. Cryobiol. Cryotech. 2021, 67, 31–40. [Google Scholar]
- Kim, Y.J.; Sah, R.L.Y.; Doong, J.Y.H.; Grodzinsky, A.J. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal. Biochem. 1988, 174, 168–176. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Miyata, S. Dielectrophoretic micro-organization of chondrocytes to regenerate mechanically anisotropic cartilaginous tissue. Micromachines 2021, 12, 1098. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S.; Tateishi, T.; Ushida, T. Influence of cartilaginous matrix accumulation on viscoelastic response of chondrocyte/agarose constructs under dynamic compressive and shear loading. J. Biomech. Eng. 2008, 130, 051016. [Google Scholar] [CrossRef]
- Frey, B.; Janko, C.; Ebel, N.; Meister, S.; Schlucker, E.; Meyer-Pittroff, R.; Fietkau, R.; Herrmann, M.; Gaipl, U. Cells Under Pressure—Treatment of Eukaryotic Cells with High Hydrostatic Pressure, from Physiologic Aspects to Pressure Induced Cell Death. Curr. Med. Chem. 2008, 15, 2329–2336. [Google Scholar] [CrossRef]
- Diehl, P.; Schmitt, M.; Blümelhuber, G.; Frey, B.; Van Laak, S.; Fischer, S.; Muehlenweg, B.; Meyer-Pittroff, R.; Gollwitzer, H.; Mittelmeier, W. Induction of tumor cell death by high hydrostatic pressure as a novel supporting technique in orthopedic surgery. Oncol. Rep. 2003, 10, 1851–1855. [Google Scholar] [CrossRef]
- Nan, J.; Zou, M.; Wang, H.; Xu, C.; Zhang, J.; Wei, B.; He, L.; Xu, Y. Effect of ultra-high pressure on molecular structure and properties of bullfrog skin collagen. Int. J. Biol. Macromol. 2018, 111, 200–207. [Google Scholar] [CrossRef]



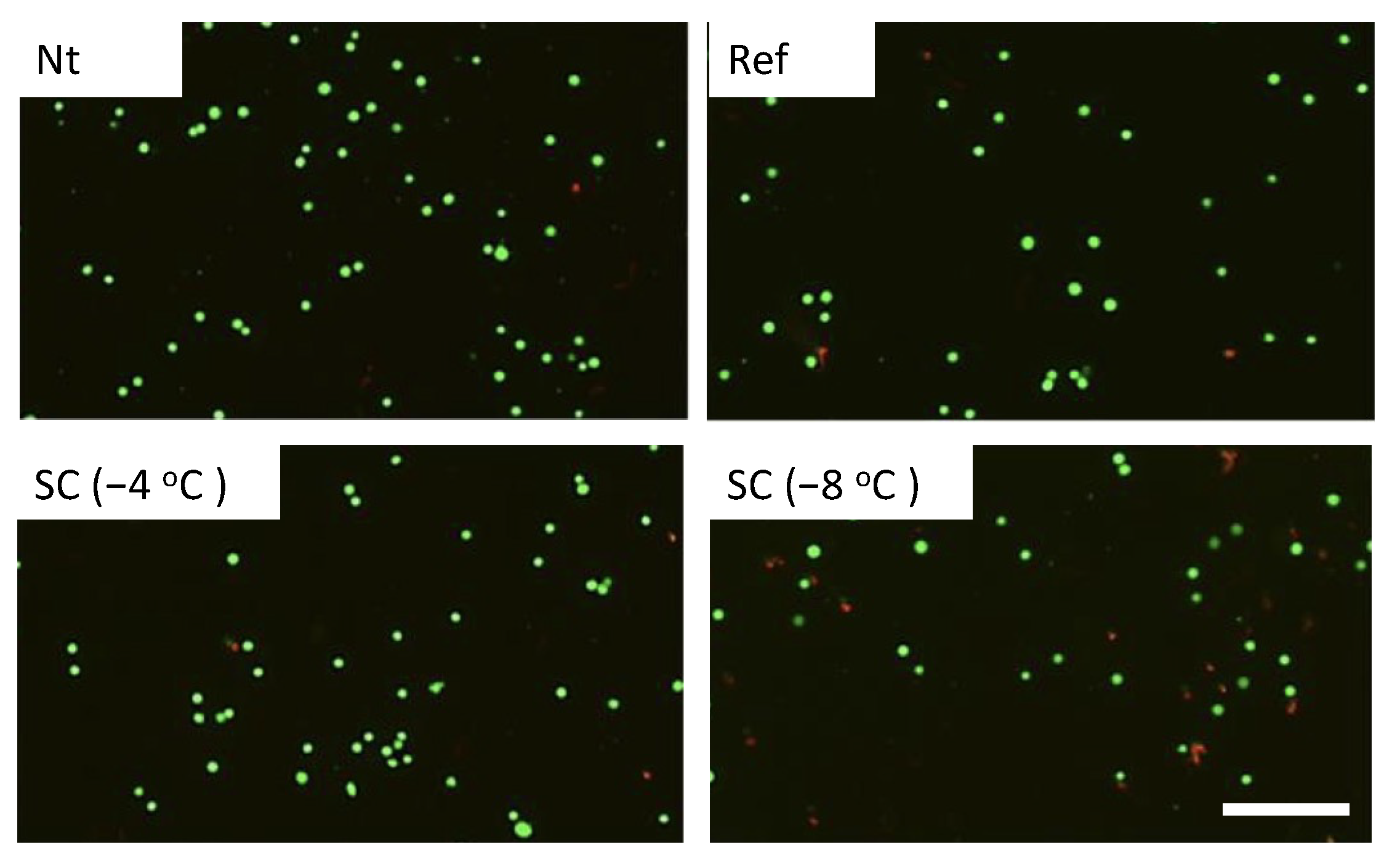
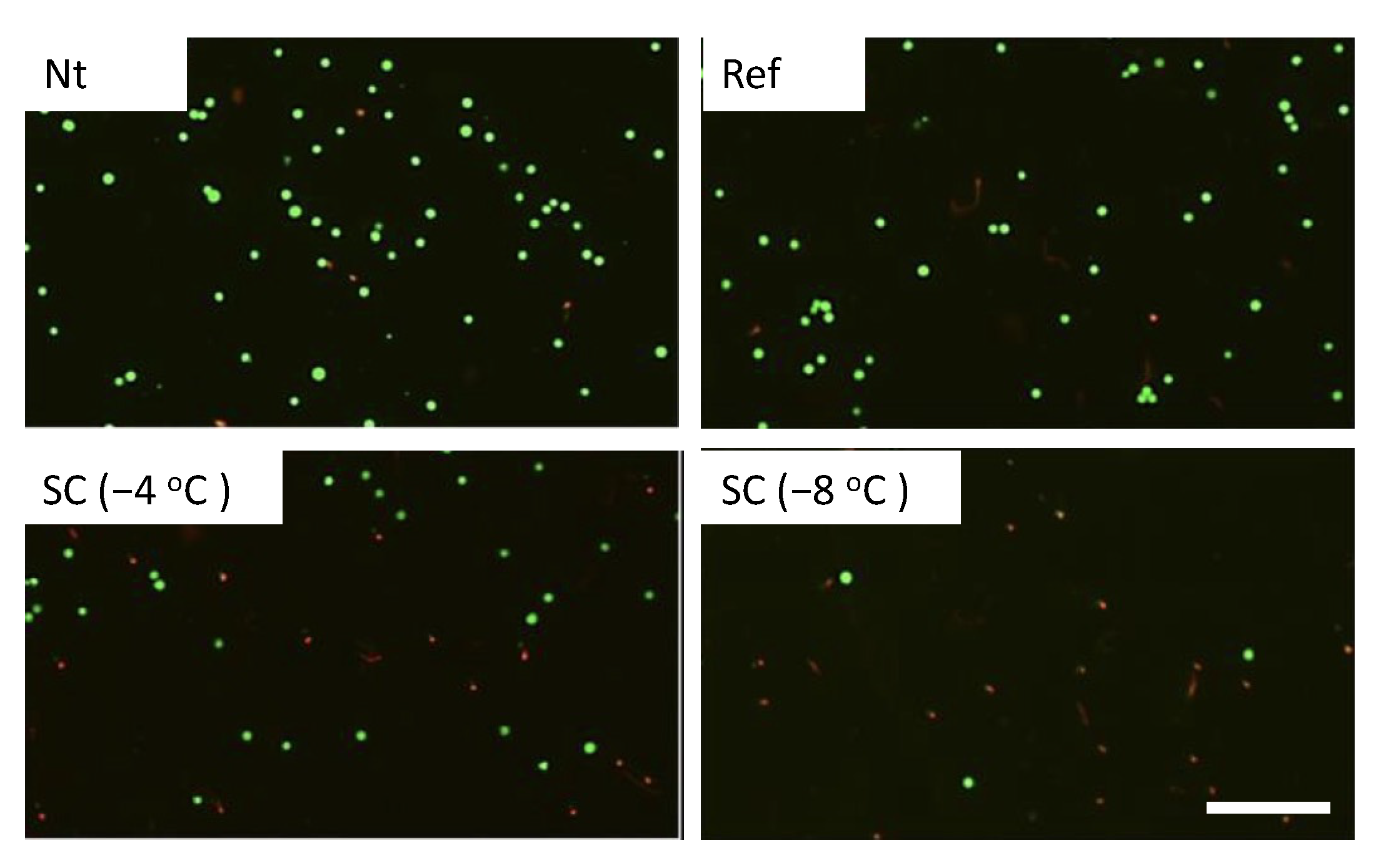
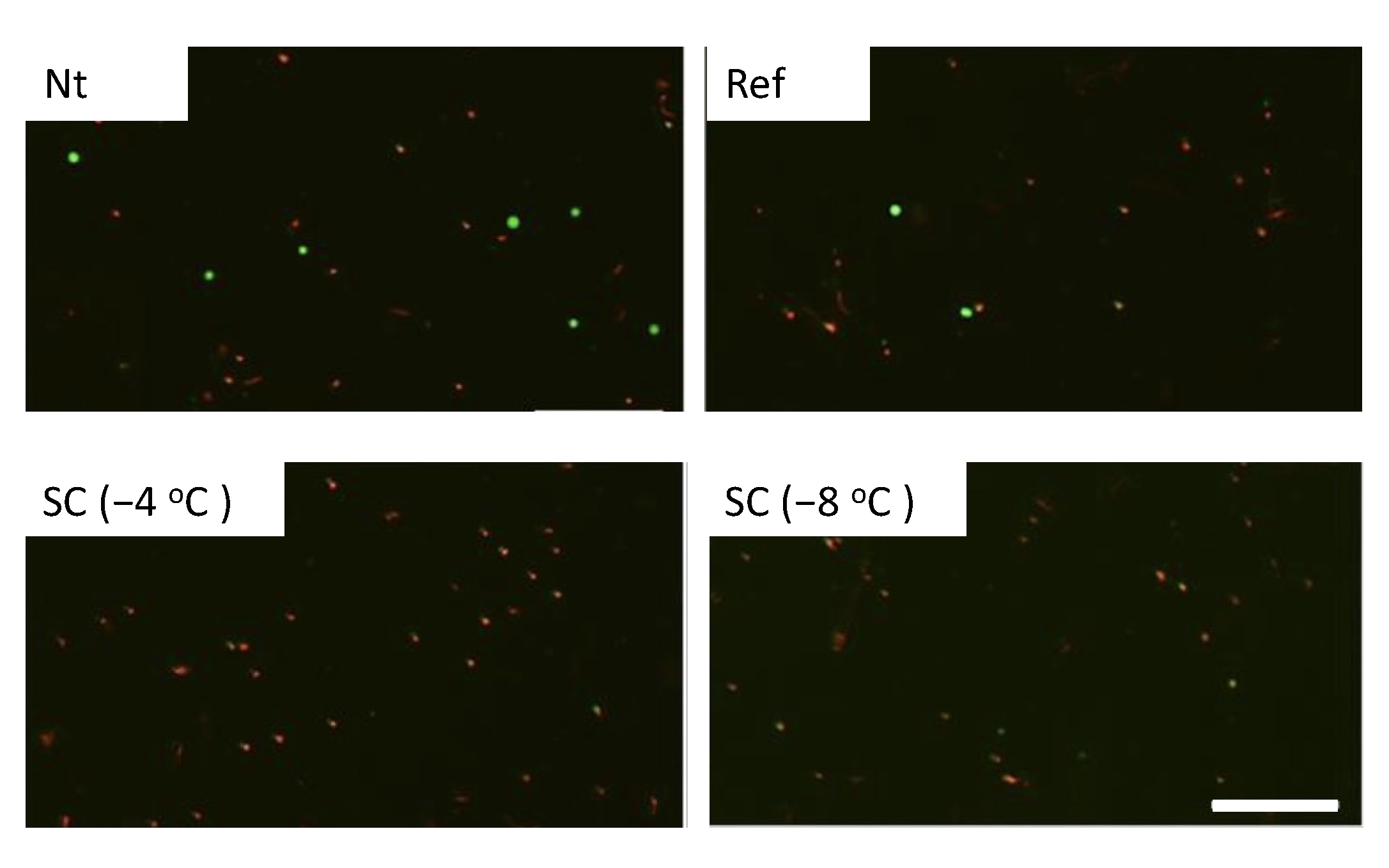



| Experimental Groups | Pretreatment | HHP |
|---|---|---|
| Nt | Non-treated | 0, 100, 150, 200 MPa for 10 min |
| Ref | Refrigerated at 4 °C | |
| SC (−4 °C) | Supercooled at −4 °C | |
| SC (−8 °C) | Supercooled at −8 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zemmyo, D.; Yamamoto, M.; Miyata, S. Efficient Decellularization by Application of Moderate High Hydrostatic Pressure with Supercooling Pretreatment. Micromachines 2021, 12, 1486. https://doi.org/10.3390/mi12121486
Zemmyo D, Yamamoto M, Miyata S. Efficient Decellularization by Application of Moderate High Hydrostatic Pressure with Supercooling Pretreatment. Micromachines. 2021; 12(12):1486. https://doi.org/10.3390/mi12121486
Chicago/Turabian StyleZemmyo, Daiki, Masashi Yamamoto, and Shogo Miyata. 2021. "Efficient Decellularization by Application of Moderate High Hydrostatic Pressure with Supercooling Pretreatment" Micromachines 12, no. 12: 1486. https://doi.org/10.3390/mi12121486
APA StyleZemmyo, D., Yamamoto, M., & Miyata, S. (2021). Efficient Decellularization by Application of Moderate High Hydrostatic Pressure with Supercooling Pretreatment. Micromachines, 12(12), 1486. https://doi.org/10.3390/mi12121486






