Four-Dimensional Stimuli-Responsive Hydrogels Micro-Structured via Femtosecond Laser Additive Manufacturing
Abstract
:1. Introduction
2. Materials and Methods
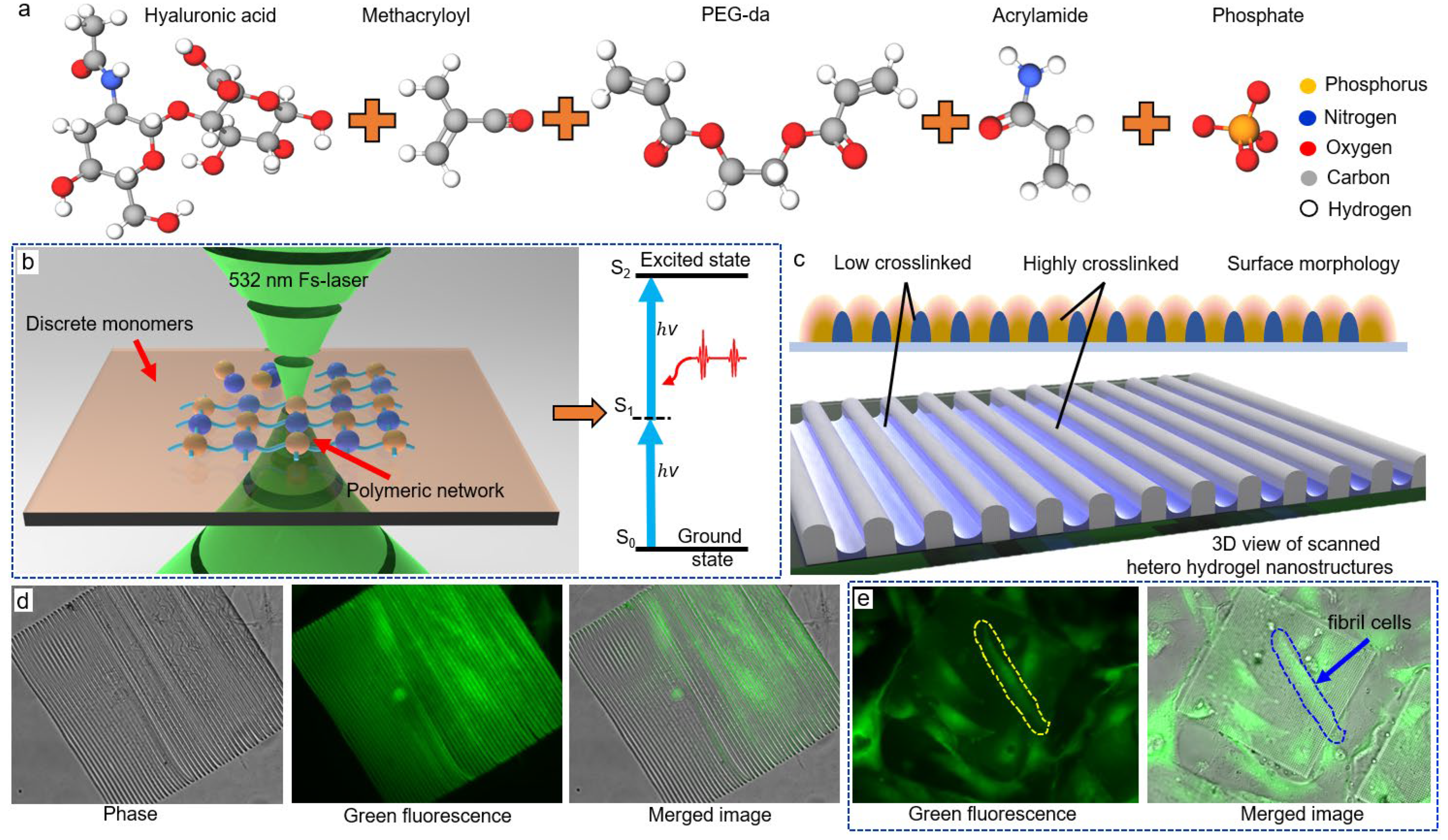
2.1. Material Preparation
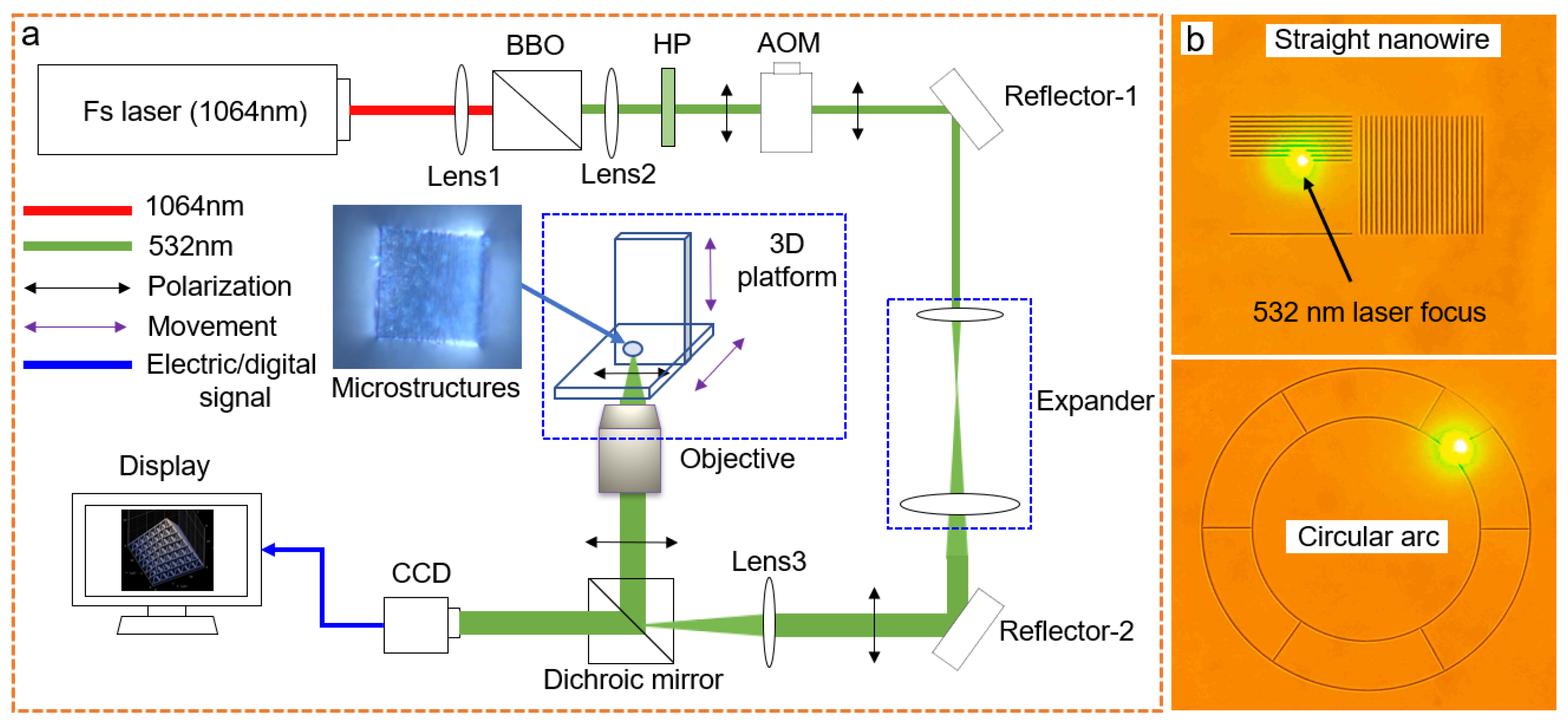
2.2. Laser System and TPP Additive Manufacturing
2.3. Measurement
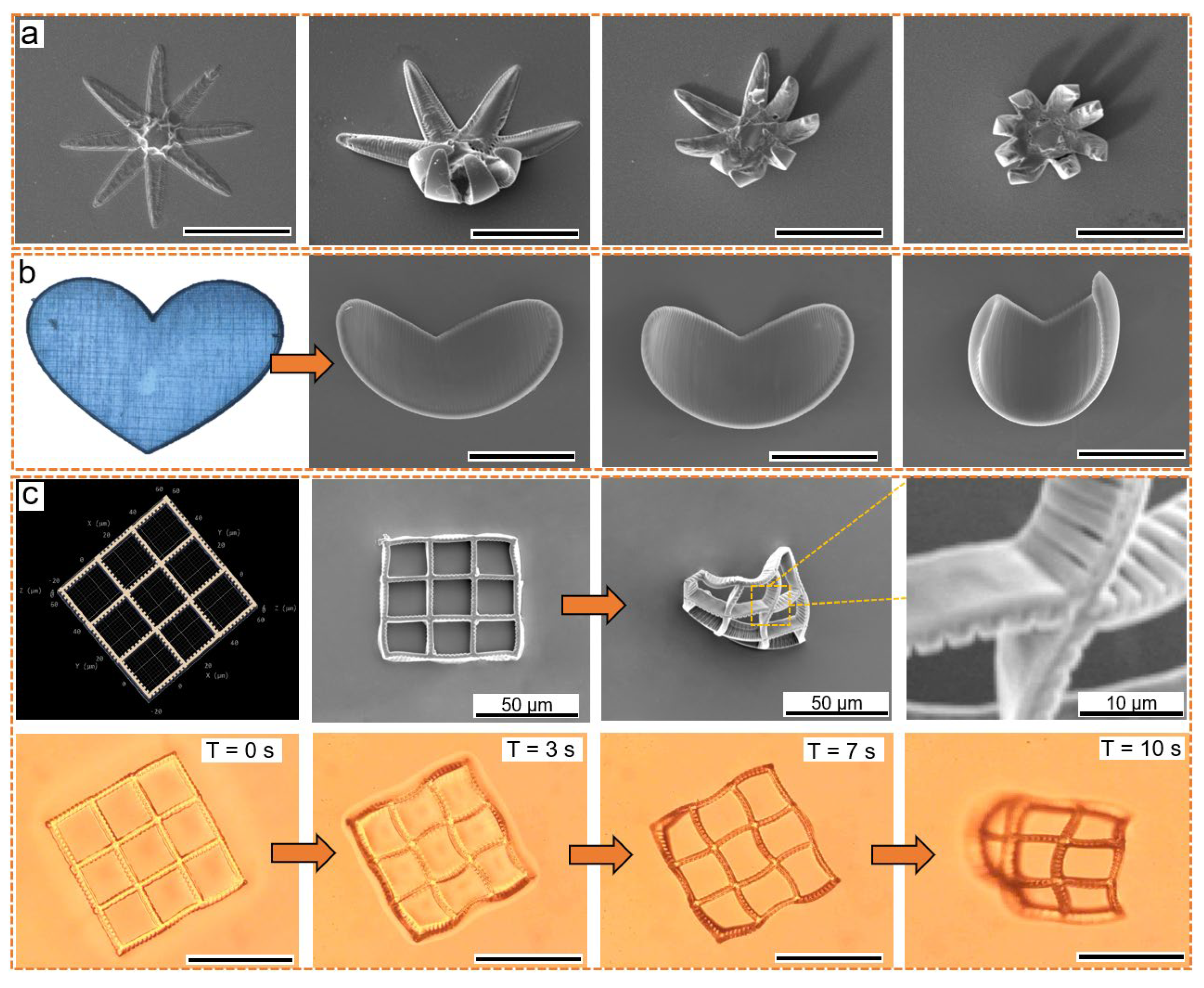
3. Results and Discussion
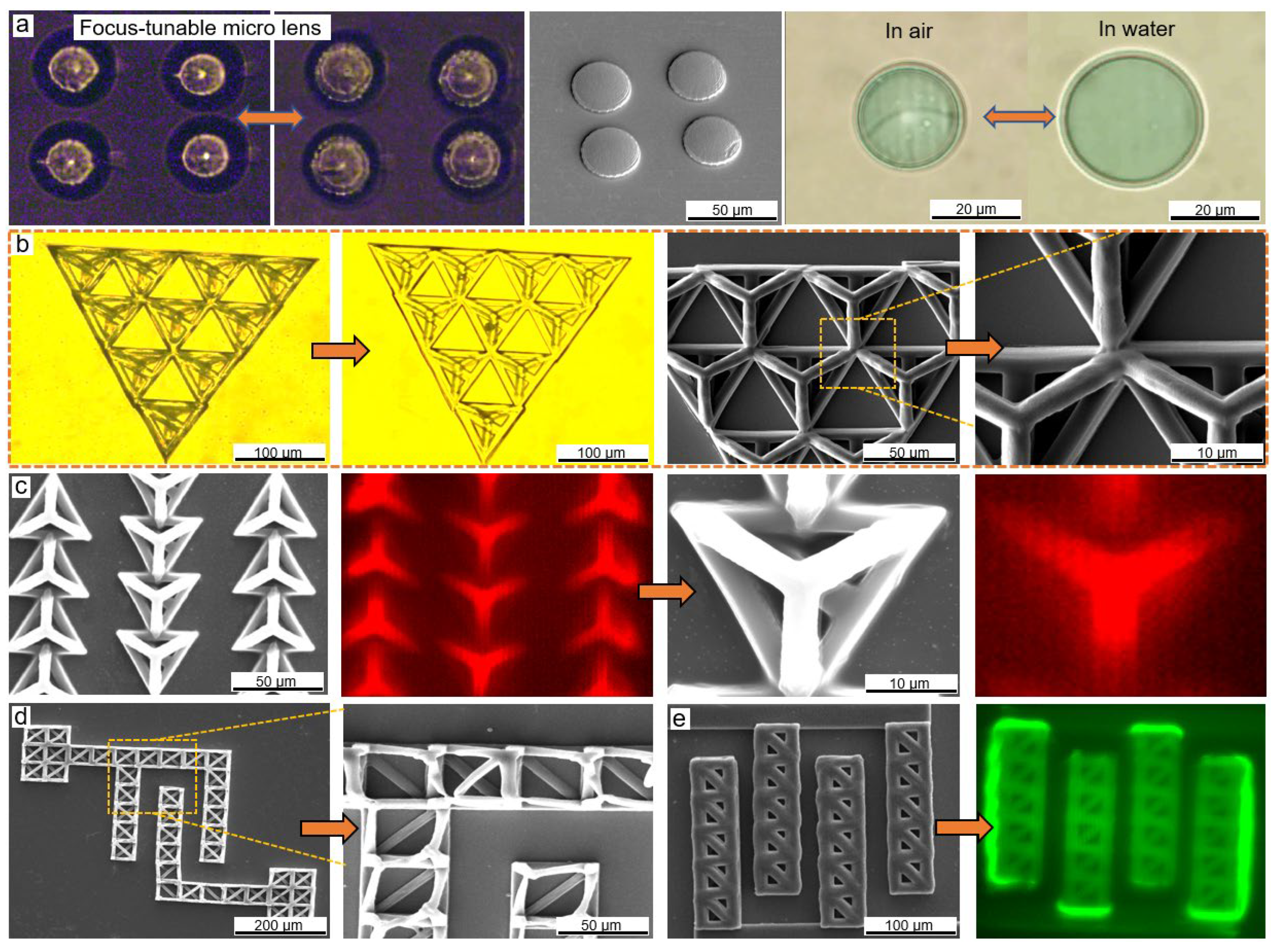
3.1. Fabricating Hollow 3D Structures with Selected Spatial Resolution
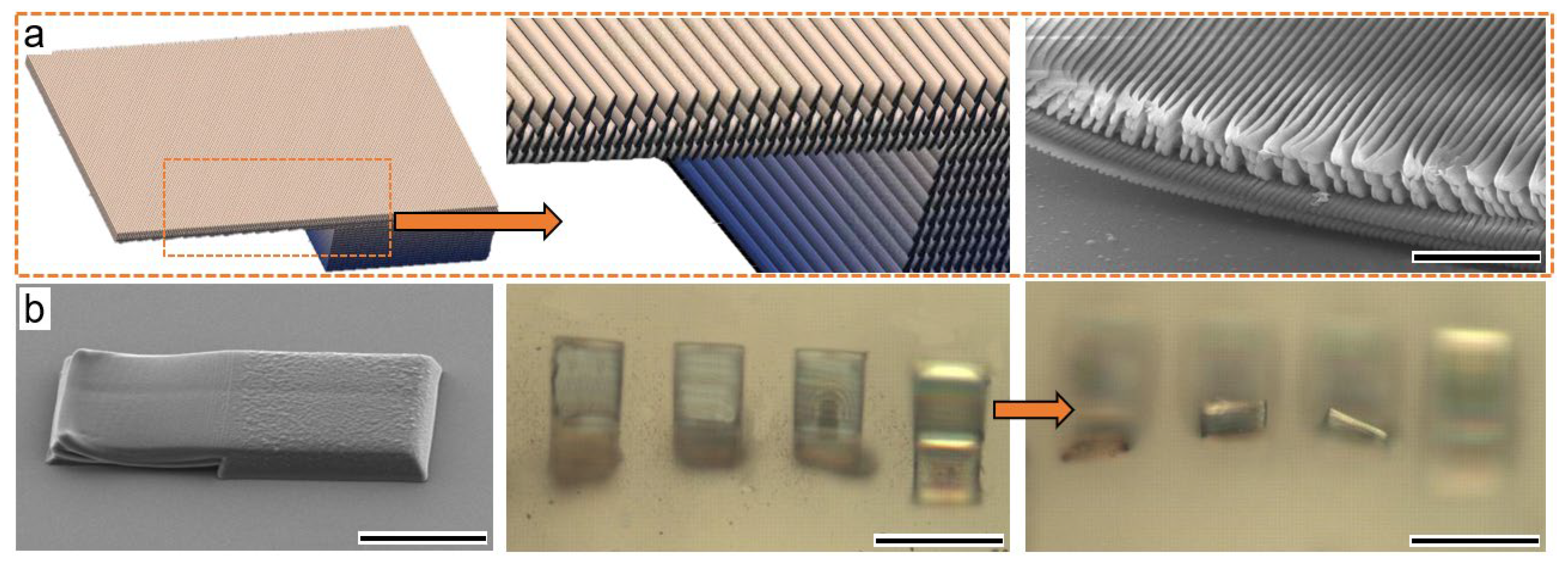
3.2. Humidity and Light-Triggered Reverse Shape Morphing
3.3. Heat-Induced Shrinkage Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, H.; Ahn, S.; Mackie, D.; Kwon, J.; Kim, S.; Choi, C.; Moon, Y.; Lee, H.B.; Ko, S. Shape morphing smart 3D actuator materials for micro soft robot. Mater. Today 2020, 41, 243–269. [Google Scholar] [CrossRef]
- Daryadela, S.; Behroozfarb, A.; Minary-Jolandan, M. A microscale additive manufacturing approach for in situ nanomechanics. Mater. Sci. Eng. C 2019, 767, 138441. [Google Scholar] [CrossRef]
- Guimarães, C.F.; Gasperini, L.; Marques, A.P.; Reis, R. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 2020, 5, 351–370. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Xin, C.; Jin, D.; Hu, Y.; Yang, L.; Li, R.; Wang, L.; Ren, Z.; Wang, D.; Ji, S.; Hu, K.; et al. Environmentally Adaptive Shape-Morphing Microrobots for Localized Cancer Cell Treatment. ACS Nano 2021, 15, 18048–18059. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, H.; Sun, Y.; Qiu, L.; Zhang, J.; Guan, G.; Zhao, X.; Qiao, M.; Cheng, L.; Cheng, L.; et al. A pH-sensitive gene delivery system based on folic acid-PEG-chitosan-PAMAM-plasmid DNA complexes for cancer cell targeting. Biomaterials 2013, 34, 10120–10132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, G.; Liu, Y.; Sun, L.; Zhao, Y. Black Phosphorus-Loaded Separable Microneedles as Responsive Oxygen Delivery Carriers for Wound Healing. ACS Nano 2020, 14, 5901–5908. [Google Scholar] [CrossRef]
- Do, A.; Worthington, K.S.; Tucker, B.A.; Salem, A.K. Controlled drug delivery from 3D printed two-photon polymerized poly (ethylene glycol) dimethacrylate devices. Int. J. Pharm. 2018, 552, 217–224. [Google Scholar] [CrossRef]
- Feliciano, A.J.; Blitterswijk, C.V.; Moroni, L.; Baker, M.B. Realizing Tissue Integration with Supramolecular Hydrogels. Acta Biomater. 2021, 124, 1–14. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Aghda, N.H.; Pillai, A.R.; Thakkar, R.; Nokhodchi, A.; Maniruzzaman, M. Emerging 3D printing technologies for drug delivery devices: Current status and future perspective. Adv. Drug Deliv. Rev. 2021, 174, 294–316. [Google Scholar] [CrossRef]
- Park, N.; Kim, J. Hydrogel-Based Artificial Muscles: Overview and Recent Progress. Advanced Intelligent Systems. Adv. Intell. Syst. 2020, 2, 1900135. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Wang, Y.; Ren, Y.Y.; Jin, G.Q.; Zhang, C.; Chen, W.Y.; Yan, F. Poly(ionic liquid) hydrogel-based anti-freezing ionic skin for a soft robotic gripper. Mater. Horiz. 2020, 7, 919–927. [Google Scholar] [CrossRef]
- Ding, M.; Jing, L.; Yang, H.; Machnicki, C.E.; Fu, X.; Li, K.; Wong, I.; Chen, P.Y. Multifunctional soft machines based on stimuli-responsive hydrogels: From freestanding hydrogels to smart integrated systems. Mater. Today Adv. 2020, 8, 100088. [Google Scholar] [CrossRef]
- Tao, Y.; Wei, C.; Liu, J.; Deng, C.; Cai, S.; Xiong, W. Nanostructured electrically conductive hydrogels via ultrafast laser processing and self-assembly. Nanoscale 2019, 11, 9176–9184. [Google Scholar] [CrossRef]
- Tao, F.; Deng, C.; Long, J.; Liu, J.; Wang, X.; Song, X.; Lu, C.; Yang, J.; Hao, H.; Wang, C.; et al. Multiprocess Laser Lifting-Off for Nanostructured Semiconductive Hydrogels. Adv. Mater. Inter. 2021, 2101250. [Google Scholar] [CrossRef]
- Economidou, S.N.; Perea, C.P.P.; Reid, A.; Uddin, M.J.; Windmill, J.F.C.; Lamproud, D.A.; Douroumis, D. 3D printed microneedle patches using stereolithography (SLA) for intradermal insulin delivery. Mater. Sci. Eng. C 2019, 743–755. [Google Scholar] [CrossRef]
- Yao, S.; Chi, J.; Wang, Y.; Zhao, Y.; Luo, Y.; Wang, Y. Zn-MOF Encapsulated Antibacterial and Degradable Microneedles Array for Promoting Wound Healing. Adv. Healthc. Mater. 2021, 10, 2100056. [Google Scholar] [CrossRef] [PubMed]
- Freedman, B.; Uzun, O.; Luna, N.; Rock, A.; Clifford, C.; Stoler, E.; Östlund-Sholars, G.; Johnson, C.; Mooney, D. Degradable and Removable Tough Adhesive Hydrogels. Adv. Mater. 2021, 33, e2008553. [Google Scholar] [CrossRef]
- Bernardeschi, I.; Ilyas, M.; Beccai, L. A Review on Active 3D Microstructures via Direct Laser Lithography. Adv. Intell. Syst. 2021, 3, 2100051. [Google Scholar] [CrossRef]
- Nishiguchi, A.; Zhang, H.; Schweizerhof, S.; Schulte, M.F.; Mourran, A.; Möller, M. 4D Printing of a Light-Driven Soft Actuator with Programmed Printing Density. ACS Appl. Mater. Interfaces 2020, 12, 12176–12185. [Google Scholar] [CrossRef] [Green Version]
- Rafiee, M.; Farahani, R.D.; Therriault, D. Multi-Material 3D and 4D Printing: A Survey. Adv. Sci. 2020, 7, 1902307. [Google Scholar] [CrossRef] [PubMed]
- Lui, Y.; Sow, W.; Tan, L.; Wu, Y.; Lai, Y.; Li, H. 4D printing and stimuli-responsive materials in biomedical aspects. Acta. Biomater. 2019, 92, 19–36. [Google Scholar] [CrossRef]
- Kufelt, O.; El-Tamer, A.; Sehring, C.; Schlie-Wolter, S.; Chichkov, B. Hyaluronic acid based materials for scaffolding via two-photon polymerization. Biomacromolecules 2014, 10, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Jambhulkar, S.; Zhu, Y.; Ravichandran, D.; Kakarla, M.; Vernon, B.; Lott, D.G.; Cornella, J.L.; Shefi, O.; Miquelard-Garnier, G.; et al. 3D printing for polymer/particle-based processing: A review. Compos. B Eng. 2021, 223, 109102. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Deiwick, A.; Vlierberghe, S.V.; Dubruel, P.; Möller, L.; Dräger, G.; Chichkov, B. Laser Fabrication of Three-Dimensional CAD Scaffolds from Photosensitive Gelatin for Applications in Tissue Engineering. Biomacromolecules 2011, 12, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Rajabasadi, F.; Schwarz, L.; Medina-Sánchez, M.; Schmidt, O. 3D and 4D lithography of untethered microrobots. Prog. Mater. Sci. 2021, 120, 100808. [Google Scholar] [CrossRef]
- Gladman, A.S.; Matsumoto, E.A.; Nuzzo, R.G.; Mahadevan, L.; Lewis, J.A. Biomimetic 4D printing. Nat. Mater. 2016, 15, 413–418. [Google Scholar] [CrossRef]
- Kawata, S.; Sun, H.B.; Tanaka, T.; Takada, K. Finer features for functional microdevices. Nature 2001, 412, 697–698. [Google Scholar] [CrossRef]
- Malinauskas, M.; Žukauskas, A.; Hasegawa, S.; Hayasaki, Y.; Mizeikis, V.; Buividas, R.; Juodkazis, S. Ultrafast laser processing of materials: From science to industry. Light-Sci. Appl. 2016, 5, e16133. [Google Scholar] [CrossRef] [Green Version]
- Ge, Q.; Qi, H.J.; Dunn, M.L. Active materials by four-dimension printing. Appl. Phys. Lett. 2013, 103, 131901. [Google Scholar] [CrossRef]
- Liu, Y.; Shaw, B.; Dickey, M.D.; Genzer, J. Sequential self-folding of polymer sheets. Sci. Adv. 2017, 3, e1602417. [Google Scholar] [CrossRef] [Green Version]
- Nishiguchi, A.; Mourran, A.; Zhang, H.; Möller, M. In-Gel Direct Laser Writing for 3D-Designed Hydrogel Composites That Undergo Complex Self-Shaping. Adv. Sci. 2017, 5, 1700038. [Google Scholar] [CrossRef]
- Maruo, S.; Ikuta, K.; Korogi, H. Force-controllable, optically driven micromachines fabricated by single-step two-photon micro stereolithography. J. Microelectromech. Syst. 2003, 12, 533–539. [Google Scholar] [CrossRef]
- Xiao, Y.; Lin, J.; Xiao, J.; Weng, M.; Zhang, W.; Zhou, P.; Luo, Z.; Chen, L. A multi-functional light-driven actuator with an integrated temperature-sensing function based on a carbon nanotube composite. Nanoscale 2021, 13, 6259–6265. [Google Scholar] [CrossRef]
- Ceylan, H.; Yasa, I.C.; Yasa, O.; Tabak, A.F.; Giltinan, J.; Sitti, M. 3D-Printed Biodegradable Microswimmer for Theranostic Cargo Delivery and Release. ACS Nano 2019, 13, 3353–3362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Li, M.; Bian, H.; Yong, J.; Zhang, F.; Hou, X.; Chen, F. Bioinspired Artificial Compound Eyes: Characteristic, Fabrication, and Application. Adv. Mater. Technol. 2021, 6, 2100091. [Google Scholar] [CrossRef]
- Urrios, A.; Parra-Cabrera, C.; Bhattacharjee, N.; Gonzalez-Suarez, A.M.; Rigat-Brugarolas, L.G. 3D-printing of transparent bio-microfluidic devices in PEG-DA. Lab Chip 2016, 16, 2287–2294. [Google Scholar] [CrossRef]
- Czich, S.; Wloka, T.; Rothe, H.; Rost, J.; Penzold, F.; Kleinsteuber, M.; Gottschaldt, M.; Schubert, U.S.; Liefeith, K. Two-Photon Polymerized Poly(2-Ethyl-2-Oxazoline) Hydrogel 3D Microstructures with Tunable Mechanical Properties for Tissue Engineering. Molecules 2020, 25, 5066. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Ding, H.; Zhang, Q.; Gu, Z.; Gu, M. Three-Dimensional Direct Laser Writing of PEGda Hydrogel Microstructures with Low Threshold Power using a Green Laser Beam. Light Adv. Manuf. 2021, 2, 3. [Google Scholar] [CrossRef]
- Vinck, E.; Cagnie, B.; Cornelissen, M.; Declercq, H.; Cambier, D. Green light emitting diode irradiation enhances fibroblast growth impaired by high glucose level. Photomed. Laser Surg. 2005, 23, 167–171. [Google Scholar] [CrossRef]
- Tao, Y.; Ren, Y.; Wang, X.; Zhao, R.; Liu, J.; Deng, C.; Wang, C.; Zhang, W.; Hao, H. A femtosecond laser-assembled SnO2 microbridge on interdigitated Au electrodes for gas sensing. Mater. Lett. 2022, 308, 131120. [Google Scholar] [CrossRef]
- Dai, Z.; Su, Q.; Wang, Y.; Qi, P.; Wang, X.; Liu, W. Fast fabrication of THz devices by femtosecond laser direct writing with a galvanometer scanner. Laser Phys. 2019, 29, 065301. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, W.; Ke, L.; Wei, H.; Wang, D.; Yang, L.; Zhu, H.; Dong, P.; Wang, G.; Zeng, X. Vaporization of alloying elements and explosion behavior during laser powder bed fusion of Cu–10Zn alloy. Int. J. Mach. Tool. Manuf. 2021, 161, 103686. [Google Scholar] [CrossRef]
- Xiong, Z.; Zheng, M.L.; Dong, X.Z.; Chen, W.Q.; Jin, F. Asymmetric microstructure of hydrogel: Two-photon micro fabrication and stimuli-responsive behavior. Soft Matter 2011, 7, 10353–10359. [Google Scholar] [CrossRef]
- Zhou, Y.; Layani, M.; Wang, S.C.; Hu, P.; Ke, Y.J.; Magdassi, S.; Long, Y. Fully Printed Flexible Smart Hybrid Hydrogels. Adv. Funct. Mater. 2018, 28, 1705365. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, C.; Peng, L.; Yu, G. Conductive “Smart” Hybrid Hydrogels with PNIPAM and Nanostructured Conductive Polymers. Adv. Funct. Mater. 2015, 25, 1219–1225. [Google Scholar] [CrossRef]
- Ji, S.; Li, X.; Chen, Q.; Lv, P.; Duan, H. Enhanced Locomotion of Shape Morphing Microrobots by Surface Coating. Adv. Intell. Syst. 2021, 3, 2000270. [Google Scholar] [CrossRef]
- Kuo, A.; Bhattacharjee, N.; Lee, Y.; Castro, K.; Kim, Y.; Folch, A. High-Precision Stereolithography of Biomicrofluidic Devices. Adv. Mater. Technol. 2019, 4, 1800395. [Google Scholar] [CrossRef]
- Zhang, X.; Pint, C.L.; Lee, M.H.; Schubert, B.E.; Jamshidi, A.; Takei, K.; Ko, H.; Gillies, A.; Bardhan, R.; Urban, J.; et al. Optically- and Thermally-Responsive Programmable Materials Based on Carbon Nanotube-Hydrogel Polymer Composites. Nano Lett. 2011, 11, 3239–3244. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Dong, B.; Sitti, M. In-air fast response and high speed jumping and rolling of a light-driven hydrogel actuator. Nat. Commun. 2020, 11, 3988. [Google Scholar] [CrossRef]
- Bauhofer, A.A.; Krödel, S.; Rys, J.; Bilal, O.R.; Constantinescu, A.; Daraio, C. Harnessing Photochemical Shrinkage in Direct Laser Writing for Shape Morphing of Polymer Sheets. Adv. Mater. 2017, 29, 1703024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guo, Y.; Hu, W.; Soon, R.H.; Davidson, Z.S.; Sitti, M. Liquid Crystal Elastomer-Based Magnetic Composite Films for Reconfigurable Shape-Morphing Soft Miniature Machines. Adv. Mater. 2021, 33, e2006191. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Li, W.; Zhou, J. Modeling programmable deformation of self-folding all-polymer structures with temperature-sensitive hydrogels. Smart Mater. Struct. 2013, 22, 115028. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, Y.; Lu, C.; Deng, C.; Long, J.; Ren, Y.; Dai, Z.; Tong, Z.; Wang, X.; Meng, S.; Zhang, W.; et al. Four-Dimensional Stimuli-Responsive Hydrogels Micro-Structured via Femtosecond Laser Additive Manufacturing. Micromachines 2022, 13, 32. https://doi.org/10.3390/mi13010032
Tao Y, Lu C, Deng C, Long J, Ren Y, Dai Z, Tong Z, Wang X, Meng S, Zhang W, et al. Four-Dimensional Stimuli-Responsive Hydrogels Micro-Structured via Femtosecond Laser Additive Manufacturing. Micromachines. 2022; 13(1):32. https://doi.org/10.3390/mi13010032
Chicago/Turabian StyleTao, Yufeng, Chengchangfeng Lu, Chunsan Deng, Jing Long, Yunpeng Ren, Zijie Dai, Zhaopeng Tong, Xuejiao Wang, Shuai Meng, Wenguang Zhang, and et al. 2022. "Four-Dimensional Stimuli-Responsive Hydrogels Micro-Structured via Femtosecond Laser Additive Manufacturing" Micromachines 13, no. 1: 32. https://doi.org/10.3390/mi13010032
APA StyleTao, Y., Lu, C., Deng, C., Long, J., Ren, Y., Dai, Z., Tong, Z., Wang, X., Meng, S., Zhang, W., Xu, Y., & Zhou, L. (2022). Four-Dimensional Stimuli-Responsive Hydrogels Micro-Structured via Femtosecond Laser Additive Manufacturing. Micromachines, 13(1), 32. https://doi.org/10.3390/mi13010032





