Droplet Microfluidics for Food and Nutrition Applications
Abstract
1. Introduction
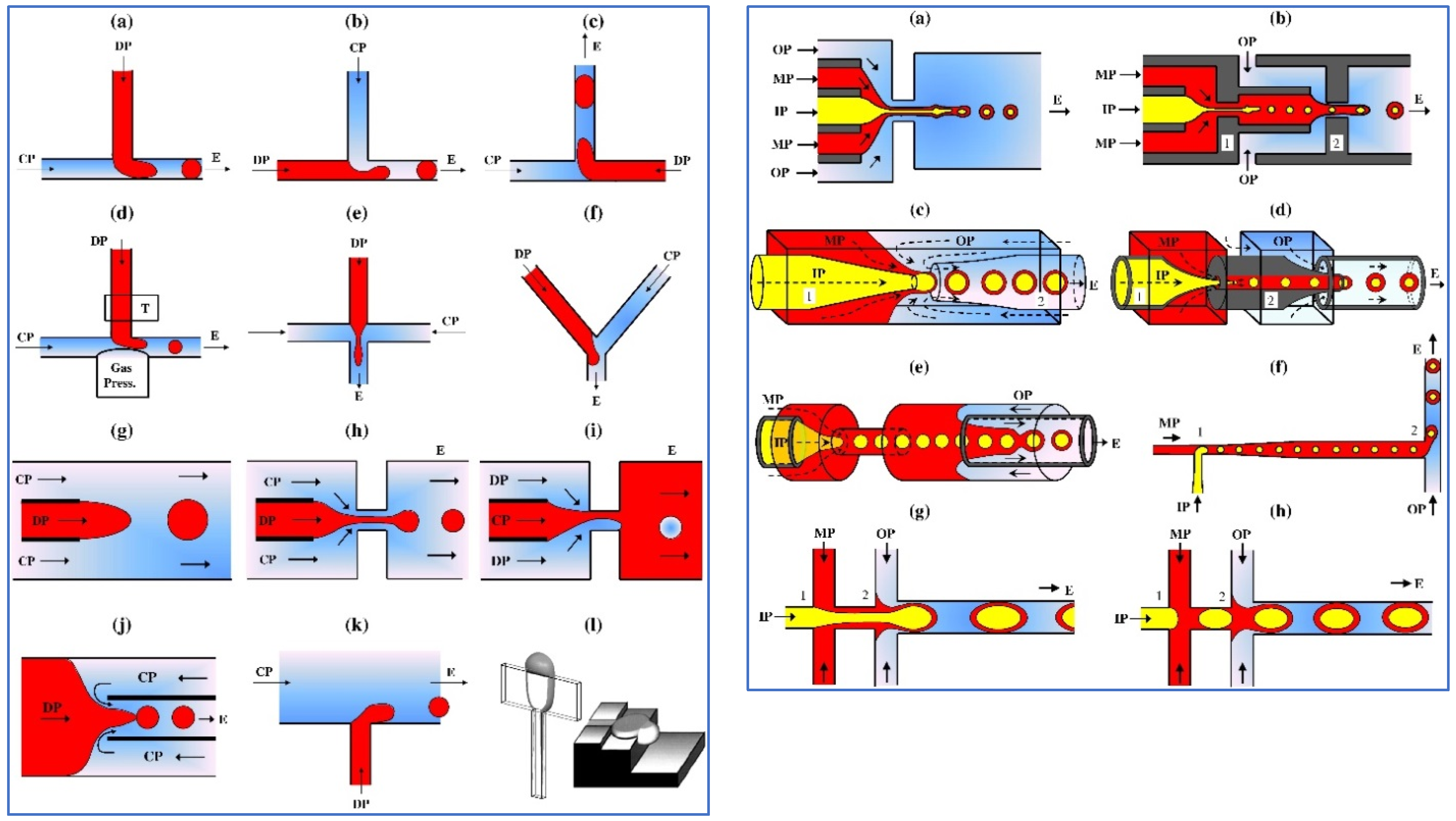
2. Droplet Microfluidics
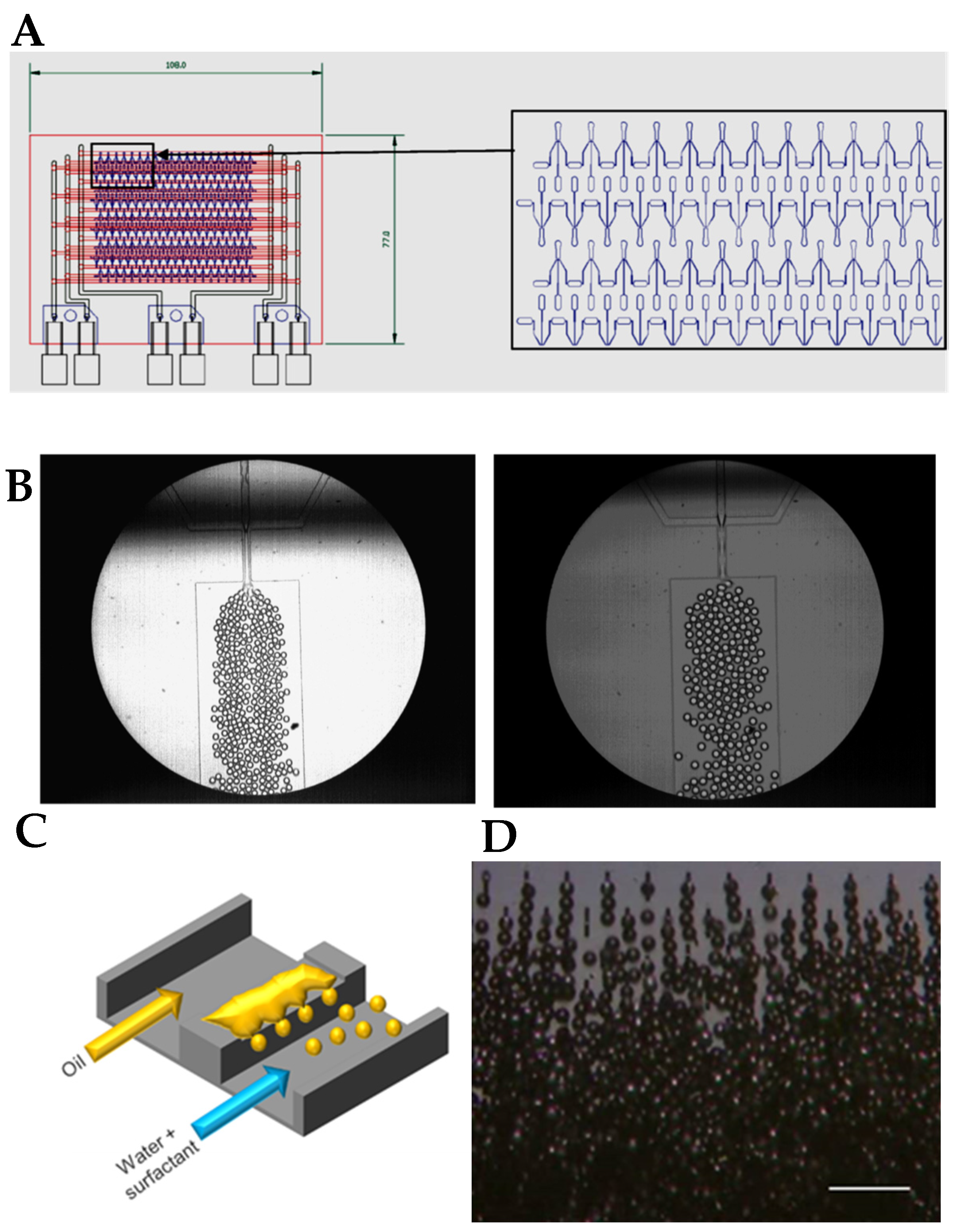
3. Microfluidic Fabrication of Food Droplets
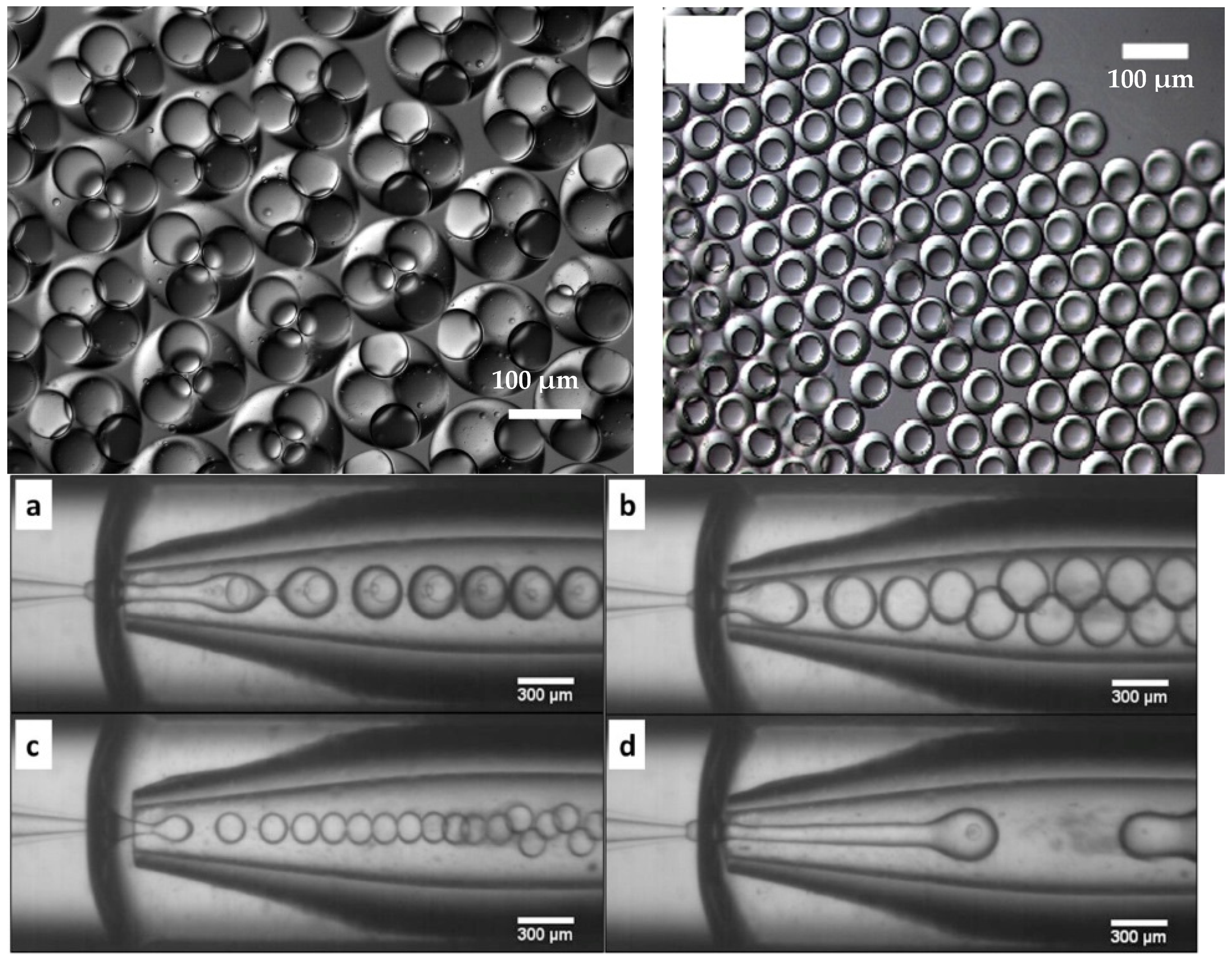
3.1. Simple Droplets and Emulsions
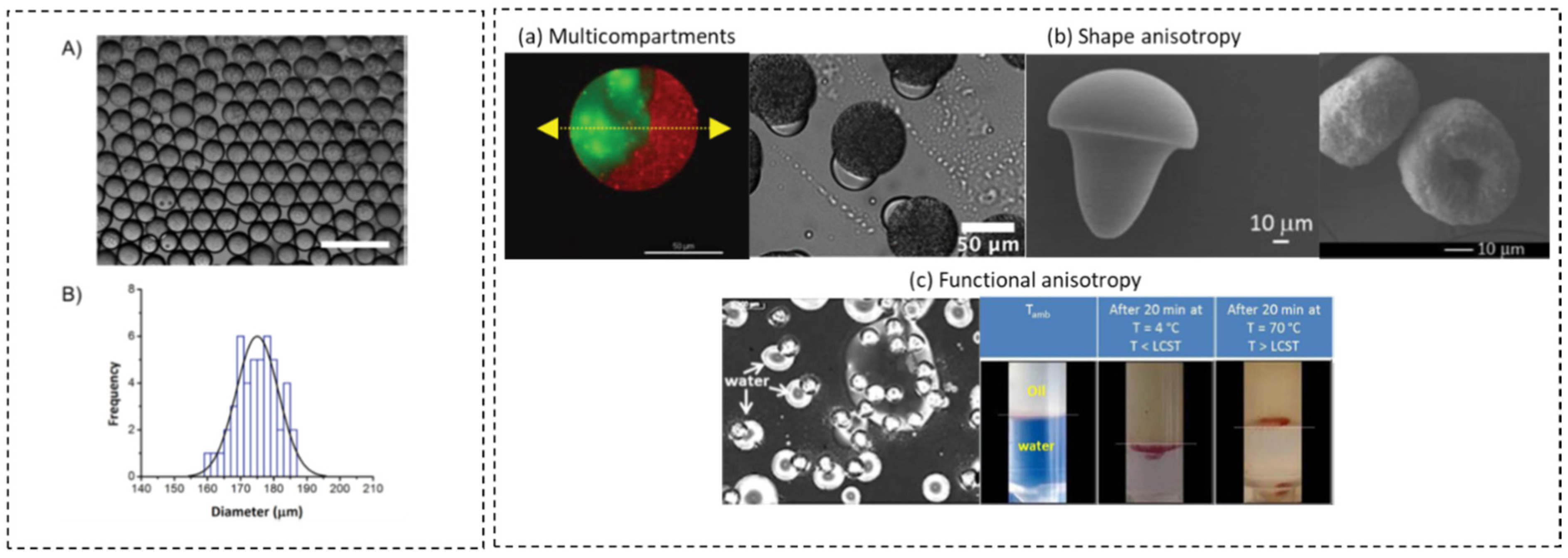
3.2. Multiple Droplets and Emulsions
3.3. Microgels and Microparticles
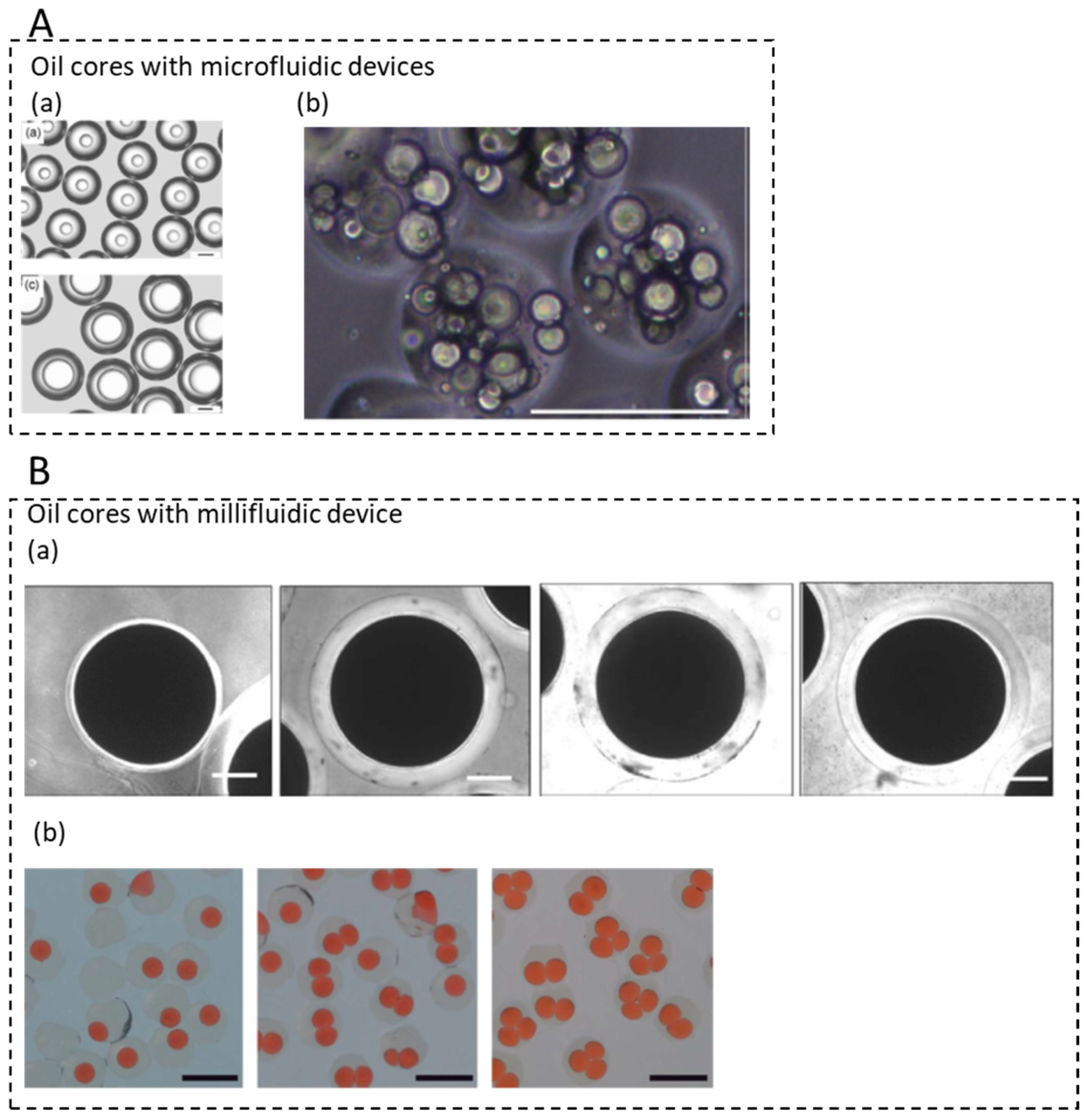
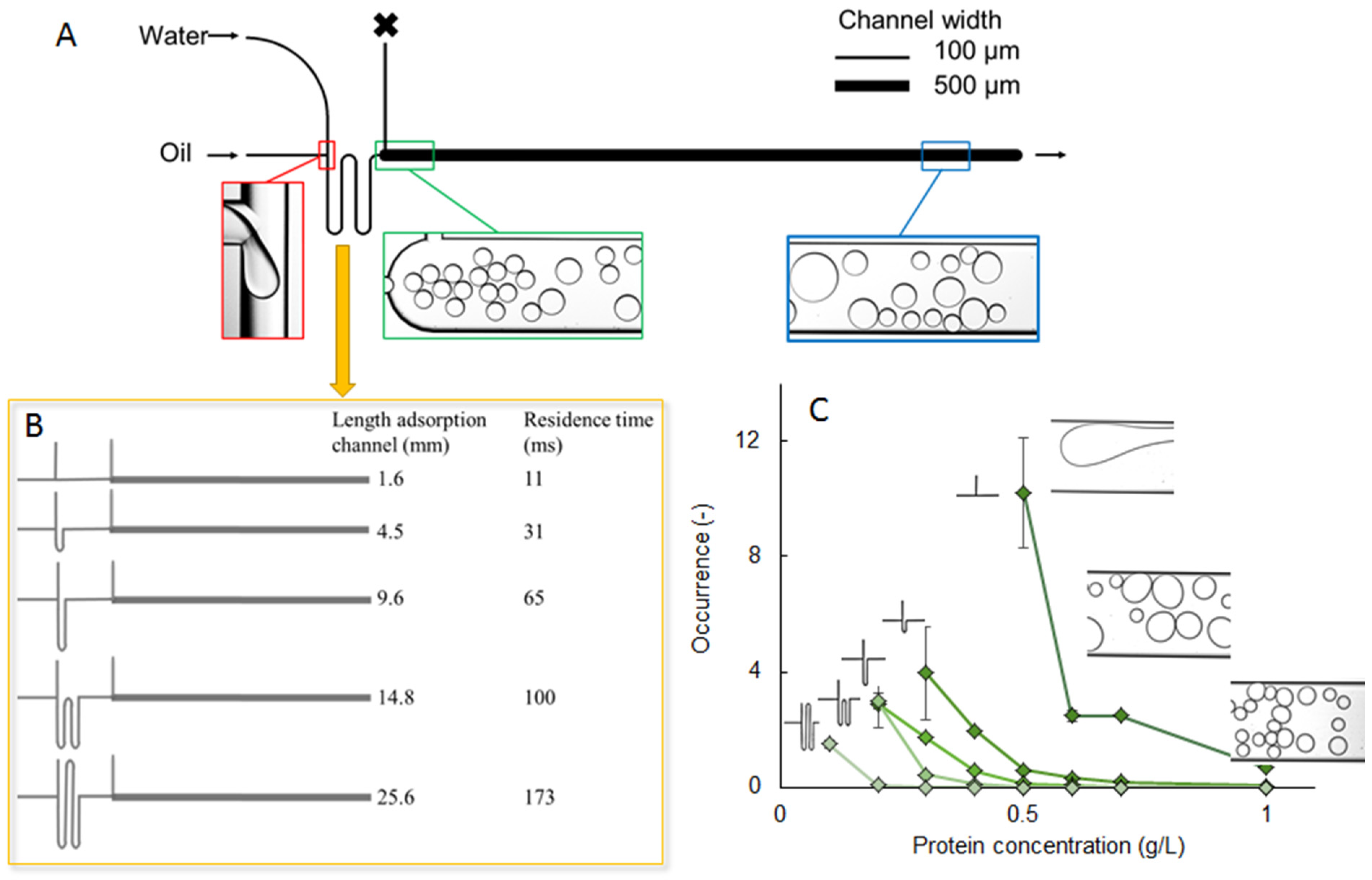
3.4. Microcapsules with Single and Multiple Oil Cores
3.5. Microcapsules from Water-in-Water Emulsions
4. On-Chip Analysis of Food Droplets
4.1. Interfacial Properties and Droplet Stability
4.1.1. Interfacial Tension
4.1.2. Coalescence Stability
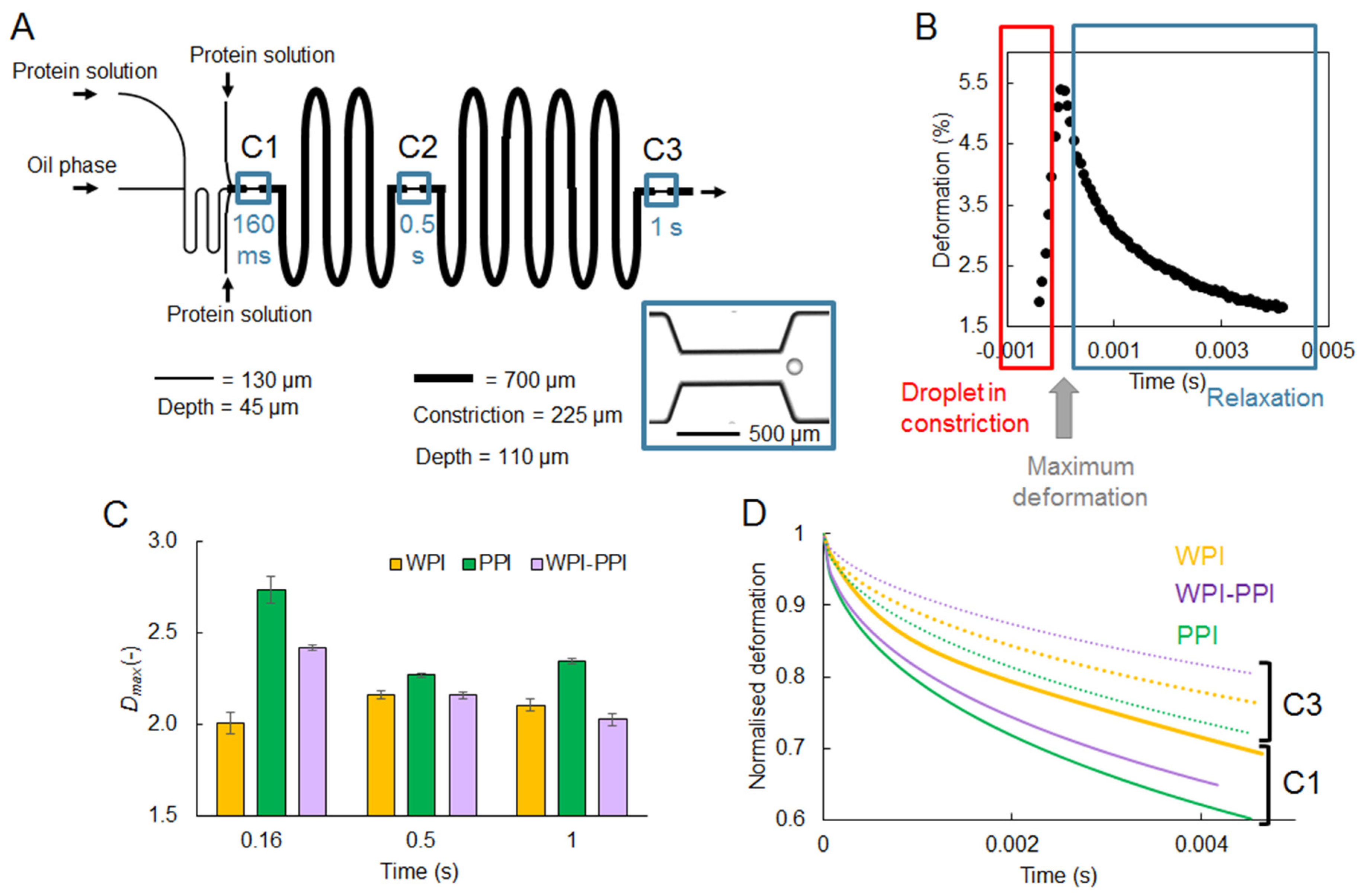
4.1.3. Interfacial Rheology
4.1.4. Temperature and Pressure Sensitivity
4.2. Screening of Biopolymer Phase Behavior Using Microfluidic Technologies
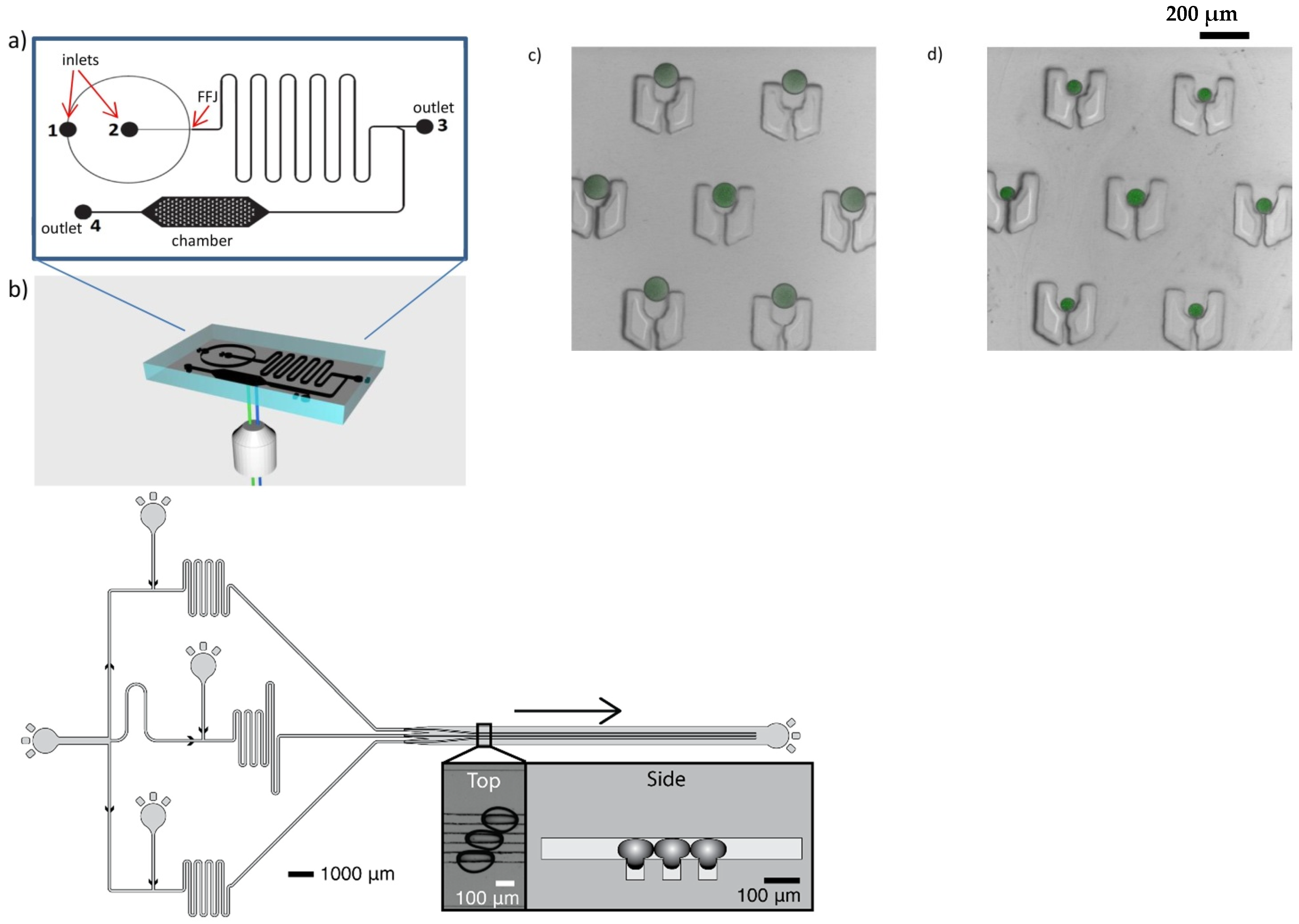
4.3. On-Chip Monitoring of Reaction Kinetics
5. Promising Developments for Food and Nutrition
5.1. Droplet Microfluidics for Biochemistry and Microbiology
5.2. Droplet Microfluidics-Analytical Instrument Coupling
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A. List of Microfluidic Producer Websites
References
- Dupont, D.; Le Feunteun, S.; Marze, S.; Souchon, I. Structuring food to control its disintegration in the gastrointestinal tract and optimize nutrient bioavailability. Innov. Food Sci. Emerg. Technol. 2018, 46, 83–90. [Google Scholar] [CrossRef]
- Stone, H.A.; Stroock, A.D.; Ajdari, A. Engineering flows in small devices: Microfluidics toward a lab-on-a-chip. Annu. Rev. Fluid Mech. 2004, 36, 381–411. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Vladisavljevic, G.T.; Khalid, N.; Neves, M.A.; Kuroiwa, T.; Nakajima, M.; Uemura, K.; Ichikawa, S.; Kobayashi, I. Industrial lab-on-a-chip: Design, applications and scale-up for drug discovery and delivery. Adv. Drug Deliv. Rev. 2013, 65, 1626–1663. [Google Scholar] [CrossRef]
- Vladisavljevic, G.T.; Williams, R.A. Recent developments in manufacturing emulsions and particulate products using membranes. Adv. Colloid Interface Sci. 2005, 113, 1–20. [Google Scholar] [CrossRef]
- Vladisavljevic, G.T.; Al Nuumani, R.; Nabavi, S.A. Microfluidic Production of Multiple Emulsions. Micromachines 2017, 8, 75. [Google Scholar] [CrossRef]
- Zhu, P.A.; Wang, L.Q. Passive and active droplet generation with microfluidics: A review. Lab Chip 2017, 17, 34–75. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.M.; Yang, Y.; Du, Y.; Pang, Y. Advances in Droplet-Based Microfluidic Technology and Its Applications. Chin. J. Anal. Chem. 2017, 45, 282–295. [Google Scholar] [CrossRef]
- Shang, L.R.; Cheng, Y.; Zhao, Y.J. Emerging Droplet Microfluidics. Chem. Rev. 2017, 117, 7964–8040. [Google Scholar] [CrossRef]
- Sohrabi, S.; Kassir, N.; Moraveji, M.K. Droplet microfluidics: Fundamentals and its advanced applications. RSC Adv. 2020, 10, 27560–27574. [Google Scholar] [CrossRef]
- Venkatesan, S.; Jerald, J.; Asokan, P.; Prabakaran, R. A Comprehensive Review on Microfluidics Technology and its Applications. In Recent Advances in Mechanical Engineering, Proceedings of the NCAME 2019, Delhi, India, 16 March 2019; Springer: Berlin/Heidelberg, Germany, 2020; pp. 235–245. [Google Scholar]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. Int. Ed. 2007, 46, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Muijlwijk, K.; Berton-Carabin, C.; Schroen, K. Cross-flow microfluidic emulsification from a food perspective. Trends Food Sci. Technol. 2016, 49, 51–63. [Google Scholar] [CrossRef]
- Maan, A.A.L.; Nazir, A.; Khan, M.K.L.; Boom, R.; Schroen, K. Microfluidic emulsification in food processing. J. Food Eng. 2015, 147, 1–7. [Google Scholar] [CrossRef]
- Schroen, K.; Bliznyuk, O.; Muijlwijk, K.; Sahin, S.; Berton-Carabin, C.C. Microfluidic emulsification devices: From micrometer insights to large-scale food emulsion production. Curr. Opin. Food Sci. 2015, 3, 33–40. [Google Scholar] [CrossRef]
- Marze, S.; Nguyen, H.T.; Marquis, M. Manipulating and studying triglyceride droplets in microfluidic devices. Biochimie 2020, 169, 88–94. [Google Scholar] [CrossRef]
- Schroen, K.; de Ruiter, J.; Berton-Carabin, C.C. Microtechnological Tools to Achieve Sustainable Food Processes, Products, and Ingredients. Food Eng. Rev. 2020, 12, 101–120. [Google Scholar] [CrossRef]
- Doufene, K.; Tourne-Peteilh, C.; Etienne, P.; Aubert-Pouessel, A. Microfluidic Systems for Droplet Generation in Aqueous Continuous Phases: A Focus Review. Langmuir 2019, 35, 12597–12612. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.H.; Issadore, D.; Lee, D. Recent developments in scale-up of microfluidic emulsion generation via parallelization. Korean J. Chem. Eng. 2016, 33, 1757–1766. [Google Scholar] [CrossRef]
- Kobayashi, I.; Neves, M.A.; Wada, Y.; Uemura, K.; Nakajima, M. Large microchannel emulsification device for mass producing uniformly sized droplets on a liter per hour scale. Green Process. Synth. 2012, 1, 353–362. [Google Scholar] [CrossRef]
- Kobayashi, I.; Nakajima, M.; Chun, K.; Kikuchi, Y.; Fukita, H. Silicon array of elongated through-holes for monodisperse emulsion droplets. Aiche J. 2002, 48, 1639–1644. [Google Scholar] [CrossRef]
- Kobayashi, I.; Lou, X.F.; Mukataka, S.; Nakajima, M. Preparation of monodisperse water-in-oil-in-water emulsions using microfluidization and straight-through microchannel emulsification. J. Am. Oil Chem. Soc. 2005, 82, 65–71. [Google Scholar] [CrossRef]
- Van Dijke, K.; Kobayashi, I.; Schroen, K.; Uemura, K.; Nakajima, M.; Boom, R. Effect of viscosities of dispersed and continuous phases in microchannel oil-in-water emulsification. Microfluid. Nanofluidics 2010, 9, 77–85. [Google Scholar] [CrossRef]
- Tetradis-Meris, G.; Rossetti, D.; de Torres, C.P.; Cao, R.; Lian, G.P.; Janes, R. Novel Parallel Integration of Microfluidic Device Network for Emulsion Formation. Ind. Eng. Chem. Res. 2009, 48, 8881–8889. [Google Scholar] [CrossRef]
- Jeong, H.H.; Yelleswarapu, V.R.; Yadavali, S.; Issadore, D.; Lee, D. Kilo-scale droplet generation in three-dimensional monolithic elastomer device (3D MED). Lab Chip 2015, 15, 4387–4392. [Google Scholar] [CrossRef] [PubMed]
- Amstad, E.; Chemama, M.; Eggersdorfer, M.; Arriaga, L.R.; Brenner, M.P.; Weitz, D.A. Robust scalable high throughput production of monodisperse drops. Lab Chip 2016, 16, 4163–4172. [Google Scholar] [CrossRef] [PubMed]
- Van Dijke, K.; de Ruiter, R.; Schroen, K.; Boom, R. The mechanism of droplet formation in microfluidic EDGE systems. Soft Matter 2010, 6, 321–330. [Google Scholar] [CrossRef]
- Sahin, S.; Bliznyuk, O.; Cordova, A.R.; Schroen, K. Microfluidic EDGE emulsification: The importance of interface interactions on droplet formation and pressure stability. Sci. Rep. 2016, 6, 26407. [Google Scholar] [CrossRef]
- Sahin, S.; Schroen, K. Partitioned EDGE devices for high throughput production of monodisperse emulsion droplets with two distinct sizes. Lab Chip 2015, 15, 2486–2495. [Google Scholar] [CrossRef]
- Visser, C.W.; Kamperman, T.; Karbaat, L.P.; Lohse, D.; Karperien, M. In-air microfluidics enables rapid fabrication of emulsions, suspensions, and 3D modular (bio)materials. Sci. Adv. 2018, 4, eaao1175. [Google Scholar] [CrossRef]
- Abate, A.R.; Weitz, D.A. High-Order Multiple Emulsions Formed in Poly(dimethylsiloxane) Microfluidics. Small 2009, 5, 2030–2032. [Google Scholar] [CrossRef]
- Hughes, E.; Maan, A.A.; Acquistapace, S.; Burbidge, A.; Johns, M.L.; Gunes, D.Z.; Clausen, P.; Syrbe, A.; Hugo, J.; Schroen, K.; et al. Microfluidic preparation and self diffusion PFG-NMR analysis of monodisperse water-in-oil-in-water double emulsions. J. Colloid Interface Sci. 2013, 389, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Comunian, T.A.; Ravanfar, R.; Alcaine, S.D.; Abbaspourrad, A. Water-in-oil-in-water emulsion obtained by glass microfluidic device for protection and heat-triggered release of natural pigments. Food Res. Int. 2018, 106, 945–951. [Google Scholar] [CrossRef]
- Michelon, M.; Huang, Y.T.; de la Torre, L.G.; Weitz, D.A.; Cunha, R.L. Single-step microfluidic production of W/O/W double emulsions as templates for beta-carotene-loaded giant liposomes formation. Chem. Eng. J. 2019, 366, 27–32. [Google Scholar] [CrossRef]
- Al Nuumani, R.; Vladisavljevic, G.T.; Kasprzak, M.; Wolf, B. In-Vitro oral digestion of microfluidically produced monodispersed W/O/W food emulsions loaded with concentrated sucrose solution designed to enhance sweetness perception. J. Food Eng. 2020, 267, 109701. [Google Scholar] [CrossRef]
- Isa, N.S.M.; El Kadri, H.; Vigolo, D.; Gkatzionis, K. Optimisation of bacterial release from a stable microfluidic-generated water-in-oil-in-water emulsion. RSC Adv. 2021, 11, 7738–7749. [Google Scholar]
- Seiffert, S. Small but Smart: Sensitive Microgel Capsules. Angew. Chem. Int. Ed. 2013, 52, 11462–11468. [Google Scholar] [CrossRef]
- Shewan, H.M.; Stokes, J.R. Review of techniques to manufacture micro-hydrogel particles for the food industry and their applications. J. Food Eng. 2013, 119, 781–792. [Google Scholar] [CrossRef]
- McClements, D.J. Designing biopolymer microgels to encapsulate, protect and deliver bioactive components: Physicochemical aspects. Adv. Colloid Interface Sci. 2017, 240, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.C.; Thom, D. Biological Interactions between Polysaccharides and Divalent Cations—Egg-Box Model. Febs Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef]
- Zhang, H.; Tumarkin, E.; Sullan, R.M.A.; Walker, G.C.; Kumacheva, E. Exploring microfluidic routes to microgels of biological polymers. Macromol. Rapid Commun. 2007, 28, 527–538. [Google Scholar] [CrossRef]
- Chau, M.; Abolhasani, M.; Therien-Aubin, H.; Li, Y.; Wang, Y.H.; Velasco, D.; Tumarkin, E.; Ramachandran, A.; Kumacheva, E. Microfluidic Generation of Composite Biopolymer Microgels with Tunable Compositions and Mechanical Properties. Biomacromolecules 2014, 15, 2419–2425. [Google Scholar] [CrossRef] [PubMed]
- Damiati, S. In Situ Microfluidic Preparation and Solidification of Alginate Microgels. Macromol. Res. 2020, 28, 1046–1053. [Google Scholar] [CrossRef]
- Marquis, M.; Renard, D.; Cathala, B. Microfluidic Generation and Selective Degradation of Biopolymer-Based Janus Microbeads. Biomacromolecules 2012, 13, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Marquis, M.; Davy, J.; Fang, A.P.; Renard, D. Microfluidics-Assisted Diffusion Self-Assembly: Toward the Control of the Shape and Size of Pectin Hydrogel Microparticles. Biomacromolecules 2014, 15, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Karakasyan, C.; Mathos, J.; Lack, S.; Davy, J.; Marquis, M.; Renard, D. Microfluidics-assisted generation of stimuli-responsive hydrogels based on alginates incorporated with thermo-responsive and amphiphilic polymers as novel biomaterials. Colloids Surf. B-Biointerfaces 2015, 135, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Jeon, K.; Ryu, S.A.; Kim, D.P.; Lee, H. Temperature-Responsive Janus Particles as Microsurfactants for On-Demand Coalescence of Emulsions. Small 2020, 16, 2005159. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Wu, J.Y.; Han, S.H.; Yadavali, S.; Issadore, D.; Stebe, K.J.; Lee, D. Scalable Synthesis of Janus Particles with High Naturality. ACS Sustain. Chem. Eng. 2020, 8, 17680–17686. [Google Scholar] [CrossRef]
- Zykwinska, A.; Marquis, M.; Sinquin, C.; Cuenot, S.; Colliec-Jouault, S. Assembly of HE800 exopolysaccharide produced by a deep-sea hydrothermal bacterium into microgels for protein delivery applications. Carbohydr. Polym. 2016, 142, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Marengo, R.C.; Olivares, M.L.; Berli, C.L.A. Generation of egg white/carrageenan microparticles by droplet-based microfluidics. J. Food Eng. 2019, 259, 21–28. [Google Scholar] [CrossRef]
- Martins, E.; Poncelet, D.; Rodrigues, R.C.; Renard, D. Oil encapsulation techniques using alginate as encapsulating agent: Applications and drawbacks. J. Microencapsul. 2017, 34, 754–771. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.W.; Ju, X.J.; Xie, R.; Chu, L.Y. Monodisperse alginate microcapsules with oil core generated from a microfluidic device. J. Colloid Interface Sci. 2010, 343, 392–395. [Google Scholar] [CrossRef]
- Datta, S.S.; Abbaspourrad, A.; Amstad, E.; Fan, J.; Kim, S.H.; Romanowsky, M.; Shum, H.C.; Sun, B.J.; Utada, A.S.; Windbergs, M.; et al. 25th Anniversary Article: Double Emulsion Templated Solid Microcapsules: Mechanics and Controlled Release. Adv. Mater. 2014, 26, 2205–2218. [Google Scholar] [CrossRef] [PubMed]
- Marquis, M.; Alix, V.; Capron, I.; Cuenot, S.; Zykwinska, A. Microfluidic Encapsulation of Pickering Oil Microdroplets into Alginate Microgels for Lipophilic Compound Delivery. ACS Biomater. Sci. Eng. 2016, 2, 535–543. [Google Scholar] [CrossRef]
- Huang, L.Y.; Wu, K.; He, X.H.; Yang, Z.J.; Ji, H.B. One-Step microfluidic synthesis of spherical and bullet-like alginate microcapsules with a core-shell structure. Colloids Surf. A-Physicochem. Eng. Asp. 2021, 608, 125612. [Google Scholar] [CrossRef]
- Martins, E.; Poncelet, D.; Marquis, M.; Davy, J.; Renard, D. Monodisperse core-shell alginate (micro)-capsules with oil core generated from droplets millifluidic. Food Hydrocoll. 2017, 63, 447–456. [Google Scholar] [CrossRef]
- Pereda, M.; Poncelet, D.; Renard, D. Characterization of Core-Shell Alginate Capsules. Food Biophys. 2019, 14, 467–478. [Google Scholar] [CrossRef]
- Schmit, A.; Courbin, L.; Marquis, M.; Renard, D.; Panizza, P. A pendant drop method for the production of calibrated double emulsions and emulsion gels. RSC Adv. 2014, 4, 28504–28510. [Google Scholar] [CrossRef]
- Madadlou, A.; Saggiomo, V.; Schroen, K.; Fogliano, V. All-aqueous emulsions as miniaturized chemical reactors in the food and bioprocess technology. Curr. Opin. Food Sci. 2020, 33, 165–172. [Google Scholar] [CrossRef]
- Ziemecka, I.; van Steijn, V.; Koper, G.J.M.; Rosso, M.; Brizard, A.M.; van Esch, J.H.; Kreutzer, M.T. Monodisperse hydrogel microspheres by forced droplet formation in aqueous two-phase systems. Lab Chip 2011, 11, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Ziemecka, I.; van Steijn, V.; Koper, G.J.M.; Kreutzer, M.T.; van Esch, J.H. All-aqueous core-shell droplets produced in a microfluidic device. Soft Matter 2011, 7, 9878–9880. [Google Scholar] [CrossRef]
- Wassen, S.; Rondeau, E.; Sott, K.; Loren, N.; Fischer, P.; Hermansson, A.M. Microfluidic production of monodisperse biopolymer particles with reproducible morphology by kinetic control. Food Hydrocoll. 2012, 28, 20–27. [Google Scholar] [CrossRef]
- Nakagawa, K.; Iwamoto, S.; Nakajima, M.; Shono, A.; Satoh, K. Microchannel emulsification using gelatin and surfactant-free coacervate microencapsulation. J. Colloid Interface Sci. 2004, 278, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Sun, X.T.; Yang, C.G.; Xu, Z.R. On-chip preparation of calcium alginate particles based on droplet templates formed by using a centrifugal microfluidic technique. J. Colloid Interface Sci. 2016, 466, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Moon, B.U.; Abbasi, N.; Jones, S.G.; Hwang, D.K.; Tsai, S.S.H. Water-in-Water Droplets by Passive Microfluidic Flow Focusing. Anal. Chem. 2016, 88, 3982–3989. [Google Scholar] [CrossRef]
- Jeyhani, M.; Thevakumaran, R.; Abbasi, N.; Hwang, D.K.; Tsai, S.S.H. Microfluidic Generation of All-Aqueous Double and Triple Emulsions. Small 2020, 16, 1906565. [Google Scholar] [CrossRef] [PubMed]
- Schroen, K.; Ferrando, M.; de Lamo-Castellvi, S.; Sahin, S.; Guell, C. Linking Findings in Microfluidics to Membrane Emulsification Process Design: The Importance of Wettability and Component Interactions with Interfaces. Membranes 2016, 6, 26. [Google Scholar] [CrossRef]
- Garstecki, P.; Fuerstman, M.J.; Stone, H.A.; Whitesides, G.M. Formation of droplets and bubbles in a microfluidic T-junction—scaling and mechanism of break-up. Lab Chip 2006, 6, 437–446. [Google Scholar] [CrossRef]
- Steegmans, M.L.J.; Schroen, K.; Boom, R.M. Characterization of Emulsification at Flat Microchannel Y Junctions. Langmuir 2009, 25, 3396–3401. [Google Scholar] [CrossRef]
- Riechers, B.; Maes, F.; Akoury, E.; Semin, B.; Gruner, P.; Baret, J.C. Surfactant adsorption kinetics in microfluidics. Proc. Natl. Acad. Sci. USA 2016, 113, 11465–11470. [Google Scholar] [CrossRef]
- Muijlwijk, K.; Hinderink, E.; Ershov, D.; Berton-Carabin, C.; Schroen, K. Interfacial tension measured at high expansion rates and within milliseconds using microfluidics. J. Colloid Interface Sci. 2016, 470, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Van der Graaf, S.; Steegmans, M.L.J.; van der Sman, R.G.M.; Schroen, C.; Boom, R.M. Droplet formation in a T-shaped microchannel junction: A model system for membrane emulsification. Colloids Surf. A-Physicochem. Eng. Asp. 2005, 266, 106–116. [Google Scholar] [CrossRef]
- Steegmans, M.L.J.; Warmerdam, A.; Schroen, K.; Boom, R.M. Dynamic Interfacial Tension Measurements with Microfluidic Y-Junctions. Langmuir 2009, 25, 9751–9758. [Google Scholar] [CrossRef] [PubMed]
- Muijlwijk, K.; Huang, W.; Vuist, J.E.; Berton-Carabin, C.; Schroen, K. Convective mass transport dominates surfactant adsorption in a microfluidic Y-junction. Soft Matter 2016, 12, 9025–9029. [Google Scholar] [CrossRef] [PubMed]
- Skhiri, Y.; Gruner, P.; Semin, B.; Brosseau, Q.; Pekin, D.; Mazutis, L.; Goust, V.; Kleinschmidt, F.; El Harrak, A.; Hutchison, J.B.; et al. Dynamics of molecular transport by surfactants in emulsions. Soft Matter 2012, 8, 10618–10627. [Google Scholar] [CrossRef]
- Schroen, K.; de Ruiter, J.; Berton-Carabin, C. The importance of interfacial tension in emulsification: Connecting scaling relations used in large scale preparation with microfluidic measurement methods. ChemEngineering 2020, 4, 63. [Google Scholar] [CrossRef]
- Guell, C.; Ferrando, M.; Trentin, A.; Schroen, K. Apparent Interfacial Tension Effects in Protein Stabilized Emulsions Prepared with Microstructured Systems. Membranes 2017, 7, 19. [Google Scholar] [CrossRef]
- Krebs, T.; Schroen, K.; Boom, R. Coalescence dynamics of surfactant-stabilized emulsions studied with microfluidics. Soft Matter 2012, 8, 10650–10657. [Google Scholar] [CrossRef]
- Krebs, T.; Schroen, C.; Boom, R.M. Separation kinetics of an oil-in-water emulsion under enhanced gravity. Chem. Eng. Sci. 2012, 71, 118–125. [Google Scholar] [CrossRef]
- Krebs, T.; Schroen, C.; Boom, R.M. Coalescence kinetics of oil-in-water emulsions studied with microfluidics. Fuel 2013, 106, 327–334. [Google Scholar] [CrossRef]
- Dudek, M.; Muijlwijk, K.; Schroen, K.; Oye, G. The effect of dissolved gas on coalescence of oil drops studied with microfluidics. J. Colloid Interface Sci. 2018, 528, 166–173. [Google Scholar] [CrossRef]
- Muijlwijk, K.; Colijn, I.; Harsono, H.; Krebs, T.; Berton-Carabin, C.; Schroen, K. Coalescence of protein-stabilised emulsions studied with microfluidics. Food Hydrocoll. 2017, 70, 96–104. [Google Scholar] [CrossRef]
- Hinderink, E.B.A.; Kaade, W.; Sagis, L.; Schroen, K.; Berton-Carabin, C.C. Microfluidic investigation of the coalescence susceptibility of pea protein-stabilised emulsions: Effect of protein oxidation level. Food Hydrocoll. 2020, 102, 105610. [Google Scholar] [CrossRef]
- Schroder, A.; Sprakel, J.; Schroen, K.; Spaen, J.N.; Berton-Carabin, C.C. Coalescence stability of Pickering emulsions produced with lipid particles: A microfluidic study. J. Food Eng. 2018, 234, 63–72. [Google Scholar] [CrossRef]
- Pipe, C.J.; McKinley, G.H. Microfluidic rheometry. Mech. Res. Commun. 2009, 36, 110–120. [Google Scholar] [CrossRef]
- Zhao, C.X.; Rondeau, E.; Cooper-White, J.J.; Middelberg, A.P.J. Microfluidic Elucidation of the Effects of Interfacial Rheology on Droplet Deformation. Ind. Eng. Chem. Res. 2012, 51, 2021–2029. [Google Scholar] [CrossRef]
- Tregouet, C.; Salez, T.; Monteux, C.; Reyssat, M. Microfluidic probing of the complex interfacial rheology of multilayer capsules. Soft Matter 2019, 15, 2782–2790. [Google Scholar] [CrossRef]
- Hinderink, E.B.A.; de Ruiter, J.; de Leeuw, J.; Schroen, K.; Sagis, L.; Berton-Carabin, C.C. Early film formation in protein-stabilised emulsions: Insights from a microfluidic approach. Food Hydrocoll. 2021, 118, 106785. [Google Scholar] [CrossRef]
- Liu, M.; Xie, S.R.; Ge, J.; Xu, Z.S.; Wu, Z.Z.; Ru, C.H.; Luo, J.; Sun, Y. Microfluidic Assessment of Frying Oil Degradation. Sci. Rep. 2016, 6, 27970. [Google Scholar] [CrossRef] [PubMed]
- Krebs, T.; Ershov, D.; Schroen, C.; Boom, R.M. Coalescence and compression in centrifuged emulsions studied with in situ optical microscopy. Soft Matter 2013, 9, 4026–4035. [Google Scholar] [CrossRef]
- Feng, H.H.; Ershov, D.; Krebs, T.; Schroen, K.; Stuart, M.A.C.; van der Gucht, J.; Sprakel, J. Manipulating and quantifying temperature-triggered coalescence with microcentrifugation. Lab Chip 2015, 15, 188–194. [Google Scholar] [CrossRef]
- Bera, B.; Khazal, R.; Schroen, K. Coalescence dynamics in oil-in-water emulsions at elevated temperatures. Sci. Rep. 2021, 11, 10990. [Google Scholar] [CrossRef]
- Assenza, S.; Mezzenga, R. Soft condensed matter physics of foods and macronutrients. Nat. Rev. Phys. 2019, 1, 551–566. [Google Scholar] [CrossRef]
- Boire, A.; Renard, D.; Bouchoux, A.; Pezennec, S.; Croguennec, T.; Lechevalier, V.; Le Floch-Fouere, C.; Bouhallab, S.; Menut, P. Soft-Matter Approaches for Controlling Food Protein Interactions and Assembly. Annu. Rev. Food Sci. Technol. 2019, 10, 521–539. [Google Scholar] [CrossRef]
- Mao, H.B.; Li, C.M.; Zhang, Y.J.; Bergbreiter, D.E.; Cremer, P.S. Measuring LCSTs by novel temperature gradient methods: Evidence for intermolecular interactions in mixed polymer solutions. J. Am. Chem. Soc. 2003, 125, 2850–2851. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.B.; Li, C.M.; Zhang, Y.J.; Furyk, S.; Cremer, P.S.; Bergbreiter, D.E. High-throughput studies of the effects of polymer structure and solution components on the phase separation of thermoresponsive polymers. Macromolecules 2004, 37, 1031–1036. [Google Scholar] [CrossRef]
- Ildefonso, M.; Guidon, N.; Veesler, S. A Cheap, Easy Microfluidic Crystallization Device Ensuring Universal Solvent Compatibility. Org. Process Res. Dev. 2012, 16, 556–560. [Google Scholar] [CrossRef]
- Zhang, S.; Ferte, N.; Candoni, N.; Veesler, S. Versatile Microfluidic Approach to Crystallization. Org. Process Res. Dev. 2015, 19, 1837–1841. [Google Scholar] [CrossRef]
- Zhang, S.H.; Gerard, C.J.J.; Ikni, A.; Ferry, G.; Vuillard, L.M.; Boutin, J.A.; Ferte, N.; Grossier, R.; Candoni, N.; Veesler, S. Microfluidic platform for optimization of crystallization conditions. J. Cryst. Growth 2017, 472, 18–28. [Google Scholar] [CrossRef]
- Amine, C.; Boire, A.; Davy, J.; Marquis, M.; Renard, D. Droplets-based millifluidic for the rapid determination of biopolymers phase diagrams. Food Hydrocoll. 2017, 70, 134–142. [Google Scholar] [CrossRef]
- Amine, C.; Boire, A.; Davy, J.; Reguerre, A.L.; Papineau, P.; Renard, D. Optimization of a Droplet-Based Millifluidic Device to Investigate the Phase Behavior of Biopolymers, Including Viscous Conditions. Food Biophys. 2020, 15, 463–472. [Google Scholar] [CrossRef]
- Davy, J.; Amine, C.; Papineau, P.; Reguerre, A.L.; Boire, A.; Renard, D. Outil millifluidique à gouttes pour déterminer les diagrammes de phase de protéines seules ou en mélange. Cah. Tech. L’inra 2020, 102, Art6. [Google Scholar]
- Song, H.; Bringer, M.R.; Tice, J.D.; Gerdts, C.J.; Ismagilov, R.F. Experimental test of scaling of mixing by chaotic advection in droplets moving through microfluidic channels. Appl. Phys. Lett. 2003, 83, 4664–4666. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Tice, J.D.; Ismagilov, R.F. A microfluidic system for controlling reaction networks in time. Angew. Chem. Int. Ed. 2003, 42, 768–772. [Google Scholar] [CrossRef]
- Yasui, T.; Omoto, Y.; Osato, K.; Kaji, N.; Suzuki, N.; Naito, T.; Watanabe, M.; Okamoto, Y.; Tokeshi, M.; Shamoto, E.; et al. Microfluidic baker’s transformation device for three-dimensional rapid mixing. Lab Chip 2011, 11, 3356–3360. [Google Scholar] [CrossRef] [PubMed]
- Lau, B.T.C.; Baitz, C.A.; Dong, X.P.; Hansen, C.L. A complete microfluidic screening platform for rational protein crystallization. J. Am. Chem. Soc. 2007, 129, 454–455. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.U.; Cristobal, G.; Link, D.R.; Thorsen, T.; Jia, Y.W.; Piattelli, K.; Fraden, S. Control and measurement of the phase behavior of aqueous solutions using microfluidics. J. Am. Chem. Soc. 2007, 129, 8825–8835. [Google Scholar] [CrossRef]
- Leng, J.; Lonetti, B.; Tabeling, P.; Joanicot, M.; Ajdari, A. Microevaporators for kinetic exploration of phase diagrams. Phys. Rev. Lett. 2006, 96, 084503. [Google Scholar] [CrossRef]
- Leng, J.; Joanicot, M.; Ajdari, A. Microfluidic exploration of the phase diagram of a surfactant/water binary system. Langmuir 2007, 23, 2315–2317. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Selimovic, S.; Gobeaux, F.; Fraden, S. Mapping and manipulating temperature-concentration phase diagrams using microfluidics. Lab Chip 2010, 10, 1696–1699. [Google Scholar] [CrossRef]
- Shangguan, Y.G.; Guo, D.M.; Feng, H.; Li, Y.; Gong, X.J.; Chen, Q.J.; Zheng, B.; Wu, C. Mapping Phase Diagrams of Polymer Solutions by a Combination of Microfluidic Solution Droplets and Laser Light-Scattering Detection. Macromolecules 2014, 47, 2496–2502. [Google Scholar] [CrossRef]
- Zheng, B.; Tice, J.D.; Roach, L.S.; Ismagilov, R.F. A droplet-based, composite PDMS/glass capillary microfluidic system for evaluating protein crystallization conditions by microbatch and vapor-diffusion methods with on-chip X-ray diffraction. Angew. Chem. Int. Ed. 2004, 43, 2508–2511. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Dehmoune, J.; Salmon, J.B.; Leng, J. Microevaporators with accumulators for the screening of phase diagrams of aqueous solutions. Appl. Phys. Lett. 2009, 95, 033108. [Google Scholar] [CrossRef]
- Leng, J.; Salmon, J.B. Microfluidic crystallization. Lab Chip 2009, 9, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Shum, H.C.; Lee, D.; Yoon, I.; Kodger, T.; Weitz, D.A. Double emulsion templated monodisperse phospholipid vesicles. Langmuir 2008, 24, 7651–7653. [Google Scholar] [CrossRef]
- Thiele, J.; Abate, A.R.; Shum, H.C.; Bachtler, S.; Forster, S.; Weitz, D.A. Fabrication of Polymersomes using Double-Emulsion Templates in Glass-Coated Stamped Microfluidic Devices. Small 2010, 6, 1723–1727. [Google Scholar] [CrossRef]
- Deshpande, S.; Caspi, Y.; Meijering, A.E.C.; Dekker, C. Octanol-assisted liposome assembly on chip. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Petit, J.; Polenz, I.; Baret, J.C.; Herminghaus, S.; Baumchen, O. Vesicles-on-a-chip: A universal microfluidic platform for the assembly of liposomes and polymersomes. Eur. Phys. J. E 2016, 39, 1–6. [Google Scholar] [CrossRef]
- Ekanem, E.E.; Zhang, Z.L.; Vladisavljevic, G.T. Facile microfluidic production of composite polymer core-shell microcapsules and crescent-shaped microparticles. J. Colloid Interface Sci. 2017, 498, 387–394. [Google Scholar] [CrossRef]
- Ugrinic, M.; Zambrano, A.; Berger, S.; Mann, S.; Tang, T.Y.D.; Demello, A. Microfluidic formation of proteinosomes. Chem. Commun. 2018, 54, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Song, L.Z.; Hobaugh, M.R.; Shustak, C.; Cheley, S.; Bayley, H.; Gouaux, J.E. Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science 1996, 274, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Noireaux, V.; Libchaber, A. A vesicle bioreactor as a step toward an artificial cell assembly. Proc. Natl. Acad. Sci. USA 2004, 101, 17669–17674. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.; Kuhn, P.; Eyer, K.; Dittrich, P.S. Microfluidic trapping of giant unilamellar vesicles to study transport through a membrane pore. Biomicrofluidics 2013, 7, 044105. [Google Scholar] [CrossRef]
- Tsuji, Y.; Kawano, R.; Osaki, T.; Kamiya, K.; Miki, N.; Takeuchi, S. Droplet-based lipid bilayer system integrated with microfluidic channels for solution exchange. Lab Chip 2013, 13, 1476–1481. [Google Scholar] [CrossRef] [PubMed]
- Gopfrich, K.; Haller, B.; Staufer, O.; Dreher, Y.; Mersdorf, U.; Platzman, I.; Spatz, J.P. One-Pot Assembly of Complex Giant Unilamellar Vesicle-Based Synthetic Cells. ACS Synth. Biol. 2019, 8, 937–947. [Google Scholar] [CrossRef]
- Deshpande, S.; Brandenburg, F.; Lau, A.; Last, M.G.F.; Spoelstra, W.K.; Reese, L.; Wunnava, S.; Dogterom, M.; Dekker, C. Spatiotemporal control of coacervate formation within liposomes. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Last, M.G.F.; Deshpande, S.; Dekker, C. pH-Controlled Coacervate-Membrane Interactions within Liposomes. ACS Nano 2020, 14, 4487–4498. [Google Scholar] [CrossRef]
- Cochereau, R.; Renard, D.; Nous, C.; Boire, A. Semi-permeable vesicles produced by microfluidics to tune the phase behaviour of encapsulated macromolecules. J. Colloid Interface Sci. 2020, 580, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Huebner, A.; Bratton, D.; Whyte, G.; Yang, M.; DeMello, A.J.; Abell, C.; Hollfelder, F. Static microdroplet arrays: A microfluidic device for droplet trapping, incubation and release for enzymatic and cell-based assays. Lab Chip 2009, 9, 692–698. [Google Scholar] [CrossRef]
- Paterson, D.J.; Reboud, J.; Wilson, R.; Tassieri, M.; Cooper, J.M. Integrating microfluidic generation, handling and analysis of biomimetic giant unilamellar vesicles. Lab Chip 2014, 14, 1806–1810. [Google Scholar] [CrossRef] [PubMed]
- Marze, S.; Algaba, H.; Marquis, M. A microfluidic device to study the digestion of trapped lipid droplets. Food Funct. 2014, 5, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Scheuble, N.; Iles, A.; Wootton, R.C.R.; Windhab, E.J.; Fischer, P.; Elvira, K.S. Microfluidic Technique for the Simultaneous Quantification of Emulsion Instabilities and Lipid Digestion Kinetics. Anal. Chem. 2017, 89, 9116–9123. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Marquis, M.; Anton, M.; Marze, S. Studying the real-time interplay between triglyceride digestion and lipophilic micronutrient bioaccessibility using droplet microfluidics. 1 lab on a chip method. Food Chem. 2019, 275, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Marquis, M.; Anton, M.; Marze, S. Studying the real-time interplay between triglyceride digestion and lipophilic micronutrient bioaccessibility using droplet microfluidics. 2 application to various oils and (pro)vitamins. Food Chem. 2019, 275, 661–667. [Google Scholar] [CrossRef]
- Nisisako, T.; Portonovo, S.A.; Schmidt, J.J. Microfluidic passive permeability assay using nanoliter droplet interface lipid bilayers. Analyst 2013, 138, 6793–6800. [Google Scholar] [CrossRef]
- Korner, J.L.; Stephenson, E.B.; Elvira, K.S. A bespoke microfluidic pharmacokinetic compartment model for drug absorption using artificial cell membranes. Lab Chip 2020, 20, 1898–1906. [Google Scholar] [CrossRef]
- Barea, J.S.; Lee, J.; Kang, D.K. Recent Advances in Droplet-based Microfluidic Technologies for Biochemistry and Molecular Biology. Micromachines 2019, 10, 412. [Google Scholar] [CrossRef]
- Negou, J.T.; Adriana Avila, L.; Li, X.; Hagos, T.M.; Easley, C.J. Automated Microfluidic Droplet-Based Sample Chopper for Detection of Small Fluorescence Differences Using Lock-In Analysis. Anal. Chem. 2017, 89, 6153–6159. [Google Scholar] [CrossRef]
- Hu, J.; Li, X.P.; Judd, R.L.; Easley, C.J. Rapid lipolytic oscillations in ex vivo adipose tissue explants revealed through microfluidic droplet sampling at high temporal resolution. Lab Chip 2020, 20, 1503–1512. [Google Scholar] [CrossRef]
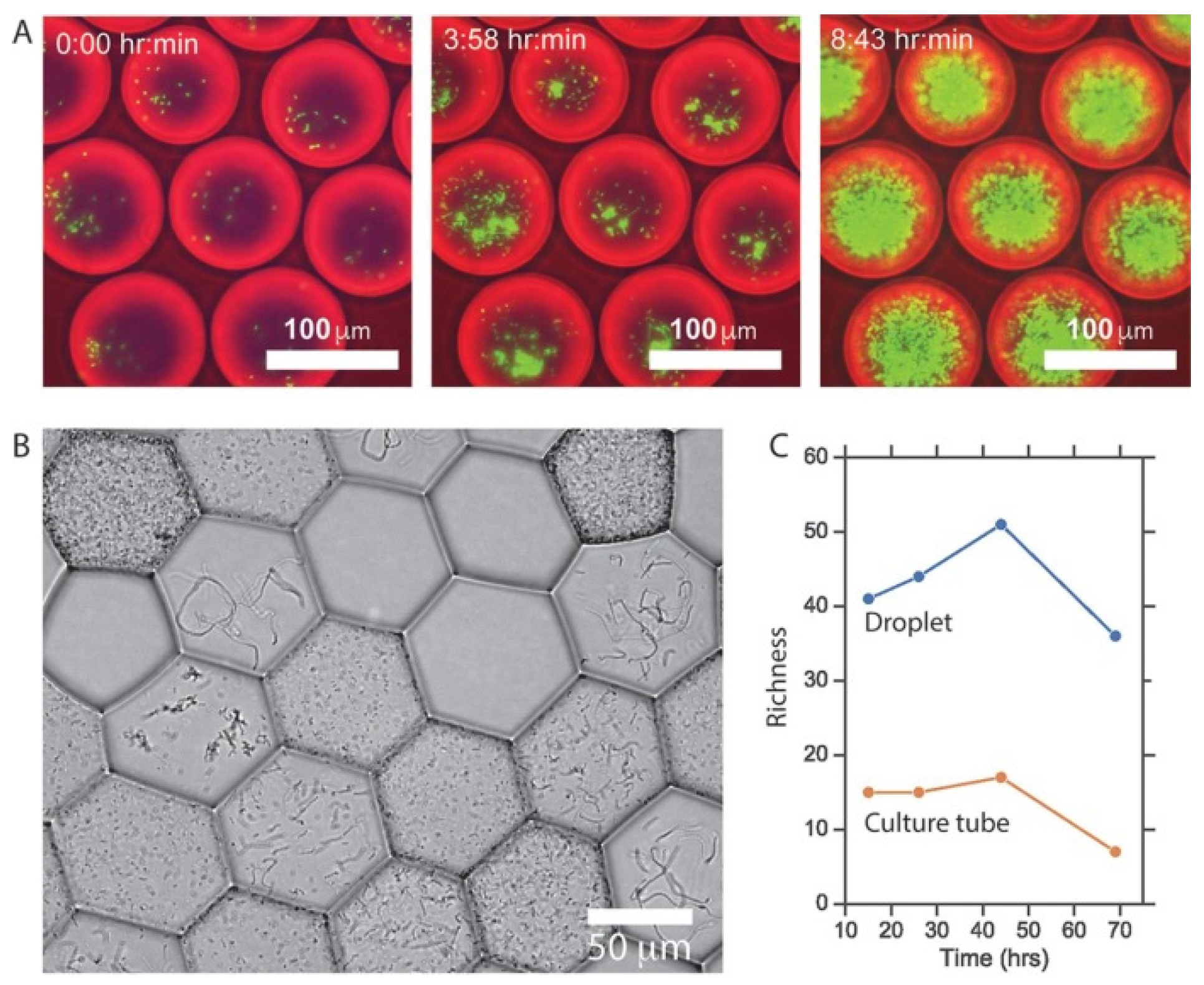
- Villa, M.M.; Bloom, R.J.; Silverman, J.D.; Durand, H.K.; Jiang, S.; Wu, A.C.; Dallow, E.P.; Huang, S.Q.; You, L.C.; David, L.A. Interindividual Variation in Dietary Carbohydrate Metabolism by Gut Bacteria Revealed with Droplet Microfluidic Culture. Msystems 2020, 5, e00864-19. [Google Scholar] [CrossRef]
- Tan, J.Y.; Wang, S.D.; Dick, G.J.; Young, V.B.; Sherman, D.H.; Burns, M.A.; Lin, X.N. Co-cultivation of microbial sub-communities in microfluidic droplets facilitates high-resolution genomic dissection of microbial ‘dark matter’. Integr. Biol. 2020, 12, 263–274. [Google Scholar] [CrossRef]
- Tauzin, A.S.; Pereira, M.R.; Van Vliet, L.D.; Colin, P.Y.; Laville, E.; Esque, J.; Laguerre, S.; Henrissat, B.; Terrapon, N.; Lombard, V.; et al. Investigating host-microbiome interactions by droplet based microfluidics. Microbiome 2020, 8, 1–20. [Google Scholar] [CrossRef]
- Knight, J.B.; Vishwanath, A.; Brody, J.P.; Austin, R.H. Hydrodynamic focusing on a silicon chip: Mixing nanoliters in microseconds. Phys. Rev. Lett. 1998, 80, 3863–3866. [Google Scholar] [CrossRef]
- Hertzog, D.E.; Michalet, X.; Jager, M.; Kong, X.X.; Santiago, J.G.; Weiss, S.; Bakajin, O. Femtomole mixer for microsecond kinetic studies of protein folding. Anal. Chem. 2004, 76, 7169–7178. [Google Scholar] [CrossRef] [PubMed]
- Hess, D.; Yang, T.J.; Stavrakis, S. Droplet-based optofluidic systems for measuring enzyme kinetics. Anal. Bioanal. Chem. 2020, 412, 3265–3283. [Google Scholar] [CrossRef]
- Renard, D.; Sanchez, C. Small Angle Scattering and ab initio modeling. In Advances in Physicochemical Properties of Biopolymers: Part 1; Masuelli, M., Renard, D., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2017. [Google Scholar]
- Kirby, N.M.; Cowieson, N.P. Time-resolved studies of dynamic biomolecules using small angle X-ray scattering. Curr. Opin. Struct. Biol. 2014, 28, 41–46. [Google Scholar] [CrossRef]
- Jain, R.; Petri, M.; Kirschbaum, S.; Feindt, H.; Steltenkamp, S.; Sonnenkalb, S.; Becker, S.; Griesinger, C.; Menzel, A.; Burg, T.P.; et al. X-ray scattering experiments with high-flux X-ray source coupled rapid mixing microchannel device and their potential for high-flux neutron scattering investigations. Eur. Phys. J. E 2013, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kathuria, S.V.; Kayatekin, C.; Barrea, R.; Kondrashkina, E.; Graceffa, R.; Guo, L.; Nobrega, R.P.; Chakravarthy, S.; Matthews, C.R.; Irving, T.C.; et al. Microsecond Barrier-Limited Chain Collapse Observed by Time-Resolved FRET and SAXS. J. Mol. Biol. 2014, 426, 1980–1994. [Google Scholar] [CrossRef] [PubMed]
- Lafleur, J.P.; Snakenborg, D.; Nielsen, S.S.; Moller, M.; Toft, K.N.; Menzel, A.; Jacobsen, J.K.; Vestergaard, B.; Arleth, L.; Kutter, J.P. Automated microfluidic sample-preparation platform for high-throughput structural investigation of proteins by small-angle X-ray scattering. J. Appl. Crystallogr. 2011, 44, 1090–1099. [Google Scholar] [CrossRef]
- Skou, M.; Skou, S.; Jensen, T.G.; Vestergaard, B.; Gillilan, R.E. In situ microfluidic dialysis for biological small-angle X-ray scattering. J. Appl. Crystallogr. 2014, 47, 1355–1366. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Jiang, L.; Mancuso, M.; Jain, A.; Oncescu, V.; Erickson, D. Optofluidic opportunities in global health, food, water and energy. Nanoscale 2012, 4, 4839–4857. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schroen, K.; Berton-Carabin, C.; Renard, D.; Marquis, M.; Boire, A.; Cochereau, R.; Amine, C.; Marze, S. Droplet Microfluidics for Food and Nutrition Applications. Micromachines 2021, 12, 863. https://doi.org/10.3390/mi12080863
Schroen K, Berton-Carabin C, Renard D, Marquis M, Boire A, Cochereau R, Amine C, Marze S. Droplet Microfluidics for Food and Nutrition Applications. Micromachines. 2021; 12(8):863. https://doi.org/10.3390/mi12080863
Chicago/Turabian StyleSchroen, Karin, Claire Berton-Carabin, Denis Renard, Mélanie Marquis, Adeline Boire, Rémy Cochereau, Chloé Amine, and Sébastien Marze. 2021. "Droplet Microfluidics for Food and Nutrition Applications" Micromachines 12, no. 8: 863. https://doi.org/10.3390/mi12080863
APA StyleSchroen, K., Berton-Carabin, C., Renard, D., Marquis, M., Boire, A., Cochereau, R., Amine, C., & Marze, S. (2021). Droplet Microfluidics for Food and Nutrition Applications. Micromachines, 12(8), 863. https://doi.org/10.3390/mi12080863






