Electrochemical Cell-Based Sensor for Detection of Food Hazards
Abstract
1. Introduction
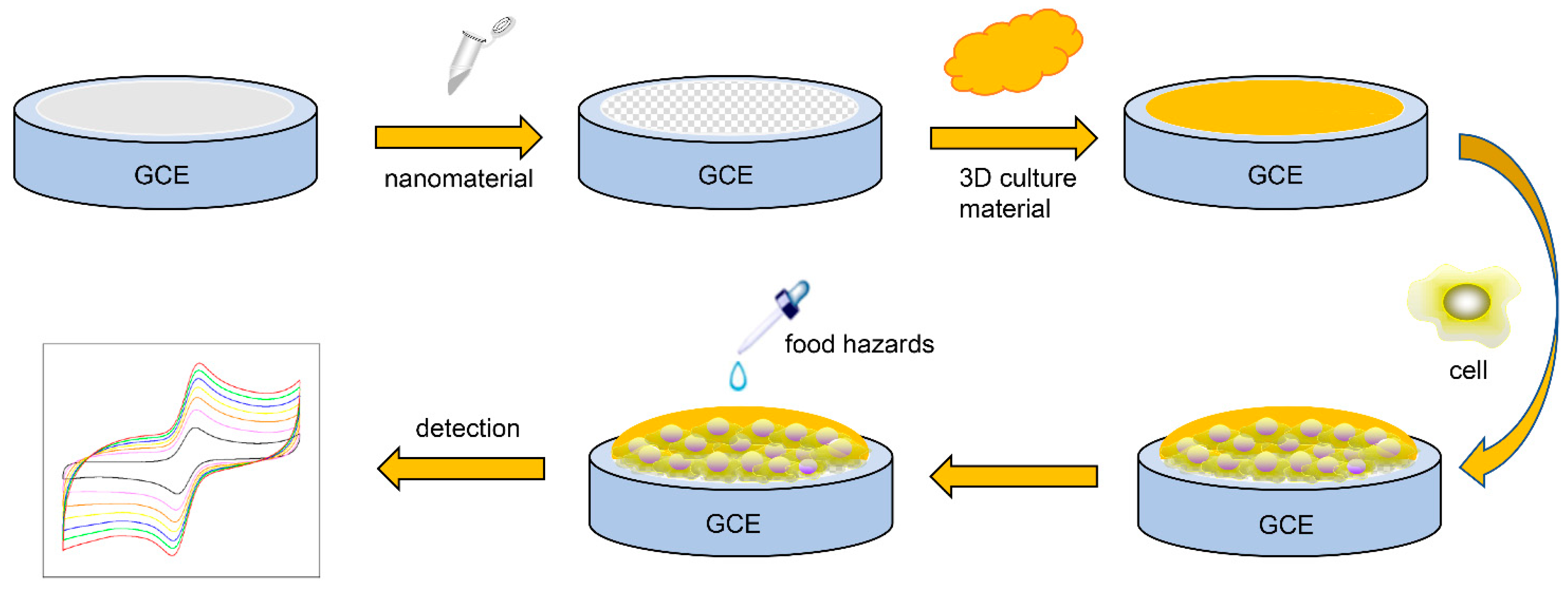
2. Cell-Based Electrochemical Sensors
2.1. Characteristics of Cell-Based Electrochemical Sensors
2.2. Electrode Surface Modification
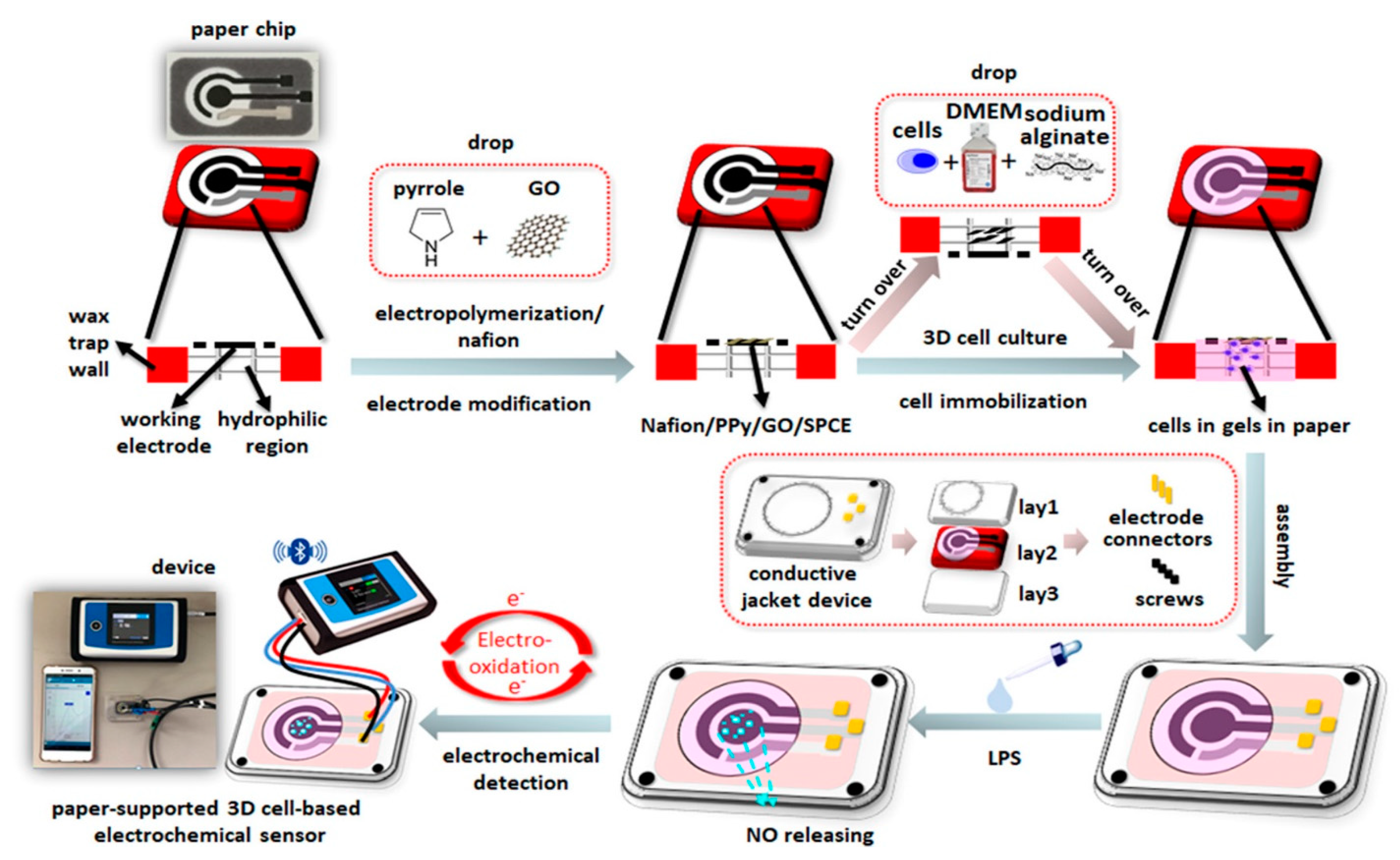
2.3. Immobilization and 3D Culture of Cells on Electrodes
3. Application of Cell-Based Electrochemical Sensor in Detection of Hazardous Substances in Food
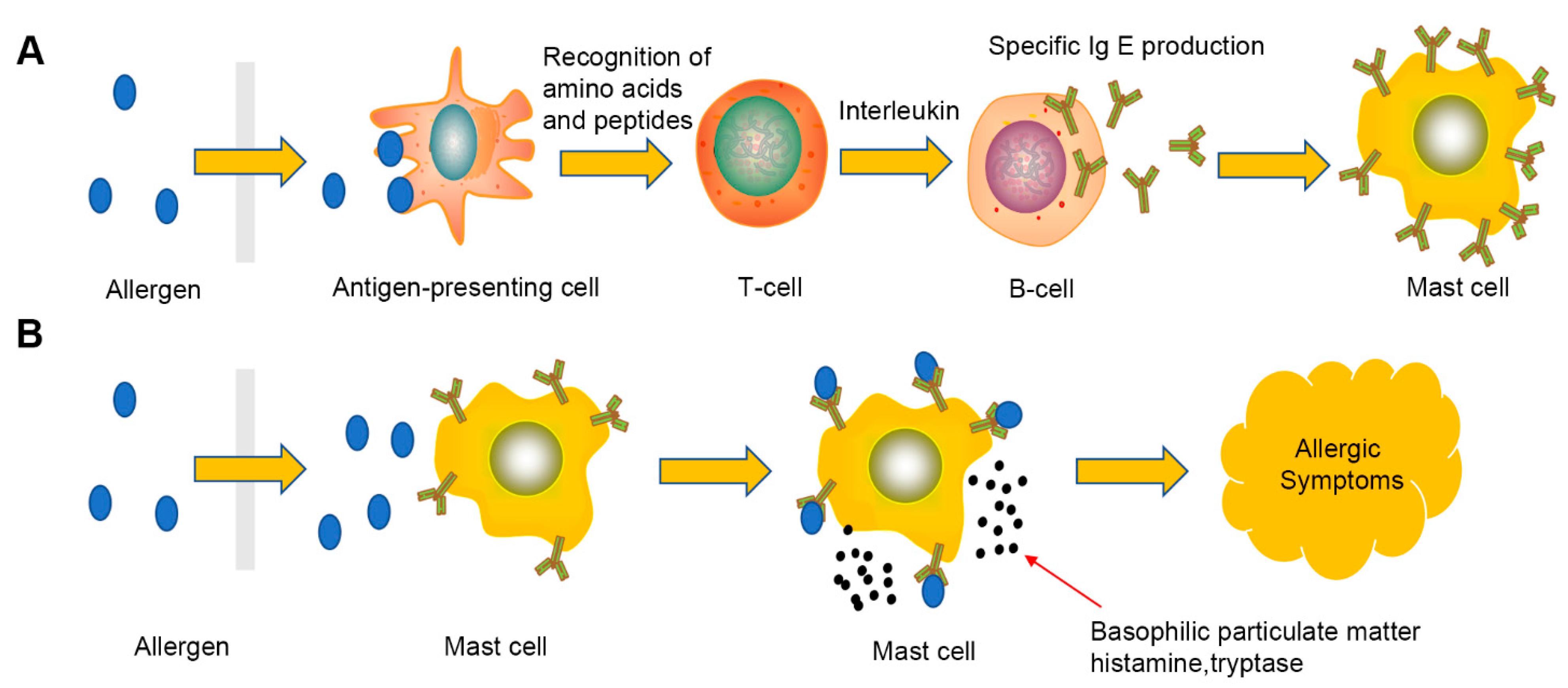
3.1. Food Allergen
3.2. Toxins in Food
3.3. Other Common Food Hazards
4. Limitations of Cell-Based Electrochemical Sensors
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Borda, D.; Mihalache, O.A.; Dumitraşcu, L.; Gafițianu, D.; Nicolau, A.I. Romanian consumers’ food safety knowledge, awareness on certified labelled food and trust in information sources. Food Control 2021, 120, 107544. [Google Scholar] [CrossRef]
- Todd, E.C.D. Foodborne Diseases: Overview of Biological Hazards and Foodborne Diseases. Encycl. Food Saf. 2014, 221–242. [Google Scholar] [CrossRef]
- Ma, Y.; Ding, S.; Fei, Y.; Liu, G.; Jang, H.; Fang, J. Antimicrobial activity of anthocyanins and catechins against foodborne pathogens Escherichia coli and Salmonella. Food Control 2019, 106, 106712. [Google Scholar] [CrossRef]
- Rivera, D.; Toledo, V.; Reyes-Jara, A.; Navarrete, P.; Tamplin, M.; Kimura, B.; Wiedmann, M.; Silva, P.; Moreno Switt, A.I. Approaches to empower the implementation of new tools to detect and prevent foodborne pathogens in food processing. Food Microbiol. 2018, 75, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Narwal, V.; Deswal, R.; Batra, B.; Kalra, V.; Hooda, R.; Sharma, M.; Rana, J.S. Cholesterol biosensors: A review. Steroids 2019, 143, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Gui, Q.; Lawson, T.; Shan, S.; Yan, L.; Liu, Y. The Application of Whole Cell-Based Biosensors for Use in Environmental Analysis and in Medical Diagnostics. Sensors 2017, 17, 1623. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Wu, Y.; Li, R.; Wang, P.; Yan, W.; Zheng, X. Cell-based biosensors:Towards the development of cellular monitoring. Chin. Sci. Bull. 2002, 1849–1856. [Google Scholar] [CrossRef]
- Banerjee, P.; Kintzios, S.; Prabhakarpandian, B. Biotoxin detection using cell-based sensors. Toxins 2013, 5, 2366–2383. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Tan, L.; Zhou, Y.; Jiang, X.; Xie, Q.; Tang, H.; Yao, S. Magnetic immobilization and electrochemical detection of leukemia K562 cells. Electrochem. Commun. 2009, 11, 141–144. [Google Scholar] [CrossRef]
- Weng, J.; Zhang, Z.; Sun, L.; Wang, J.A. High sensitive detection of cancer cell with a folic acid-based boron-doped diamond electrode using an AC impedimetric approach. Biosens. Bioelectron. 2011, 26, 1847–1852. [Google Scholar] [CrossRef]
- Zhong, X.; Bai, H.-J.; Xu, J.-J.; Chen, H.-Y.; Zhu, Y.-H. A Reusable Interface Constructed by 3-Aminophenylboronic Acid-Functionalized Multiwalled Carbon Nanotubes for Cell Capture, Release, and Cytosensing. Adv. Funct. Mater. 2010, 20, 992–999. [Google Scholar] [CrossRef]
- Jing-Jing, Z.; Fang-Fang, C.; Ting-Ting, Z.; Jun-Jie, Z. Design and implementation of electrochemical cytosensor for evaluation of cell surface carbohydrate and glycoprotein. Anal. Chem. 2010, 82, 3547–3555. [Google Scholar]
- Nonner, W. Electrodiffusion in ionic channels of biological membranes. J. Mol. Liqs. 2000, 87, 149–162. [Google Scholar] [CrossRef]
- Wegener, J.; Keese, C.R.; Giaever, I. Electric cell-substrate impedance sensing (ECIS) as a noninvasive means to monitor the kinetics of cell spreading to artificial surfaces. Exp. Cell Res. 2000, 259, 158–166. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Nordin, A.N.; Li, F.; Jang, S.; Voiculescu, I. The influence of the electrode dimension on the detection sensitivity of electric cell–substrate impedance sensing (ECIS) and its mathematical modeling. Sens. Actuators B Chem. 2017, 247, 780–790. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, K.; Li, B.; Wang, C.; Ding, Y.; Li, C.; Mao, L.; Lin, Y. Fabrication of a Flexible and Stretchable Nanostructured Gold Electrode Using a Facile Ultraviolet-Irradiation Approach for the Detection of Nitric Oxide Released from Cells. Anal. Chem. 2018, 90, 7158–7163. [Google Scholar] [CrossRef]
- Lin, Y.; Bariya, M.; Nyein, H.Y.Y.; Kivimäki, L.; Uusitalo, S.; Jansson, E.; Ji, W.; Yuan, Z.; Happonen, T.; Liedert, C.; et al. Porous Enzymatic Membrane for Nanotextured Glucose Sweat Sensors with High Stability toward Reliable Noninvasive Health Monitoring. Adv. Funct. Mater. 2019, 29, 1902521. [Google Scholar] [CrossRef]
- Kokkinos, C.; Economou, A.; Giokas, D. Paper-based device with a sputtered tin-film electrode for the voltammetric determination of Cd(II) and Zn(II). Sens. Actuators B: Chem 2018, 260, 223–226. [Google Scholar] [CrossRef]
- Justino, C.I.L.; Gomes, A.R.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A.P. Graphene based sensors and biosensors. TrAC Trends Anal. Chem. 2017, 91, 53–66. [Google Scholar] [CrossRef]
- Gu, W.; Zhu, P.; Jiang, D.; He, X.; Li, Y.; Ji, J.; Zhang, L.; Sun, Y.; Sun, X. A novel and simple cell-based electrochemical impedance biosensor for evaluating the combined toxicity of DON and ZEN. Biosens. Bioelectron. 2015, 70, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Xian, G.C.; Rui, N.S.; Yun, K.S.; Xinting, Z.; Peng, C.; Ming, L.C. RGD-peptide functionalized graphene biomimetic live-cell sensor for real-time detection of nitric oxide molecules. ACS Nano 2012, 6, 6944–6951. [Google Scholar]
- Viswanathan, S.; Radecka, H.; Radecki, J. Electrochemical biosensors for food analysis. Mon. Chem. Chem. Mon. 2009, 140, 891–899. [Google Scholar] [CrossRef]
- Tilmaciu, C.M.; Morris, M.C. Carbon nanotube biosensors. Front. Chem. 2015, 3, 59. [Google Scholar] [CrossRef]
- Cellot, G.; Cilia, E.; Cipollone, S.; Rancic, V.; Sucapane, A.; Giordani, S.; Gambazzi, L.; Markram, H.; Grandolfo, M.; Scaini, D.; et al. Carbon nanotubes might improve neuronal performance by favouring electrical shortcuts. Nat. Nanotechnol. 2009, 4, 126–133. [Google Scholar] [CrossRef]
- Taale, M.; Schutt, F.; Zheng, K.; Mishra, Y.K.; Boccaccini, A.R.; Adelung, R.; Selhuber-Unkel, C. Bioactive Carbon-Based Hybrid 3D Scaffolds for Osteoblast Growth. ACS Appl. Mater. Interfaces 2018, 10, 43874–43886. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, R.; Ali, M.; Lee, H.; Ko, M.J. In Situ Grown MWCNTs/MXenes Nanocomposites on Carbon Cloth for High-Performance Flexible Supercapacitors. Adv. Funct. Mater. 2020, 30, 2002739. [Google Scholar] [CrossRef]
- Yao, L.; Teng, J.; Zhu, M.; Zheng, L.; Zhong, Y.; Liu, G.; Xue, F.; Chen, W. MWCNTs based high sensitive lateral flow strip biosensor for rapid determination of aqueous mercury ions. Biosens. Bioelectron. 2016, 85, 331–336. [Google Scholar] [CrossRef]
- Zhang, R.; Ying, C.; Gao, H.; Liu, Q.; Fu, X.; Hu, S. Highly flexible strain sensors based on polydimethylsiloxane/carbon nanotubes (CNTs) prepared by a swelling/permeating method and enhanced sensitivity by CNTs surface modification. Compos. Sci. Technol. 2019, 171, 218–225. [Google Scholar] [CrossRef]
- Guo, J.W.; Zhang, B.; Hou, Y.; Yang, S.; Yang, X.H.; Yang, H.G. A sulfur-assisted strategy to decorate MWCNTs with highly dispersed Pt nanoparticles for counter electrode in dye-sensitized solar cells. J. Mater. Chem. A 2013, 1, 1982–1986. [Google Scholar] [CrossRef]
- Li, J.; Huang, X.; Shi, W.; Jiang, M.; Tian, L.; Su, M.; Wu, J.; Liu, Q.; Yu, C.; Gu, H. Pt nanoparticle decorated carbon nanotubes nanocomposite based sensing platform for the monitoring of cell-secreted dopamine. Sens. Actuators B Chem. 2021, 330, 129311. [Google Scholar] [CrossRef]
- Vlasceanu, G.M.; Amarandi, R.M.; Ionita, M.; Tite, T.; Iovu, H.; Pilan, L.; Burns, J.S. Versatile graphene biosensors for enhancing human cell therapy. Biosens. Bioelectron. 2018, 117, 283–302. [Google Scholar] [CrossRef]
- Taniselass, S.; Arshad, M.K.M.; Gopinath, S.C.B. Graphene-based electrochemical biosensors for monitoring noncommunicable disease biomarkers. Biosens. Bioelectron. 2019, 130, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yang, J.; Wan, K.; Jiang, D.; Jin, C. Miniaturized Paper-Supported 3D Cell-Based Electrochemical Sensor for Bacterial Lipopolysaccharide Detection. ACS Sens. 2020, 5, 1325–1335. [Google Scholar] [CrossRef]
- Minati, L.; Antonini, V.; Torrengo, S.; Serra, M.D.; Boustta, M.; Leclercq, X.; Migliaresi, C.; Vert, M.; Speranza, G. Sustained in vitro release and cell uptake of doxorubicin adsorbed onto gold nanoparticles and covered by a polyelectrolyte complex layer. Int. J. Pharm. 2012, 438, 45–52. [Google Scholar] [CrossRef]
- Jiang, D.; Ji, J.; An, L.; Sun, X.; Zhang, Y.; Zhang, G.; Tang, L. Mast cell-based electrochemical biosensor for quantification of the major shrimp allergen Pen a 1 (tropomyosin). Biosens. Bioelectron. 2013, 50, 150–156. [Google Scholar] [CrossRef]
- Li, J.; Xie, J.; Gao, L.; Li, C.M. Au nanoparticles-3D graphene hydrogel nanocomposite to boost synergistically in situ detection sensitivity toward cell-released nitric oxide. ACS Appl. Mater. Interfaces 2015, 7, 2726–2734. [Google Scholar] [CrossRef]
- Fan, W.T.; Qin, Y.; Hu, X.B.; Yan, J.; Wu, W.T.; Liu, Y.L.; Huang, W.H. Stretchable Electrode Based on Au@Pt Nanotube Networks for Real-Time Monitoring of ROS Signaling in Endothelial Mechanotransduction. Anal Chem. 2020, 92, 15639–15646. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, C.; Cai, H.; Hu, N.; Zhou, J.; Wang, P. Cell-Based Biosensors and Their Application in Biomedicine. Chem. Rev. 2014, 114, 6423–6461. [Google Scholar] [CrossRef]
- Deng, Y.; Zheng, H.; Yi, X.; Shao, C.; Xiang, B.; Wang, S.; Zhao, Z.; Zhang, X.; Hui, G. Paralytic shellfish poisoning toxin detection based on cell-based sensor and non-linear signal processing model. Int. J. Food Prop. 2019, 22, 890–897. [Google Scholar] [CrossRef]
- Xia, S.; Zhu, P.; Pi, F.; Zhang, Y.; Li, Y.; Wang, J.; Sun, X. Development of a simple and convenient cell-based electrochemical biosensor for evaluating the individual and combined toxicity of DON, ZEN, and AFB1. Biosens. Bioelectron. 2017, 97, 345–351. [Google Scholar] [CrossRef]
- Li, Y.; Yu, C. RGD peptide doped polypyrrole film as a biomimetic electrode coating for impedimetric sensing of cell proliferation and cytotoxicity. J. Appl. Biomed. 2017, 15, 256–264. [Google Scholar] [CrossRef]
- Hitoshi, A.; Katsumi, M.; Tetsuya, H. Seamless signal transduction from live cells to an NO sensor via a cell-adhesive sensing matrix. Anal. Chem. 2008, 80, 1505–1511. [Google Scholar]
- Asphahani, F.; Thein, M.; Veiseh, O.; Edmondson, D.; Kosai, R.; Veiseh, M.; Xu, J.; Zhang, M. Influence of cell adhesion and spreading on impedance characteristics of cell-based sensors. Biosens. Bioelectron. 2008, 23, 1307–1313. [Google Scholar] [CrossRef]
- Wu, Y.; Lian, J.; Goncales, V.R.; Pardehkhorram, R.; Tang, W.; Tilley, R.D.; Gooding, J.J. Patterned Molecular Films of Alkanethiol and PLL-PEG on Gold-Silicate Interfaces: How to Add Functionalities while Retaining Effective Antifouling. Langmuir 2020, 36, 5243–5250. [Google Scholar] [CrossRef] [PubMed]
- Foglietta, F.; Canaparo, R.; Muccioli, G.; Terreno, E.; Serpe, L. Methodological aspects and pharmacological applications of three-dimensional cancer cell cultures and organoids. Life Sci. 2020, 254, 117784. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dai, X.; Zhang, X.; Li, X.; Xu, T.; Lan, Q. Enrichment of glioma stem cell-like cells on 3D porous scaffolds composed of different extracellular matrix. Biochem. Biophys. Res. Commun. 2018, 498, 1052–1057. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhou, M.; Zhang, M.; Liu, W.; Zhou, Y.; Lang, M. Hepatocyte culture on 3D porous scaffolds of PCL/PMCL. Colloids Surf. B Biointerfaces 2019, 173, 185–193. [Google Scholar] [CrossRef]
- Liu, T.; Yi, S.; Liu, G.; Hao, X.; Du, T.; Chen, J.; Meng, T.; Li, P.; Wang, Y. Aqueous two-phase emulsions-templated tailorable porous alginate beads for 3D cell culture. Carbohydr. Polym. 2021, 258, 117702. [Google Scholar] [CrossRef]
- Jiang, D.; Ge, P.; Wang, L.; Jiang, H.; Yang, M.; Yuan, L.; Ge, Q.; Fang, W.; Ju, X. A novel electrochemical mast cell-based paper biosensor for the rapid detection of milk allergen casein. Biosens. Bioelectron. 2019, 130, 299–306. [Google Scholar] [CrossRef]
- Liang, L.; Su, M.; Li, L.; Lan, F.; Yang, G.; Ge, S.; Yu, J.; Song, X. Aptamer-based fluorescent and visual biosensor for multiplexed monitoring of cancer cells in microfluidic paper-based analytical devices. Sens. Actuators B Chem. 2016, 229, 347–354. [Google Scholar] [CrossRef]
- Roberts, I.S.D.; Brenchley, P.E.C. Mast cells: The forgotten cells of renal fibrosis. J. Clin. Pathol. 2000, 53, 858–862. [Google Scholar] [CrossRef]
- Becker, M.; Lemmermann, N.A.; Ebert, S.; Baars, P.; Renzaho, A.; Podlech, J.; Stassen, M.; Reddehase, M.J. Mast cells as rapid innate sensors of cytomegalovirus by TLR3/TRIF signaling-dependent and -independent mechanisms. Cell. Mol. Immunol. 2015, 12, 192–201. [Google Scholar] [CrossRef][Green Version]
- Jiang, D.; Zhu, P.; Jiang, H.; Ji, J.; Sun, X.; Gu, W.; Zhang, G. Fluorescent magnetic bead-based mast cell biosensor for electrochemical detection of allergens in foodstuffs. Biosens. Bioelectron. 2015, 70, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Kumazawa, S.; Matsunaga, T. In vitro electrochemical detection of wheat allergen using rat basophilic leukaemia (RBL-1) cells. J. Appl. Microbiol. Biotechnol. 1995, 43, 622–625. [Google Scholar] [CrossRef]
- Jiang, H.; Jiang, D.; Zhu, P.; Pi, F.; Ji, J.; Sun, C.; Sun, J.; Sun, X. A novel mast cell co-culture microfluidic chip for the electrochemical evaluation of food allergen. Biosens. Bioelectron. 2016, 83, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current situation of mycotoxin contamination and co-occurrence in animal feed--focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef]
- Jiang, D.; Feng, D.; Jiang, H.; Yuan, L.; Yongqi, Y.; Xu, X.; Fang, W. Preliminary study on an innovative, simple mast cell-based electrochemical method for detecting foodborne pathogenic bacterial quorum signaling molecules (N-acyl-homoserine-lactones). Biosens. Bioelectron. 2017, 90, 436–442. [Google Scholar] [CrossRef]
- Jiang, D.; Liu, Y.; Jiang, H.; Rao, S.; Fang, W.; Wu, M.; Yuan, L.; Fang, W. A novel screen-printed mast cell-based electrochemical sensor for detecting spoilage bacterial quorum signaling molecules (N-acyl-homoserine-lactones) in freshwater fish. Biosens. Bioelectron. 2018, 102, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Wu, C.; Wang, Q.; Zhou, J.; Su, K.; Li, H.; Hu, N.; Wang, P. An improved sensitive assay for the detection of PSP toxins with neuroblastoma cell-based impedance biosensor. Biosens. Bioelectron. 2015, 67, 458–464. [Google Scholar] [CrossRef]
- Bouafsoun, A.; Othmane, A.; Jaffrézic-Renault, N.; Kerkeni, A.; Thoumire, O.; Prigent, A.F.; Ponsonnet, L. Impedance endothelial cell biosensor for lipopolysaccharide detection. Mater. Sci. Eng. C 2008, 28, 653–661. [Google Scholar] [CrossRef]
- Zou, L.; Wang, Q.; Tong, M.; Li, H.; Wang, J.; Hu, N.; Wang, P. Detection of diarrhetic shellfish poisoning toxins using high-sensitivity human cancer cell-based impedance biosensor. Sens. Actuators B Chem. 2016, 222, 205–212. [Google Scholar] [CrossRef]
- McDermott, P.F.; Zhao, S.; Wagner, D.D.; Simjee, S.; Walker, R.D.; White, D.G. The food safety perspective of antibiotic resistance. Anim. Biotechnol. 2002, 13, 71–84. [Google Scholar] [CrossRef]
- Bird, S.B.; Sutherland, T.D.; Gresham, C.; Oakeshott, J.; Scott, C.; Eddleston, M. OpdA, a bacterial organophosphorus hydrolase, prevents lethality in rats after poisoning with highly toxic organophosphorus pesticides. Toxicology 2008, 247, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Ipte, P.R.; Satpati, A.K. Probing the interaction of ciprofloxacin and E. coli by electrochemistry, spectroscopy and atomic force microscopy. Biophys. Chem. 2020, 266, 106456. [Google Scholar] [CrossRef]
- Singh, J.; Mittal, S.K. Whole cell based amperometric sensor with relative selectivity for zinc ions. Anal. Methods 2012, 4, 1326–1331. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, M.; Yang, Q.; Wei, H.; Xia, A.; Wang, L.; Ben, Y.; Zhou, Q.; Yang, Z.; Huang, X. A living plant cell-based biosensor for real-time monitoring invisible damage of plant cells under heavy metal stress. Sci. Total Environ. 2019, 697, 134097. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.Z.; Fang, Z.; Yu, Y.Y.; Yong, Y.C. Bioelectrochemical biosensor for water toxicity detection: Generation of dual signals for electrochemical assay confirmation. Anal. Bioanal. Chem. 2018, 410, 1231–1236. [Google Scholar] [CrossRef]
- Curtis, T.M.; Widder, M.W.; Brennan, L.M.; Schwager, S.J.; van der Schalie, W.H.; Fey, J.; Salazar, N. A portable cell-based impedance sensor for toxicity testing of drinking water. Lab Chip. 2009, 9, 2176–2183. [Google Scholar] [CrossRef]
- Tekaya, N.; Saiapina, O.; Ouada, H.B.; Lagarde, F.; Namour, P.; Ouada, H.B.; Jaffrezic-Renault, N. Bi-Enzymatic Conductometric Biosensor for Detection of Heavy Metal Ions and Pesticides in Water Samples Based on Enzymatic Inhibition in Arthrospira platensis. J. Environ. Prot. 2014, 05, 441–453. [Google Scholar] [CrossRef]
- Kumar, S.; Kundu, S.; Pakshirajan, K.; Dasu, V.V. Cephalosporins determination with a novel microbial biosensor based on permeabilized Pseudomonas aeruginosa whole cells. Appl. Biochem. Biotechnol. 2008, 151, 653–664. [Google Scholar] [CrossRef]
- Curulli, A. Electrochemical Biosensors in Food Safety: Challenges and Perspectives. Molecules 2021, 26, 2940. [Google Scholar] [CrossRef]
- Kim, T.E.; Park, S.W.; Cho, N.Y.; Choi, S.Y.; Yong, T.S.; Nahm, B.H.; Lee, S.; Noh, G. Quantitative Measurement of Serum Allergen-Specific IgE on Protein Chip. Exp. Mol. Med. 2002, 34, 9–158. [Google Scholar] [CrossRef]

| Immobilization Materials | Electrode Interface | Cell Type | Ref |
|---|---|---|---|
| Collagen | Gold electrode | Mast cell | [35] |
| Laminin | Screen-printed electrode | Mouse tongue isolated taste bud (MTITB) cells and human embryonic kidney 293 cell lines (HEK293) | [39] |
| Laminin | Screen-printed electrode | Hep G2 cells | [40] |
| RGD peptide | Graphene film | Human umbilical vein endothelial cells | [21] |
| RGD peptide | ITO electrode | Human lung cancer cell A549 | [41] |
| MAST peptide | Gold electrode | Human umbilical vein endothelial cells | [42] |
| lysine–arginine–glycine–aspartic acid peptide | Gold electrode | Mouse fibroblast cells | [43] |
| Alkanethiols and polymeric poly-L-lysine-grafted-poly (ethylene glycol) | Gold–silicate interfaces | MCF-7 cells | [44] |
| Cell Types | Analyst | Food | Methods | Performance | Ref. |
|---|---|---|---|---|---|
| RBL-2H3 mast cell (RBL-2H3) | tropomyosin | shrimp | EIS | Linear range: 0.5–0.25 μg/mL Detection limit: 0.15 μg/mL | [35] |
| RBL-2H3 | casein | milk | differential pulse voltammetry (DPV) | Linear range: 1 × 10−7–1 × 10−6 g/mL Detection limit: 3.2 × 10−8 g/mL | [49] |
| RBL-2H3 | tropomyosin parvalbumin | shrimp fish | EIS | Detection limit: 0.03 μg/mL Detection limit: 0.16 ng/mL | |
| [53] | |||||
| RBL-2H3, ANA-1macrophages | dinitrophenylated bovine serum albumin | - | EIS | Cell co-culture model Detection limit: 10−1 ng/mL | [55] |
| RBL-1 | wheat protein | wheat | cyclic voltammetry (CV) | Linear range: 0.01–0.5 μg/mL | [54] |
| Cell Types | Analyst | Food | Methods | Performance | Ref. |
|---|---|---|---|---|---|
| RBL-2H3 | N-acyl-homoserine-lactones | fish | EIS | Linear range: 0.1–1 μmol/L Detection limit: 0.034 μmol/L | [57] |
| HeLa & HepG2 cell | okadaic acid | shellfish | Electrical cell-substrate impedance sensing (ECIS) | Detection limit: 10.2 μg/L | [61] |
| Neuroblastoma cell | saxitoxin, ouabain, veratridine | shellfish | EIS | Detection limit: 0.03 ng/mL | [59] |
| BEL-7402 cell | DON, ZEN | - | EIS | Linear range: 0.1–20 μg/Ml, 0.1–50 μg/mL Detection limit: 0.03 μg/mL, 0.05 μg/mL | [20] |
| Hep G2 cell | DON, ZEN, AFB1 | - | EIS | Linear range: 0.01–20, 0.1–50 and 0.1–3.5 μg/mL | [40] |
| Raw264.7 macrophage cells | lipopolysaccharide | juice | DPV | Linear range: 1 × 10−2–3 nmol/L Detection limit: 3.5 × 10−3 ng/mL | [33] |
| Cell Types | Analyst | Food | Methods | Performance | Ref. |
|---|---|---|---|---|---|
| S. oneidensis MR-1 | 3,5-dichlorophenol | water | Amperometric i–t curve | half maximal inhibitory concentration: 14.5 mg/L | [67] |
| microalgae Chlorella sp. | Zn2+ | water | DPV | Linear range: 10−12–10−10 mol/L Detection limit: 10−11 mol/L | [65] |
| plant cell (protoplasts) | Cd2+, Pb2+ | soybean | EIS | Linear range: 45–210 μmol/L, 120–360 μmol/L Detection limit: 18.5 nmol/L, 25.6 nmol/L | [66] |
| E. coli cell | ciprofloxacin | - | DPV | the binding constant of E. coli membrane protein F and CIP log Kf = 12.1 | [64] |
| BPAECs | lindane | drinking water | ECIS | Detection limit: 0.1 mmol/L | [68] |
| Arthrospira platensis cell | Cd2+, Hg+ | water | Lock-in amplifier method | Linear range: 10−20–10−6 mol/L Detection limit: 10−20 mol/L | [69] |
| P. aeruginosa cell | cephalosporin group of antibiotics | - | - | Linear range: 0.1–11 mmol/L | [70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Lu, L.; Zhang, Z.; Zang, L. Electrochemical Cell-Based Sensor for Detection of Food Hazards. Micromachines 2021, 12, 837. https://doi.org/10.3390/mi12070837
Zhang J, Lu L, Zhang Z, Zang L. Electrochemical Cell-Based Sensor for Detection of Food Hazards. Micromachines. 2021; 12(7):837. https://doi.org/10.3390/mi12070837
Chicago/Turabian StyleZhang, Jiancheng, Lixia Lu, Zhenguo Zhang, and Liguo Zang. 2021. "Electrochemical Cell-Based Sensor for Detection of Food Hazards" Micromachines 12, no. 7: 837. https://doi.org/10.3390/mi12070837
APA StyleZhang, J., Lu, L., Zhang, Z., & Zang, L. (2021). Electrochemical Cell-Based Sensor for Detection of Food Hazards. Micromachines, 12(7), 837. https://doi.org/10.3390/mi12070837






