Generation of Photopolymerized Microparticles Based on PEGDA Using Microfluidic Devices. Part 1. Initial Gelation Time and Mechanical Properties of the Material
Abstract
1. Introduction
2. Materials
2.1. Preparation of Eosin “Y” Solution
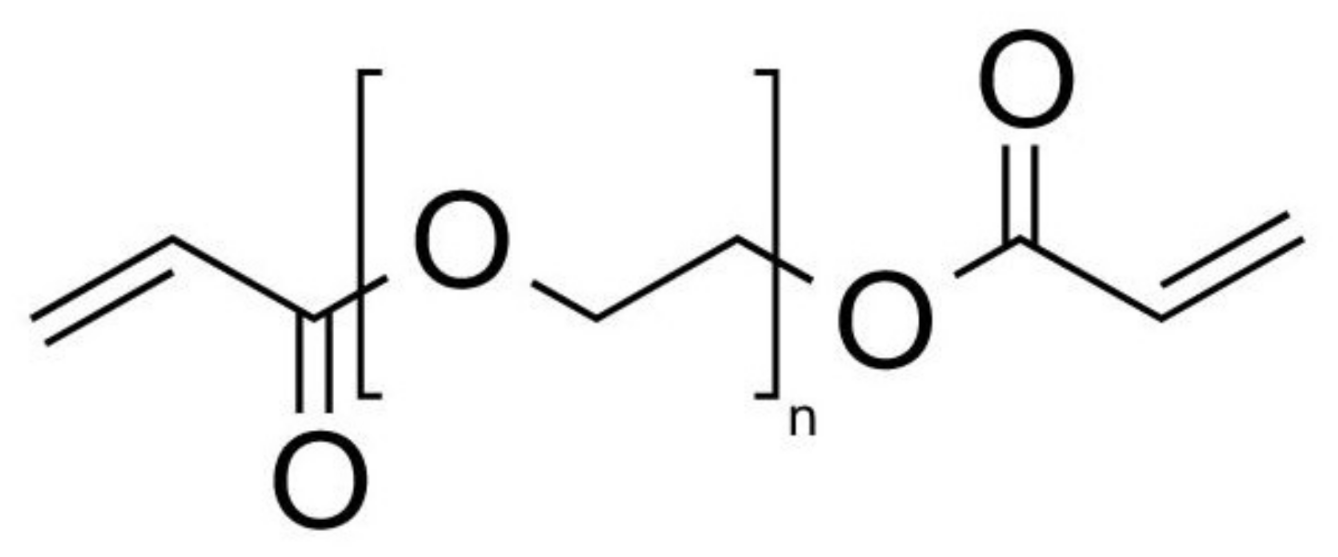
2.2. Hydrogel Solution Synthesis
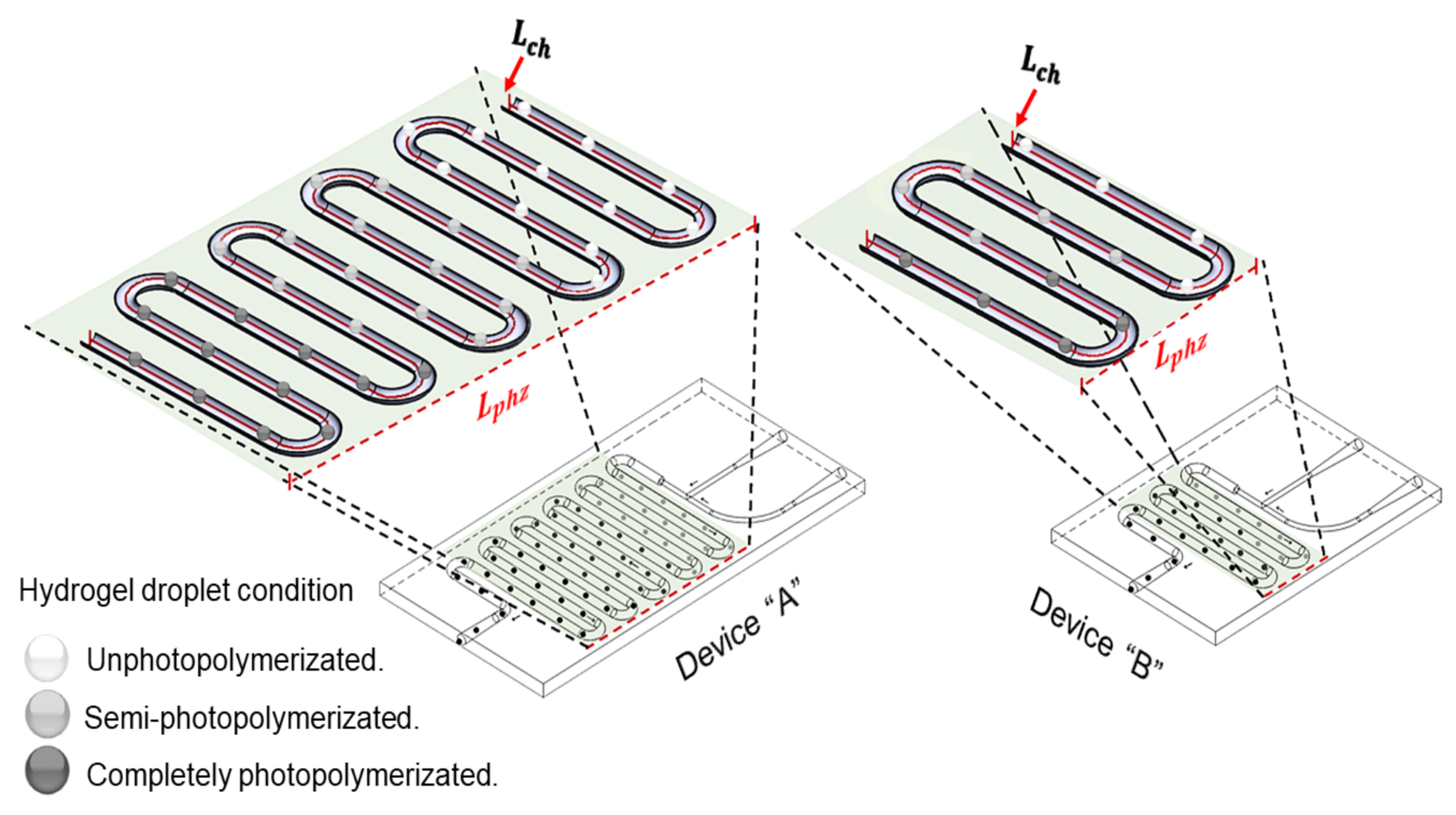
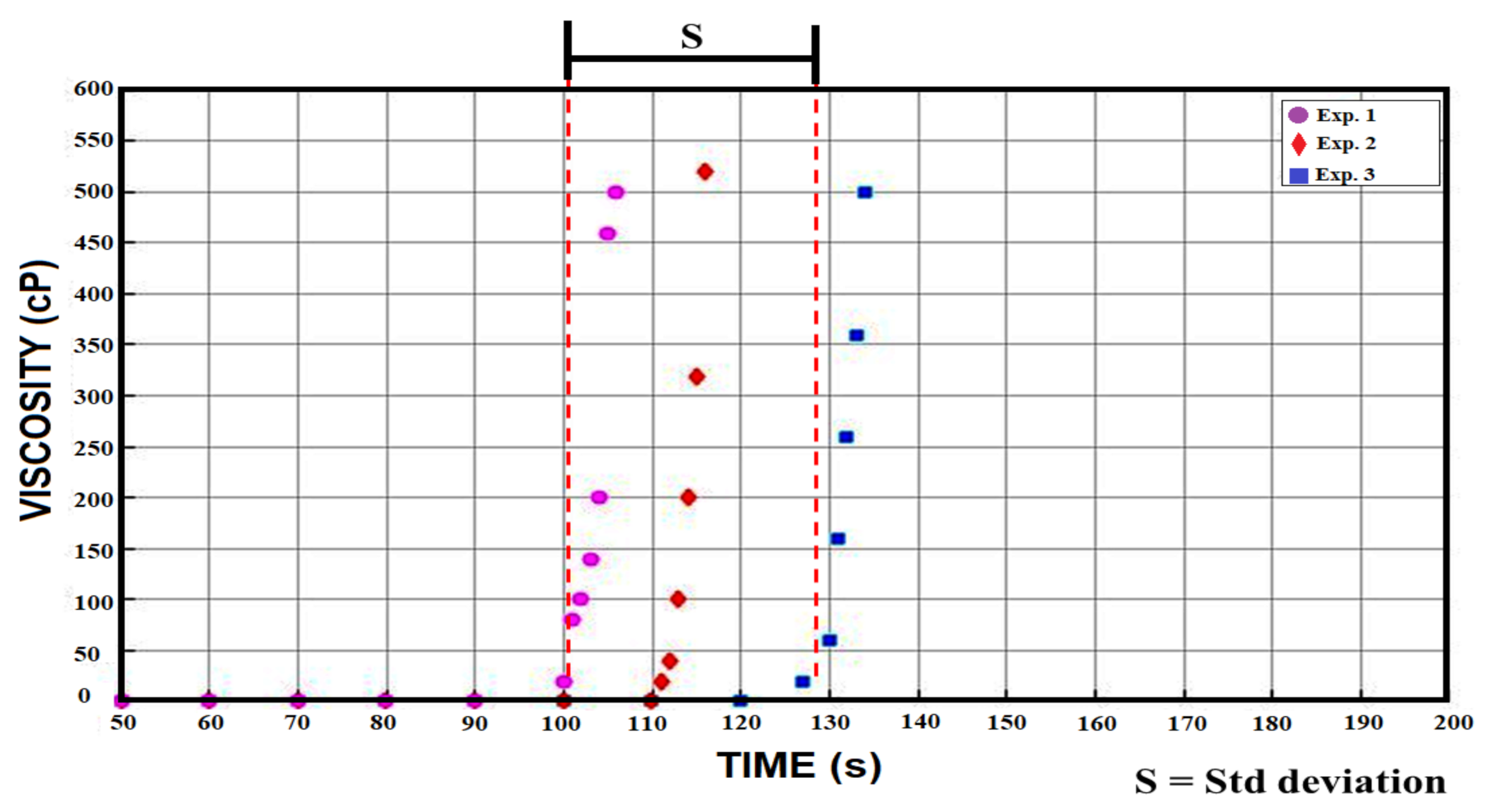
2.3. Initial Gelation Time
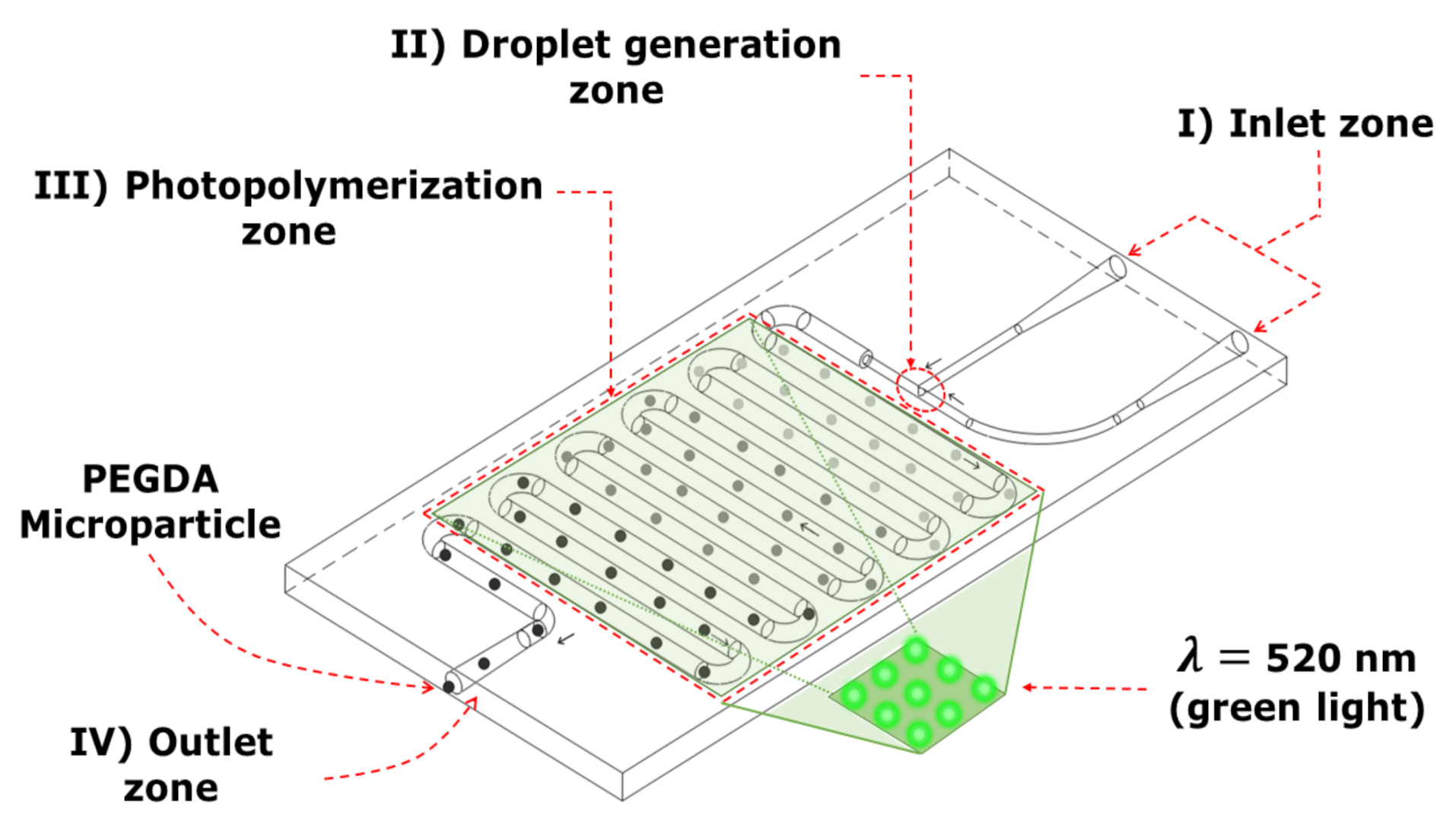
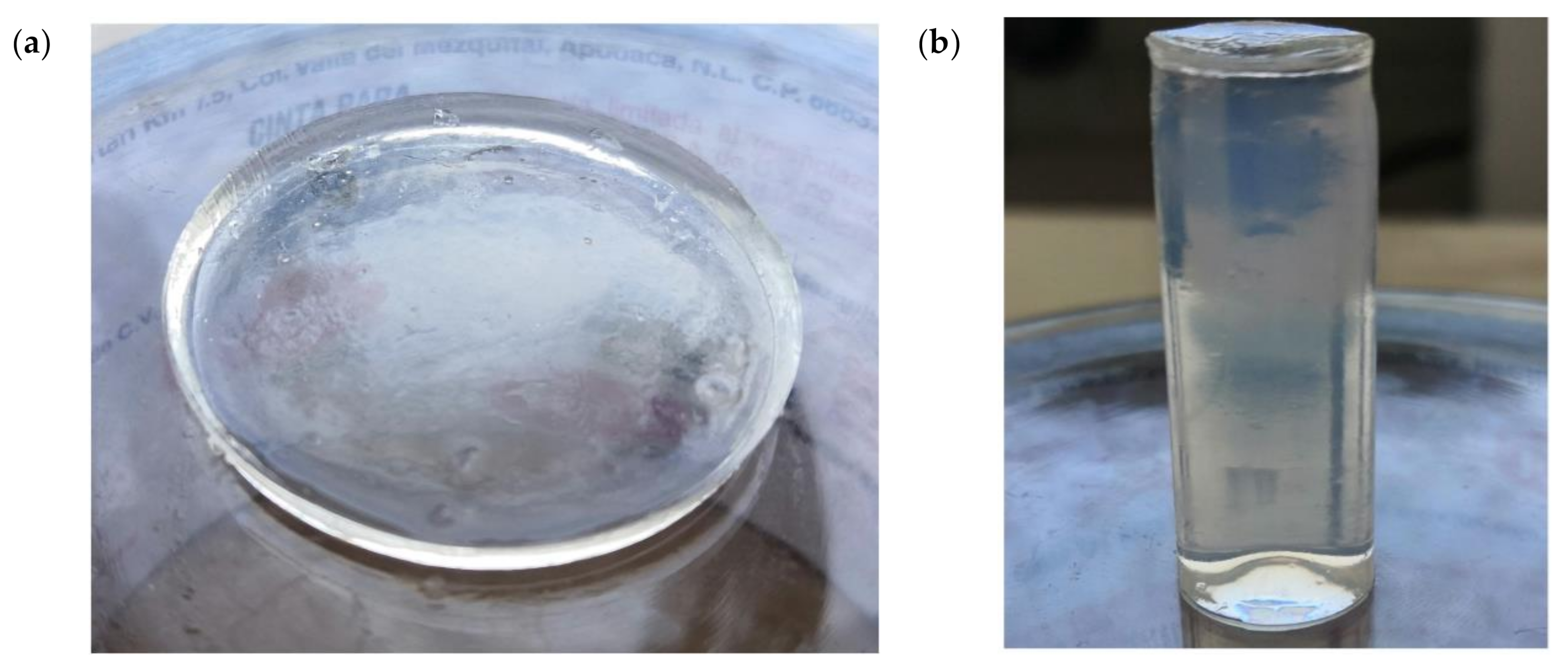
2.4. Sample Preparation
2.5. Swelling Test
2.6. Compression Tests
2.7. Young’s Module
3. Results and Discussion
3.1. Formulation
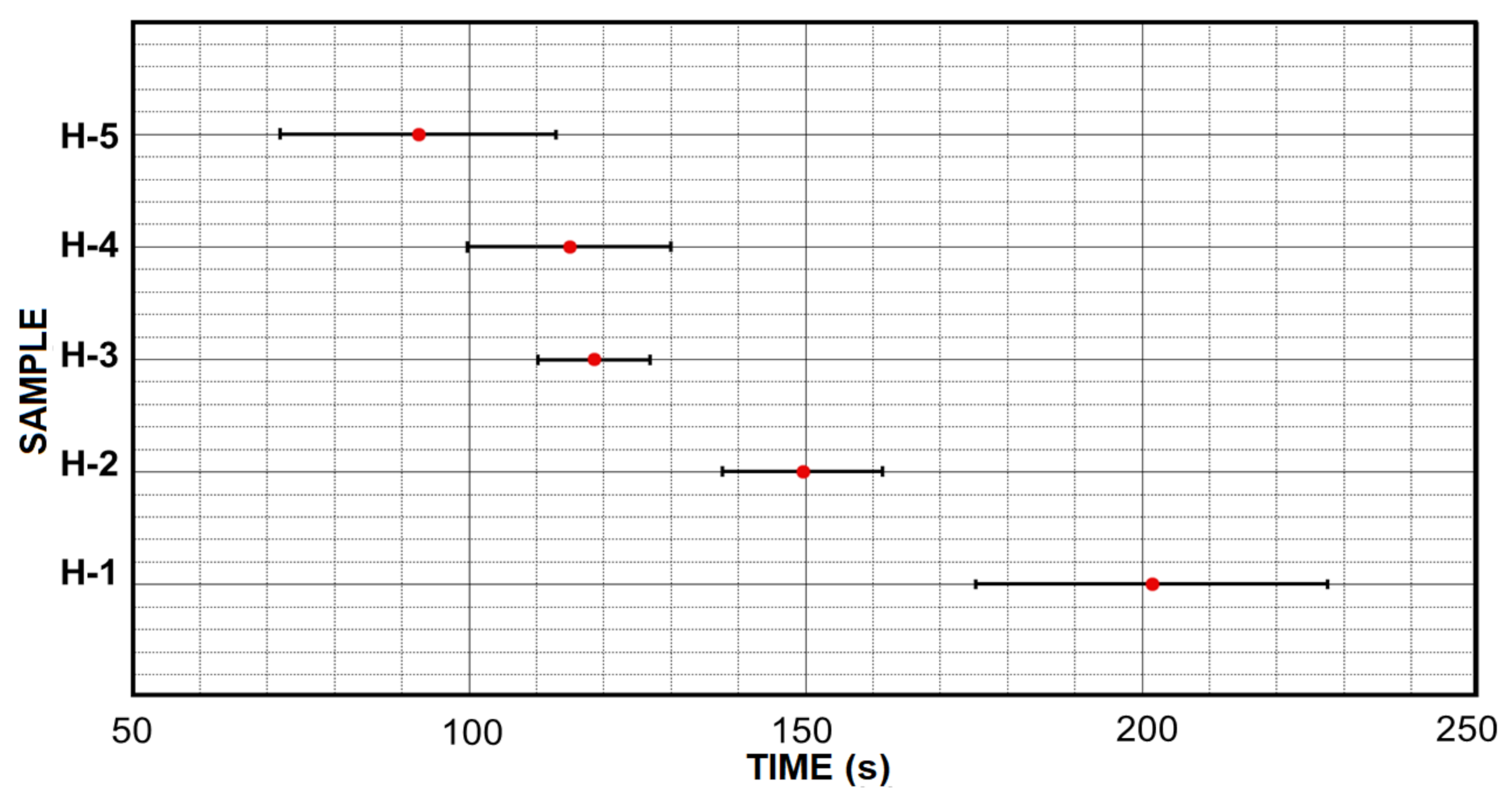
3.2. Initial Gelation Time
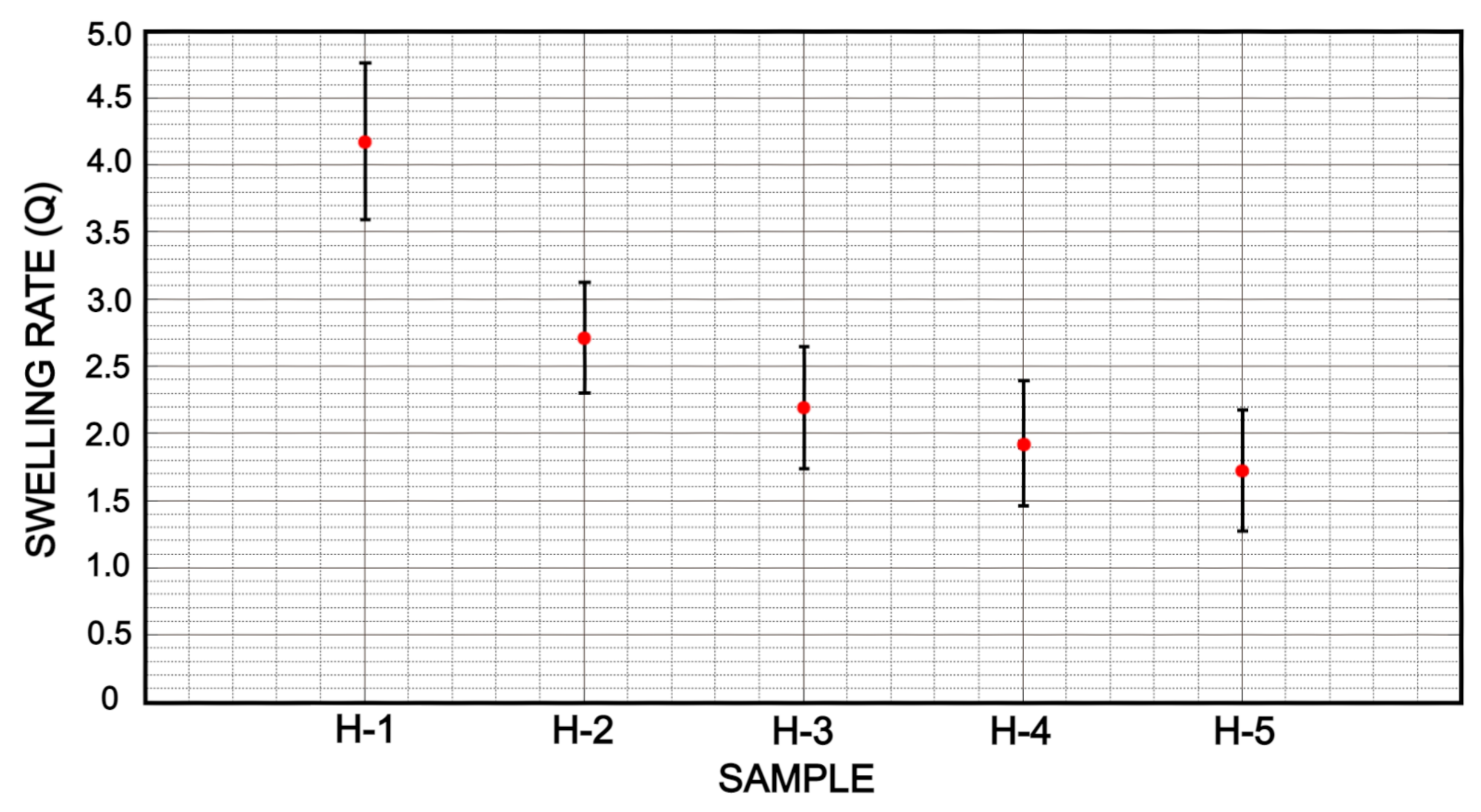
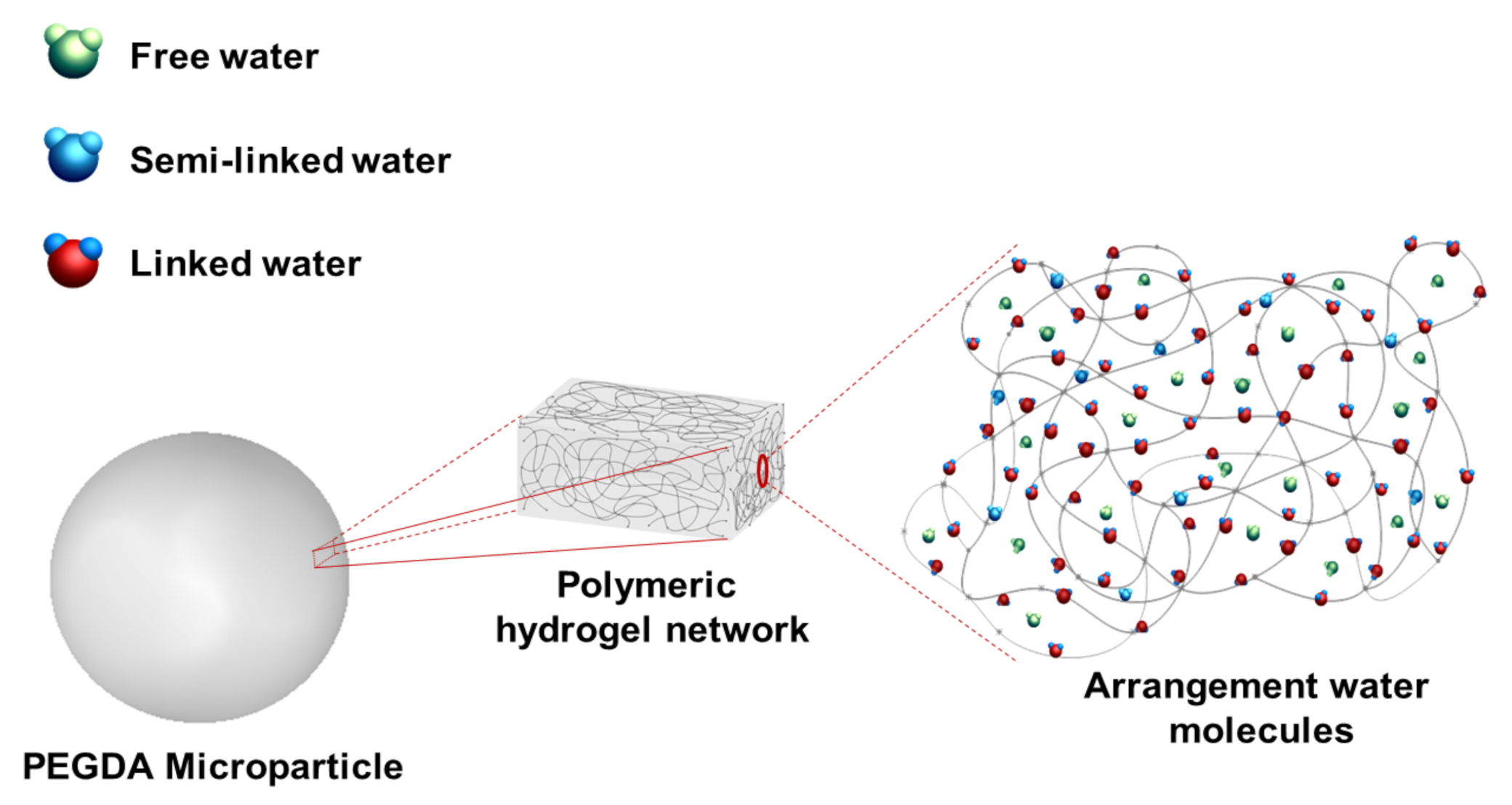
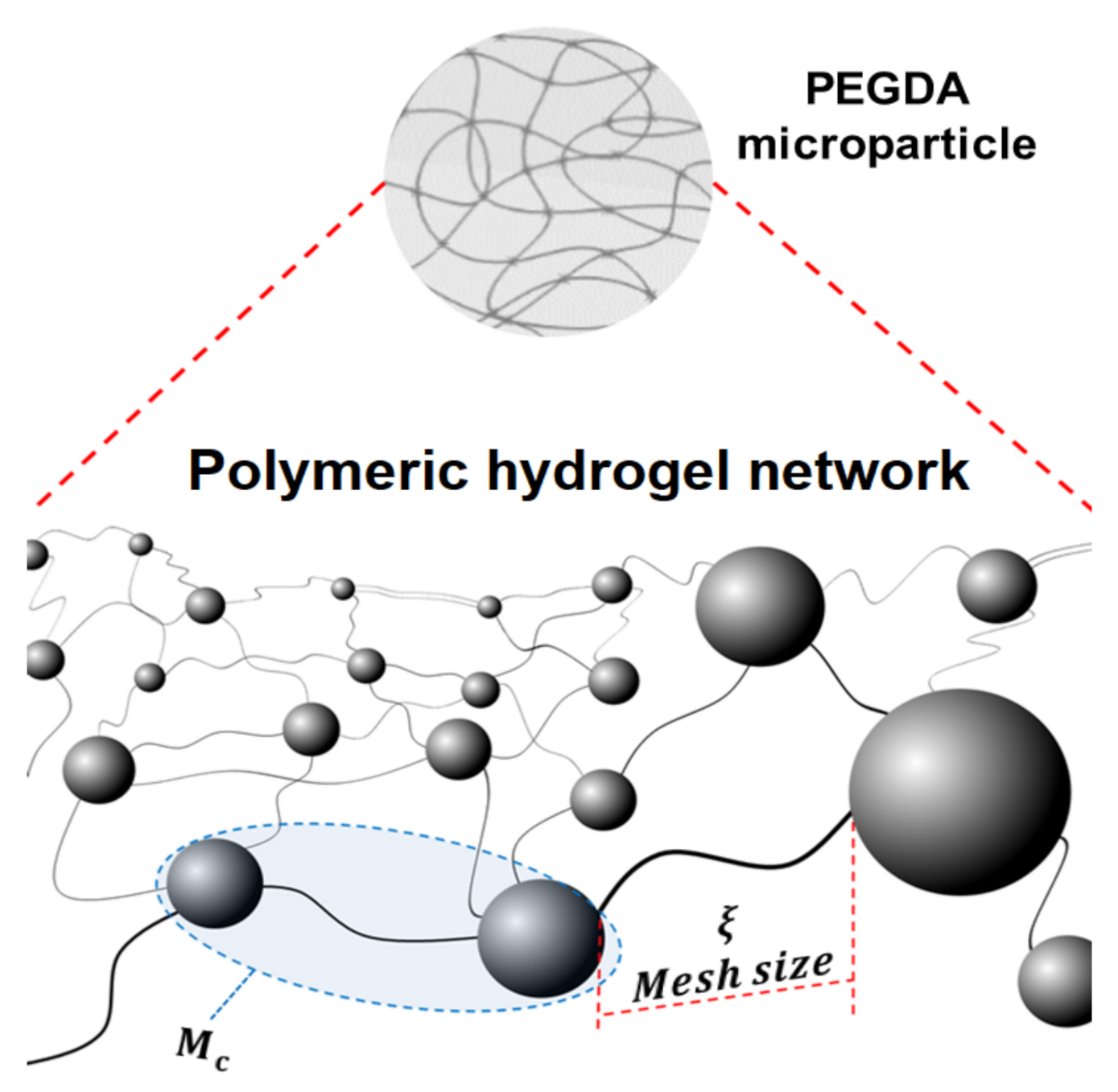
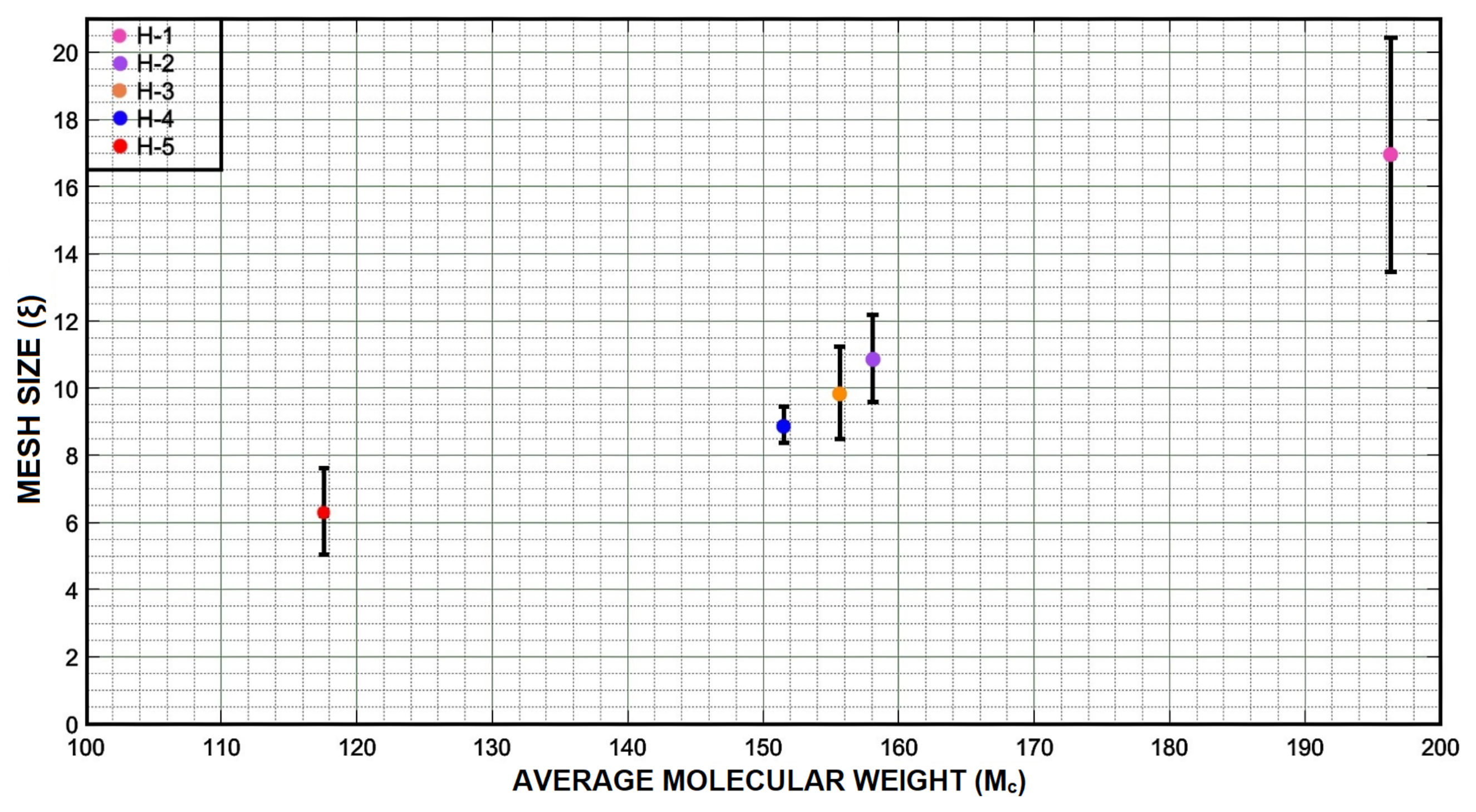
3.3. Swelling and Cross-Linking Density
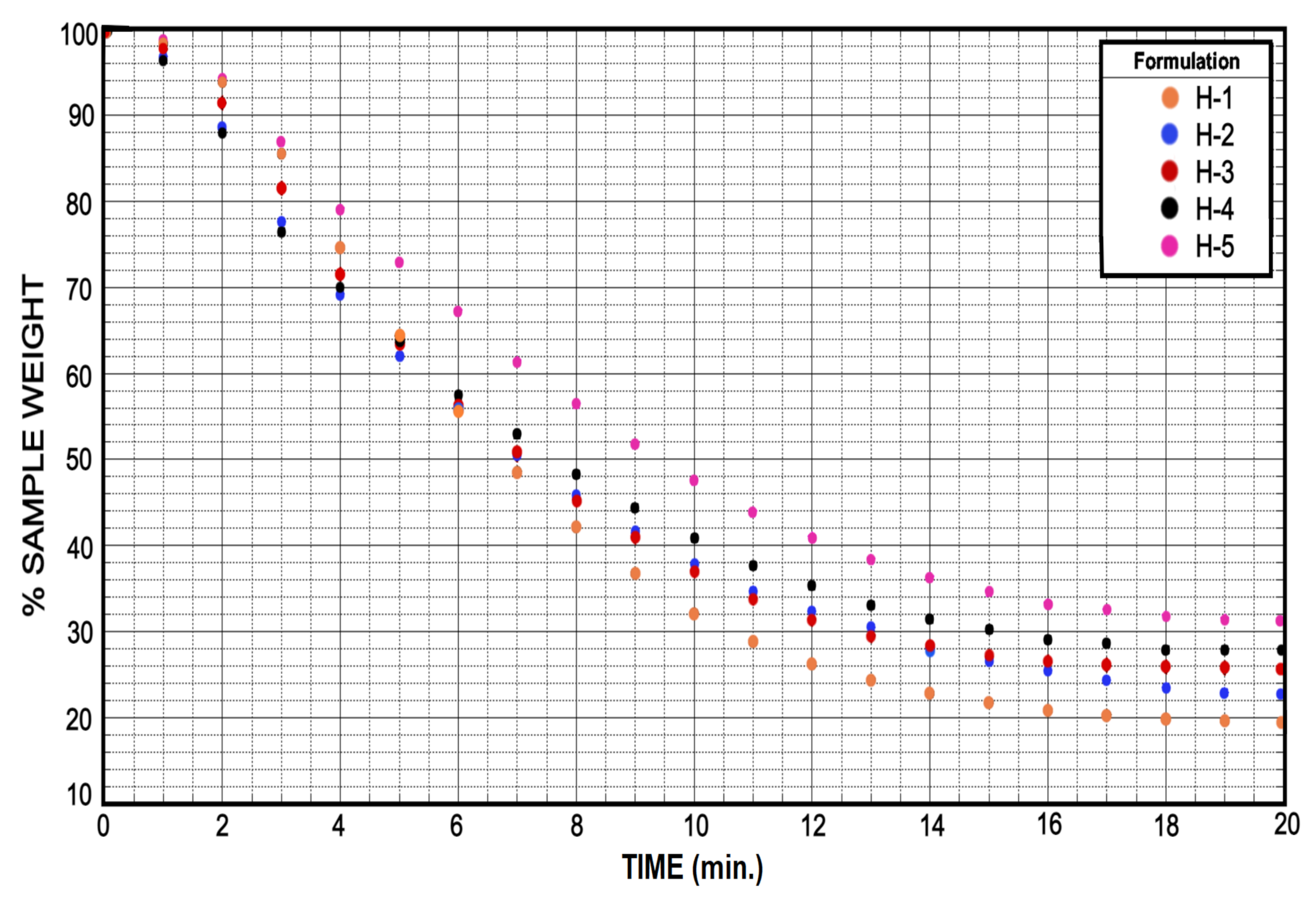
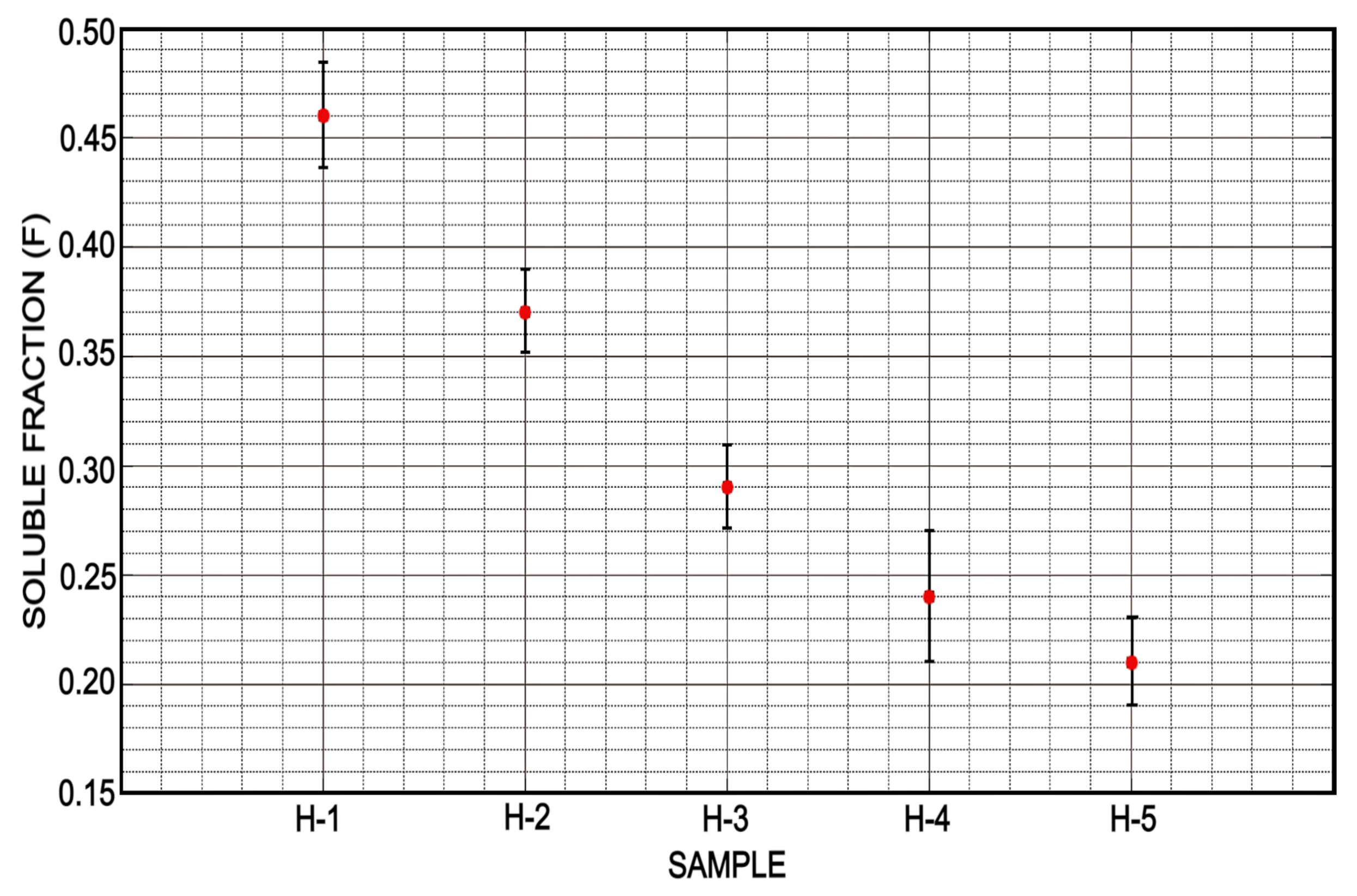
3.4. Soluble Fraction and Drying
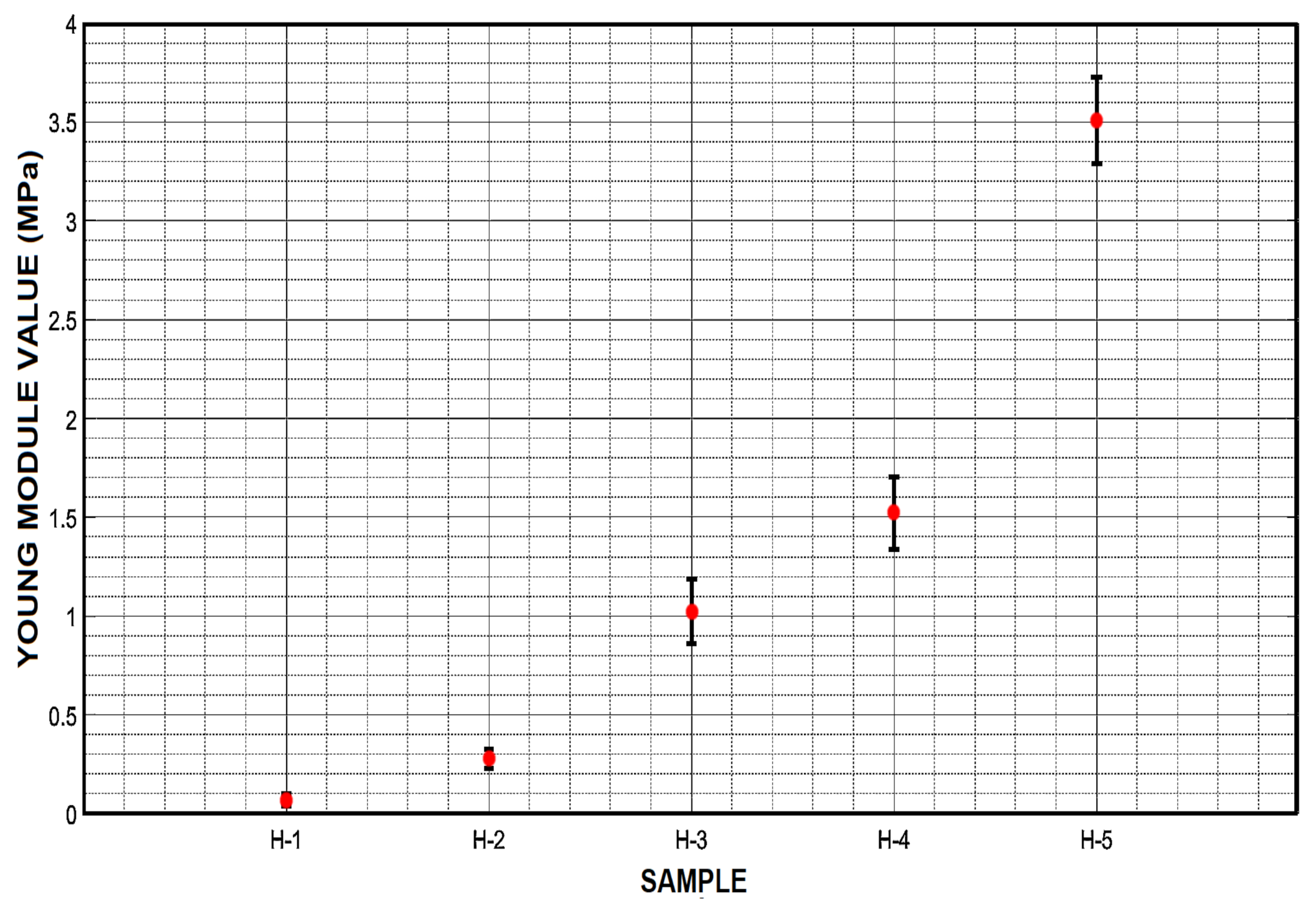
3.5. Compression Test and Young’s Module
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Theberge, A.B.; Courtois, F.; Schaerli, Y.; Fischlechner, M.; Abell, C.; Hollfelder, F.; Huck, W.T. Microdroplets in microfluidics: An evolving platform for discoveries in chemistry and biology. Angew. Chem. Int. Ed. 2010, 49, 5846–5868. [Google Scholar] [CrossRef]
- Islam, M.; Natu Rucha, M.R. A study on the limits and advantages of using a desktop cutter plotter to fabricate microfluidic networks. Microfluid Nanofluid 2015, 19, 973–985. [Google Scholar] [CrossRef]
- Primiceri, E.; Chiriacò, M.S.; Rinaldi, R.; Maruccio, G. Cell chips as new tools for cell biology-results, perspectives and opportunities. Lab Chip 2013, 13, 3789–3802. [Google Scholar] [CrossRef] [PubMed]
- Hattori, S.; Tang, C.; Tanaka, D.; Yoon, D.H.; Nozaki, Y. Development of Microdroplet Generation Method for Organic Solvents Used in Chemical Synthesis. Molecules 2020, 25, 5360. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zheng, D.; Li, N.; Wang, X.; Liu, Y.; Sun, M.; Zhao, X. Size-adjustable microdroplets generation based on microinjection. Micromachines 2017, 8, 88. [Google Scholar] [CrossRef]
- Yang, W.; Wang, H. X-Mechanics—An endless frontier. Sci. China 2019, 62, 1–8. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Xie, Z.; Jing, X.; Bellotti, A.; Gu, Z. Stimuli-Responsive Polymersomes for Biomedical Applications. Biomacromolecules 2017, 18, 649–673. [Google Scholar] [CrossRef]
- Cai, G.; Wang, J.; Qian, K.; Chen, J.; Li, S.; Lee, P.S. Extremely Stretchable Strain Sensors Based on Conductive Self-Healing Dynamic Cross-Links Hydrogels for Human-Motion Detection. Adv. Sci. 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Mannoor, M.S.; Jiang, Z.; James, T.; Kong, Y.L.; Malatesta, K.A.; Soboyejo, W.O.; Verma, N.; Gracias, D.H.; McAlpine, M.C. 3D printed bionic ears. Nano Lett. 2013, 13, 2634–2639. [Google Scholar] [CrossRef]
- Leong, T.G.; Randall, C.L.; Benson, B.R.; Bassik, N.; Stern, G.M.; Gracias, D.H. Tetherless thermobiochemically actuated microgrippers. Proc. Natl. Acad. Sci. USA 2009, 106, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Shang, L.; Chen, Z.; Yu, Y.; Zhao, Y. Bioinspired living structural color hydrogels. Sci. Robot. 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.; Lin, S.; Ma, C.; Takaffoli, M.; Fang, N.X.; Zhao, X. Hydraulic hydrogel actuators and robots optically and sonically camouflaged in water. Nat. Commun. 2017, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Liu, M.; Chen, X.; Sun, J.; Du, C.; Zhang, Y.; Zhai, J.; Hu, W.; Wang, Z.L. Ultrastretchable, transparent triboelectric nanogenerator as electronic skin for biomechanical energy harvesting and tactile sensing. Sci. Adv. 2017, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guo, X.; Wu, J.; Fang, D.; Zhang, Y. Soft mechanical metamaterials with unusual swelling behavior and tunable stress-strain curves. Sci. Adv. 2018, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hao, X.; Xiao, R.; Lin, J.; Wu, Z.L.; Yin, J.; Qian, J. Controllable Bending of Bi-hydrogel Strips with Differential Swelling. Acta Mech. Solida Sin. 2019, 32, 652–662. [Google Scholar] [CrossRef]
- Lee, J.B.; Peng, S.; Yang, D.; Roh, Y.H.; Funabashi, H.; Park, N.; Rice, E.J.; Chen, L.; Long, R.; Wu, M.; et al. A mechanical metamaterial made from a DNA hydrogel. Nat. Nanotechnol. 2012, 7, 816–820. [Google Scholar] [CrossRef]
- Ning, X.; Wang, X.; Zhang, Y.; Yu, X.; Choi, D.; Zheng, N.; Kim, D.S.; Huang, Y.; Zhang, Y.; Rogers, J.A. Assembly of Advanced Materials into 3D Functional Structures by Methods Inspired by Origami and Kirigami: A Review. Adv. Mater. Interfaces 2018, 5, 1–13. [Google Scholar] [CrossRef]
- Peng, X.; Wang, H. Shape changing hydrogels and their applications as soft actuators. J. Polym. Sci. Part Polym. Phys. 2018, 56, 1314–1324. [Google Scholar] [CrossRef]
- Ionov, L. Hydrogel-based actuators: Possibilities and limitations. Mater. Today 2014, 17, 494–503. [Google Scholar] [CrossRef]
- Kolewe, K.W.; Kalasin, S.; Shave, M.; Schiffman, J.D.; Santore, M.M. Mechanical Properties and Concentrations of Poly(ethylene glycol) in Hydrogels and Brushes Direct the Surface Transport of Staphylococcus aureus. ACS Appl. Mater. Interfaces 2019, 11, 320–330. [Google Scholar] [CrossRef]
- Zhang, J.M.; Ji, Q.; Duan, H. Three-Dimensional Printed Devices in Droplet Microfluidic. Micromachines 2019, 10, 754. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.; Samanipour, R.; Ahmadi, A.; Kim, K. Rapid fabrication of circular channel microfluidic flow-focusing devices for hydrogel droplet generation. Micro Nano Lett. 2016, 11, 41–45. [Google Scholar] [CrossRef]
- Wang, X.; Jing, S.; Hou, Z.; Liu, Y.; Qiu, X.; Liu, Y.; Tan, Y. Permeable, robust and magnetic hydrogel beads: Water droplet templating synthesis and utilization for heavy metal ions removal. J. Mater. Sci. 2018, 53, 15009–15024. [Google Scholar] [CrossRef]
- Xue, P.; Wu, Y.; Menon, N.V. Microfluidic synthesis of monodisperse PEGDA microbeads for sustained release of 5-fluorouracil. Microfluid Nanofluid 2014, 1–10. [Google Scholar] [CrossRef]
- Lopez-Juárez, B.P.; Garcia-Ramirez, M.A.; Perez-Luna, V.H.; Gonzalez-Reynoso, O. Formation of PEG-DA polymerized microparticles by different microfluidics devices: A T-junction device and a flow focusing device. Mater. Today Proc. 2019, 13, 374–378. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Singh, T.R.R.; Laverty, G.; Donnelly, R.F. Hydrogels: Design, Synthesis and Application in Drug Delivery and Regenerative Medicine; CRC Press: Boca Raton, FL, USA, 2018; p. 344. [Google Scholar]
- Pietro, M.; Franco, A.; Coviello, T. Polysaccharide Hydrogels: Characterization and Biomedical Applications, Number 1. arXiv 2016, arXiv:1011.1669v3. [Google Scholar]
- Chaplin, M.F. A proposal for the structuring of water. Biophys. Chem. 2000, 83, 211–221. [Google Scholar] [CrossRef]
- Wiggins, P. Life depends upon two kinds of water. PLoS ONE 2008, 3, e1406. [Google Scholar] [CrossRef]
- Gun’ko, V.; Savina, I.; Mikhalovsky, S. Properties of Water Bound in Hydrogels. Gels 2017, 3, 37. [Google Scholar] [CrossRef]
- Daniela Pasqui, M.D.C.; Barbucci, R. Polysaccharide-based hydrogels: The key role of water in affecting mechanical properties. Polymers 2012, 4, 1517–1534. [Google Scholar] [CrossRef]
- Neethirajan, S.; Kobayashi, I.; Nakajima, M.; Wu, D.; Lin, F. Lab on a Chip. R. Soc. Chem. 2011, 11, 1574–1586. [Google Scholar] [CrossRef]
- Seo, J.W.; Shin, S.R.; Park, Y.J.; Bae, H. Hydrogel Production Platform with Dynamic Movement Using Photo-Crosslinkable/ Temperature Reversible Chitosan Polymer and Stereolithography 4D Printing Technology. Tissue Eng. Regen. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.N.; Smith, C.K.; Patrick, C.W., Jr. Rheological and recovery properties of poly(ethylene glycol) diacrylate hydrogels and human adipose tissue. J. Biomed. Mater 2005, 73, 313–319. [Google Scholar] [CrossRef]
- Khetan, S.; Burdick, J. Cellular Encapsulation in 3D Hydrogels for Tissue Engineering. J. Vis. Exp. 2009, 3–7. [Google Scholar] [CrossRef]
- Vermonden, T.; Censi, R.; Hennink, W.E. Hydrogels for Protein Delivery. ACS Publ. 2012, 5, 2853–2888. [Google Scholar] [CrossRef]
- Aranzana, S.P.; Mendel Rezusta, F.J.; Sánchez Somolinos, C. Modulación Mecánica en Hidrogeles de Polietilenglicol. Master’s Tesis, Universidad de Zaragoza (ICMA), Zaragoza, Spain, 2016; p. 40. [Google Scholar]
- Kim, H.J.; Cho, S.; Oh, S.J.; Shin, S.G.; Ryu, H.W.; Jeong, J.H. Tuning the hydrophobicity of a hydrogel using self-assembled domains of polymer cross-linkers. Materials 2019, 12, 1635. [Google Scholar] [CrossRef] [PubMed]
- Cruise, G.M.; Hegre, O.D.; Scharp, D.S.; Hubbell, J.A. A sensitivity study of the key parameters in the interfacial photopolymerization of poly(ethylene glycol) diacrylate upon porcine islets. Biotechnol. Bioeng. 1998, 57, 655–665. [Google Scholar] [CrossRef]
- Perez-Puyana, V.; Jiménez-Rosado, M.; Romero, A.; Guerrero, A. Fabrication and characterization of hydrogels based on gelatinised collagen with potential application in tissue engineering. Polymers 2020, 12, 1146. [Google Scholar] [CrossRef] [PubMed]
- Dogan, E.M.; Zalluhoglu, F.S.; Orbey, N. Effect of Potassium Ion on the Stability and Release Rate of Hydrogen Peroxide Encapsulated in Silica Hydrogels. AIChE J. 2017, 63, 215–228. [Google Scholar] [CrossRef]
- Park, H.; Guo, X.; Temenoff, J.S.; Tabata, Y.; Caplan, A.I.; Kasper, F.K.; Mikos, A.G. Effect of Swelling Ratio of Injectable Hydrogel Composites on Chondrogenic Differentiation of Encapsulated Rabbit Marrow Mesenchymal Stem Cells In Vitro. Biomacromolecules 2010, 10, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Merrill, E.W.; Dennison, K.A.; Sung, C. Partitioning and diffusion of solutes in hydrogels of poly(ethylene oxide). Biomaterials 1993, 14, 1117–1126. [Google Scholar] [CrossRef]
- Cavallo, A.; Madaghiele, M.; Masullo, U.; Lionetto, M.G.; Sannino, A. Photo-crosslinked poly(ethylene glycol) diacrylate (PEGDA) hydrogels from low molecular weight prepolymer: Swelling and permeation studies. Appl. Polym. Sci. 2016, 44380, 1–9. [Google Scholar] [CrossRef]
- Mazzoccoli, J.P.; Feke, D.L.; Baskaran, H.; Pintauro, P.N. Mechanical and cell viability properties of crosslinked low- And high-molecular weight poly(ethylene glycol) diacrylate blends. J. Biomed. Mater. Res. Part A 2010, 93, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Bal, T.; Kepsutlu, B.; Kizilel, S. Characterization of protein release from poly(ethylene glycol) hydrogels with crosslink density gradients. J. Biomed. Mater. Res. Part A 2014, 102, 487–495. [Google Scholar] [CrossRef]
- Lee, C.y.; Chiu, Y.C.; Nenawe, P.; Ozturk, M.; Vadlamudi, S.; Teymour, F.; Papavasiliou, G.; Turturro, M.; James, J. Mathematical Modeling Of Poly (Ethylene Glycol) Diacrylate Hydrogel Synthesis Via Visible Light Free-Radical Photopolymerization For Tissue Engineering Applications; ProQuest LLC.: Ann Arbor, MI, USA, 2013; Volume 1, p. 197. [Google Scholar]
- Carrasco, R.; Cordero, E.; Goldberg, I.; Martínez, B.; Pacheco, C. Evaluación de la presión arterial media durante la cirugía de implante dental. Rev. Esp. Cir. Oral Maxilofac. 2014, 36, 95–98. [Google Scholar] [CrossRef]





| Volume (mL) | |||||
|---|---|---|---|---|---|
| Substance | H-1 | H-2 | H-3 | H-4 | H-5 |
| Triethanolamine | 018.0 | 018.0 | 018.0 | 018.0 | 018.0 |
| 1-Vinyl-2-pyrrolidinone | 002.0 | 002.0 | 002.0 | 002.0 | 002.0 |
| PEGDA | 057.5 | 086.5 | 115.0 | 144.0 | 172.5 |
| Eosin “Y” | 005.0 | 005.0 | 005.0 | 005.0 | 005.0 |
| Distilled water | 417.5 | 388.5 | 360.0 | 331.0 | 302.5 |
| Volume (mL) | |||||
|---|---|---|---|---|---|
| Substance | H-1 | H-2 | H-3 | H-4 | H-5 |
| Triethanolamine | 0.36 | 0.36 | 0.36 | 0.36 | 0.36 |
| 1-Vinyl-2-pyrrolidinone | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 |
| PEGDA | 1.15 | 1.73 | 2.30 | 2.88 | 3.45 |
| Eosin “Y” | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Distilled water | 8.35 | 7.77 | 7.20 | 6.62 | 6.05 |
| & Values | ||||||
|---|---|---|---|---|---|---|
| Formulation | Average Q () | V | V | Value () | (r)(Å) | (Å) |
| H-1 | 4.18 | 0.18 | 0.12 | 196.33 | 8.89 | 16.93 |
| H-2 | 2.85 | 0.24 | 0.17 | 158.08 | 8.03 | 10.85 |
| H-3 | 2.50 | 0.26 | 0.23 | 155.70 | 7.97 | 09.83 |
| H-4 | 2.13 | 0.30 | 0.29 | 151.50 | 7.87 | 08.88 |
| H-5 | 1.53 | 0.37 | 0.35 | 117.57 | 6.90 | 06.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta-Cuevas, J.M.; González-García, J.; García-Ramírez, M.; Pérez-Luna, V.H.; Cisneros-López, E.O.; González-Nuñez, R.; González-Reynoso, O. Generation of Photopolymerized Microparticles Based on PEGDA Using Microfluidic Devices. Part 1. Initial Gelation Time and Mechanical Properties of the Material. Micromachines 2021, 12, 293. https://doi.org/10.3390/mi12030293
Acosta-Cuevas JM, González-García J, García-Ramírez M, Pérez-Luna VH, Cisneros-López EO, González-Nuñez R, González-Reynoso O. Generation of Photopolymerized Microparticles Based on PEGDA Using Microfluidic Devices. Part 1. Initial Gelation Time and Mechanical Properties of the Material. Micromachines. 2021; 12(3):293. https://doi.org/10.3390/mi12030293
Chicago/Turabian StyleAcosta-Cuevas, José M., José González-García, Mario García-Ramírez, Víctor H. Pérez-Luna, Erick Omar Cisneros-López, Rubén González-Nuñez, and Orfil González-Reynoso. 2021. "Generation of Photopolymerized Microparticles Based on PEGDA Using Microfluidic Devices. Part 1. Initial Gelation Time and Mechanical Properties of the Material" Micromachines 12, no. 3: 293. https://doi.org/10.3390/mi12030293
APA StyleAcosta-Cuevas, J. M., González-García, J., García-Ramírez, M., Pérez-Luna, V. H., Cisneros-López, E. O., González-Nuñez, R., & González-Reynoso, O. (2021). Generation of Photopolymerized Microparticles Based on PEGDA Using Microfluidic Devices. Part 1. Initial Gelation Time and Mechanical Properties of the Material. Micromachines, 12(3), 293. https://doi.org/10.3390/mi12030293