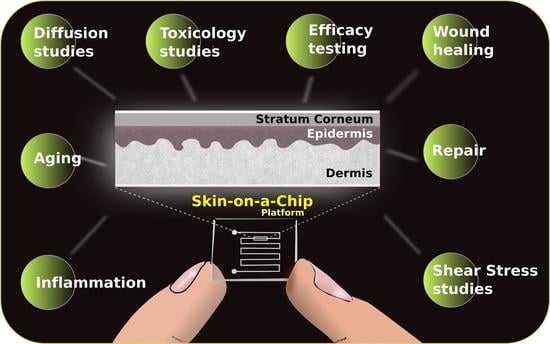
Development of Skin-On-A-Chip Platforms for Different Utilizations: Factors to Be Considered
Abstract
1. Introduction
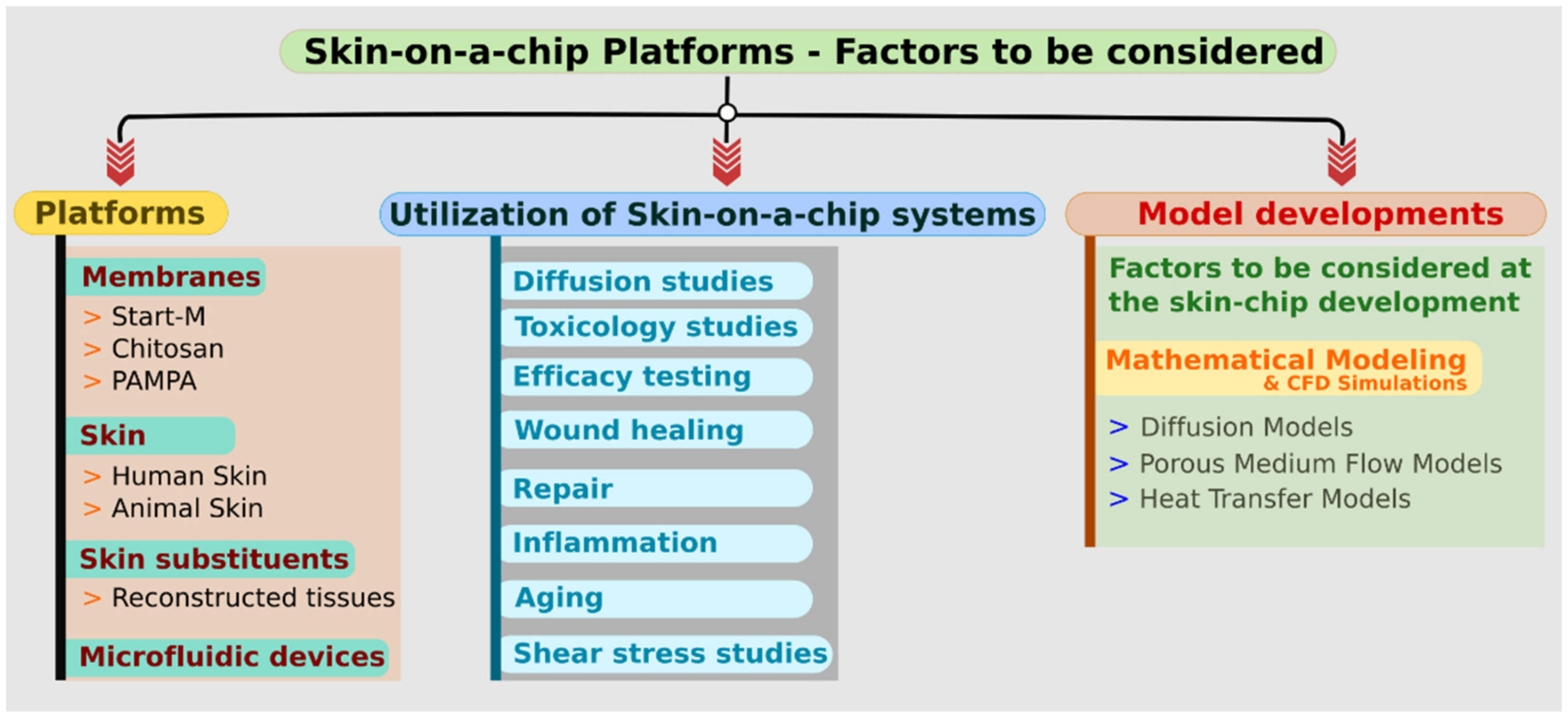
2. About the Platforms
2.1. Membranes
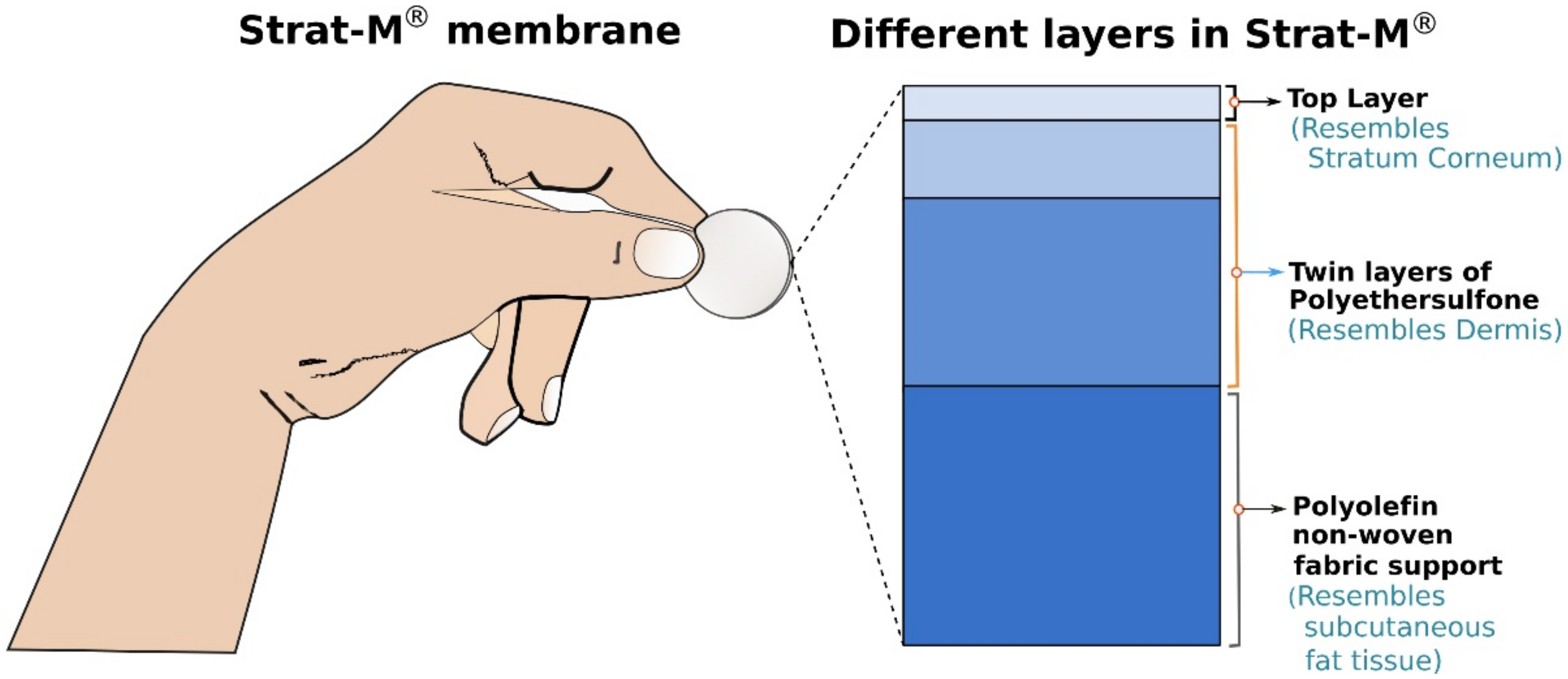
2.1.1. Strat-M®
2.1.2. Chitosan
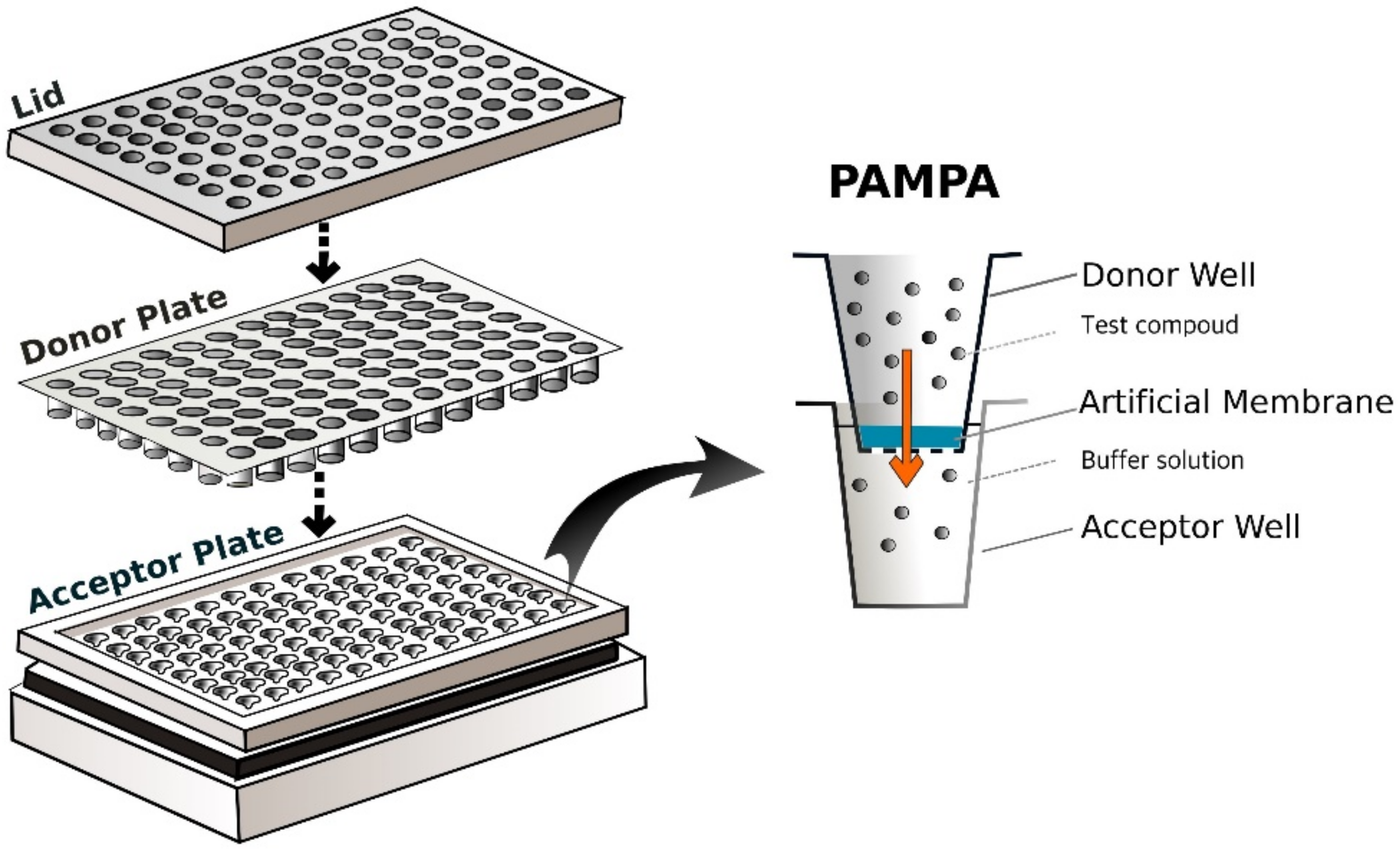
2.1.3. PAMPA
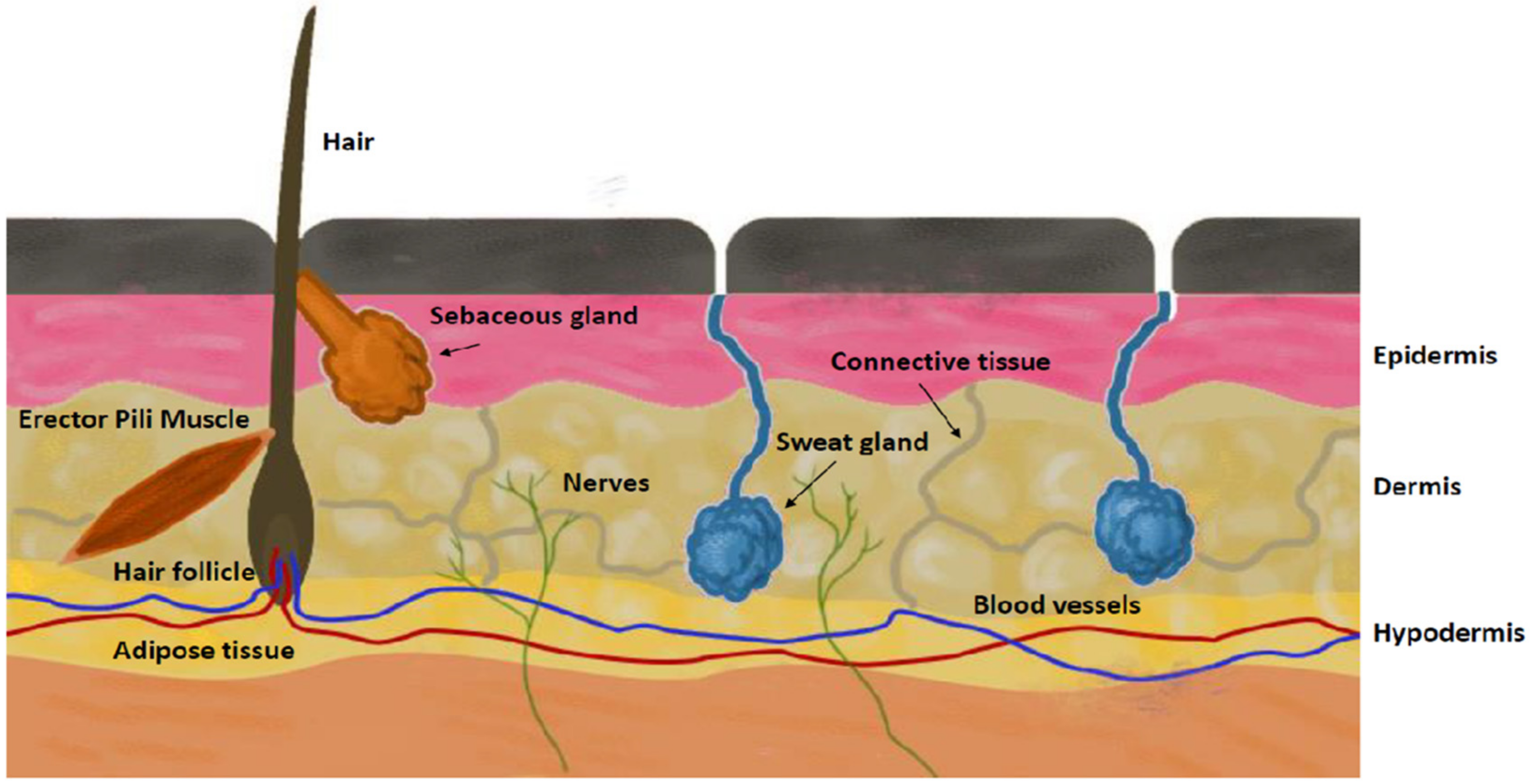
2.2. Skins
2.2.1. Human Skins
2.2.2. Animal Skins
2.3. Skin Substituents
Reconstructed Tissues
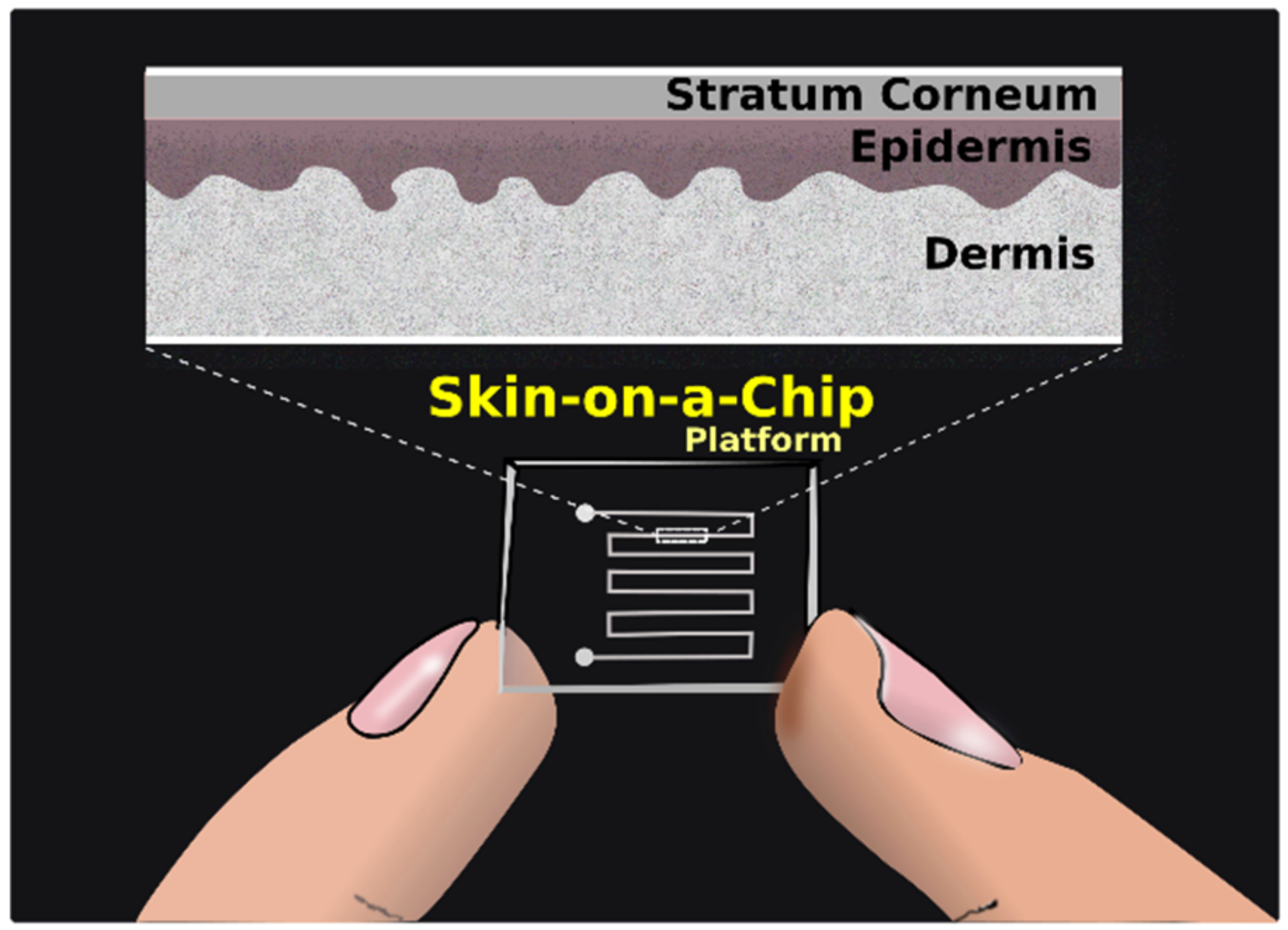
2.4. Microfluidic Devices
3. Utilization of Skin-On-A-Chip Systems
3.1. Diffusion Studies
3.2. Toxicology Studies
3.3. Efficacy Testing
3.4. Wound Healing
3.5. Repair
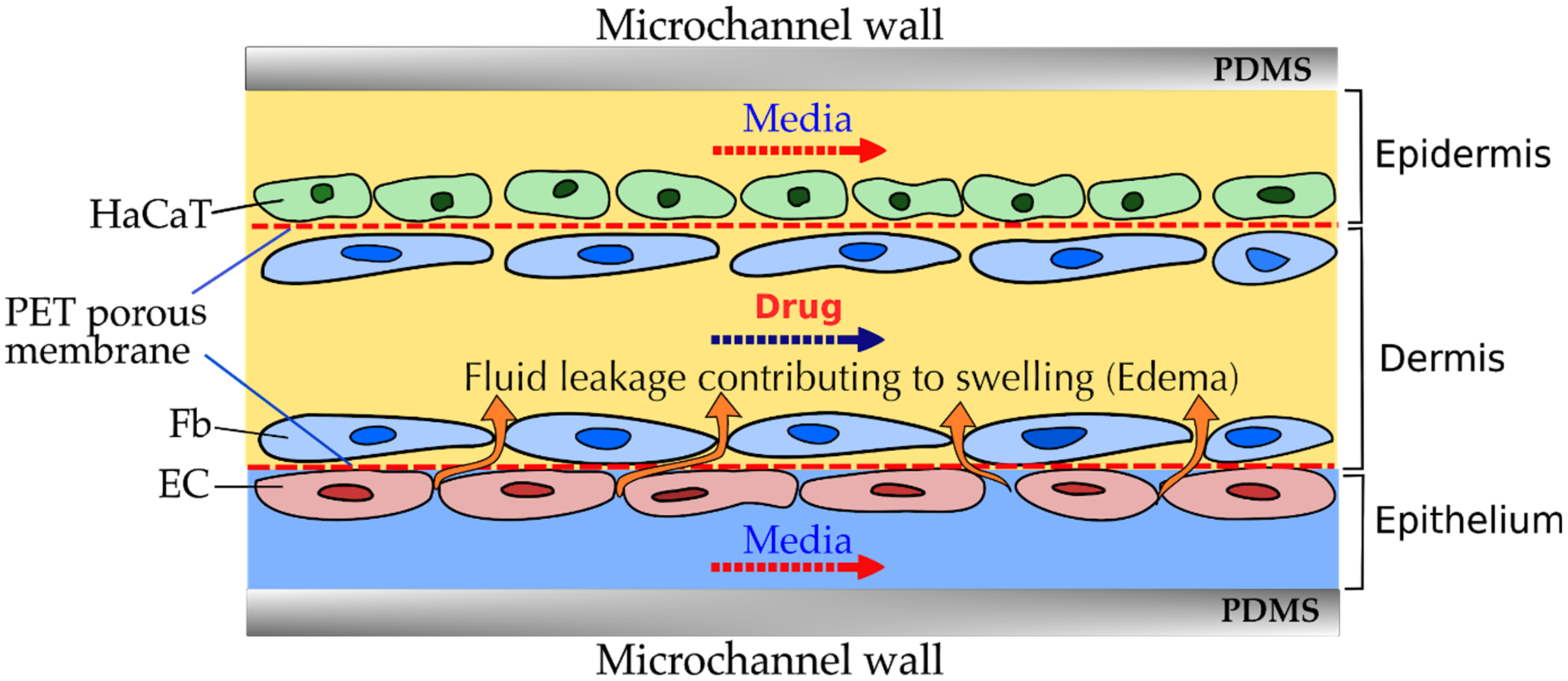
3.6. Inflammation
3.7. Aging
3.8. Shear Stress Studies
4. Model Developments
4.1. Factors to Be Considered at the Skin-Chip Development
4.1.1. Mathematical Modelling and CFD Simulations
4.1.2. Diffusion Model for Dermal Layers
4.1.3. Fluid Flow Model for the Dermis
Darcy Model
Darcy-Brinkman-Forchheimer Model
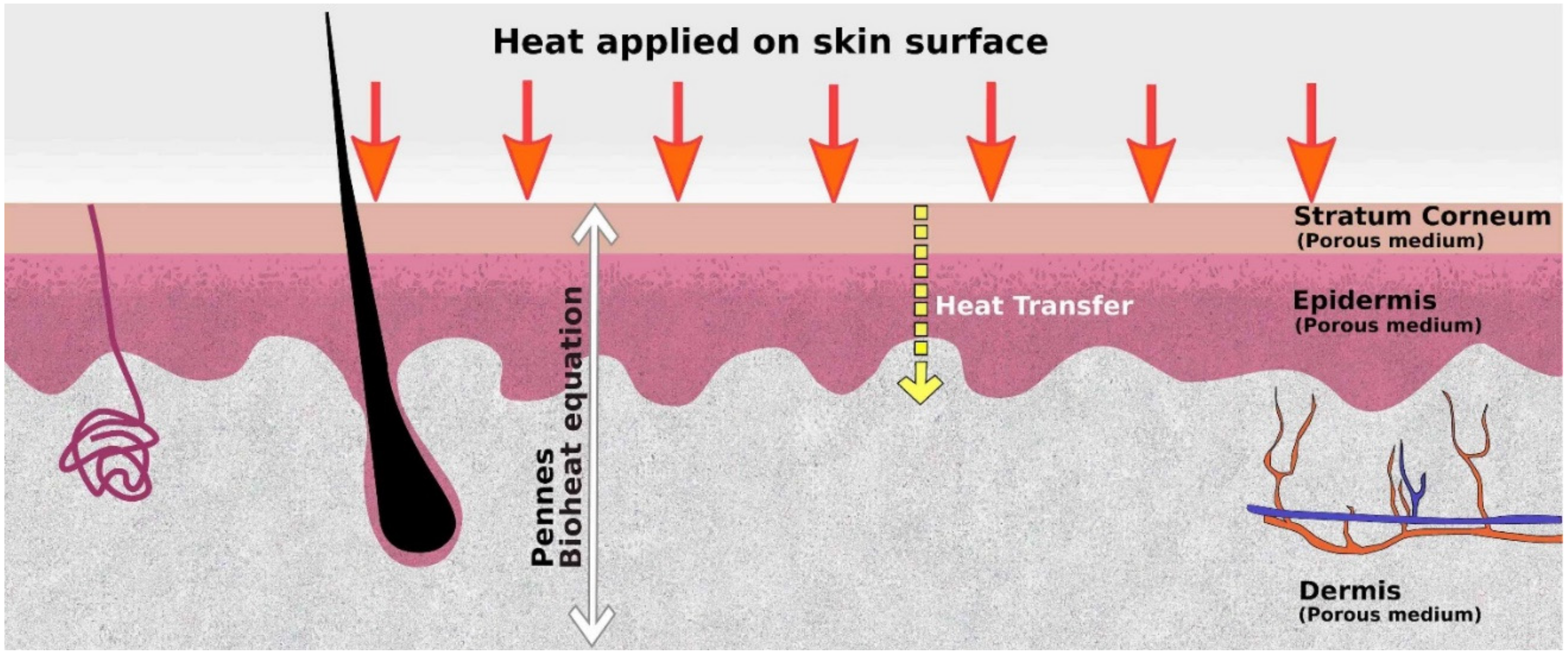
4.2. Heat Transfer Model for the Skin
5. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Notes
- Neupane, R.; Boddu, S.H.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to Biological Skin in Permeation Studies: Current Trends and Possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Waters, L.J. Recent Developments in Skin Mimic Systems to Predict Transdermal Permeation. Curr. Pharm. Des. 2015, 21, 2725–2732. [Google Scholar] [CrossRef] [PubMed]
- Ameri, M.; Lewis, H.; Lehman, P. Effect of Skin Model on In Vitro Performance of an Adhesive Dermally Applied Microarray Coated with Zolmitriptan. J. Pharm. 2018, 2018, 1–5. [Google Scholar] [CrossRef]
- Farner, F.; Bors, L.; Bajza, Á.; Karvaly, G.; Antal, I.; Erdő, F. Validation of an In Vitro-in Vivo Assay System for Evaluation of Transdermal Delivery of Caffeine. Drug Deliv. Lett. 2019, 9, 15–20. [Google Scholar] [CrossRef]
- Ng, S.F. Investigation into the Use of Synthetic Membranes for Drug Diffusion in Franz Cells. Ph.D. Thesis, University of Strathclyde, Glasgow, UK, 2007. [Google Scholar]
- Karadzovska, D.; Riviere, J.E. Assessing vehicle effects on skin absorption using artificial membrane assays. Eur. J. Pharm. Sci. 2013, 50, 569–576. [Google Scholar] [CrossRef]
- Carrer, V.; Guzmán, B.; Martí, M.; Alonso, C.; Coderch, L. Lanolin-Based Synthetic Membranes as Percutaneous Absorption Models for Transdermal Drug Delivery. Pharmaceutics 2018, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Grillo, R.; Dias, F.V.; Querobino, S.M.; Alberto-Silva, C.; Fraceto, L.F.; de Paula, E.; de Araujo, D.R. Influence of hybrid polymeric nanoparticle/thermosensitive hydrogels systems on formulation tracking and in vitro artificial membrane permeation: A promising system for skin drug-delivery. Colloids Surf. B Biointerfaces 2019, 174, 56–62. [Google Scholar] [CrossRef]
- Ng, S.-F.; Rouse, J.J.; Sanderson, F.D.; Eccleston, G.M. The relevance of polymeric synthetic membranes in topical formulation assessment and drug diffusion study. Arch. Pharmacal Res. 2012, 35, 579–593. [Google Scholar] [CrossRef]
- Haq, A.; Dorrani, M.; Goodyear, B.; Joshi, V.; Michniak-Kohn, B. Membrane properties for permeability testing: Skin versus synthetic membranes. Int. J. Pharm. 2018, 539, 58–64. [Google Scholar] [CrossRef]
- Bierhalz, A.C.; Westin, C.B.; Moraes, Â.M. Comparison of the properties of membranes produced with alginate and chitosan from mushroom and from shrimp. Int. J. Biol. Macromol. 2016, 91, 496–504. [Google Scholar] [CrossRef]
- Geinoz, S.; Guy, R.H.; Testa, B.; Carrupt, P.-A. Quantitative structure-permeation relationships (QSPeRs) to predict skin permeation: A critical evaluation. Pharm. Res. 2004, 21, 83–92. [Google Scholar] [CrossRef]
- Ottaviani, G.; Martel, A.S.; Carrupt, P.-A. Parallel Artificial Membrane Permeability Assay: A New Membrane for the Fast Prediction of Passive Human Skin Permeability. J. Med. Chem. 2006, 49, 3948–3954. [Google Scholar] [CrossRef] [PubMed]
- Sinkó, B.; Garrigues, T.M.; Balogh, G.T.; Nagy, Z.K.; Tsinman, O.; Avdeef, A.; Takács-Novák, K. Skin–PAMPA: A new method for fast prediction of skin penetration. Eur. J. Pharm. Sci. 2012, 45, 698–707. [Google Scholar] [CrossRef]
- Luo, L.; Patel, A.; Sinko, B.; Bell, M.; Wibawa, J.; Hadgraft, J.; Lane, M.E. A comparative study of the in vitro permeation of ibuprofen in mammalian skin, the PAMPA model and silicone membrane. Int. J. Pharm. 2016, 505, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Kadhum, W.R.; Kanai, S.; Todo, H.; Oshizaka, T.; Sugibayashi, K. Prediction of skin permeation by chemical compounds using the artificial membrane, Strat-M™. Eur. J. Pharm. Sci. 2015, 67, 113–118. [Google Scholar] [CrossRef] [PubMed]
- De Wever, B.; Kurdykowski, S.; Descargues, P. Human Skin Models for Research Applications in Pharmacology and Toxicology: Introducing NativeSkin, the “Missing Link” Bridging Cell Culture and/or Reconstructed Skin Models and Human Clinical Testing. Appl. Vitr. Toxicol. 2015, 1, 26–32. [Google Scholar] [CrossRef]
- Danso, M.O.; Berkers, T.; Mieremet, A.; Hausil, F.; Bouwstra, J.A. Anex vivo humanskin model for studying skin barrier repair. Exp. Dermatol. 2014, 24, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.C.; Maas, W.J.M.; Nielsen, J.B.; Greaves, L.C.; Van De Sandt, J.J.M.; Williams, F.M. Interactions of skin thickness and physicochemical properties of test compounds in percutaneous penetration studies. Int. Arch. Occup. Environ. Health 2006, 79, 405–413. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Plant Protection Products and their Residues (PPR). Scientific Opinion on the Science behind the Revision of the Guidance Document on Dermal Absorption. EFSA J. 2011, 9, 2294. [Google Scholar] [CrossRef]
- EFSA Panel on Plant Protection Products and their Residues (PPR). Guidance on Dermal Absorption. EFSA J. 2012, 10, 2665. [Google Scholar] [CrossRef]
- Publishing Series on Testing and Assessment, No. 156; ENV/JM/MONO(2011)36; IOMC, Environment Directorate Organisation For Economic Co-Operation and Development: Paris, France, 2011.
- Herkenne, C.; Naik, A.; Kalia, Y.N.; Hadgraft, J.; Guy, R.H. Pig Ear Skin ex Vivo as a Model for in Vivo Dermatopharmacokinetic Studies in Man. Pharm. Res. 2006, 23, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Bajza, Á.; Kocsis, D.; Berezvai, O.; Laki, A.J.; Lukács, B.; Imre, T.; Iván, K.; Szabó, P.; Erdő, F. Verification of P-Glycoprotein Function at the Dermal Barrier in Diffusion Cells and Dynamic “Skin-On-A-Chip” Microfluidic Device. Pharmaceutics 2020, 12, 804. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Mano, Y.; Terasaka, S.; Sakurai, T.; Furuya, A.; Urano, H.; Sugibayashi, K. Usefulness of rat skin as a substitute for human skin in the in vitro skin permeation study. Exp. Anim. 2011, 60, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Lukács, B.; Bajza, Á.; Kocsis, D.; Csorba, A.; Antal, I.; Iván, K.; Laki, A.J.; Erdő, F. Skin-on-a-Chip Device for Ex Vivo Monitoring of Transdermal Delivery of Drugs—Design, Fabrication, and Testing. Pharmaceutics 2019, 11, 445. [Google Scholar] [CrossRef] [PubMed]
- Van Ravenzwaay, B.; Leibold, E. A comparison between in vitro rat and human and in vivo rat skin absorption studies. Hum. Exp. Toxicol. 2004, 23, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Bartek, M.J.; LaBudde, J.A.; Maibach, H.I. Skin Permeability In Vivo: Comparison in Rat, Rabbit, Pig and Man. J. Investig. Dermatol. 1972, 58, 114–123. [Google Scholar] [CrossRef]
- Oesch, F.; Fabian, E.; Guth, K.; Landsiedel, R. Xenobiotic-metabolizing enzymes in the skin of rat, mouse, pig, guinea pig, man, and in human skin models. Arch. Toxicol. 2014, 88, 2135–2190. [Google Scholar] [CrossRef]
- Jung, E.C.; Maibach, H.I. Animal models for percutaneous absorption. J. Appl. Toxicol. 2015, 35, 1–10. [Google Scholar] [CrossRef]
- NAFTA Detailed Review and Harmonization of Dermal Absorption Practices—Position Paper on Use of In Vitro Dermal Absorption Data in Risk Assessment; NAFTA Dermal Absorption Group: 2009.
- Liu, J.; Kim, D.; Brown, L.; Madsen, T.; Bouchard, G. Comparison of Human, Porcine, and Rodent Wound Healing with New Miniature Swine Study Data. J. Am. Assoc. Lab. Anim. Sci. JAALAS 2009, 48, 581. [Google Scholar]
- Ponec, M. In vitro cultured human skin cells as alternatives to animals for skin irritancy screening. Int. J. Cosmet. Sci. 1992, 14, 245–264. [Google Scholar] [CrossRef]
- Van Gele, M.; Geusens, B.; Brochez, L.; Speeckaert, R.; Lambert, J. Three-dimensional skin models as tools for transdermal drug delivery: Challenges and limitations. Expert Opin. Drug Deliv. 2011, 8, 705–720. [Google Scholar] [CrossRef]
- De Wever, B.; Petersohn, D.; Mewes, K.R. Overview of Human Three-Dimensional (3D) Skin Models Used for Dermal Toxicity Assessment. HPC Today 2013, 8, 18–22. [Google Scholar]
- Welss, T.; Basketter, D.A.; Schröder, K.R. In vitro skin irritation: Facts and future. State of the art review of mechanisms and models. Toxicol. Vitr. 2004, 18, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, S.; Meng, T.; Zhang, L.; Dai, T.; Xiang, Q.; Su, Z.; Zhang, Q.; Huang, Y. HSP27 as a biomarker for predicting skin irritation in human skin and reconstructed organotypic skin model. Toxicol. Lett. 2014, 226, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Broek, L.J.V.D.; Bergers, L.I.J.C.; Reijnders, C.M.A.; Gibbs, S. Progress and Future Prospectives in Skin-on-Chip Development with Emphasis on the use of Different Cell Types and Technical Challenges. Stem Cell Rev. Rep. 2017, 13, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Desprez, B.; Barroso, J.; Griesinger, C.; Kandárová, H.; Alépée, N.; Fuchs, H.W. Two novel prediction models improve predictions of skin corrosive sub-categories by test methods of OECD Test Guideline No. 431. Toxicol. Vitr. 2015, 29, 2055–2080. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, S.; Corsini, E.; Spiekstra, S.W.; Galbiati, V.; Fuchs, H.W.; DeGeorge, G.; Troese, M.; Hayden, P.; Deng, W.; Roggen, E. An epidermal equivalent assay for identification and ranking potency of contact sensitizers. Toxicol. Appl. Pharmacol. 2013, 272, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, S.; Murli, S.; De Boer, G.; Mulder, A.; Mommaas, A.M.; Ponec, M. Melanosome Capping of Keratinocytes in Pigmented Reconstructed Epidermis—Effect of Ultraviolet Radiation and 3-Isobutyl-1-Methyl-Xanthine on Melanogenesis. Pigment. Cell Res. 2000, 13, 458–466. [Google Scholar] [CrossRef]
- Choi, W.; Wolber, R.; Gerwat, W.; Mann, T.; Batzer, J.; Smuda, C.; Liu, H.; Kolbe, L.; Hearing, V.J. The fibroblast-derived paracrine factor neuregulin-1 has a novel role in regulating the constitutive color and melanocyte function in human skin. J. Cell Sci. 2010, 123, 3102–3111. [Google Scholar] [CrossRef] [PubMed]
- Hayden, P.J.; Bachelor, M.; Ayehunie, S.; Letasiova, S.; Kaluzhny, Y.; Klausner, M.; Kandárová, H. Application of MatTekIn VitroReconstructed Human Skin Models for Safety, Efficacy Screening, and Basic Preclinical Research. Appl. Vitr. Toxicol. 2015, 1, 226–233. [Google Scholar] [CrossRef]
- Bojar, R.A. Studying the Human Skin Microbiome Using 3DIn VitroSkin Models. Appl. Vitr. Toxicol. 2015, 1, 165–171. [Google Scholar] [CrossRef]
- Ackermann, K.; Borgia, S.L.; Korting, H.C.; Mewes, K.R.; Schäfer-Korting, M. The Phenion® Full-Thickness Skin Model for Percutaneous Absorption Testing. Ski. Pharmacol. Physiol. 2010, 23, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Kosten, I.J.; Buskermolen, J.K.; Spiekstra, S.W.; de Gruijl, T.D.; Gibbs, S. Gingiva Equivalents Secrete Negligible Amounts of Key Chemokines Involved in Langerhans Cell Migration Compared to Skin Equivalents. J. Immunol. Res. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Trottier, V.; Marceau-Fortier, G.; Germain, L.; Vincent, C.; Fradette, J. IFATS Collection: Using Human Adipose-Derived Stem/Stromal Cells for the Production of New Skin Substitutes. Stem Cells 2008, 26, 2713–2723. [Google Scholar] [CrossRef] [PubMed]
- Bellas, E.; Seiberg, M.; Garlick, J.; Kaplan, D.L. In vitro 3D Full-Thickness Skin-Equivalent Tissue Model Using Silk and Collagen Biomaterials. Macromol. Biosci. 2012, 12, 1627–1636. [Google Scholar] [CrossRef]
- Monfort, A.; Izeta, A.; Soriano-Navarro, M.; García-Verdugo, J.M. Production of human tissue-engineered skin trilayer on a plasma-based hypodermis. J. Tissue Eng. Regen. Med. 2012, 7, 479–490. [Google Scholar] [CrossRef]
- Shepherd, B.R.; Enis, D.R.; Wang, F.; Suarez, Y.; Pober, J.S.; Schechner, J.S.; Scheduier, J.S. Vascularization and engraftment of a human skin substitute using circulating progenitor cell-derived endothelial cells. FASEB J. 2006, 20, 1739–1741. [Google Scholar] [CrossRef] [PubMed]
- Supp, D.M.; Wilson-Landy, K.; Boyce, S.T. Human dermal microvascular endothelial cells form vascular analogs in cultured skin substitutes after grafting to athymic mice. FASEB J. 2002, 16, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Kosten, I.J.; Spiekstra, S.W.; De Gruijl, T.D.; Gibbs, S. MUTZ-3 derived Langerhans cells in human skin equivalents show differential migration and phenotypic plasticity after allergen or irritant exposure. Toxicol. Appl. Pharmacol. 2015, 287, 35–42. [Google Scholar] [CrossRef]
- Ouwehand, K.; Spiekstra, S.W.; Waaijman, T.; Scheper, R.J.; De Gruijl, T.D.; Gibbs, S. Technical Advance: Langerhans cells derived from a human cell line in a full-thickness skin equivalent undergo allergen-induced maturation and migration. J. Leukoc. Biol. 2011, 90, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Beissert, S.; Cavazzana, I.; Mascia, F.; Meroni, P.; Pastore, S.; Tessari, G.; Girolomoni, G. Mechanisms of Immune-Mediated Skin Diseases: An Overview. Clin. Exp. Rheumatol. 2006, 24, S1–S6. [Google Scholar] [PubMed]
- Demetrulias, J.; Donnelly, T.; Morhenn, V.; Jessee, B.; Hainsworth, S.; Casterton, P.; Bernhofer, L.; Martin, K.; Decker, D. Skin2®—An in vitro human skin model: The correlation betweenin vivoandin Vitrotesting of surfactants. Exp. Dermatol. 1998, 7, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Picarles, V.; Chibout, S.; Kolopp, M.; Médina, J.; Burtin, P.; Ebelin, M.; Osborne, S.; Mayer, F.K.; Spake, A.; Rosdy, M.; et al. Predictivity of an in vitro model for acute and chronic skin irritation (SkinEthic) applied to the testing of topical vehicles. Cell Biol. Toxicol. 1999, 15, 121–135. [Google Scholar] [CrossRef]
- Hoffmann, S.; Saliner, A.G.; Patlewicz, G.; Eskes, C.; Zuang, V.; Worth, A.P. A feasibility study developing an integrated testing strategy assessing skin irritation potential of chemicals. Toxicol. Lett. 2008, 180, 9–20. [Google Scholar] [CrossRef]
- Jírová, D.; Basketter, D.; Liebsch, M.; Bendová, H.; Kejlová, K.; Marriott, M.; Kandárová, H. Comparison of human skin irritation patch test data with in vitro skin irritation assays and animal data. Contact Dermat. 2010, 62, 109–116. [Google Scholar] [CrossRef]
- Abaci, H.E.; Gledhill, K.; Guo, Z.; Christiano, A.M.; Shuler, M.L. Pumpless microfluidic platform for drug testing on human skin equivalents. Lab Chip 2015, 15, 882–888. [Google Scholar] [CrossRef]
- Ataç, B.; Wagner, I.; Horland, R.; Lauster, R.; Marx, U.; Tonevitsky, A.G.; Azar, R.P.; Lindner, G. Skin and hair on-a-chip: In vitro skin models versus ex vivo tissue maintenance with dynamic perfusion. Lab Chip 2013, 13, 3555–3561. [Google Scholar] [CrossRef] [PubMed]
- Maschmeyer, I.; Hasenberg, T.; Jaenicke, A.; Lindner, M.; Lorenz, A.K.; Zech, J.; Garbe, L.-A.; Sonntag, F.; Hayden, P.; Ayehunie, S.; et al. Chip-based human liver–intestine and liver–skin co-cultures—A first step toward systemic repeated dose substance testing in vitro. Eur. J. Pharm. Biopharm. 2015, 95, 77–87. [Google Scholar] [CrossRef]
- Maschmeyer, I.; Lorenz, A.K.; Schimek, K.; Hasenberg, T.; Ramme, A.P.; Hübner, J.; Lindner, M.; Drewell, C.; Bauer, S.; Thomas, A.; et al. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip 2015, 15, 2688–2699. [Google Scholar] [CrossRef] [PubMed]
- Alberti, M.; Dancik, Y.; Sriram, G.; Wu, B.; Teo, Y.L.; Feng, Z.; Bigliardi-Qi, M.; Wu, R.G.; Wang, Z.P.; Bigliardi, P.L. Multi-chamber microfluidic platform for high-precision skin permeation testing. Lab Chip 2017, 17, 1625–1634. [Google Scholar] [CrossRef]
- Lee, S.; Jin, S.-P.; Kim, Y.K.; Sung, G.Y.; Chung, J.H.; Sung, J.H. Construction of 3D multicellular microfluidic chip for an in vitro skin model. Biomed. Microdevices 2017, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Sutterby, E.; Thurgood, P.; Baratchi, S.; Khoshmanesh, K.; Pirogova, E. Microfluidic Skin-on-a-Chip Models: Toward Biomimetic Artificial Skin. Small 2020, 16, e2002515. [Google Scholar] [CrossRef]
- O’Neill, A.T.; Monteiro-Riviere, N.A.; Walker, G.M. Characterization of microfluidic human epidermal keratinocyte culture. Cytotechnology 2008, 56, 197–207. [Google Scholar] [CrossRef]
- Wagner, I.; Materne, E.-M.; Brincker, S.; Süssbier, U.; Frädrich, C.; Busek, M.; Sonntag, F.; Sakharov, D.A.; Trushkin, E.V.; Tonevitsky, A.G.; et al. A dynamic multi-organ-chip for long-term cultivation and substance testing proven by 3D human liver and skin tissue co-culture. Lab Chip 2013, 13, 3538–3547. [Google Scholar] [CrossRef]
- Kim, J.J.; Ellett, F.; Thomas, C.N.; Jalali, F.; Anderson, R.R.; Irimia, D.; Raff, A.B. A microscale, full-thickness, human skin on a chip assay simulating neutrophil responses to skin infection and antibiotic treatments. Lab Chip 2019, 19, 3094–3103. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.Y.; Kim, J.; Song, H.J.; Kim, K.; Choi, K.C.; Park, S.; Sung, G.Y. Development of wrinkled skin-on-a-chip (WSOC) by cyclic uniaxial stretching. J. Ind. Eng. Chem. 2018, 68, 238–245. [Google Scholar] [CrossRef]
- Jeon, H.M.; Kim, K.; Choi, K.C.; Sung, G.Y. Side-effect test of sorafenib using 3-D skin equivalent based on microfluidic skin-on-a-chip. J. Ind. Eng. Chem. 2020, 82, 71–80. [Google Scholar] [CrossRef]
- Wufuer, M.; Lee, G.; Hur, W.; Jeon, B.; Kim, B.J.; Choi, T.H.; Lee, S. Skin-on-a-chip model simulating inflammation, edema and drug-based treatment. Sci. Rep. 2016, 6, 37471. [Google Scholar] [CrossRef] [PubMed]
- Jusoh, N.; Ko, J.; Jeon, N.L. Microfluidics-based skin irritation test using in vitro 3D angiogenesis platform. APL Bioeng. 2019, 3, 036101. [Google Scholar] [CrossRef] [PubMed]
- Sriram, G.; Alberti, M.; Dancik, Y.; Wu, B.; Wu, R.; Feng, Z.; Ramasamy, S.; Bigliardi, P.L.; Bigliardi-Qi, M.; Wang, Z. Full-thickness human skin-on-chip with enhanced epidermal morphogenesis and barrier function. Mater. Today 2018, 21, 326–340. [Google Scholar] [CrossRef]
- Sasaki, N.; Tsuchiya, K.; Kobayashi, H. Photolithography-Free Skin-on-a-Chip for Parallel Permeation Assays. Sens. Mater. 2019, 31, 107. [Google Scholar] [CrossRef]
- Ramadan, Q.; Ting, F.C.W. In vitro micro-physiological immune-competent model of the human skin. Lab Chip 2016, 16, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Morimoto, Y.; Takeuchi, S. Perfusable and stretchable 3D culture system for skin-equivalent. Biofabrication 2018, 11, 011001. [Google Scholar] [CrossRef]
- Mori, N.; Morimoto, Y.; Takeuchi, S. Skin integrated with perfusable vascular channels on a chip. Biomaterials 2017, 116, 48–56. [Google Scholar] [CrossRef]
- Kim, B.S.; Gao, G.; Kim, J.Y.; Cho, D. 3D Cell Printing of Perfusable Vascularized Human Skin Equivalent Composed of Epidermis, Dermis, and Hypodermis for Better Structural Recapitulation of Native Skin. Adv. Health Mater. 2018, 8, e1801019. [Google Scholar] [CrossRef]
- Heringa, M.B.; Park, M.V.D.Z.; Kienhuis, A.S.; Vandebriel, R.J. The value of organs-on-chip for regulatory safety assessment. ALTEX 2019, 37, 208–222. [Google Scholar] [CrossRef]
- Hardwick, R.N.; Betts, C.J.; Whritenour, J.; Sura, R.; Thamsen, M.; Kaufman, E.H.; Fabre, K. Drug-induced skin toxicity: Gaps in preclinical testing cascade as opportunities for complex in vitro models and assays. Lab Chip 2019, 20, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Han, X.; Wang, Y.; Chen, Z.; Lu, Y.; Liu, T.; Wu, Z.; Jin, Y.; Luo, Y.; Zhang, X. Drug Toxicity Evaluation Based on Organ-on-a-chip Technology: A Review. Micromachines 2020, 11, 381. [Google Scholar] [CrossRef]
- Jeon, B.; Lee, G.; Wufuer, M.; Huang, Y.; Choi, Y.; Kim, S.; Choi, T.H. Enhanced predictive capacity using dual-parameter chip model that simulates physiological skin irritation. Toxicol. Vitr. 2020, 68, 104955. [Google Scholar] [CrossRef] [PubMed]
- Tavares, R.S.N.; Maria-Engler, S.S.; Colepicolo, P.; Debonsi, H.M.; Schäfer-Korting, M.; Marx, U.; Gaspar, L.R.; Zoschke, C. Skin Irritation Testing beyond Tissue Viability: Fucoxanthin Effects on Inflammation, Homeostasis, and Metabolism. Pharmaceutics 2020, 12, 136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sito, L.; Mao, M.; He, J.; Zhang, Y.S.; Zhao, X. Current advances in skin-on-a-chip models for drug testing. Microphysiol. Syst. 2018, 1, 1. [Google Scholar] [CrossRef]
- Suhail, S.; Sardashti, N.; Jaiswal, D.; Rudraiah, S.; Misra, M.; Kumbar, S.G. Engineered Skin Tissue Equivalents for Product Evaluation and Therapeutic Applications. Biotechnol. J. 2019, 14, e1900022. [Google Scholar] [CrossRef]
- Biglari, S.; Le, T.Y.L.; Tan, R.P.; Wise, S.G.; Zambon, A.; Codolo, G.; De Bernard, M.; Warkiani, M.; Schindeler, A.; Naficy, S.; et al. Simulating Inflammation in a Wound Microenvironment Using a Dermal Wound-on-a-Chip Model. Adv. Health Mater. 2019, 8, e1801307. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Huang, X.; Jiang, B.; Zhang, P.; Guo, L.; Cui, X.; Zhou, S.; Ren, L.; Zhang, M.; Zeng, J.; et al. linc00174-EZH2-ZNF24/Runx1-VEGFA Regulatory Mechanism Modulates Post-burn Wound Healing. Mol. Ther. Nucleic Acids 2020, 21, 824–836. [Google Scholar] [CrossRef]
- Jensen, J.-M.; Schütze, S.; Förl, M.; Krönke, M.; Proksch, E. Roles for tumor necrosis factor receptor p55 and sphingomyelinase in repairing the cutaneous permeability barrier. J. Clin. Investig. 1999, 104, 1761–1770. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.; Sung, G.Y. Coenzyme Q10 Efficacy Test for Human Skin Equivalents Using a Pumpless Skin-On-A-Chip System. Int. J. Mol. Sci. 2020, 21, 8475. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-P.; Schunck, M.; Kallen, K.-J.; Neumann, C.; Trautwein, C.; Rose-John, S.; Proksch, E. The Interleukin-6 Cytokine System Regulates Epidermal Permeability Barrier Homeostasis. J. Investig. Dermatol. 2004, 123, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Giacomoni, P.U.; Rein, G. A mechanistic model for the aging of human skin. Micron 2004, 35, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, T.; Merola, J.; Catarino, C.; Xie, C.B.; Kirkiles-Smith, N.C.; Lee, V.; Hotta, S.; Dai, G.; Xu, X.; Ferreira, F.C.; et al. Three Dimensional Bioprinting of a Vascularized and Perfusable Skin Graft Using Human Keratinocytes, Fibroblasts, Pericytes, and Endothelial Cells. Tissue Eng. Part A 2020, 26, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Yan, W.-C.; Davoodi, P.; Vijayavenkataraman, S.; Tian, Y.; Ng, W.C.; Fuh, J.Y.; Robinson, K.S.; Wang, C.-H. 3D bioprinting of skin tissue: From pre-processing to final product evaluation. Adv. Drug Deliv. Rev. 2018, 132, 270–295. [Google Scholar] [CrossRef] [PubMed]
- Phua, Q.H.; Han, H.A.; Soh, B.-S. Translational stem cell therapy: Vascularized skin grafts in skin repair and regeneration. J. Transl. Med. 2021, 19, 1–11. [Google Scholar] [CrossRef]
- Kwak, B.S.; Jin, S.-P.; Kim, S.J.; Kim, E.J.; Chung, J.H.; Sung, J.H. Microfluidic skin chip with vasculature for recapitulating the immune response of the skin tissue. Biotechnol. Bioeng. 2020, 117, 1853–1863. [Google Scholar] [CrossRef]
- Kim, K.; Jeon, H.M.; Choi, K.C.; Sung, G.Y. Testing the Effectiveness of Curcuma longa Leaf Extract on a Skin Equivalent Using a Pumpless Skin-on-a-Chip Model. Int. J. Mol. Sci. 2020, 21, 3898. [Google Scholar] [CrossRef] [PubMed]
- Pauty, J.; Nakano, S.; Usuba, R.; Nakajima, T.; Johmura, Y.; Omori, S.; Sakamoto, N.; Kikuchi, A.; Nakanishi, M.; Matsunaga, Y.T. A 3D tissue model-on-a-chip for studying the effects of human senescent fibroblasts on blood vessels. Biomater. Sci. 2021, 9, 199–211. [Google Scholar] [CrossRef]
- Lawrence, B.J.; Madihally, S.V. Cell colonization in degradable 3D porous matrices. Cell Adhes. Migr. 2008, 2, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, N.; Ohashi, T.; Sato, M. Effect of Shear Stress on Permeability of Vascular Endothelial Monolayer Cocultured with Smooth Muscle Cells. JSME Int. J. Ser. C 2004, 47, 992–999. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Lo, K.-Y.; Sun, Y.-S. A microfluidics-based wound-healing assay for studying the effects of shear stresses, wound widths, and chemicals on the wound-healing process. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Davies, P.F.; Remuzzi, A.; Gordon, E.J.; Dewey, C.F.; Gimbrone, M.A. Turbulent fluid shear stress induces vascular endothelial cell turnover in vitro. Proc. Natl. Acad. Sci. USA 1986, 83, 2114–2117. [Google Scholar] [CrossRef] [PubMed]
- Ruze, A.; Zhao, Y.; Li, H.; Gulireba, X.; Li, J.; Lei, D.; Dai, H.; Wu, J.; Zhao, X.; Nie, Y. Low shear stress upregulates the expression of fractalkine through the activation of mitogen-activated protein kinases in endothelial cells. Blood Coagul. Fibrinolysis 2018, 29, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lin, Y.-S.; Haynes, C.L. On-Chip Evaluation of Shear Stress Effect on Cytotoxicity of Mesoporous Silica Nanoparticles. Anal. Chem. 2011, 83, 8377–8382. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Mao, S.; Zhang, Q.; Li, W.; Lin, J.-M. Online Analysis of Drug Toxicity to Cells with Shear Stress on an Integrated Microfluidic Chip. ACS Sens. 2019, 4, 521–527. [Google Scholar] [CrossRef]
- Hou, L.; Hagen, J.; Wang, X.; Papautsky, I.; Naik, R.; Kelley-Loughnane, N.; Heikenfeld, J. Artificial microfluidic skin for in vitro perspiration simulation and testing. Lab Chip 2013, 13, 1868. [Google Scholar] [CrossRef]
- Agarwal, T.; Narayana, G.H.; Banerjee, I. Keratinocytes are mechanoresponsive to the microflow-induced shear stress. Cytoskeleton 2019, 76, 209–218. [Google Scholar] [CrossRef]
- Vahav, I.; Broek, L.J.V.D.; Thon, M.; Monsuur, H.N.; Spiekstra, S.W.; Atac, B.; Scheper, R.J.; Lauster, R.; Lindner, G.; Marx, U.; et al. Reconstructed human skin shows epidermal invagination towards integrated neopapillae indicating early hair follicle formation in vitro. J. Tissue Eng. Regen. Med. 2020, 14, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Suhas, V. Patankar Numerical Heat Transfer and Fluid Flow; CRC Press: New Delhi, India, 2018; ISBN 978-1-315-27513-0. [Google Scholar]
- Blazek, J. Computational Fluid Dynamics—Principles and Applications; Butterworth-Heinemann: Oxford, UK, 2015. [Google Scholar]
- Garcia, J.; Rios, I.; Fonthal, F. Structural and microfluidic analysis of microneedle array for drug delivery. In Proceedings of the 2016 31st Symposium on Microelectronics Technology and Devices (SBMicro), Belo Horizonte, Brazil, 29 August–3 September 2016; pp. 1–4. [Google Scholar]
- Jain, S.K.; Verma, A.; Jain, A.; Hurkat, P. Transfollicular drug delivery: Current perspectives. Res. Rep. Transdermal Drug Deliv. 2016, 5, 1–17. [Google Scholar] [CrossRef]
- Kitson, N.; Thewalt, J.L. Hypothesis: The epidermal permeability barrier is a porous medium. Acta Derm. Venereol. 2000, 80, 12–15. [Google Scholar] [CrossRef]
- Narasimhan, A.; Joseph, A. Porous Medium Modeling of Combined Effects of Cell Migration and Anisotropicity of Stratum Corneum on Transdermal Drug Delivery. J. Heat Transf. 2015, 137, 121007. [Google Scholar] [CrossRef]
- Nield, D.A.; Bejan, A. Convection in Porous Media, 4th ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Marquez-Lago, T.T.; Allen, D.M.; Thewalt, J. A novel approach to modelling water transport and drug diffusion through the stratum corneum. Theor. Biol. Med. Model. 2010, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, H.C. A Calculation of the Viscous Forced Exerted by a Flowing Fluid on a Dense Swarm of Particles. Appl. Sci. Res. 1949, 1, 27–34. [Google Scholar] [CrossRef]
- Brinkman, H.C. On the permeability of media consisting of closely packed porous particles. Flow Turbul. Combust. 1949, 1, 81–86. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Dhinakaran, S.; Khalili, A. Fluid motion around and through a porous cylinder. Chem. Eng. Sci. 2006, 61, 4451–4461. [Google Scholar] [CrossRef]
- Dhinakaran, S.; Ponmozhi, J. Heat transfer from a permeable square cylinder to a flowing fluid. Energy Convers. Manag. 2011, 52, 2170–2182. [Google Scholar] [CrossRef]
- Pennes, H.H. Analysis of Tissue and Arterial Blood Temperatures in the Resting Human Forearm. J. Appl. Physiol. 1948, 1, 93–122. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Ghosh, P.; Li, S.K.; Newman, B.; Kasting, G.B.; Raney, S.G. Heat effects on drug delivery across human skin. Expert Opin. Drug Deliv. 2016, 13, 755–768. [Google Scholar] [CrossRef]
- Silva, M.; Freitas, B.; Andrade, R.; Espregueira-Mendes, J.; Silva, F.; Carvalho, Ó.; Flores, P. Computational Modelling of the Bioheat Transfer Process in Human Skin Subjected to Direct Heating and/or Cooling Sources: A Systematic Review. Ann. Biomed. Eng. 2020, 48, 1616–1639. [Google Scholar] [CrossRef] [PubMed]
- Abaci, H.; Guo, Z.; Doucet, Y.; Jacków, J.; Christiano, A. Next generation human skin constructs as advanced tools for drug development. Exp. Biol. Med. 2017, 242, 1657–1668. [Google Scholar] [CrossRef]
- Bechetoille, N.; Vachon, H.; Gaydon, A.; Boher, A.; Fontaine, T.; Decossas, M.; André-Frei, V.; Schaeffer, E.; Mueller, C.G. A new organotypic model containing dermal-type macrophages. Exp. Dermatol. 2011, 20, 1035–1037. [Google Scholar] [CrossRef] [PubMed]


| Membranes | Materials | Pros | Cons |
|---|---|---|---|
| Silicon based | Silastic, Polydimethylsiloxane (PDMS), Silatos | Cost effective, good storage conditions, good reproducibility, low variability | Fails to incorporate components like metabolism, distribution, and excretion |
| Cellulose-based | Pure cellulose, Cellulose-acetate, cellulose nitrate (glycerin and preservatives can be added for better flexibility) | Cost effective, good storage conditions, good reproducibility, low variability, very low protein binding capacity, hydrophilic, improved solvent resistance | Fails to incorporate components like metabolism, distribution, and excretion, lubrication is needed, not lipophilic |
| Synthetic polymer based | Nylon (aliphatic polyamides) (hydrophobic), polysulfone, polycarbonates (high flux membranes) | Low protein binding, chemical inertness, cost-effective, lack of tortuosity of the pores, good chemical stability | Higher cost, lower availability, fails to incorporate components like metabolism, distribution, and excretion |
| Strat-M® | Multilayer polyester sulfone polyolefin | Multiple layers with different permeability good storage conditions good reproducibility low variability, good correlation with excised skin | Fails to incorporate components like metabolism, distribution, and excretion |
| Chitosan | Chitosan-alginate Poloxamer 188 | Porosity can be varied based on molecular weight and origin (fungal or animal) good physicochemical properties, thickness, roughness, opacity, liquid uptake, and water vapor permeability can be modified, non-toxic | Fails to incorporate components like metabolism, distribution, and excretion |
| Mouse | Rat | Porcine | Human | |
|---|---|---|---|---|
| Skin thickness | 0.4–1 mm | 1–2 mm | 1.5–2 mm | 2–3 mm |
| Epidermal thickness | 9.4–13.3 µm | 21.7 µm | 52–100 µm | 50–100 µm |
| Stratum corneum | 2.9 µm | 5 µm | 12.28 µm | 10–12.5 µm |
| Fixed skin | no | no | Yes | Yes |
| Hair follicles | 658 hairs/cm2 | 289 hairs/cm2 | 11 hairs/cm2 | 11 hairs/cm2 |
| Sources | laboratory animals | laboratory animals | veterinary education, food industry | cadaver, tissue bank, biopsy |
| Reconstructed Human Epidermis Models (RHE) | Full-Thickness Human Skin Models (LSE) | ||
|---|---|---|---|
| EpiDerm | MatTek Corporation, Ashland, MA, USA | EpiDermFT | MatTek Corporation, Ashland, MA, USA |
| EpiSkin | L’Oréal, Lyon, France | StrataTest | Stratatech, Madison, WI, USA |
| reconstructed human epidermis | SkinEthic, Lyon, France | Phenion Full-Thick-ness Skin | Phenion, Düsseldorf, Germany |
| EpiCs | CellSystems, Troisdorf, Germany | GraftSkin | Apligraf; Organogenesis, MI, USA |
| open source reconstructed epidermis model | Phenion, Düsseldorf, Germany | Vitrolife-Skin | Kyoto, Japan |
| Straticell | Straticell, Les Isnes, Belgium | ||
| Labcyte | Gamagori, Japan | ||
| Model | Commercially Available | Advantages/Disadvantages | Ref. |
|---|---|---|---|
| Reconstructed epidermis | Yes: EpiDerm™, EpiSkin™, SkinEthic™, epiCS® No: in house models | +: differentiated epidermis from keratinocytes −: only keratinocytes, no dermal compartment present, or immune cells | [39,40] |
| Pigmented Reconstructed epidermis | Yes: MelanoDerm No: in house models | +: pigmented differentiated epidermis from keratinocytes and melanocytes −: no living dermal compartment, immune cells, adipose tissue, appendages, or blood vessels present | [41,42] |
| Full-thickness skin models | Yes: EpiDerm-FT, Phenion-FT, LabSkin No: in house models | +: differentiated epidermis on the fibroblast-populated dermis −: no immune cells, adipose tissue, appendages, or blood vessels | [43,44,45,46] |
| Three-layered skin model | No: in house models | +: differentiated epidermis on fibroblast-populated dermis on an adipocyte/ASC populated hypodermis −: no immune cells or appendages | [47,48,49] |
| Full-thickness skin model containing EC | No: in house models | +: differentiated epidermis on fibroblast and endothelial cell (show vessel-like structures) populated dermis −: no immune cells, adipose tissue, appendages, or perfused blood vessels | [50,51] |
| Skin equivalent with integrated Langerhans Cells | No: in house model | +: pigmented skin model containing functional MUTZ-3 derived Langerhans −: no adipose tissue, appendages, or blood vessels | [52,53] |
| Traditional Diffusion Devices | Skin-On-A-Chip Devices |
|---|---|
| high tissue need | low tissue need |
| high active ingredient need | low active ingredient need |
| high formulation need | low formulation need |
| macroscale size | microscale size |
| static system | dynamic system |
| poor reproducibility | good reproducibility |
| only ex vivo (or in vitro membranes) | ex vivo and in vitro membranes or cell cultures |
| high sample volumes | low sample volumes |
| high cost | low cost |
| controlled parameters | precisely controlled parameters |
| Materials of the Chip | Fabrication Technology | Testing Features | Reference |
|---|---|---|---|
| PDMS | lithography | toxicity testing, high throughput | [66] |
| PDMS, PDMS membrane, natural ECM | lithography | multiorgan chip optimization of the parameters | [67] |
| PDMS, natural ECM | lithography | efficacy testing ex vivo using skin micro-biopsy | [68] |
| PDMS, collagen ECM | lithography | skin wrinkling cosmetic testing | [69] |
| PDMS, PET membrane, collagen ECM | lithography | drug testing pump free system | [59] |
| PDMS, collagen ECM | lithography | multiple collagen sources were compared toxicity testing | [70] |
| PDMS, collagen ECM | lithography | ex vivo skin and hair, validation study | [60] |
| PDMS, PET membrane, fibronectin ECM | lithography | edema and inflammation | [71] |
| PDMS, fibrin with collagen | lithography | skin irritation | [72] |
| PDMS, polycarbonate membrane, collagen ECM | lithography | pump free system, multicell skin model | [64] |
| PMMA, polycarbonate membrane, fibrin ECM | CNC micro milling | micro-milling | [73] |
| PDMS, PET membrane | Laser cutting | three parallels, diffusion study | [74] |
| PDMS, PMMA, PET membrane | Laser cutting | three parallels and TEER sensor integrated, immune study | [75] |
| silicon rubber, collagen ECM | 3D printing | blood vessels, diffusion study | [76,77] |
| PCL, skin-derived dECM | 3D printing | fabricated with vascular channels, validation study | [78] |
Undisturbed Laminar Flow | Disturbed Laminar Flow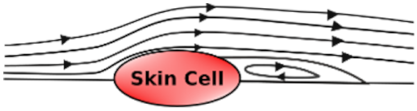 | Turbulent Flow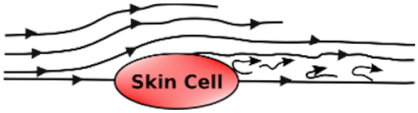 | |
|---|---|---|---|
| Porosity | Controlled porosity [100] | Mixed porosity | - |
| Permeability | Decreases [101] | Low | - |
| Wound Repair | Healing speed increases [102] | Healing speed is low | Healing speed is very low |
| Turnover rate | Low | High [103] | Very high |
| Inflammation | Very low [104] | High | Very high |
| Toxicology studies | Good toxicity results compared to static conditions [105,106] | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponmozhi, J.; Dhinakaran, S.; Varga-Medveczky, Z.; Fónagy, K.; Bors, L.A.; Iván, K.; Erdő, F. Development of Skin-On-A-Chip Platforms for Different Utilizations: Factors to Be Considered. Micromachines 2021, 12, 294. https://doi.org/10.3390/mi12030294
Ponmozhi J, Dhinakaran S, Varga-Medveczky Z, Fónagy K, Bors LA, Iván K, Erdő F. Development of Skin-On-A-Chip Platforms for Different Utilizations: Factors to Be Considered. Micromachines. 2021; 12(3):294. https://doi.org/10.3390/mi12030294
Chicago/Turabian StylePonmozhi, J., S. Dhinakaran, Zsófia Varga-Medveczky, Katalin Fónagy, Luca Anna Bors, Kristóf Iván, and Franciska Erdő. 2021. "Development of Skin-On-A-Chip Platforms for Different Utilizations: Factors to Be Considered" Micromachines 12, no. 3: 294. https://doi.org/10.3390/mi12030294
APA StylePonmozhi, J., Dhinakaran, S., Varga-Medveczky, Z., Fónagy, K., Bors, L. A., Iván, K., & Erdő, F. (2021). Development of Skin-On-A-Chip Platforms for Different Utilizations: Factors to Be Considered. Micromachines, 12(3), 294. https://doi.org/10.3390/mi12030294