Microfluidic Separation of Blood Cells Based on the Negative Dielectrophoresis Operated by Three Dimensional Microband Electrodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of the Fluidic Device for Dielectrophoresis (DEP) Separation
2.2. Preparation of Mouse Erythrocytes and Human Acute Monocytic Leukemia Cell Line
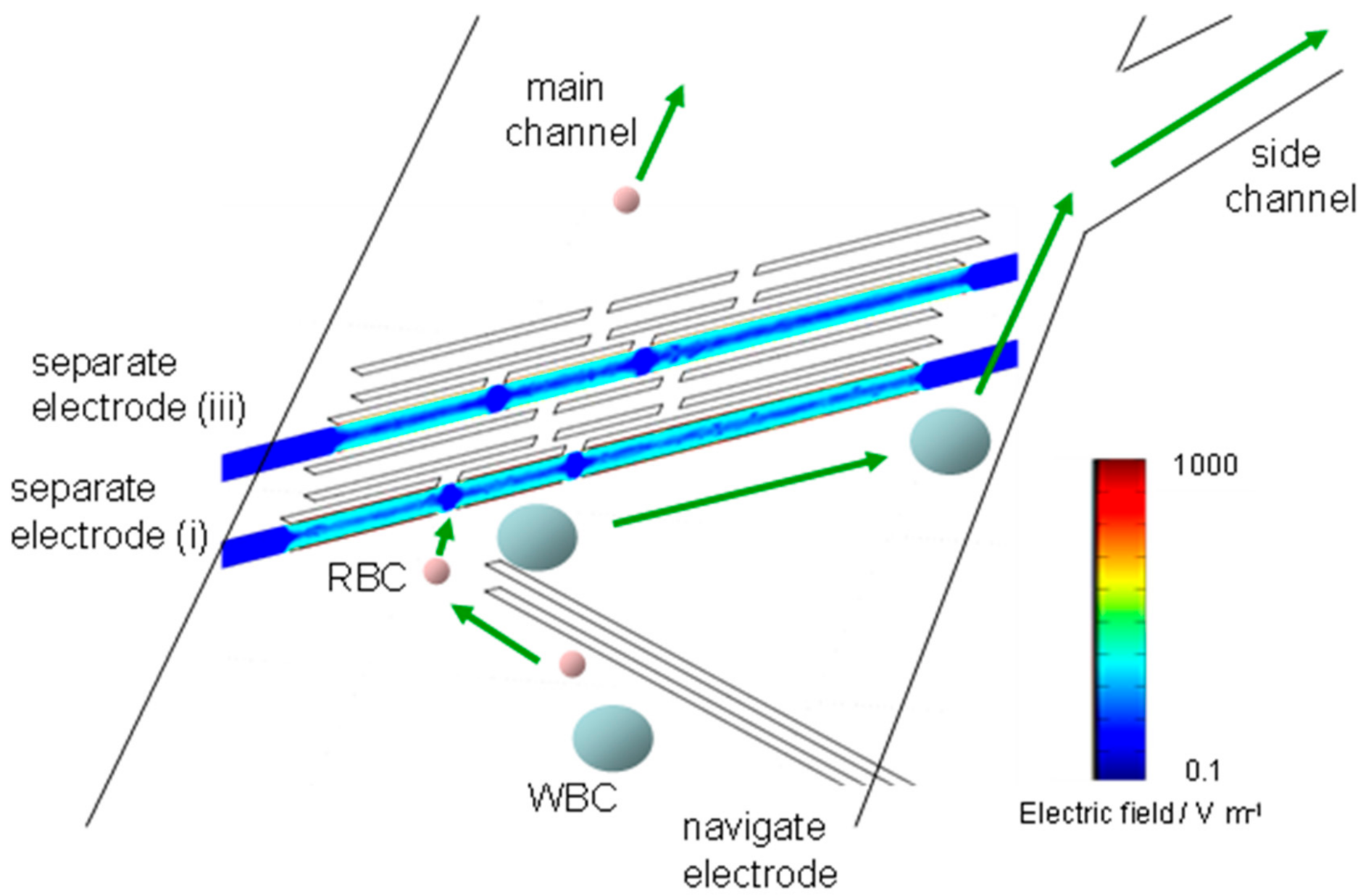
2.3. Regulation of Cell Position in The Fluid Channel
3. Results
3.1. DEP Behavior of Red Blood Cells (RBCs) by Using Castellated Electrode
3.2. Numerical Calculation of Electric Field for the Separator
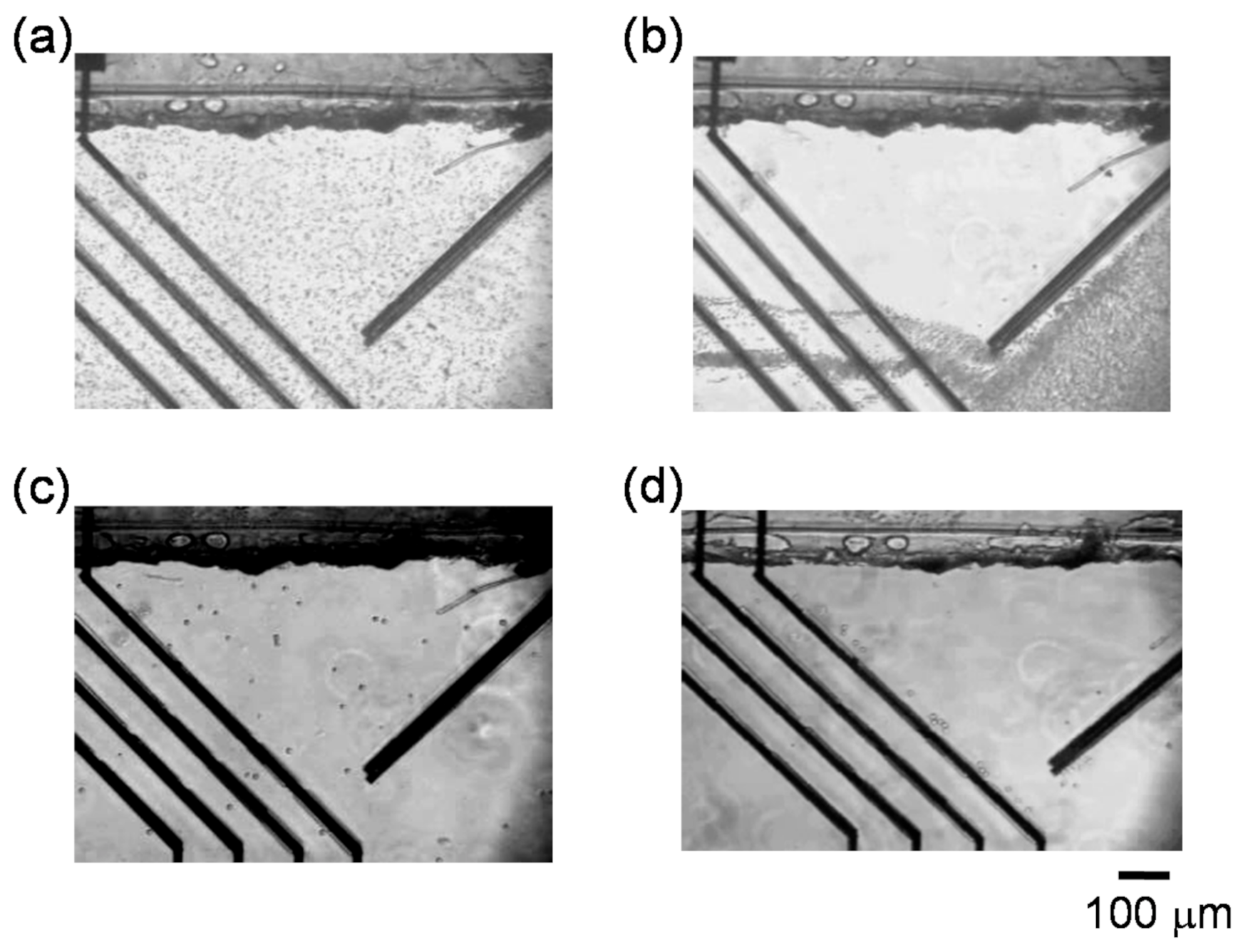
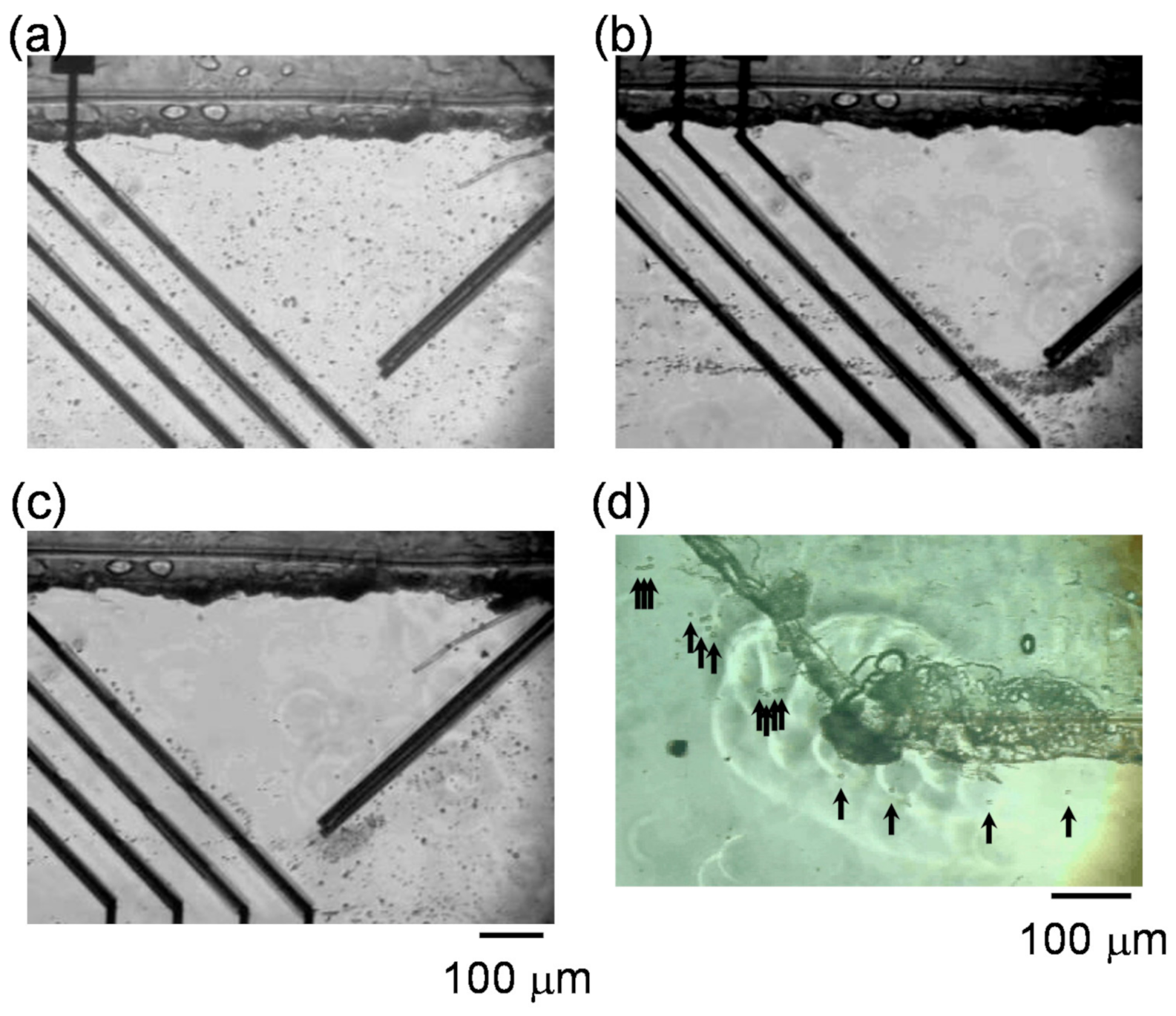
3.3. Manipulation of RBCs and Human Acute Monocytic Leukemia Cell Line (THP-1 Cells) by n-DEP
3.4. Separation of THP-1 Cells and RBCs from Mixtures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Páez-Avilés, C.; Juanola-Feliu, E.; Punter-Villagrasa, J.; Del Moral Zamora, B.; Homs-Corbera, A.; Colomer-Farrarons, J.; Miribel-Català, P.L.; Samitier, J. Combined Dielectrophoresis and Impedance Systems for Bacteria Analysis inMicrofluidic On-Chip Platforms. Sensors 2016, 16, 1514. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Yang, X.; Wang, J.; Wang, Y.; Yang, W.; Liu, L. Determination of Dielectric Properties of Cells using AC Electrokinetic-based Microfluidic Platform: A Review of Recent Advances. Micromachines 2020, 11, 513. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Suzuki, M.; Arimoto, S.; Korenaga, T.; Yasukawa, T. Determination of membrane capacitance and cytoplasm conductivity by the simultaneous electrorotation. Analyst 2020, 145, 4188–4195. [Google Scholar] [CrossRef] [PubMed]
- Yahya, W.N.W.; Kadri, N.A.; Ibrahim, F. Cell Patterning for Liver Tissue Engineering via Dielectrophoretic Mechanisms. Sensors 2014, 14, 11714–11734. [Google Scholar] [CrossRef]
- Yasukawa, T.; Morishima, A.; Suzuki, M.; Yoshioka, J.; Yoshimoto, K.; Mizutani, F. Rapid formation of aggregates with uniform numbers of cells based on three-dimensional dielectrophoresis. Anal. Sci. 2019, 35, 895–901. [Google Scholar] [CrossRef]
- Okayama, H.; Tomita, M.; Suzuki, M.; Yasukawa, T. Rapid formation of arrayed cells on an electrode with microwells by a scanning electrode based on positive dielectrophoresis. Anal. Sci. 2019, 35, 701–704. [Google Scholar] [CrossRef]
- Sugano, T.; Sasaki, Y.; Mizutani, F.; Yasukawa, T. Simple Formation of Cell Arrays Embedded in Hydrogel Sheets and Cubes. Anal. Sci. 2018, 34, 127–130. [Google Scholar] [CrossRef]
- Yoshioka, J.; Yoshitomi, T.; Yasukawa, T.; Yoshimoto, K. Alternation of Gene Expression Levels in Mesenchymal Stem Cells by Applying Positive Dielectrophoresis. Anal. Sci. 2016, 32, 1213–1216. [Google Scholar] [CrossRef]
- Abd Rahman, N.; Ibrahim, F.; Yafouz, B. Dielectrophoresis for Biomedical Sciences Applications: A Review. Sensors 2017, 17, 449. [Google Scholar] [CrossRef]
- Zhang, H.; Chang, H.; Neuzil, P. DEP-on-a-Chip: Dielectrophoresis Applied to Microfluidic Platforms. Micromachines 2019, 10, 423. [Google Scholar] [CrossRef]
- Voldman, J. Electrical forces for microscale cell manipulation. Annu. Rev. Biomed. Eng. 2006, 8, 425–454. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Roh, S.M.; Lee, E.; Park, Y.; Lee, B.C.; Kwon, Y.; Kim, H.J.; Kim, J. Applications of Converged Various Forces for Detection of Biomolecules and Novelty of Dielectrophoretic Force in the Applications. Sensors 2020, 20, 3242. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, X.-B.; Becker, F.F.; Gascoyne, P.C.R. Introducing dielectrophoresis as a new force field for field-flow fractionation. Biophys. J. 1997, 73, 1118–1129. [Google Scholar] [CrossRef]
- Markx, G.H.; Rousselet, J.; Pethig, R. DEP-FFF Field-flow fractionation using non-uniform electric fields. J. Liq. Chromatogr. Relat. Technol. 1997, 20, 2857–2872. [Google Scholar] [CrossRef]
- Hughes, M.P. Strategies for dielectrophoretic separation in laboratory—on—a—chip systems. Electrophoresis 2002, 23, 2569–2582. [Google Scholar] [CrossRef]
- Wang, X.-B.; Yang, J.; Huang, Y.; Vykoukal, J.; Becker, F.F.; Gascoyne, P.R.C. Cell separation by dielectrophoretic field-flow-fractionation. Anal. Chem. 2000, 72, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Gascoyne, P.R.C.; Noshari, J.; Anderson, T.J.; Becker, F.F. Isolation of rare cells from cell mixtures by dielectrophoresis. Electrophoresis 2009, 30, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Flanagan, L.A.; Jeon, N.L.; Monuki, E.; Lee, A.P. Dielectrophoresis switching with vertical sidewall electrodes for microfluidic flow cytometry. Lab Chip 2007, 7, 1114–1120. [Google Scholar] [CrossRef]
- Fiedler, S.; Shirley, S.G.; Schnelle, T.; Fuhr, G. Dielectrophoretic Sorting of Particles and Cells in a Microsystem. Anal. Chem. 1998, 70, 1909–1915. [Google Scholar] [CrossRef]
- Han, K.-H.; Frazier, A.B. Lateral-driven continuous dielectrophoretic microseparators for blood cells suspended in a highly conductive medium. Lab Chip 2008, 8, 1079–1086. [Google Scholar] [CrossRef]
- Pommer, M.S.; Zhang, Y.; Keerthi, N.; Chen, D.; Thomson, J.A.; Meinhart, C.D.; Soh, H.T. Dielectrophoretic separation of platelets from diluted whole blood in microfluidic channels. Electrophoresis 2008, 29, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.-S.; Kwon, K.; Kim, S.-I.; Han, H.; Sohn, J.; Lee, S.; Jung, H.-I. Continuous separation of breast cancer cells from blood samples using multi-orifice flow fractionation (MOFF) and dielectrophoresis (DEP). Lab Chip 2011, 11, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Rosano, J.M.; Wang, Y.; Garson, C.J.; Prabhakarpandian, B.; Pant, K.; Klarmann, G.J.; Perantoni, A.; Alvarez, L.M.; Lai, E. Continuous-flow sorting of stem cells and differentiation products based on dielectrophoresis. Lab Chip 2015, 15, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Dalili, A.; Taatizadeh, E.; Tahmooressi, H.; Tasnim, N.; Rellstab-Sanchez, P.I.; Shaunessy, M.; Najjaran, H.; Hoorfar, M. Parametric study on the geometrical parameters of a lab—on—a—chip platform with tilted planar electrodes for continuous dielectrophoretic manipulation of microparticles. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dürr, M.; Kentsch, J.; Müller, T.; Schnelle, T.; Stelzle, M. Microdevices for manipulation and accumulation of micro-and nanoparticles by dielectrophoresis. Electrophoresis 2003, 24, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.F.; Du, H.; Li, W.H. A 3D paired microelectrode array for accumulation and separation of microparticles. J. Micromech. Microeng. 2006, 16, 1162–1169. [Google Scholar] [CrossRef]
- Hu, X.; Bessette, P.H.; Qian, J.; Meinhart, C.D.; Daugherty, P.S.; Soh, H.T. Marker-specific sorting of rare cells using dielectrophoresis. Proc. Natl. Acad. Sci. USA 2005, 102, 15757–15761. [Google Scholar] [CrossRef] [PubMed]
- Bessette, P.H.; Hu, X.; Soh, H.T.; Daugherty, P.S. Microfluidic Library Screening for Mapping Antibody Epitopes. Anal. Chem. 2007, 79, 2174–2178. [Google Scholar] [CrossRef]
- Yasukawa, T.; Suzuki, M.; Sekiya, T.; Shiku, H.; Matsue, T. Flow sandwich-type immunoassay in microfluidic devices based on negative dielectrophoresis. Biosens. Bioelectron. 2007, 22, 2730–2736. [Google Scholar] [CrossRef] [PubMed]
- Ramón-Azcón, J.; Kunikata, R.; Sanchez, F.-J.; Marco, M.-P.; Shiku, H.; Yasukawa, T.; Matsue, T. Detection of pesticide residues using an immunodevice based on negative dielectrophoresis. Biosens. Bioelectron. 2009, 24, 1592–1597. [Google Scholar]
- Kim, U.; Qian, J.; Kenrick, S.A.; Daugherty, P.S.; Soh, H.T. Multitarget Dielectrophoresis Activated Cell Sorter. Anal. Chem. 2008, 80, 8656–8661. [Google Scholar] [CrossRef] [PubMed]
- Han, K.-H.; Han, S.-I.; Frazier, A.B. Lateral displacement as a function of particle size using a piecewise curved planar interdigitated electrode array. Lab Chip 2009, 9, 2958–2964. [Google Scholar] [CrossRef]
- Yasukawa, T.; Suzuki, M.; Shiku, H.; Matsue, T. Control of the microparticle position in the channel based on dielectrophoresis. Sens. Actuator. B Chem. 2009, 142, 400–403. [Google Scholar] [CrossRef]
- Yasukawa, T.; Yamada, J.; Shiku, H.; Mizutani, F.; Matsue, T. Negative Dielectrophoretic particle positioning in a fluidic flow. Intell. Autom. Soft Comput. 2012, 18, 201–211. [Google Scholar] [CrossRef]
- Schnelle, T.; Muller, T.; Fiedler, S.; Fuhr, G. The influence of higher moments on particle behaviour in dielectrophoretic field cages. J. Electrostat. 1999, 46, 13–28. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Tomita, M.; Mizutani, F.; Yasukawa, T. Cell Pairing Using Microwell Array Electrodes Based on Dielectrophoresis. Anal. Chem. 2014, 86, 6818–6822. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Mizutani, F.; Yasukawa, T. Dielectrophoretic Tweezers for Pickup and Relocation of Individual Cells Using Microdisk Electrodes with a Microcavity. Electrochemistry 2016, 84, 361–363. [Google Scholar] [CrossRef][Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasukawa, T.; Yamada, J.; Shiku, H.; Matsue, T.; Suzuki, M. Microfluidic Separation of Blood Cells Based on the Negative Dielectrophoresis Operated by Three Dimensional Microband Electrodes. Micromachines 2020, 11, 833. https://doi.org/10.3390/mi11090833
Yasukawa T, Yamada J, Shiku H, Matsue T, Suzuki M. Microfluidic Separation of Blood Cells Based on the Negative Dielectrophoresis Operated by Three Dimensional Microband Electrodes. Micromachines. 2020; 11(9):833. https://doi.org/10.3390/mi11090833
Chicago/Turabian StyleYasukawa, Tomoyuki, Junko Yamada, Hitoshi Shiku, Tomokazu Matsue, and Masato Suzuki. 2020. "Microfluidic Separation of Blood Cells Based on the Negative Dielectrophoresis Operated by Three Dimensional Microband Electrodes" Micromachines 11, no. 9: 833. https://doi.org/10.3390/mi11090833
APA StyleYasukawa, T., Yamada, J., Shiku, H., Matsue, T., & Suzuki, M. (2020). Microfluidic Separation of Blood Cells Based on the Negative Dielectrophoresis Operated by Three Dimensional Microband Electrodes. Micromachines, 11(9), 833. https://doi.org/10.3390/mi11090833




