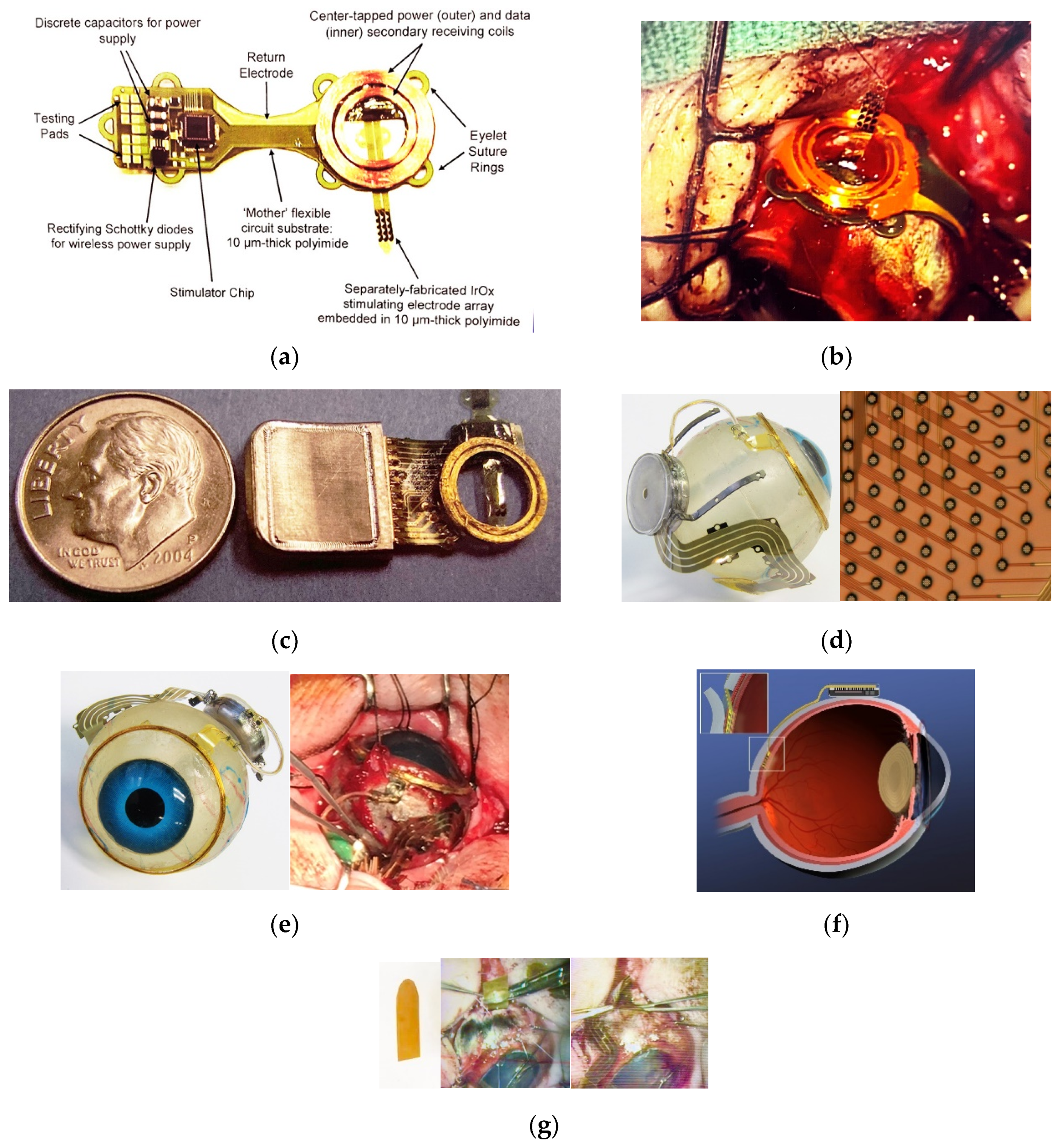
The fabrication related challenges that our team addressed in building implantable visual prostheses are outlined below. We have constructed three generations of retinal implants, and the implantable components can be seen in
Figure 1. The complete visual prosthesis system contains an external controller with a radio frequency transceiver, but that system does not make use of any unique micro-fabrication technology, and is not reported on here. Likewise, the application-specific integrated circuits (ASICs) for providing charge-balanced stimuli to retinal tissue were sourced from established mixed-signal chip foundry services, and have been described elsewhere [
23]. The ASICs were mounted on internal, high-density multilayer flexible circuit boards using flip-chip/ surface-mount assembly methods that are well established in the industry. Our approach was to attach the multi-layered, flexible internal circuit board to the interior surface of a high temperature, co-fired (HTCC) alumina ceramic feedthrough that formed the lid of our round hermetic package (see
Figure 1). The package materials (alumina, ceramic, and platinum feedthroughs) and our gold brazing methods are in common use in the implantable medical device industry, and are outlined in [
24]. Here, we will focus on the flexible multi-electrode arrays that deliver stimuli from the implanted, sealed wirelessly powered stimulator units to the retina, and the microfabricated supporting structures for the secondary radio frequency coils.
2.1. Mechanical Properties and Design Considerations for Retinal Prostheses
Each fabrication and surgical challenge that we encountered along the way influenced not only the CAD design of the components and their multilayered construction, but also the order of fabrication and the choices of materials for processing. As well, we obtained practical feedback on both the layout and the stiffness and handling of the components by performing many pre-clinical animal surgical procedures in miniature pig models, and these too informed our design choices; see e.g., [
16]. Further feedback came from in vitro testing, particularly accelerated lifetime testing in biological saline, which influenced our material selection. The design elements and challenges that we addressed also interacted with one another; the mechanical design problems facing the creation of micro-fabricated implantable components for a sub-retinal visual prosthesis are, in fact, multi-faceted. For example, our multi-electrode arrays for retinal stimulation must cross the sclera and the choroid for their leads to convey signals from a peri-ocular, hermetically packaged implanted stimulator unit to the distal electrodes in the sub-retinal space. The peri-ocular portion of our implantable visual prosthesis is subject to different mechanical stresses during and after the surgical procedure than the intra-ocular portion. For instance, the extra-ocular portion may rub against and possibly erode overlying tissue such as the conjunctiva and Tenon’s capsule, which we observed early in the development of our second-generation device [
25]. This outcome resulted in modifications to the most anterior part of the peri-ocular implant to reduce its profile as much as possible. For example, the 19 mm diameter secondary radio frequency coil, which in our case is implanted and sutured around the cornea, was at one time encapsulated in a ~2 mm thick layer of polydimethylsiloxane (PDMS). This gave rise to undue tension on the overlying conjunctiva at the end of the procedure. This recurrent outcome led to an effort to micro-fabricate the coils using a SU-8 epoxy mold and gold electroplating techniques. However, ultimately we found that the best solution to mechanical stresses on the RF coil and the surrounding tissue was to wind the coils from 75 μm diameter gold wire on a spherical form of the same diameter as the target eye. By careful attention to the layering of the turns, we were able to reduce the height of the coil assembly to <0.5 mm, which provided long-term biocompatibility. We later elected in our third-generation device to microfabricate a coil support structure from electroplated Au and polyimide that provided attachment points for suturing the coil to the sclera. Even this simple structure, shown assembled in
Figure 1 and discussed further below, had mechanical design considerations that were resolved through trial-and-error in multiple implantations in mini-pig animal models. For example, eye size impacts design and materials requirements, not only in the animal models but also among the (frequently myopic) human patient candidates whom we hope to treat [
26]. If the secondary radio frequency coil were to be maintained at a fixed distance from the anterior edge of the hermetic electronics package among the peri-ocular components, e.g., using a fixed-length micro-fabricated connecting structure, a smaller-diameter host eye would force the posterior edge of that electronics package far back into the orbit, thus creating a challenging trade-off for the surgeon during implantation. This design element was optimized through collaboration between our surgical and engineering teams. The micro-fabricated coil support structure shown in
Figure 1 was built to accommodate varied host eye shapes by encapsulating the RF coil’s leads in flexible silicone surgical tubing, and by fixing the ends of that tubing segment to the coil support with sutures and to the electronics package via silicone over-molding. These modifications allowed considerable flexibility in the relative placement of the electronics package and the RF coil because of the tubing’s flexibility, while at the same time allowing the surgical field to be less crowded, since the coil assembly could be “flipped over” out of harm’s way until it became time to affix the coil to the sclera.
Turning to considerations for microfabricated intra-ocular components, there are two competing needs. On the one hand, our electrode array must be stiff enough to pass through a flap created in the sclera and an incision in the choroid, which is one of the most vascular tissues in the body. The electrodes must also be able to be advanced to the target sub-retinal location. On the other hand, once the array is advanced to its final resting spot, it is desirable for the array to be flexible, so as to conform to the contour of the sub-retinal space. This segment of the device must be stiff enough to breach an incision in the choroid, yet at the same time, if the distal end of the electrode array retained that same level of stiffness, it might cause long-term biocompatibility issues for the device, including potentially a retinal tear. Our solution to this problem was in part to keep the intra-ocular portion of the flexible electrode array to <15 μm in thickness, and to perforate the electrode array by design using reactive ion etching (RIE) so as to allow fluid exchange through the openings that rest within the sub-retinal space. Further processing details are below. Additionally, to assist in the surgical procedure, we employed a custom-fabricated 75 μm-thick shaped polyimide surgical guide that was laser-cut from a DuPont Kapton® sheet. The distal aspect of the guide was dull (i.e., had rounded edges) but semicircular in shape, which aided implantation of the flexible multi-electrode array through the flap and into the sub-retinal space (see
Figure 1). The procedure proceeded by first introducing the stiffer polyimide guide through the cauterized choroidal incision, and then implanting the electrode array by sliding it over and along the guide into the eye, having first raised the retina out of harm’s way by creating a bleb (by injection through a small retinotomy from inside of the eye). The guide was then removed, the electrode array was sutured in place to anchor it to the sclera outside the incision site; the bleb was slowly resorbed. In this manner, a flexible multi-electrode array with or without penetrating posts was deployed through an incision that the array would not otherwise have had enough stiffness to pass through. Further details about the implantation procedure have been recently presented [
16].
A final mechanical design consideration for a retinal prosthetic’s electrode array and other micro-fabricated implantable components is the selection of encapsulating materials to protect the leads and other structures from ingress of moisture. This consideration also intersects with other factors, such as biocompatibility in the sub-retinal space, and compatibility of the deposition process for the encapsulating material in question with the previously existing component materials that are deposited earlier in the process flow. These intersecting needs may be better understood through examination of
Table 2 below. Biocompatibility data are drawn in part from our team’s earlier studies in mini-pig animal models [
27] and are supplemented with more recent data from pre-clinical testing of our team’s retinal prosthetic device.
From the development experiences of earlier generations of our team’s visual prosthesis, we gradually came to appreciate the unique and intersecting challenges that were summarized in
Table 2 concerning the impact of added encapsulating films on the bio-stability and biocompatibility of the devices in the eye environment. Early non-hermetically encapsulated visual prosthesis components micro-fabricated by our team had vapor-deposited protective coatings of Parylene-C applied (
Figure 1a,b) including the use of organosilane adhesion promoters and careful surface preparation before coating and patterning using oxygen reactive ion etching. Despite these precautions, we found that Parylene-C coatings alone had useful lifetimes in saline environments of at best 1–2 years at 37 °C. This was adequate, for example, for some in vitro and early in vivo testing, but fell short of our ultimate design lifetime target of 10+ years. After evaluating a number of potential encapsulants [
27], we eventually settled on “wrapping” the conductors in our arrays with plasma enhanced chemical vapor deposition (PECVD) of amorphous silicon carbide (a-SiC:H) at 325 °C, which showed excellent long-term material and electrical stability in accelerated life tests at 87 °C (see e.g.,
Figure 2). These inorganic encapsulating layers were used above and below each layer of conductors, which we kept near to the neutral axis of the arrays to minimize any effects of internal film stress. The presence of a-SiC:H layers in the multi-layered electrode array structure also contributed to their overall stiffness, which was compensated for in part by adjusting the thickness of the outer polymer layers of the micro-fabricated components for scratch protection.
While our combined inorganic and organic encapsulation strategy was evaluated through numerous tests, a systematic study of the effects of coating thickness (and thus stiffness) on long-term device lifetime was not performed from first principles. Rather, in the case of inorganic encapsulation layers deposited e.g., by plasma enhanced CVD, their natural stiffness was so much greater than that of the flexible polyimide substrates that we had microfabricated that we limited the thickness of such layers to only that which was necessary to have reasonable confidence of pinhole-free coatings. In the case of the a-SiC:H coatings used for the test devices of
Figure 2, this thickness was 0.5 μm, but later process optimization led to the reduction of the required SiC layer thickness to 0.1 μm for top or bottom layers, and 0.25 μm for any middle layers. Other materials that we investigated in
Table 2 either lacked sufficient biocompatibilty, biostability, or both.
Another matter relating to mechanical properties of our implantable multi-electrode array components in particular was the channel count needed in order to be able to stimulate sufficient numbers of sites to have a reasonable likelihood of restoring “useful” vision. We estimated for our third-generation retinal prosthesis that at least 256 channels would be required, based on the findings of a number of teams in the field over the last 20+ years that have recently been reviewed [
3,
4]. Since no de-multiplexer was included in our system in a distal region near the stimulation sites beneath the retina, this meant that >256 individual traces or leads would need to be incorporated along the length of the array from the stimulator package containing the circuitry, to the electrode locations within the eye. A quick calculation revealed that even for a modest center-to-center spacing of adjacent conducting traces in the leads of 20 μm, a single micro-fabricated layer containing side-by-side leads to each electrode would need to be >5 mm wide. Not only would a multi-electrode array of this width likely not conform well to the approximately spherical contour of the eye, but it would also require a relatively large incision to access the sub-retinal space; broadly speaking, our surgical experience has shown that reliable insertion into the sub-retinal space can be achieved using electrode arrays that are <5 mm wide that are implanted through sclera flaps that are ~6 mm in width [
16]. The pitch between electrode sites at the distal end of the array, then, was determined largely due to surgical factors. Smaller- or larger-pitch arrays would have been feasible to micro-fabricate, but may not have been as feasible to implant. “Pitch” between electrode sites here refers to the nearest-neighbor distance in a nominal hexagonally close packed configuration, which was used to save space. In contrast, the rigid, disc-shaped hermetic package containing our stimulating electronics was limited in size as well, since a larger package would likewise have been difficult to implant behind the eye in the orbit. The pitch between signal feedthroughs in that package in turn determined the overall package’s 11.5 mm diameter. In that case, it was the limitations of the fabrication process for creating high temperature co-fired ceramic parts that determined the spacing. While it is true that high channel counts can and have been achieved, e.g., by the use of alternative, photodiode-based visual prosthesis designs, those approaches are entirely different, make use of the capabilities of silicon foundries used to create such devices, and have been reviewed elsewhere [
3,
4].
Given the channel count desired for our design, then, it was concluded that for a high-density retinal prosthetic, the large number of independent sites involved required that the leads be printed on multiple layers of metal. Each layer of conductors required inorganic encapsulation above and below the metal traces to insulate them from one another, which also increased the overall required silicon carbide thickness and thus, the stiffness of the multi-electrode arrays. We also studied the feasibility of increasing the number of layers of conducting traces to as many as 4, which would be required in the case of very high channel counts unless a distributed system involving the use of signal multiplexing and de-multiplexing were employed. In
Figure 3, test structures and cross sections of sample four-layer devices that were fabricated are shown [
30]. The electrode arrays shown in
Figure 1, however, were fabricated with just two layers of conductors, again using vias between the layers; this process is outlined in
Table 3. Using two layers of traces, we were able to accommodate twice the channel count in the same electrode array width, and we were also able to “cross over” or under a trace on an adjacent layer by creating conductive vias between the layers on either side of the area that was desired to pass beneath (or over).
The final issue concerning mechanical properties of the implanted components concerned the fit of the as-fabricated 2-D micro-fabricated components to the real, 3-D features of the eye. This was a particularly challenging issue on several fronts. First, there are natural variations in anatomy of the eye including its length (which correlates with the natural refractive state of the eye) and the insertion sites of the extra-ocular muscles, which differ between porcine and human subjects.
Specifically, we sought a design that could accommodate variations in our animal model (needed for pre-clinical testing) that also would be suitable for human eyes that also have variations. And, we wished to build electrodes that would be usable across a range of eye sizes without having to customize arrays for individual patients. Thus, a degree of adjustability was desirable in our design. After much trial-and-error over the course of a number of early surgical trials, we developed two array designs that were workable. A first design evolved which employed a medical grade silicone tube as a physical support for a helically wound coiled-cable type lead, which was inspired in part by flexible, implantable helix type wire-based leads that are in common use in other medical devices. The use of the coiled arrays required a much longer physical length of the arrays, because much of the length was consumed in the coiling process but the coiled arrays were easily re-positioned without significant creasing or sharp bending of the coiled structures. And, the coiled assemblies could easily be passed beneath the eye muscles by simply elevating them with a muscle hook and passing the array beneath with the free hand. An example of an inactive coiled-cable array mockup fitted around a human cadaver eye can be seen in
Figure 4.
Ultimately, our ~4 mm wide, two-dimensional implementation of a 2-layered multi-electrode array was refined through pre-clinical testing. This required dealing with a fundamental problem that micro-fabricated structures for medical applications often encounter, and that is particularly true for organs such as the eye. The micro-fabricated structures are frequently created on planar surfaces, such as silicon wafers, for ease of handling and processing. However, the host tissue, i.e., the mini-pig eye in the case of our pre-clinical tests, had a complex 3-D shape and a diameter of ~23 ± 2 mm. The issues that were related to laying our fabricated 2-D structures down onto 3-D surfaces can easily be visualized by wrapping a strip of paper around a ball. There is a natural tendency for the 2-D object, such as the paper in this example, to buckle or to protrude in some manner when portions of 2-D objects are affixed to the 3-D surface, and the edges of the very thin 2-D parts, such as the flexible electrode arrays here, can be sharp. This deformation with a sharp leading edge can lead to erosion of the conjunctiva when thin, flat prosthesis components are implanted beneath it, which we in fact initially did experience [
25]. To facilitate a degree of adjustability to different host eye sizes, and to allow the 2-D multi-electrode arrays the ability to lay flat on the sclera surface, we eventually adopted a serpentine shape for the arrays, which can be seen in
Figure 1. Even these structures, which lay relatively flat on the eye surface, required segmentation of the two-layered multi-lead structure into strips or groupings of 10 μm wide metal traces that were interconnected by thin polyimide “bridges.” In this way, the natural tendency to buckle was minimized, since a segmented structure lays flatter than one which crowds all of its leads into a single group. We also incorporated suture loops along the perimeter of the electrode arrays’ length to facilitate the attachment of the arrays at key stress points—again, in the service of fixing the arrays in position with minimal protrusion from the sclera surface. Further, we found that the order of the surgical procedure for implanting a device in the orbit was also important. For example, it is possible to prevent the need to compress, bunch the arrays, or stretch the arrays to span the gap between the sclera incision site and the location of the packaged electronics, by first marking on the surface of the eye the desired location of the insertion site for the electrode array, which then facilitates selecting a compatible location for the hermetic package to which it is attached.
2.2. Process Flows for Microfabrication
Our multi-electrode array fabrication processes needed to consider the positioning of the distal retina-facing side of the array, in relation to the contact pads on the proximal side of the micro-fabricated structure that connect with the signal feedthroughs in the hermetic electronics package. This decision had significant manufacturing ramifications. A sub-retinal electrode array fabricated with the contact pads for thermosonic bonding to feedthroughs on the same side of the array as the iridium oxide stimulating electrodes required that the package’s feedthrough disc face away from the eye surface. Consequently, the multi-electrode array’s flexible leads exit the package at some elevation (in our case, about 2.5 mm) above the sclera surface, and the silicone strain relief that was designed for that joint needed to take that into account. The volume of the elevated electronics case and the attached, micro-fabricated leads necessitated placement away from the delicate conjunctival tissue in the front of the eye and around the back of the eye where these structures could be easily accommodated by the compliant fatty tissue of the orbit. Even with this posterior placement, the relative bulk of these structures produced some tension, which we buttressed by placing the anterior edge below the sturdier Tenon’s capsule.
Thus, a simplification of the micro-fabrication complexity (by fabricating multi-electrode arrays with features patterned on one side only) needed to be “traded off” with a slightly more nuanced step in the surgery. Our team also tried a different micro-fabrication approach that placed the electrodes on the reverse side of the array from the pads that connect to the stimulator package, but this substantially increased the difficulty and number of process steps required. This modification is illustrated below in
Figure 5; both single- and double-sided multi-electrode arrays were fabricated, but our current-generation retinal prosthesis uses the simplified fabrication sequence outlined in
Table 3 and in
Figure 5b.
The process flows of
Figure 5 for single- or double-sided fabrication of electrodes had certain critical properties in common which were important for successful device fabrication. First, it was essential that the flexible components could be nondestructively removed from their host wafers once all process steps were completed. This required that the base layer(s) of the structures cling sufficiently to the host wafer to allow for reliable alignment of the mask layers to one another and for maintenance of planarity during processing. At the same time, we wanted to be able to peel the completed parts from the surface of the host wafer at will. This required careful attention to the moisture levels on the host silicon wafers prior to the application of the first layer of HD Microsystems PI-2611 polyimide, taking care not to unduly expose the wafers to water for extended periods during the fabrication sequence. In this way, the natural, gradual loss of adhesion of polyimide to Si wafers upon soaking in DI H
2O can be used to advantage; soaking for 24 h was usually sufficient to loosen the completed/singulated devices from their host substrates.
As mentioned above, another feature of the planar electrode manufacturing process that was common to either single- or double-sided arrays was the need to “wrap” each conducting trace on the multiple layers with an inorganic encapsulant, for example amorphous SiC:H in this case. Polyimide or Parylene-C polymers alone were not sufficient to electrically insulate the traces from one another throughout long-term in vitro accelerated lifetime tests, and moreover, we found that such structures would blister and de-laminate over time in saline environments despite careful cleaning before coating. The use of an inorganic primary encapsulant, however, has enabled our devices to survive for a projected lifetime of over 10 years at 37 °C (see e.g.,
Figure 2); the outer polymeric encapsulants primarily serve as scratch protection, improve the ability to handle the arrays in surgery, and also perform the release function from the host wafer.
The evaporated metallization that we patterned using a standard photoresist lift-off method likewise served multiple functions in the microfabrication process (see
Table 3). The need to deliver current to the electrodes with negligible series resistance due to the leads was primarily addressed by using thermally evaporated gold that comprised the bulk of the trace volume. The end-to-end resistance of the 0.425 μm thick traces was <500 Ohms, which was deemed acceptable, since the electrode-tissue interface impedance for retinal prostheses was generally an order of magnitude higher, and the in vivo impedance was found to be even greater, often in the range of 10–20 kOhms. On the top and bottom of each metallization stack was a Titanium adhesion layer of 0.025 μm thickness. It was found when curing the upper polyimide layers at 350 °C in a N
2 ambient that the Titanium and Gold formed an inter-layer which altered the appearance of the traces and could also give rise to abnormally high impedances. In part to prevent this phenomenon, and in part to prevent accidental exposure of the Gold metallization in the SF
6/O
2 reactive ion etching process during etching of the overlying a-SiC:H layer, a thin 0.05 μm-thick film of Platinum was added by electron beam evaporation between the Gold and the Titanium layers on the upper side.
The sputtered iridium oxide (SIROF) electrode material was deposited by reactive sputtering of an Iridium metal target in the presence of moist N
2 carrier gas; process details have been summarized elsewhere [
31]. The nature of sputtered films is that they arrive at the substrate in an isotropic manner, which is excellent for providing uniform coverage but can sometimes present a challenge for conventional patterning techniques such as lift-off. In practice, the SIROF material did lift off readily using ordinary photoresist processing methods, but the sidewalls of the SIROF-coated electrodes would at times separate from the portion of the electrode that was deposited in the open areas that had been patterned to receive the film—particularly after a period of hydration and repetitive pulsing. While this altered the effective electrode geometric surface area somewhat, there appeared to be no deleterious effects in vitro or in vivo. We also noted that the as-fabricated SIROF films did not have the full charge storage capacity that they are capable of immediately after fabrication; rather, a break-in period significantly improved the charge capacity of both planar and penetrating SIROF-coated electrodes, and this is discussed further below.
The process for creating the supports for the wire-wound gold radio frequency coils differed substantially from that for fabricating the multi-electrode arrays above, but retained certain features in common. For example, the same method for releasing the completed coil supports from the host substrate was used, with a base layer of HD Microsystems PI-2611 polyimide. In the case of the coil supports, their primary function that they served was mechanical and not electrical, in that the coil wires terminated on connection pads prepared for that purpose on the proximal end of the electrode arrays, and no conductors were used to carry current in or through the coil supports themselves. Rather, electroplated Au metallization was instead used for its ability to strengthen the suture holes that were etched around the perimeter for affixing the coil to the sclera. Once the coil supports were freed from the host wafer, the wire-wound coils were attached to them using Kwik-cast, a medical grade silicone adhesive. Completed coil supports are shown in
Figure 6.
Most recently, our team has made advances in microfabricating and implanting subretinal multi-electrode arrays that contain both planar electrodes, and electrodes atop penetrating posts for stimulation of the inner retinal layers, each of which are SIROF-coated; these have been well tolerated in a number of animal trials [
16]. The process for creating “hybrid” penetrating and planar electrode arrays differed from the basic planar multi-electrode array process in that prior to sputtering the SIROF electrode material (but after the exposure of the metal underlying the electrode sites), SU-8 photo-imageable epoxy was applied to the entire wafer surface and patterned; special care needed to be taken in the extended soft baking step. On the one hand, this prevented adhesion between the photomask with the desired post pattern and the coated wafer surface, and on the other hand, the soft baking process improved the adhesion of the posts themselves to the host surface after developing the pattern. A SIROF film was sputtered onto the passive penetrating posts, creating a conducting path from the base of the posts to the tips; the 50 μm and 100 μm-tall posts had a top-to-bottom impedance of <40 Ω, which introduced negligible additional series resistance to the sites. After the SIROF patterning, the arrays were coated in 1 μm of Parylene-C by vapor deposition, and the tops of the posts were exposed by reactive ion etching in an O
2 plasma. Special care was needed to properly protect the posts and the surrounding electrodes during this etching step with photoresist, and applying the resist using a spraying technique with a Suss Microtec Gamma Cluster Tool with a spray coating module provided this protection. A schematic cross section diagram of a multi-electrode array with an incorporated penetrating post and a micrograph of a completed hybrid array with mixed planar- and penetrating electrode sizes is in
Figure 7.