Modeling and Analysis of the Two-Dimensional Axisymmetric Acoustofluidic Fields in the Probe-Type and Substrate-Type Ultrasonic Micro/Nano Manipulation Systems
Abstract
1. Introduction
2. Basic Theories and Simulation Methods of 2D Axisymmetric Acoustic Streaming Fields
2.1. The Perturbation Method (PM)
2.2. The Reynolds Stress Method (RSM)
3. Numerical Models
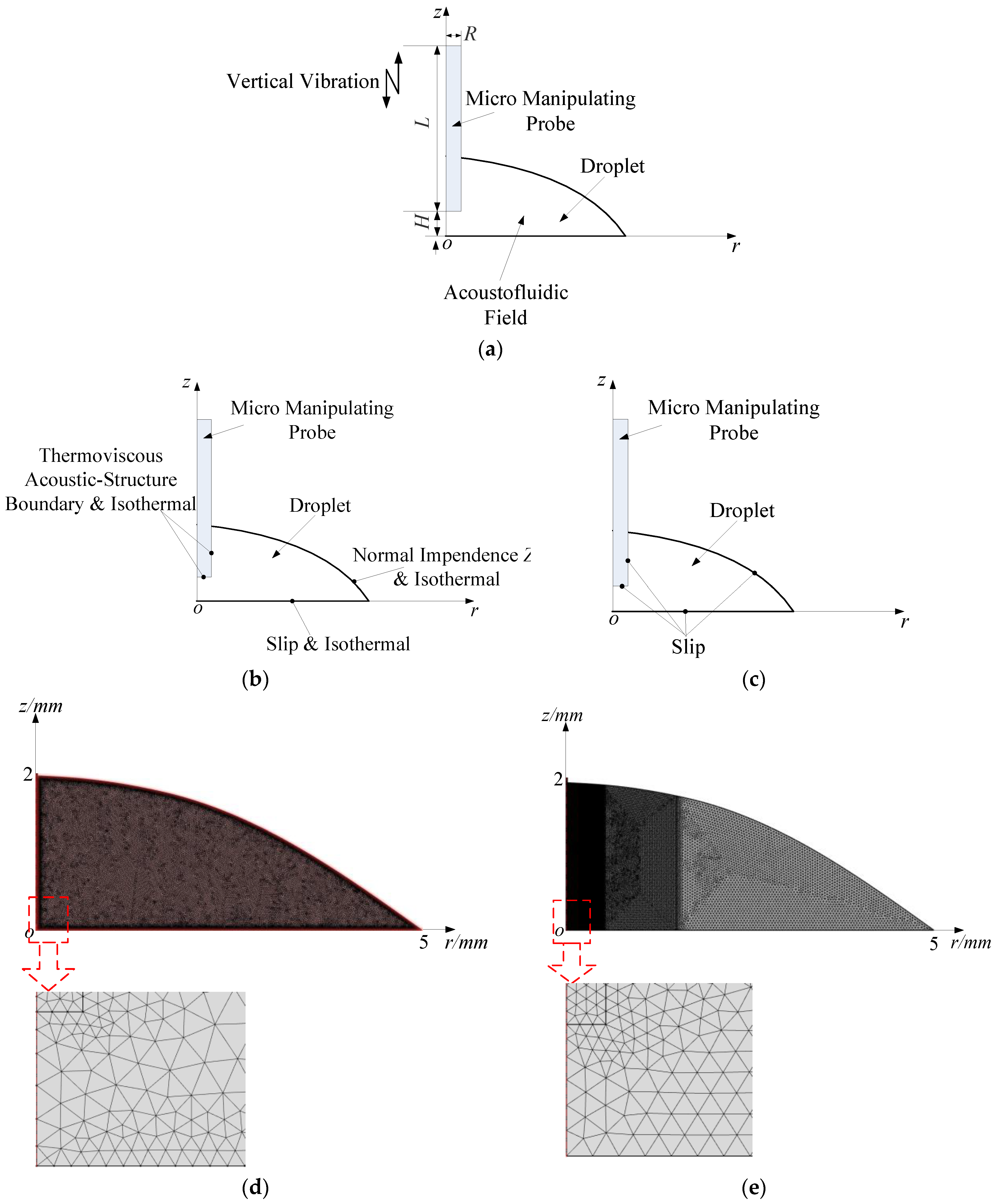
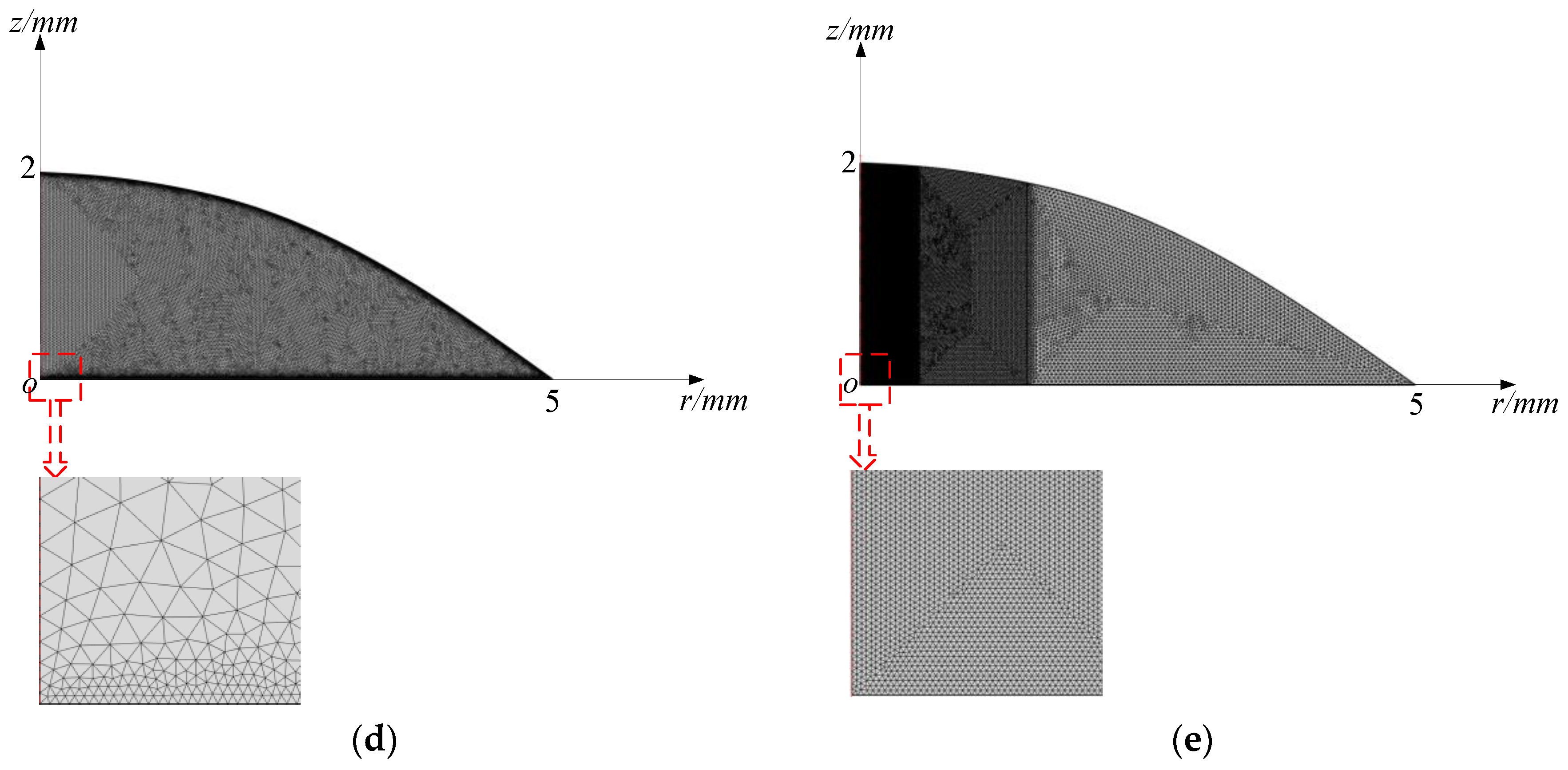
3.1. The Ultrasonic Probe-Droplet-Substrate System for Micro/Nanoscale Particle Removal
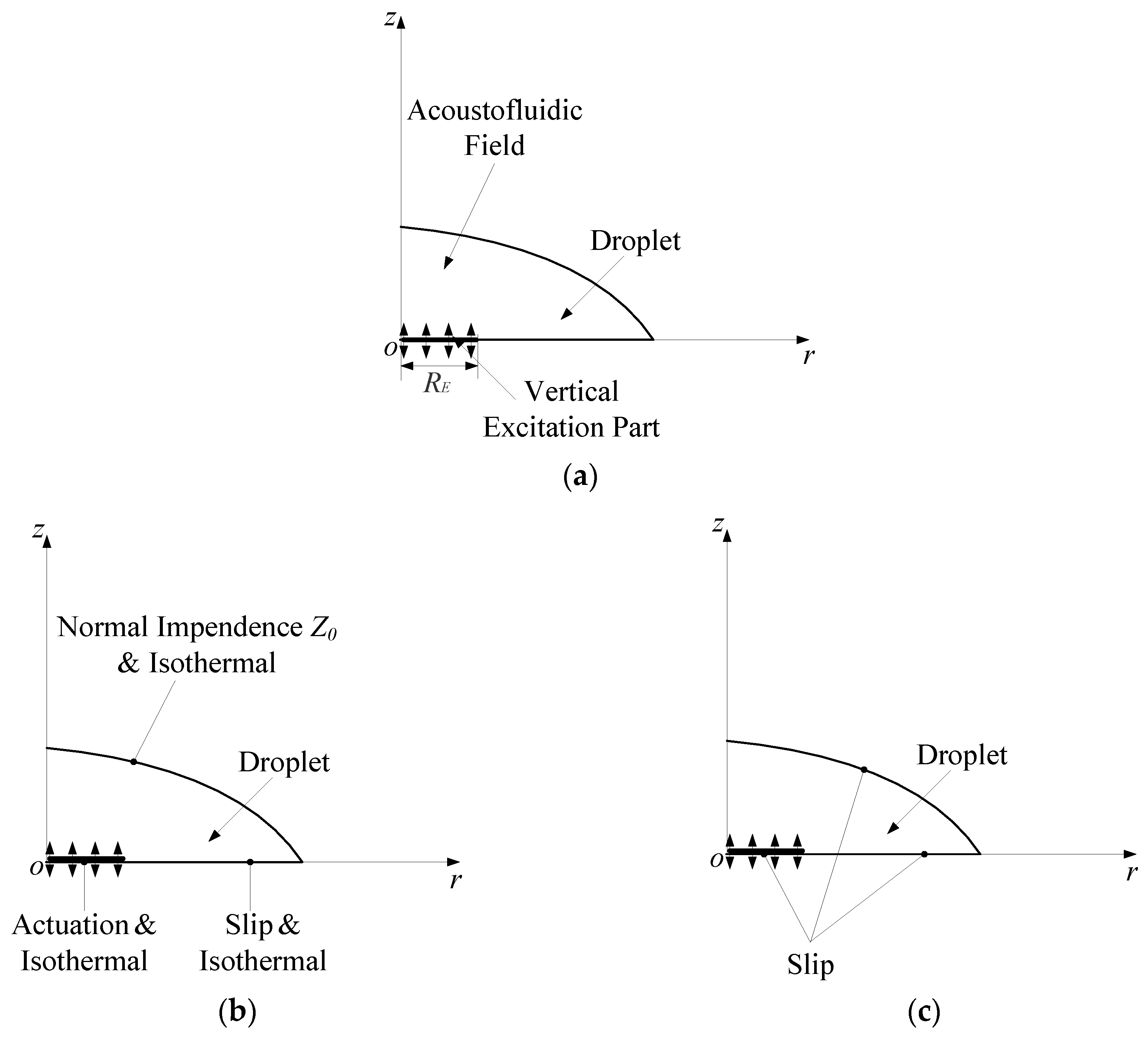
3.2. The Droplet-Ultrasonic Substrate System for Micro/Nanoscale Particle Concentration
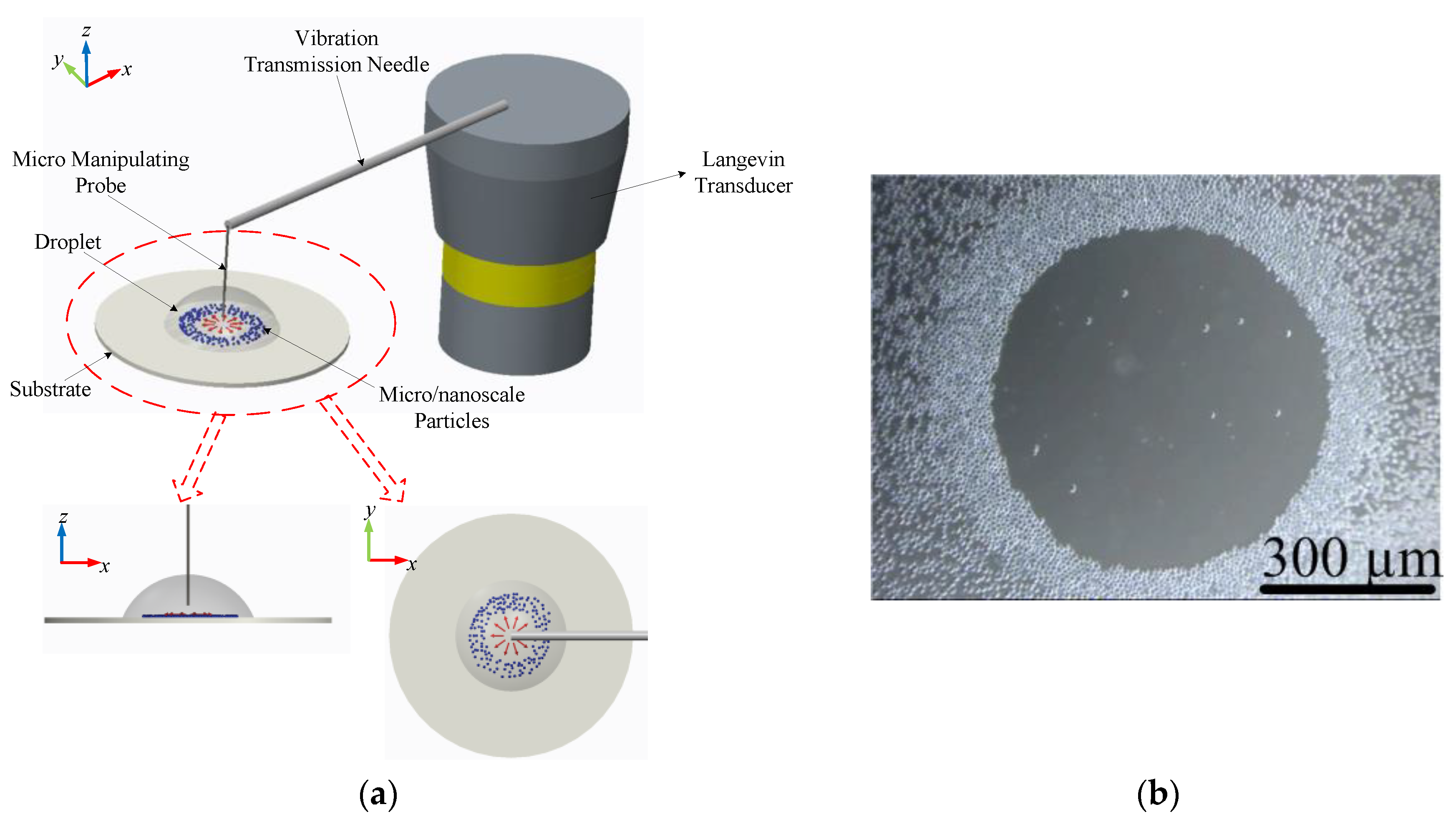
4. Experimental Verification
4.1. The Ultrasonic Probe-Droplet-Substrate System-Based Micro/Nanoscale Particle Removal
4.2. The Droplet-Ultrasonic Substrate System-Based Micro/Nanoscale Particle Concentration
5. Results and Discussion
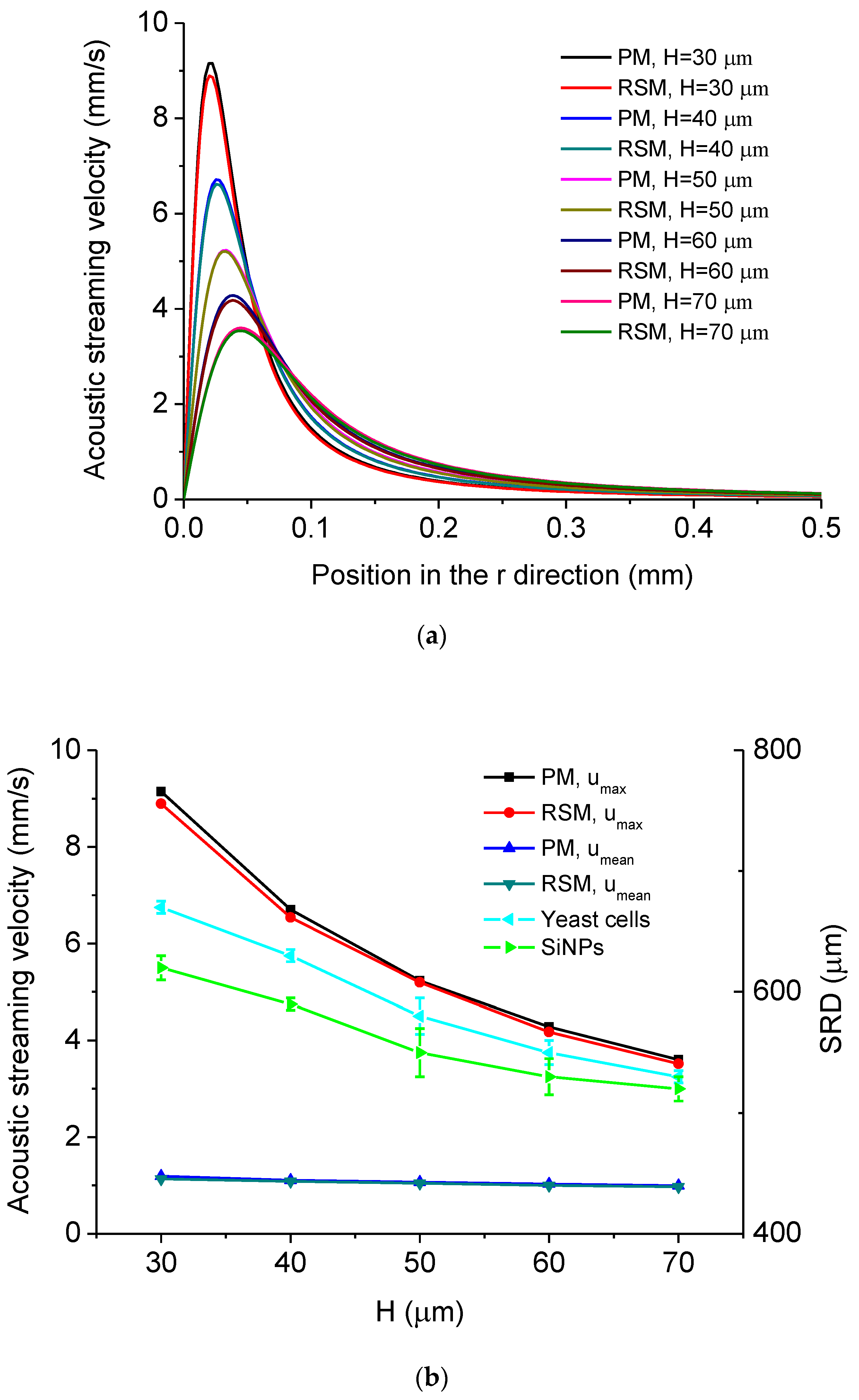
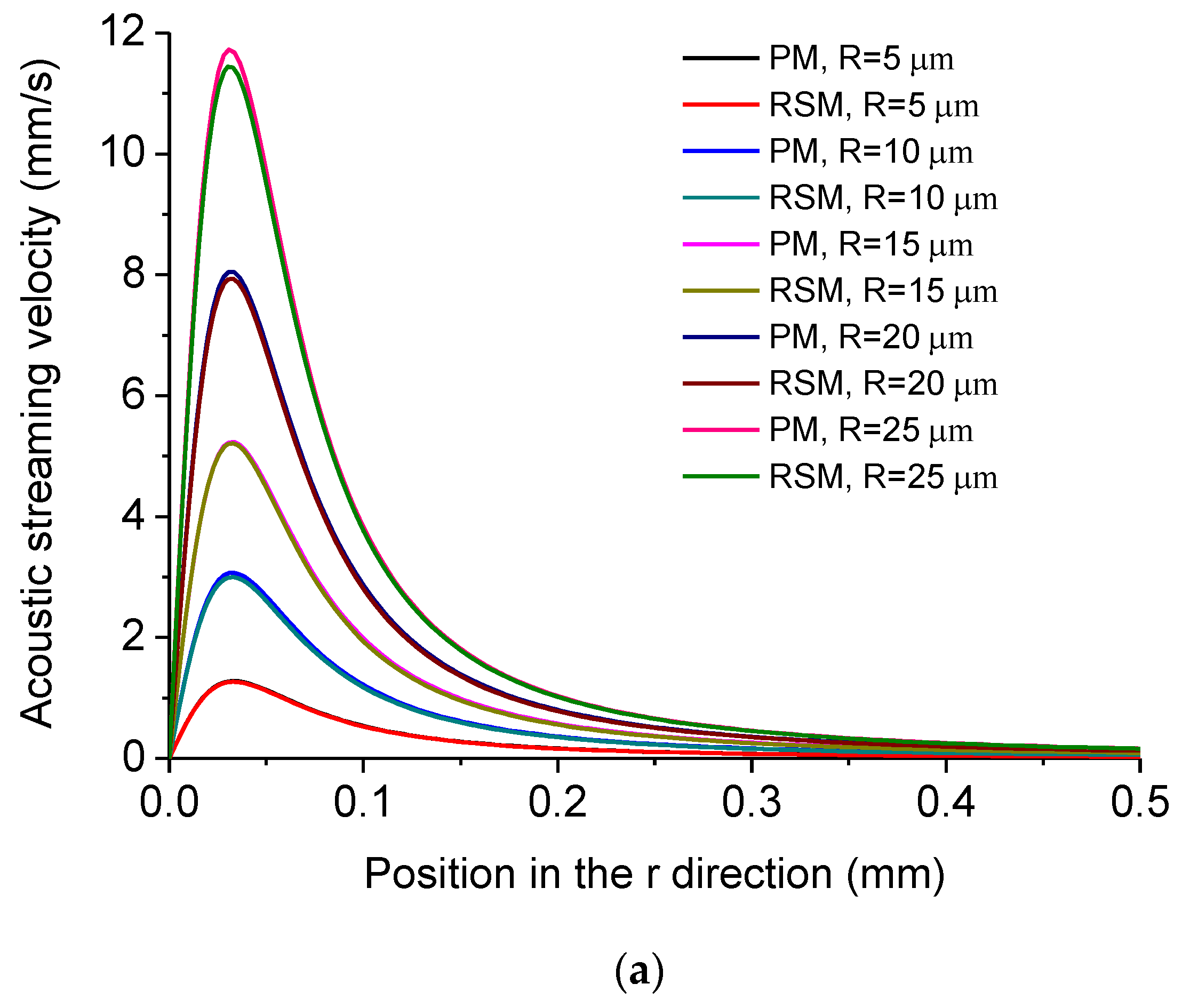
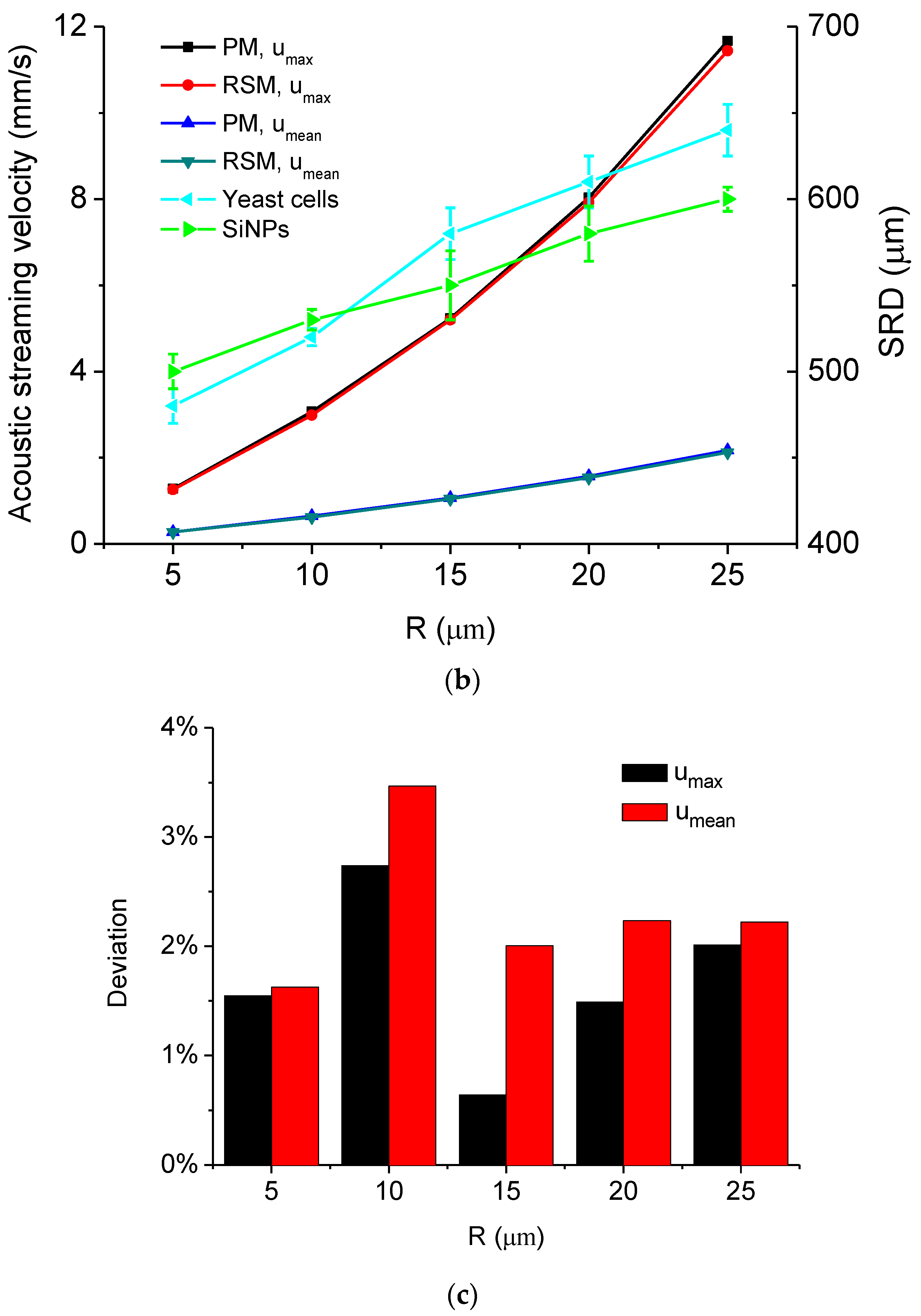
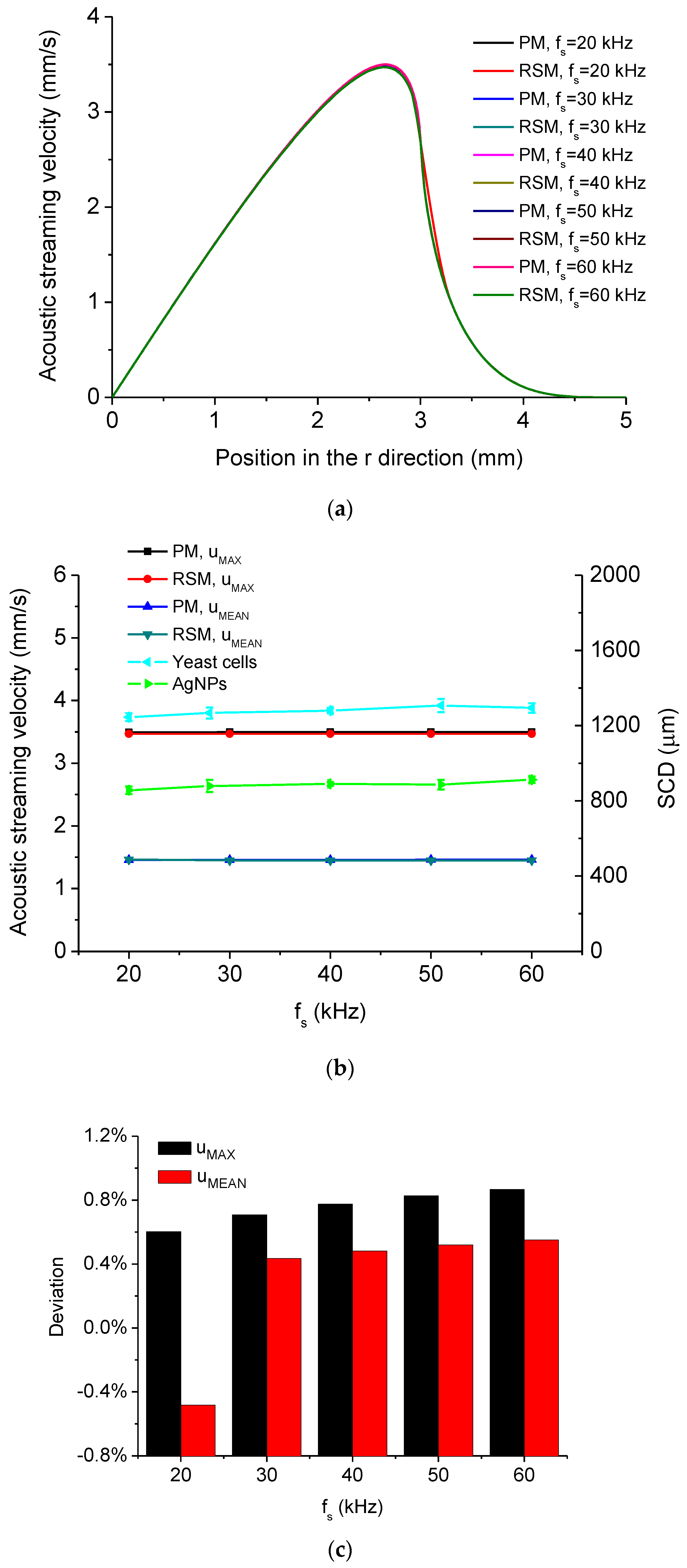
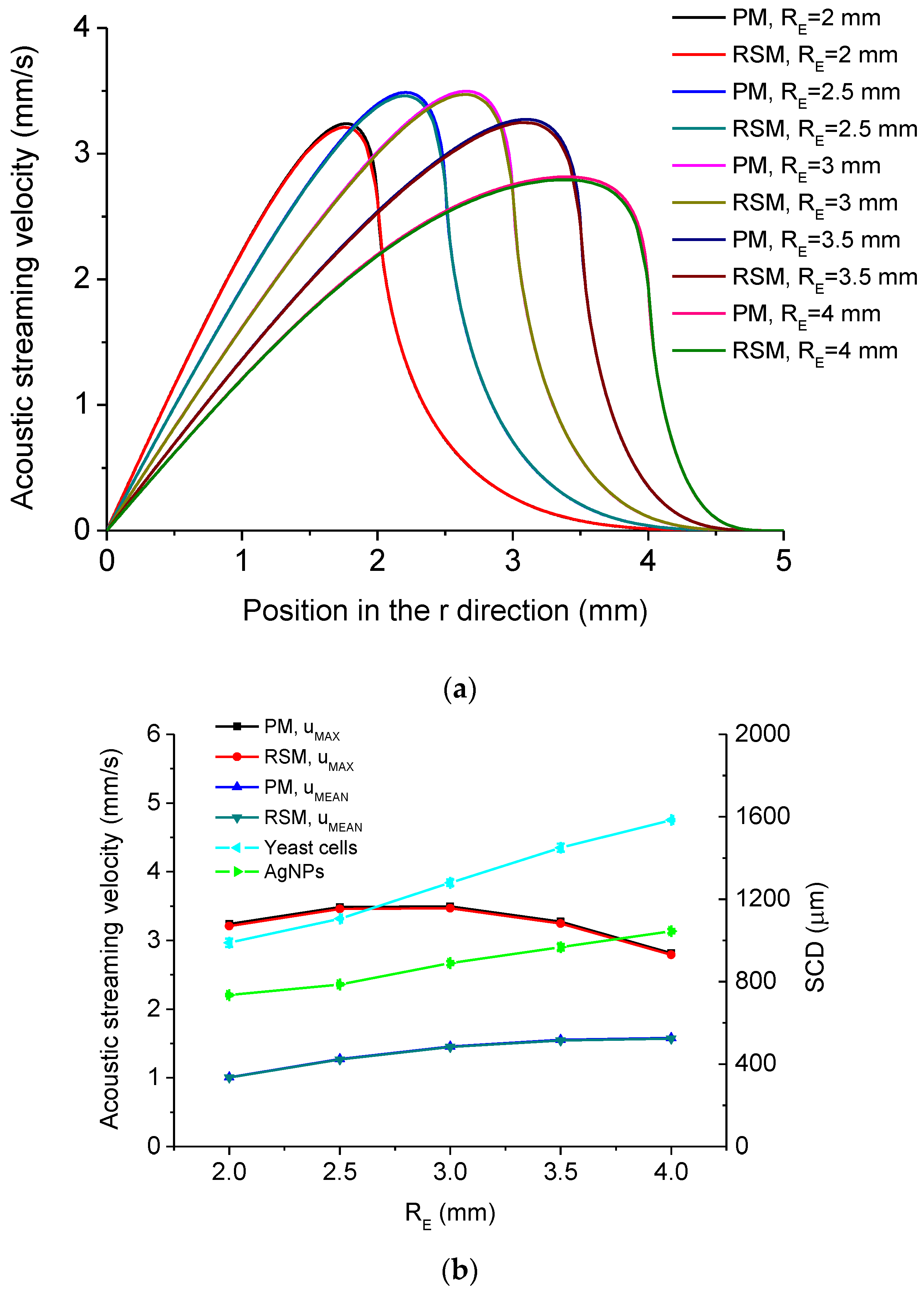
5.1. Simulated Acoustofluidic Fields in the Ultrasonic Probe-Droplet-Substrate System for Micro/Nanoscale Particle Removal
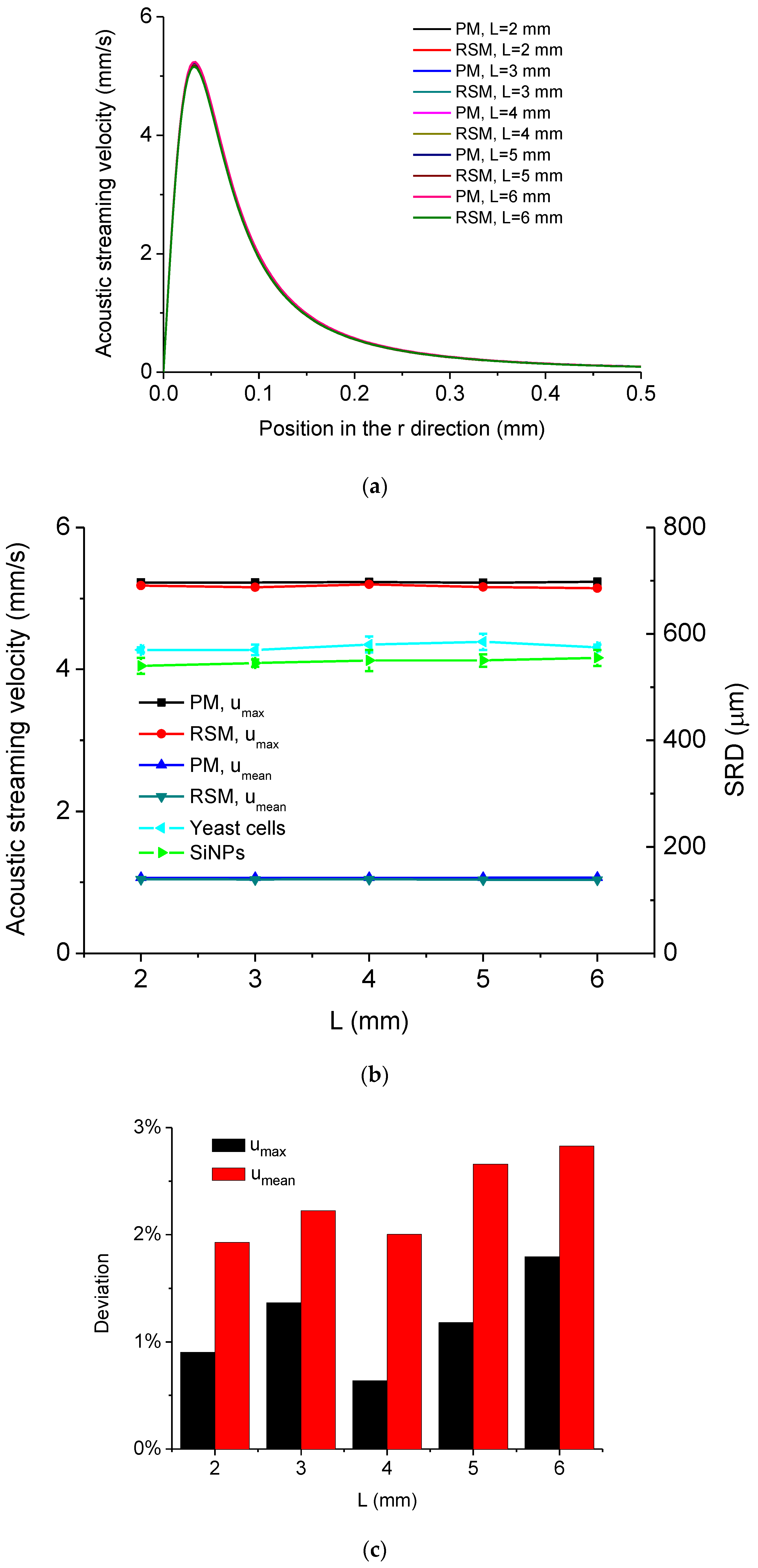
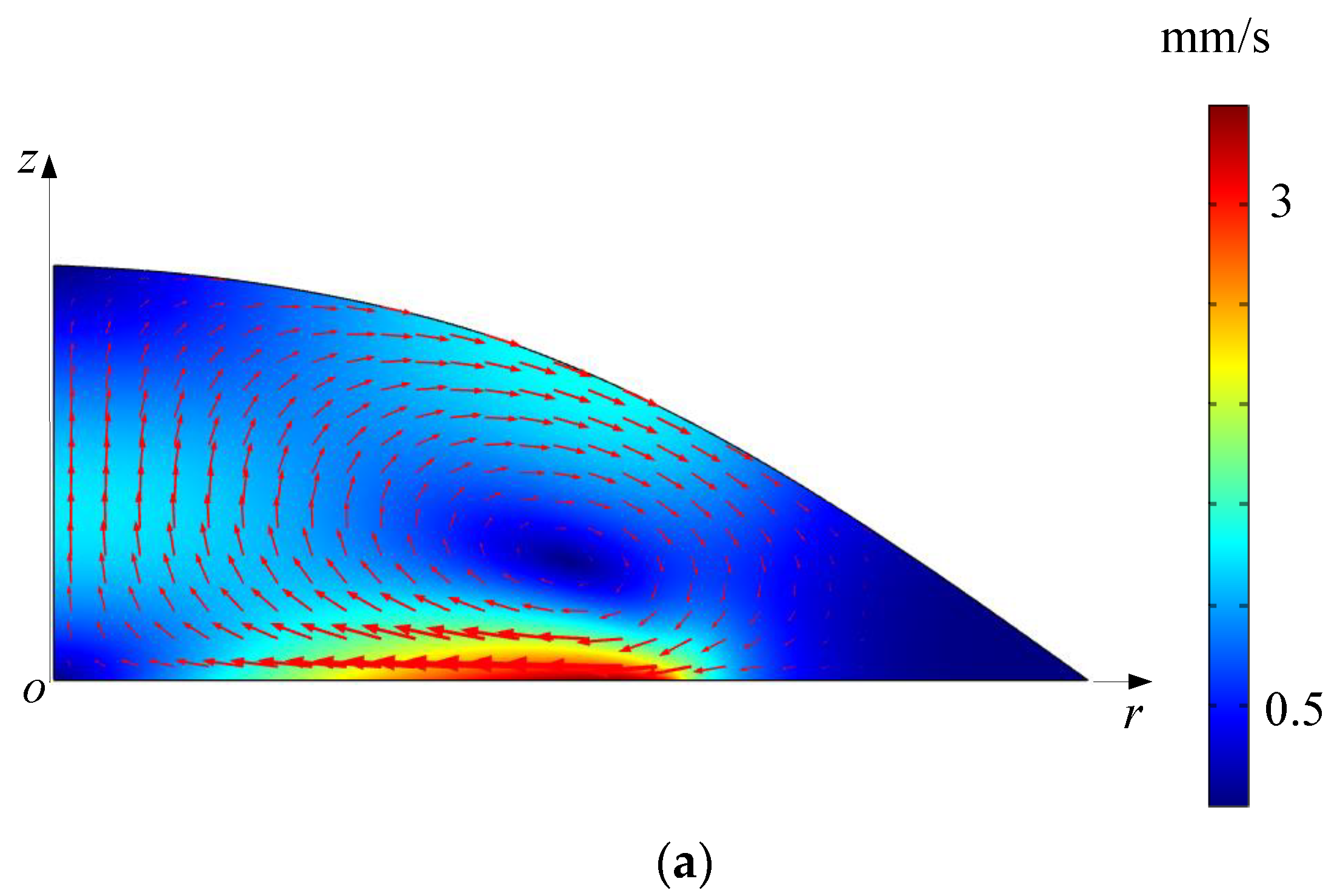
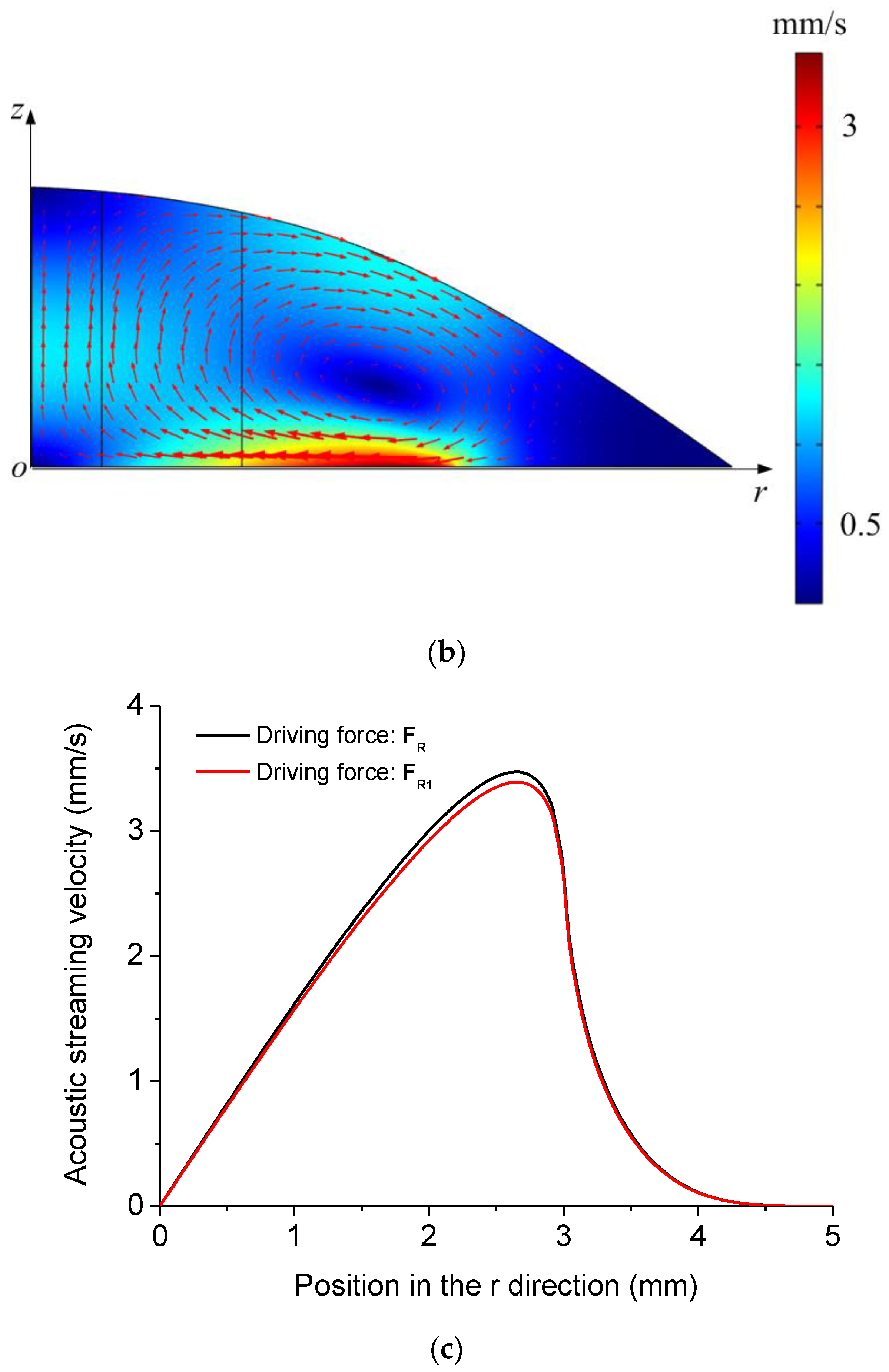
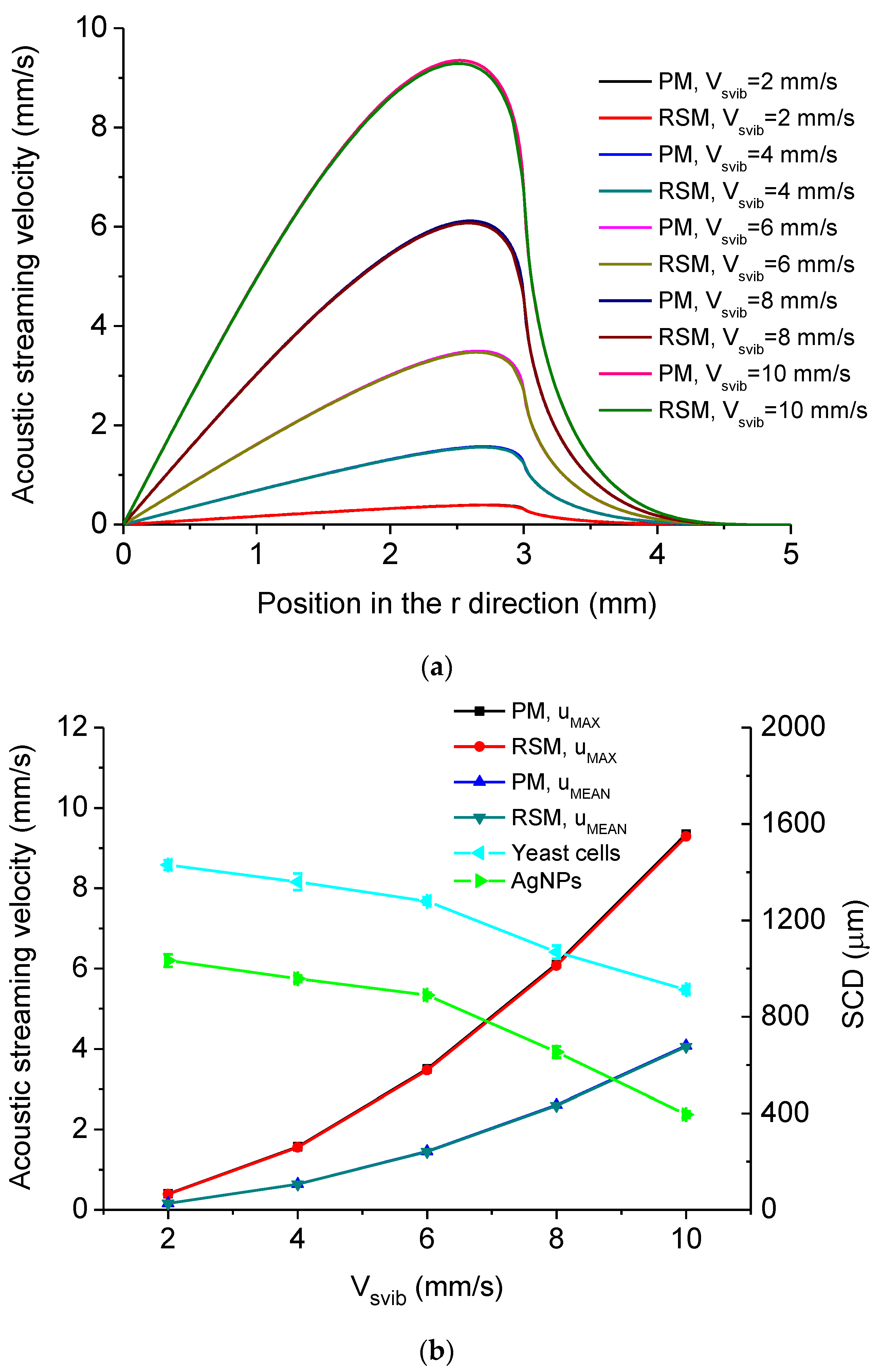
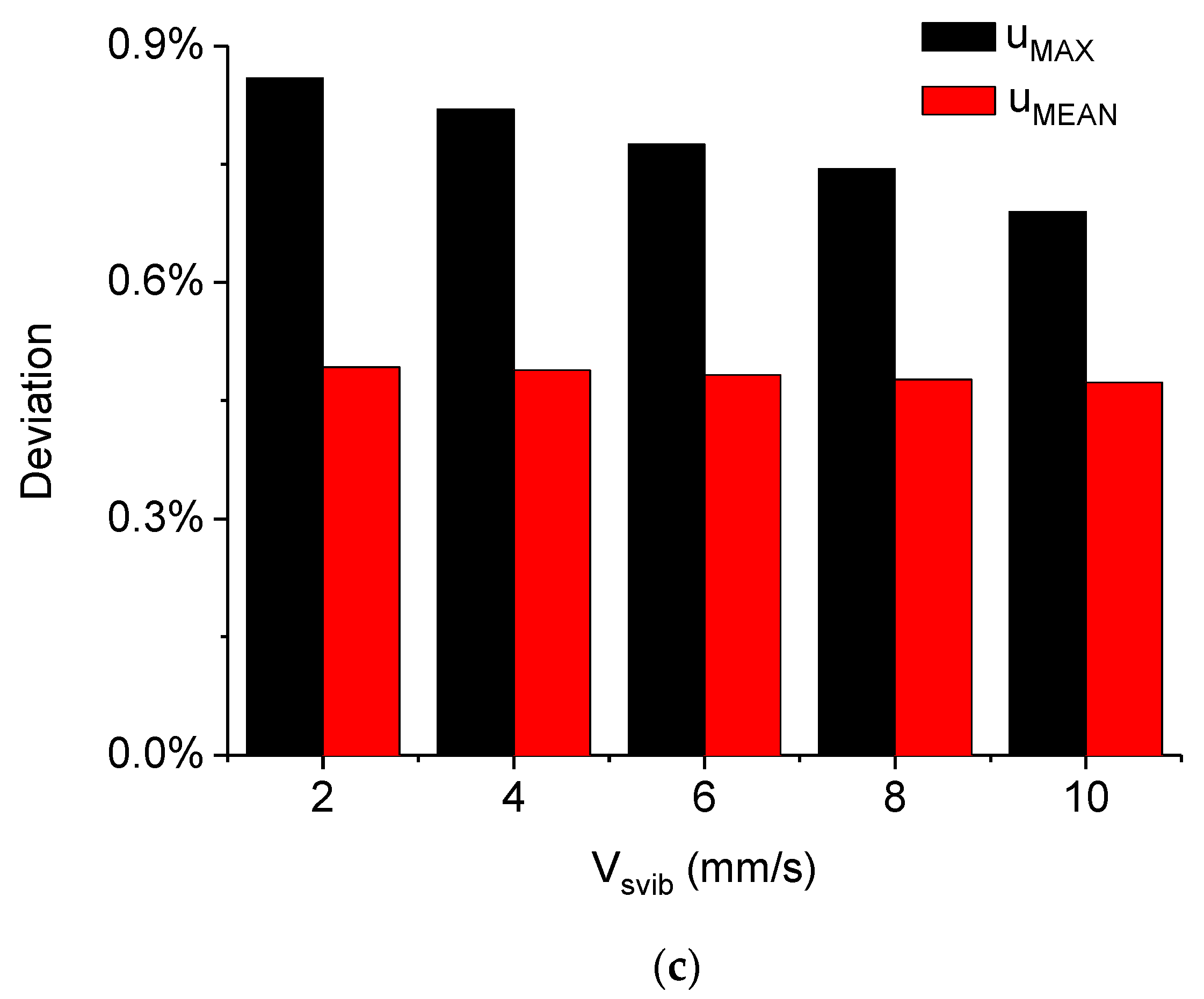
5.2. Simulated Acoustofluidic Fields in the Droplet-Ultrasonic Substrate System for Micro/Nanoscale Particle Concentration
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, J. Ultrasonic Micro/Nano Manipulations: Principles and Examples; World Scientific: Singapore, 2014. [Google Scholar]
- Friend, J.; Yeo, L.Y. Microscale acoustofluidics: Microfluidics driven via acoustics and ultrasonics. Rev. Mod. Phys. 2011, 83, 647–704. [Google Scholar] [CrossRef]
- Connacher, W.; Zhang, N.Q.; Huang, A.; Mei, J.Y.; Zhang, S.; Gopesh, T.; Friend, J. Micro/nano acoustofluidics: Materials, phenomena, design, devices, and applications. Lab Chip 2018, 18, 1952–1996. [Google Scholar] [CrossRef] [PubMed]
- Ozcelik, A.; Rufo, J.; Guo, F.; Gu, Y.Y.; Li, P.; Lata, J.; Huang, T.J. Acoustic tweezers for the life sciences. Nat. Methods 2018, 15, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Yeo, L.Y.; Chang, H.C.; Chan, P.P.Y.; Friend, J.R. Microfluidic Devices for Bioapplications. Small 2011, 7, 12–48. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Huang, T.J. Applications of Acoustofluidics in Bioanalytical Chemistry. Anal. Chem. 2019, 91, 757–767. [Google Scholar] [CrossRef]
- Fu, Y.Q.; Luo, J.K.; Nguyen, N.T.; Walton, A.J.; Flewitt, A.J.; Zu, X.T.; Li, Y.; McHale, G.; Matthews, A.; Iborra, E.; et al. Advances in piezoelectric thin films for acoustic biosensors, acoustofluidics and lab-on-chip applications. Prog. Mater. Sci. 2017, 89, 31–91. [Google Scholar] [CrossRef]
- Qi, A.S.; Chan, P.; Ho, J.; Rajapaksa, A.; Friend, J.; Yeo, L.L. Template-free Synthesis and Encapsulation Technique for Layer-by-Layer Polymer Nanocarrier Fabrication. ACS Nano 2011, 5, 9583–9591. [Google Scholar] [CrossRef]
- Sazan, H.; Piperno, S.; Layani, M.; Magdassi, S.; Shpaisman, H. Directed assembly of nanoparticles into continuous microstructures by standing surface acoustic waves. J. Colloid. Interf. Sci. 2019, 536, 701–709. [Google Scholar] [CrossRef]
- Chen, Y.C.; Ding, X.Y.; Lin, S.C.S.; Yang, S.K.; Huang, P.H.; Nama, N.; Zhao, Y.H.; Nawaz, A.A.; Guo, F.; Wang, W.; et al. Tunable Nanowire Patterning Using Standing Surface Acoustic Waves. ACS Nano 2013, 7, 3306–3314. [Google Scholar] [CrossRef]
- Gadkari, S.A.; Nayfeh, T.H. Micro fabrication using electro deposition and ultrasonic acoustic liquid manipulation. Int. J. Adv. Manuf. Technol. 2008, 39, 107–117. [Google Scholar] [CrossRef]
- Barani, A.; Paktinat, H.; Janmaleki, M.; Mohammadi, A.; Mosaddegh, P.; Fadaei-Tehrani, A.; Sanati-Nezhad, A. Microfluidic integrated acoustic waving for manipulation of cells and molecules. Biosens. Bioelectron. 2016, 85, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.D.; Zheng, H.; Guo, W.; Ganan-Calvo, A.M.; Ai, Y.; Tsao, C.W.; Zhou, J.; Li, W.H.; Huang, Y.Y.; Nguyen, N.T.; et al. Active droplet sorting in microfluidics: A review. Lab Chip 2017, 17, 751–771. [Google Scholar] [CrossRef] [PubMed]
- Ashkin, A.; Dziedzic, J.M. Optical Trapping and Manipulation of Viruses and Bacteria. Science 1987, 235, 1517–1520. [Google Scholar] [CrossRef] [PubMed]
- Ashkin, A.; Dziedzic, J.M.; Yamane, T. Optical Trapping and Manipulation of Single Cells Using Infrared-Laser Beams. Nature 1987, 330, 769–771. [Google Scholar] [CrossRef]
- Agiotis, L.; Theodorakos, I.; Samothrakitis, S.; Papazoglou, S.; Zergioti, I.; Raptis, Y.S. Magnetic manipulation of superparamagnetic nanoparticles in a microfluidic system for drug delivery applications. J. Magn. Magn. Mater. 2016, 401, 956–964. [Google Scholar] [CrossRef]
- Zhu, T.T.; Cheng, R.; Sheppard, G.R.; Locklin, J.; Mao, L.D. Magnetic-Field-Assisted Fabrication and Manipulation of Nonspherical Polymer Particles in Ferrofluid-Based Droplet Microfluidics. Langmuir 2015, 31, 8531–8534. [Google Scholar] [CrossRef]
- Feng, H.F.; Xu, X.; Hao, W.C.; Du, Y.; Tian, D.L.; Jiang, L. Magnetic field actuated manipulation and transfer of oil droplets on a stable underwater superoleophobic surface. Phys. Chem. Chem. Phys. 2016, 18, 16202–16207. [Google Scholar] [CrossRef]
- Schiwek, S.; Heim, L.O.; Stark, R.W.; Dietz, C. Manipulation of polystyrene nanoparticles on a silicon wafer in the peak force tapping mode in water: pH-dependent friction and adhesion force. J. Appl. Phys. 2015, 117, 104303. [Google Scholar] [CrossRef]
- Zhou, P.L.; Yu, H.B.; Yang, W.G.; Wen, Y.D.; Wang, Z.D.; Li, W.J.; Liu, L.Q. Spatial Manipulation and Assembly of Nanoparticles by Atomic Force Microscopy Tip-Induced Dielectrophoresis. ACS Appl. Mater. Interfaces 2017, 9, 16715–16724. [Google Scholar] [CrossRef]
- Freer, E.M.; Grachev, O.; Duan, X.F.; Martin, S.; Stumbo, D.P. High-yield self-limiting single-nanowire assembly with dielectrophoresis. Nat. Nanotechnol. 2010, 5, 525–530. [Google Scholar] [CrossRef]
- Hermanson, K.D.; Lumsdon, S.O.; Williams, J.P.; Kaler, E.W.; Velev, O.D. Dielectrophoretic assembly of electrically functional microwires from nanoparticle suspensions. Science 2001, 294, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.W.; Lin, Y.H.; Lee, G.B. Manipulation and patterning of carbon nanotubes utilizing optically induced dielectrophoretic forces. Microfluid. Nanofluid. 2010, 8, 609–617. [Google Scholar] [CrossRef]
- Yeo, L.Y.; Friend, J.R. Surface Acoustic Wave Microfluidics. Annu. Rev. Fluid Mech. 2014, 46, 379–406. [Google Scholar] [CrossRef]
- Gantner, A.; Hoppe, R.H.W.; Köster, D.; Siebert, K.; Wixforth, A. Numerical simulation of piezoelectrically agitated surface acoustic waves on microfluidic biochips. Comput. Vis. Sci. 2007, 10, 145–161. [Google Scholar] [CrossRef]
- Guo, F.; Mao, Z.M.; Chen, Y.C.; Xie, Z.W.; Lata, J.P.; Li, P.; Ren, L.Q.; Liu, J.Y.; Yang, J.; Dao, M.; et al. Three-dimensional manipulation of single cells using surface acoustic waves. Proc. Natl. Acad. Sci. USA 2016, 113, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.J.; Devendran, C.; Ma, Z.C.; Ng, J.W.; Neild, A.; Ai, Y. Acoustic tweezers via sub-time-of-flight regime surface acoustic waves. Sci. Adv. 2016, 2, e1600089. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Liu, X.; Feng, P.X.L. Manipulating and Patterning Micro/Nanoparticles in Liquid Using Multimode Membrane Resonators. In Proceedings of the 2018 IEEE Biomedical Circuits and Systems Conference (BioCAS), Cleveland, OH, USA, 17–19 October 2018; pp. 4314–4334. [Google Scholar]
- Zheng, T.F.; Wang, C.H.; Xu, C.P.; Hu, Q.; Wei, S.P. Patterning microparticles into a two-dimensional pattern using one column standing surface acoustic waves. Sens. Actuators A Phys. 2018, 284, 168–171. [Google Scholar] [CrossRef]
- Sehgal, P.; Kirby, B.J. Separation of 300 and 100 nm Particles in Fabry-Perot Acoustofluidic Resonators. Anal. Chem. 2017, 89, 12192–12200. [Google Scholar] [CrossRef]
- Gautam, G.P.; Gurung, R.; Fencl, F.A.; Piyasena, M.E. Separation of sub-micron particles from micron particles using acoustic fluid relocation combined with acoustophoresis. Anal. Bioanal. Chem. 2018, 410, 6561–6571. [Google Scholar] [CrossRef]
- Mao, Z.M.; Li, P.; Wu, M.X.; Bachman, H.; Mesyngier, N.; Guo, X.S.; Liu, S.; Costanzo, F.; Huang, T.J. Enriching Nanoparticles via Acoustofluidics. ACS Nano 2017, 11, 603–612. [Google Scholar] [CrossRef]
- Bourquin, Y.; Syed, A.; Reboud, J.; Ranford-Cartwright, L.C.; Barrett, M.P.; Cooper, J.M. Rare-Cell Enrichment by a Rapid, Label-Free, Ultrasonic Isopycnic Technique for Medical Diagnostics. Angew. Chem. Int. Ed. 2014, 53, 5587–5590. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Lim, C.S. Micromixing using swirling induced by three-dimensional dual surface acoustic waves (3D-dSAW). Sens. Actuators B Chem. 2018, 255, 3434–3440. [Google Scholar] [CrossRef]
- Rezk, A.R.; Qi, A.; Friend, J.R.; Li, W.H.; Yeo, L.Y. Uniform mixing in paper-based microfluidic systems using surface acoustic waves. Lab Chip 2012, 12, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Bernard, I.; Doinikov, A.A.; Marmottant, P.; Rabaud, D.; Poulain, C.; Thibault, P. Controlled rotation and translation of spherical particles or living cells by surface acoustic waves. Lab Chip 2017, 17, 2470–2480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, S.; Chen, C.; Hartman, J.H.; Huang, P.-H.; Wang, L.; Tian, Z.; Zhang, P.; Faulkenberry, D.; Meyer, J.N.; et al. Surface acoustic waves enable rotational manipulation of Caenorhabditis elegans. Lab Chip 2019, 19, 984–992. [Google Scholar] [CrossRef]
- Andrade, M.A.B.; Camargo, T.S.A.; Marzo, A. Automatic contactless injection, transportation, merging, and ejection of droplets with a multifocal point acoustic levitator. Rev. Sci. Instrum. 2018, 89, 125105. [Google Scholar] [CrossRef]
- Bouyer, C.; Chen, P.; Guven, S.; Demirtas, T.T.; Nieland, T.J.F.; Padilla, F.; Demirci, U. A Bio-Acoustic Levitational (BAL) Assembly Method for Engineering of Multilayered, 3D Brain-Like Constructs, Using Human Embryonic Stem Cell Derived Neuro-Progenitors. Adv. Mater. 2016, 28, 161–167. [Google Scholar] [CrossRef]
- Chen, P.; Luo, Z.Y.; Guven, S.; Tasoglu, S.; Ganesan, A.V.; Weng, A.; Demirci, U. Microscale Assembly Directed by Liquid-Based Template. Adv. Mater. 2014, 26, 5936–5941. [Google Scholar] [CrossRef]
- Owens, C.E.; Shields, C.W.; Cruz, D.F.; Charbonneau, P.; Lopez, G.P. Highly parallel acoustic assembly of microparticles into well-ordered colloidal crystallites. Soft. Matter. 2016, 12, 717–728. [Google Scholar] [CrossRef]
- Gao, L.; Shields, C.W.; Johnson, L.M.; Graves, S.W.; Yellen, B.B.; Lopez, G.P. Two-dimensional spatial manipulation of microparticles in continuous flows in acoustofluidic systems. Biomicrofluidics 2015, 9, 014105. [Google Scholar] [CrossRef]
- Shu, X.; Liu, H.Q.; Zhu, Y.Z.; Cai, B.; Jin, Y.X.; Wei, Y.C.; Zhou, F.L.; Liu, W.; Guo, S.S. An improved bulk acoustic waves chip based on a PDMS bonding layer for high-efficient particle enrichment. Microfluid. Nanofluid. 2018, 22, 32. [Google Scholar] [CrossRef]
- Reichert, P.; Deshmukh, D.; Lebovitz, L.; Dual, J. Thin film piezoelectrics for bulk acoustic wave (BAW) acoustophoresis. Lab Chip 2018, 18, 3655–3667. [Google Scholar] [CrossRef] [PubMed]
- Gor’kov, L.P. On the Forces Acting on a Small Particle in an Acoustical Field in an Ideal Fluid. Sov. Phys. Dokl. 1962, 6, 773–775. [Google Scholar]
- Nyborg, W.L. Acoustic Streaming Due to Attenuated Plane Waves. J. Acoust. Soc. Am. 1953, 25, 68–75. [Google Scholar] [CrossRef]
- Lighthill, J. Acoustic Streaming. J. Sound Vib. 1978, 61, 391–418. [Google Scholar] [CrossRef]
- Riley, N. Steady streaming. Annu. Rev. Fluid Mech. 2001, 33, 43–65. [Google Scholar] [CrossRef]
- Antil, H.; Glowinski, R.; Hoppe, R.H.W.; Linsenmann, C.; Pan, T.-W.; Wixforth, A. Modeling, Simulation, and Optimaization of Surface Acoustic Wave Driven Microfluidic Biochips. J. Comput. Math. 2010, 28, 149–169. [Google Scholar] [CrossRef]
- Riley, N. Acoustic Streaming. Theor. Comput. Fluid Dyn. 1998, 10, 349–356. [Google Scholar] [CrossRef]
- Barnkob, R.; Augustsson, P.; Laurell, T.; Bruus, H. Acoustic radiation- and streaming-induced microparticle velocities determined by microparticle image velocimetry in an ultrasound symmetry plane. Phys. Rev. E 2012, 86, 056307. [Google Scholar] [CrossRef]
- Barnkob, R.; Iranmanesh, I.; Wiklund, M.; Bruus, H. Measuring acoustic energy density in microchannel acoustophoresis using a simple and rapid light-intensity method. Lab Chip 2012, 12, 2337–2344. [Google Scholar] [CrossRef]
- Nama, N.; Huang, T.J.; Costanzo, F. Acoustic streaming: An arbitrary Lagrangian-Eulerian perspective. J. Fluid Mech. 2017, 825, 600–630. [Google Scholar] [CrossRef] [PubMed]
- Barnkob, R.; Nama, N.; Ren, L.Q.; Huang, T.J.; Costanzo, F.; Kahler, C.J. Acoustically Driven Fluid and Particle Motion in Confined and Leaky Systems. Phys. Rev. Appl. 2018, 9, 014027. [Google Scholar] [CrossRef]
- Barnkob, R.; Augustsson, P.; Laurell, T.; Bruus, H. Measuring the local pressure amplitude in microchannel acoustophoresis. Lab Chip 2010, 10, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Frampton, K. The scaling of acoustic streaming for application in microfluidic devices. J. Acoust. Soc. Am. 2001, 109, 2348. [Google Scholar] [CrossRef]
- Shamloo, A.; Boodaghi, M. Design and simulation of a microfluidic device for acoustic cell separation. Ultrasonics 2018, 84, 234–243. [Google Scholar] [CrossRef]
- Xu, D.; Cai, F.; Chen, M.; Li, F.; Wang, C.; Meng, L.; Xu, D.; Wang, W.; Wu, J.; Zheng, H. Acoustic manipulation of particles in a cylindrical cavity: Theoretical and experimental study on the effects of boundary conditions. Ultrasonics 2019, 93, 18–25. [Google Scholar] [CrossRef]
- Lei, J.J.; Glynne-Jones, P.; Hill, M. Acoustic streaming in the transducer plane in ultrasonic particle manipulation devices. Lab Chip 2013, 13, 2133–2143. [Google Scholar] [CrossRef]
- Lei, J.J.; Hill, M.; Glynne-Jones, P. Numerical simulation of 3D boundary-driven acoustic streaming in microfluidic devices. Lab Chip 2014, 14, 532–541. [Google Scholar] [CrossRef]
- Chen, C.Y.; Zhang, S.P.; Mao, Z.M.; Nama, N.; Gu, Y.Y.; Huang, P.H.; Jing, Y.; Guo, X.S.; Costanzo, F.; Huang, T.J. Three-dimensional numerical simulation and experimental investigation of boundary-driven streaming in surface acoustic wave microfluidics. Lab Chip 2018, 18, 3645–3654. [Google Scholar] [CrossRef]
- Muller, P.B.; Barnkob, R.; Jensen, M.J.H.; Bruus, H. A numerical study of microparticle acoustophoresis driven by acoustic radiation forces and streaming-induced drag forces. Lab Chip 2012, 12, 4617–4627. [Google Scholar] [CrossRef]
- Catarino, S.O.; Miranda, J.M.; Lanceros-Mendez, S.; Minas, G. Numerical Prediction of Acoustic Streaming in a Microcuvette. Can. J. Chem. Eng. 2014, 92, 1988–1998. [Google Scholar] [CrossRef]
- Catarino, S.O.; Minas, G.; Miranda, J.M. Evaluation of the successive approximations method for acoustic streaming numerical simulations. J. Acoust. Soc. Am. 2016, 139, 2269–2279. [Google Scholar] [CrossRef] [PubMed]
- Sadhal, S.S. Acoustofluidics 13: Analysis of acoustic streaming by perturbation methods Foreword. Lab Chip 2012, 12, 2292–2300. [Google Scholar] [CrossRef] [PubMed]
- Koster, D. Numerical simulation of acoustic streaming on surface acoustic wave-driven biochips. SIAM J. Sci. Comput. 2007, 29, 2352–2380. [Google Scholar] [CrossRef]
- Tang, Q.; Hu, J.H. Diversity of acoustic streaming in a rectangular acoustofluidic field. Ultrasonics 2015, 58, 27–34. [Google Scholar] [CrossRef]
- Lei, J.J.; Glynne-Jones, P.; Hill, M. Modal Rayleigh-like streaming in layered acoustofluidic devices. Phys. Fluids 2016, 28, 012004. [Google Scholar] [CrossRef]
- Eckart, C. Vortices and Streams Caused by Sound Waves. Phys. Rev. 1948, 73, 68–76. [Google Scholar] [CrossRef]
- Boluriaan, S.; Morris, P.J. Acoustic streaming: From Rayleigh to today. Int. J. Aeroacoust. 2003, 2, 255–292. [Google Scholar] [CrossRef]
- Wiklund, M.; Green, R.; Ohlin, M. Acoustofluidics 14: Applications of acoustic streaming in microfluidic devices. Lab Chip 2012, 12, 2438–2451. [Google Scholar] [CrossRef]
- Nama, N.; Barnkob, R.; Mao, Z.M.; Kahler, C.J.; Costanzo, F.; Huang, T.J. Numerical study of acoustophoretic motion of particles in a PDMS microchannel driven by surface acoustic waves. Lab Chip 2015, 15, 2700–2709. [Google Scholar] [CrossRef]
- Lei, J.J.; Glynne-Jones, P.; Hill, M. Comparing methods for the modelling of boundary-driven streaming in acoustofluidic devices. Microfluid. Nanofluid. 2017, 21, 23. [Google Scholar] [CrossRef]
- Tang, Q.; Hu, J.H.; Qian, S.Z.; Zhang, X.Y. Eckart acoustic streaming in a heptagonal chamber by multiple acoustic transducers. Microfluid. Nanofluid. 2017, 21, 28. [Google Scholar] [CrossRef]
- Li, N.; Hu, J.H.; Li, H.Q.; Bhuyan, S.; Zhou, Y.J. Mobile acoustic streaming based trapping and 3-dimensional transfer of a single nanowire. Appl. Phys. Lett. 2012, 101, 093113. [Google Scholar] [CrossRef]
- Wang, X.; Hu, J.H. An ultrasonic manipulator with noncontact and contact-type nanowire trapping functions. Sens. Actuators A Phys. 2015, 232, 13–19. [Google Scholar] [CrossRef]
- Zhu, X.B.; Hu, J.H. Ultrasonic drive of small mechanical components on a tapered metal strip. Ultrasonics 2013, 53, 417–422. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Li, H.Q.; Hu, J.H. An ultrasonic stage for controlled spin of micro particles. Rev. Sci. Instrum. 2012, 83, 045004. [Google Scholar] [CrossRef]
- Li, N.; Hu, J.H. Sound-Controlled Rotary Driving of a Single Nanowire. IEEE Trans. Nanotechnol. 2014, 13, 437–441. [Google Scholar] [CrossRef]
- Liu, P.Z.; Hu, J.H. Controlled removal of micro/nanoscale particles in submillimeter-diameter area on a substrate. Rev. Sci. Instrum. 2017, 88, 105003. [Google Scholar] [CrossRef]
- Qi, X.M.; Tang, Q.; Liu, P.Z.; Minin, I.V.; Minin, O.V.; Hu, J.H. Controlled concentration and transportation of nanoparticles at the interface between a plain substrate and droplet. Sens. Actuators B Chem. 2018, 274, 381–392. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Hu, J.H.; Bhuyan, S. Manipulations of Silver Nanowires in a Droplet on a Low-Frequency Ultrasonic Stage. IEEE Trans. Ultrason. Ferr. 2013, 60, 622–629. [Google Scholar] [CrossRef]
- Lighthill, J. Waves in Fluids; Cambridge University Press: Cambridge, UK, 1978. [Google Scholar]
- Lei, J.J.; Hill, M.; Albarran, C.P.D.; Glynne-Jones, P. Effects of micron scale surface profiles on acoustic streaming. Microfluid. Nanofluid. 2018, 22, 140. [Google Scholar] [CrossRef]
- Tang, Q.; Hu, J.H. Analyses of acoustic streaming field in the probe-liquid-substrate system for nanotrapping. Microfluid. Nanofluid. 2015, 19, 1395–1408. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, X.F.; Hu, J.H. Nano concentration by acoustically generated complex spiral vortex field. Appl. Phys. Lett. 2017, 110, 104105. [Google Scholar] [CrossRef]
- Tang, Q.; Liu, P.Z.; Hu, J.H. Analyses of acoustofluidic field in ultrasonic needle-liquid-substrate system for micro-/nanoscale material concentration. Microfluid. Nanofluid. 2018, 22, 46. [Google Scholar] [CrossRef]
- Nama, N.; Huang, P.H.; Huang, T.J.; Costanzo, F. Investigation of micromixing by acoustically oscillated sharp-edges. Biomicrofluidics 2016, 10, 024124. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Hu, J.H. Ultrasonic removal of coarse and fine droplets in air. Sep. Purif. Technol. 2015, 153, 156–161. [Google Scholar] [CrossRef]
- Hattori, T. Non-Aqueous Cleaning Challenges for Preventing Damage to Fragile Nano-Structures: A Review. ECS J. Solid State Sci. 2014, 3, N3054–N3059. [Google Scholar] [CrossRef]
- Blackstock, D.T. Fundamentals of Physical Acoustics; Wiley-Interscience: Hoboken, NJ, USA, 2000. [Google Scholar]


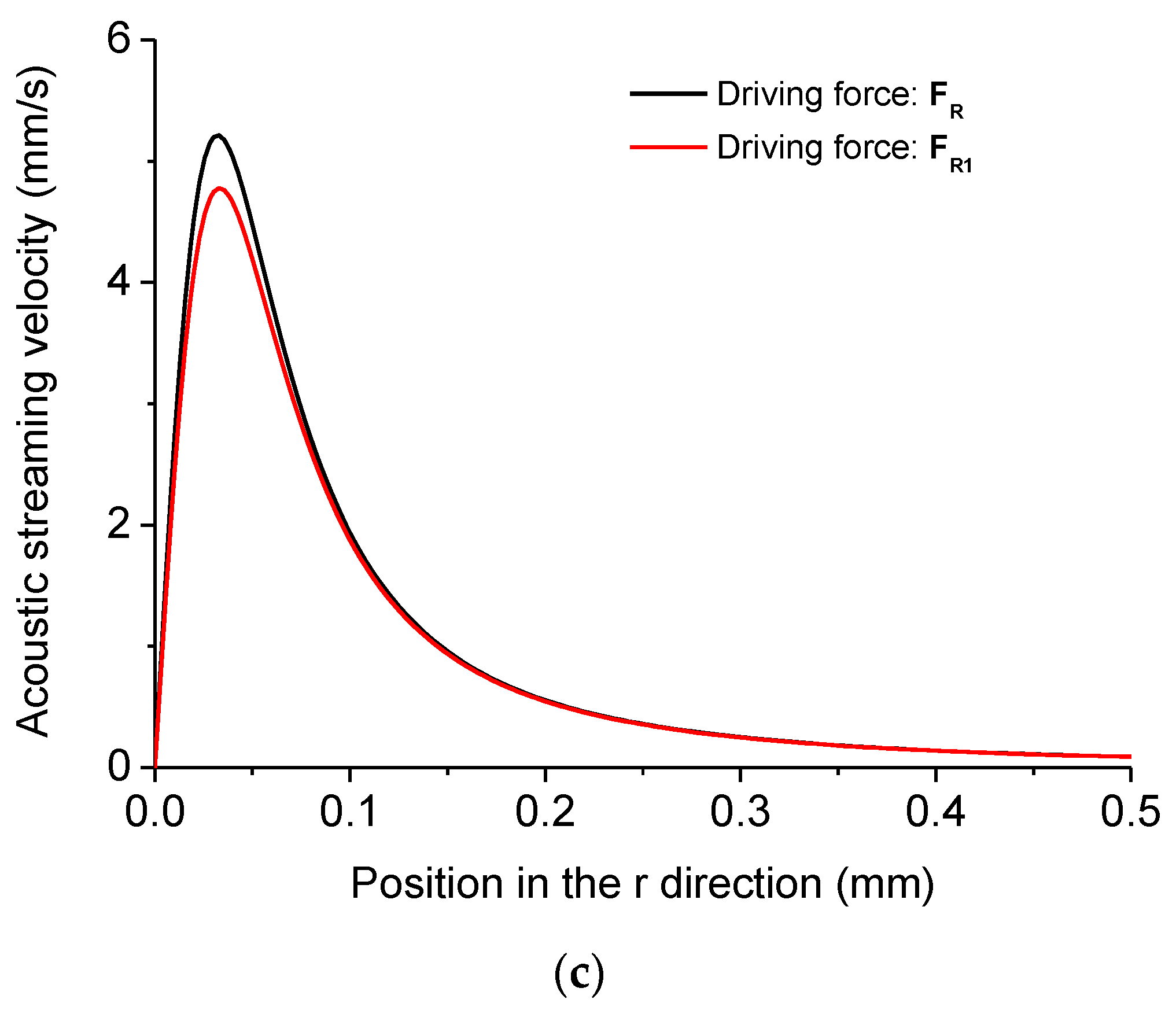
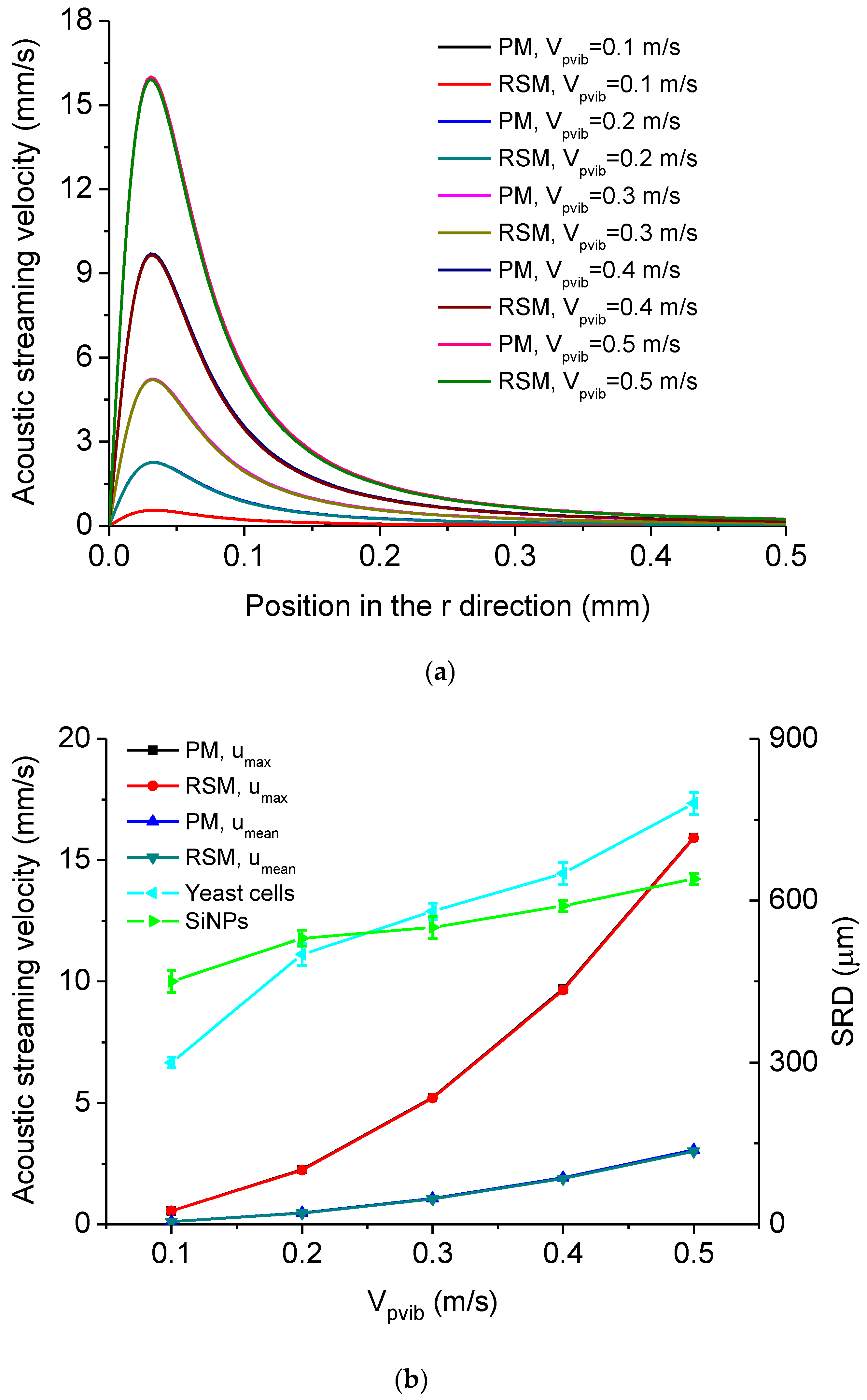
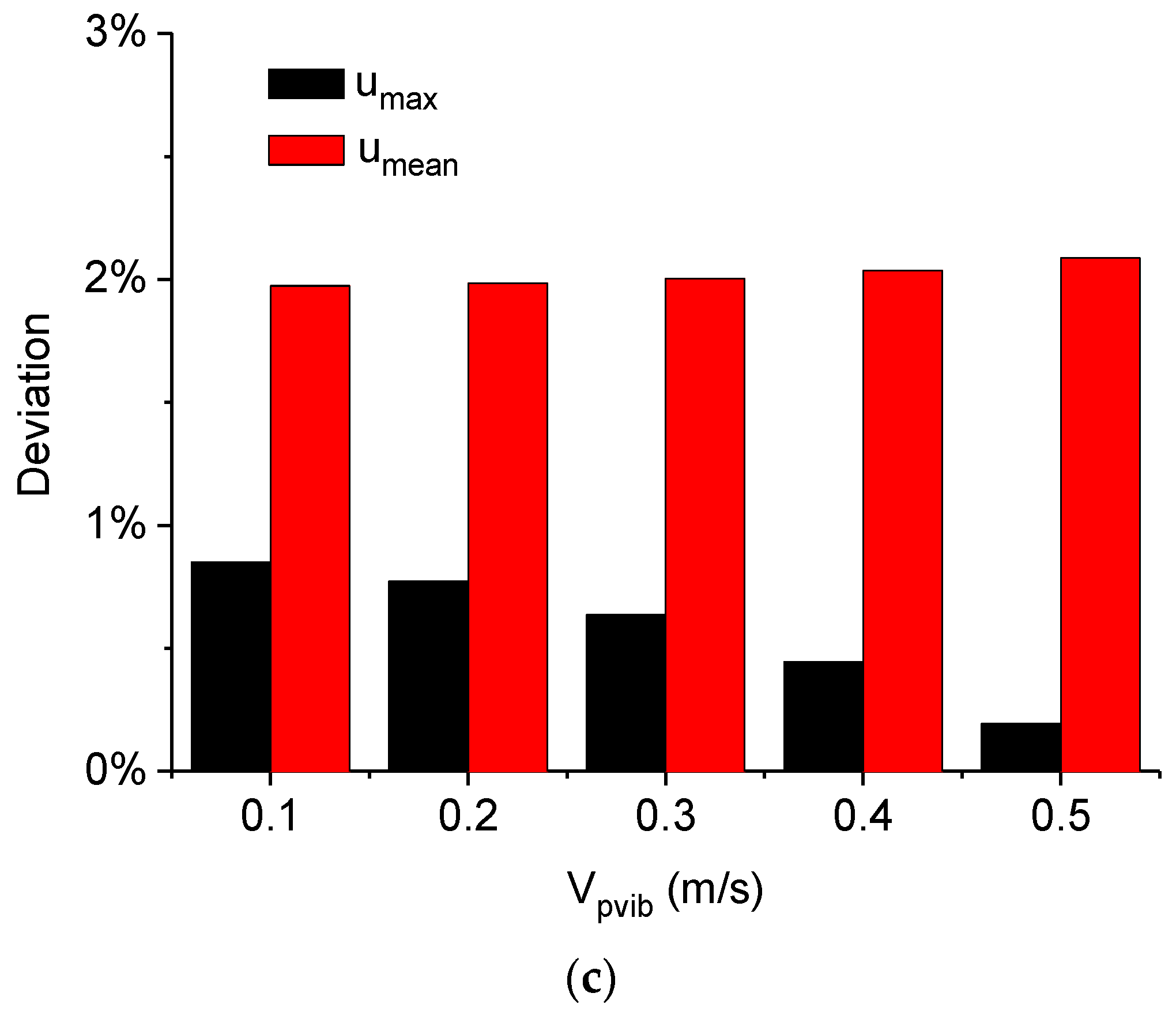
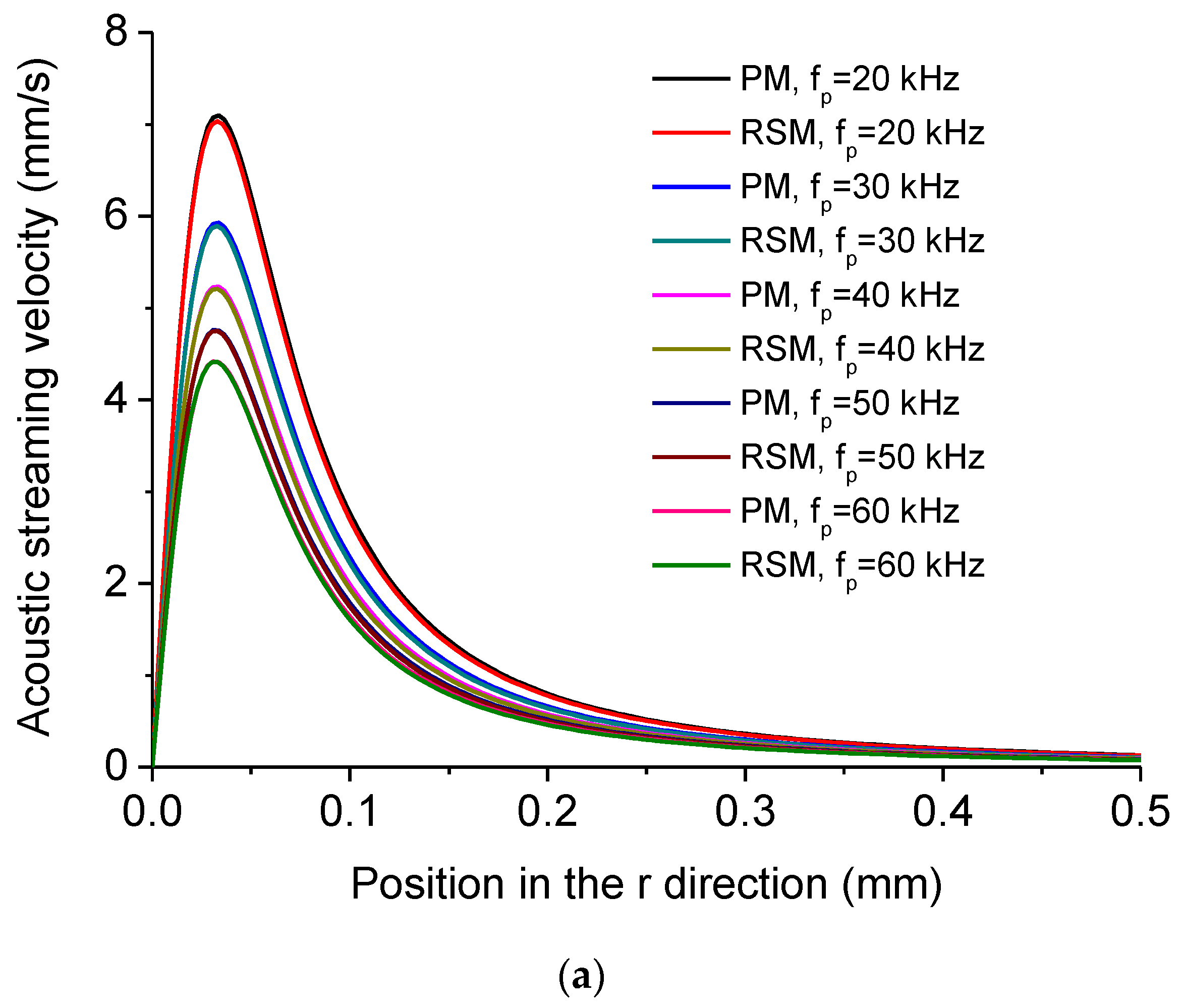



| Height of the Droplet (mm) | Radius of the Droplet (mm) | Density of Water ρ0 (kg/m3) |
|---|---|---|
| 2 | 5 | 998 |
| Sound speed in water c0 (m/s) | Shear viscosity of water μ (Pa∙s) | Bulk-to-shear viscosity ratio in water μb/μ |
| 1482 | 0.001 | 2.1 |
| Driving frequency of the ultrasonic probe-droplet-substrate system fp (kHz) | Vibration velocity of the MMP Vpvib (m/s) | Distance between the MMP’s tip and substrate surface H (μm) |
| 40 | 0.3 | 50 |
| Radius of the MMP R (μm) | Length of the MMP L (mm) | Density of the MMP (kg/m3) |
| 15 | 4 | 2210 |
| Young’s modulus of the MMP (Pa) | Poisson ratio of the MMP | Driving frequency of the droplet-ultrasonic substrate system fs (kHz) |
| 7.2 × 1010 | 0.2 | 40 |
| Excitation velocity of the droplet-ultrasonic substrate system Vsvib (mm/s) | Radius of the excitation part RE (mm) | Density of yeast cells (kg/m3) |
| 6 | 3 | 1114 |
| Sound speed in yeast cells (m/s) | Density of SiNPs (kg/m3) | Sound speed in SiNPs (m/s) |
| 1606 | 2330 | 5664 |
| Density of AgNPs (kg/m3) | Sound speed in AgNPs (m/s) | Sound speed in air cair (m/s) |
| 10,500 | 2600 | 343 |
| Density of air ρair (kg/m3) | ||
| 1.205 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Tang, Q.; Su, S.; Hu, J.; Yu, Y. Modeling and Analysis of the Two-Dimensional Axisymmetric Acoustofluidic Fields in the Probe-Type and Substrate-Type Ultrasonic Micro/Nano Manipulation Systems. Micromachines 2020, 11, 22. https://doi.org/10.3390/mi11010022
Liu P, Tang Q, Su S, Hu J, Yu Y. Modeling and Analysis of the Two-Dimensional Axisymmetric Acoustofluidic Fields in the Probe-Type and Substrate-Type Ultrasonic Micro/Nano Manipulation Systems. Micromachines. 2020; 11(1):22. https://doi.org/10.3390/mi11010022
Chicago/Turabian StyleLiu, Pengzhan, Qiang Tang, Songfei Su, Jie Hu, and Yang Yu. 2020. "Modeling and Analysis of the Two-Dimensional Axisymmetric Acoustofluidic Fields in the Probe-Type and Substrate-Type Ultrasonic Micro/Nano Manipulation Systems" Micromachines 11, no. 1: 22. https://doi.org/10.3390/mi11010022
APA StyleLiu, P., Tang, Q., Su, S., Hu, J., & Yu, Y. (2020). Modeling and Analysis of the Two-Dimensional Axisymmetric Acoustofluidic Fields in the Probe-Type and Substrate-Type Ultrasonic Micro/Nano Manipulation Systems. Micromachines, 11(1), 22. https://doi.org/10.3390/mi11010022






