An Integrated Portable Multiplex Microchip Device for Fingerprinting Chemical Warfare Agents
Abstract
1. Introduction
2. Methodology
3. Experimental Details
3.1. Chemicals
3.2. Microchip Fabrication
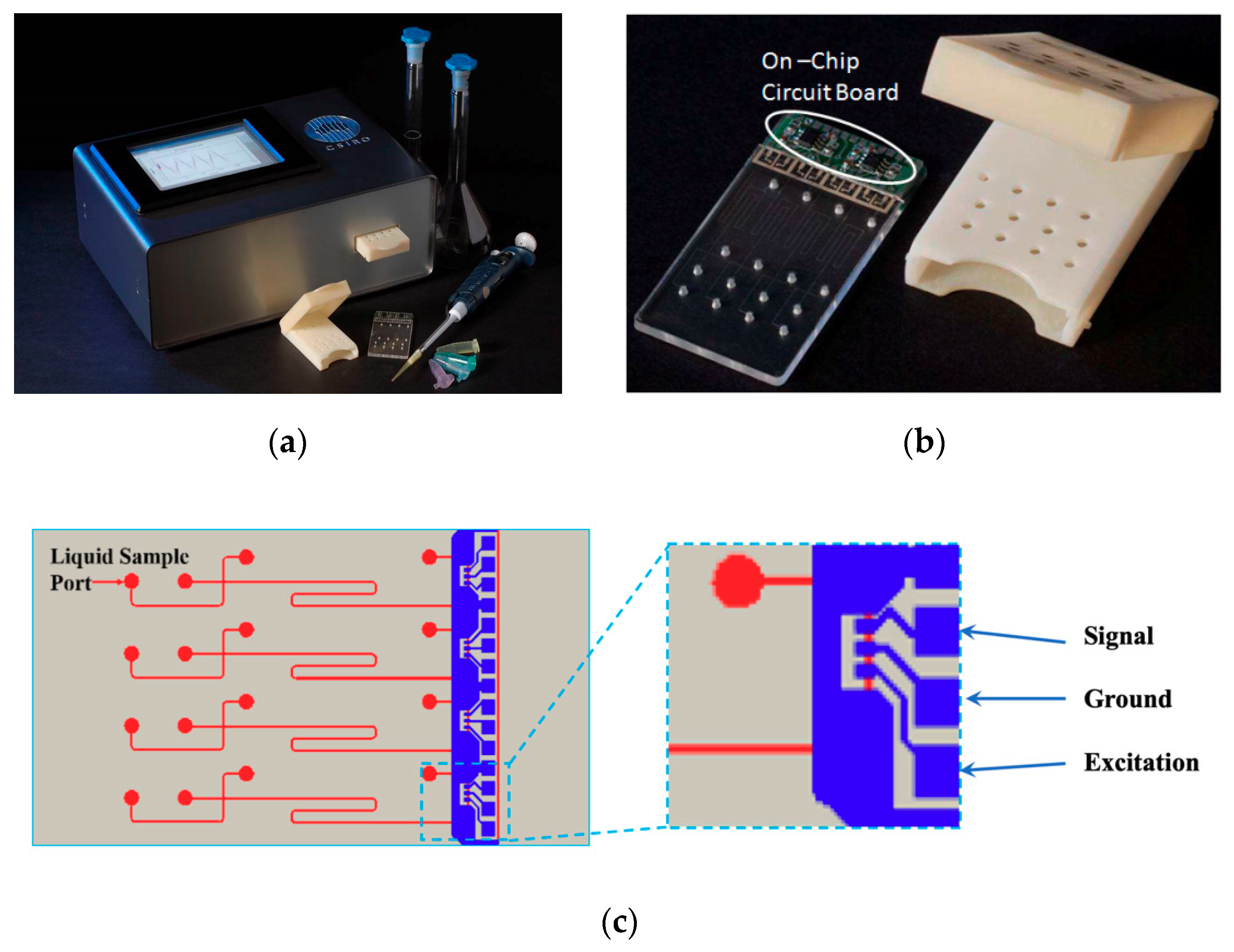
3.3. C4D Sensor Fabrication
3.4. Microchip Electrophoresis
4. Device Development
4.1. Development of Portable Multiplex Microfluidics Device
4.2. Microfluidics and High Voltage Power Supply Module
4.3. Display Module and Graphic User Interface (GUI)
5. Results
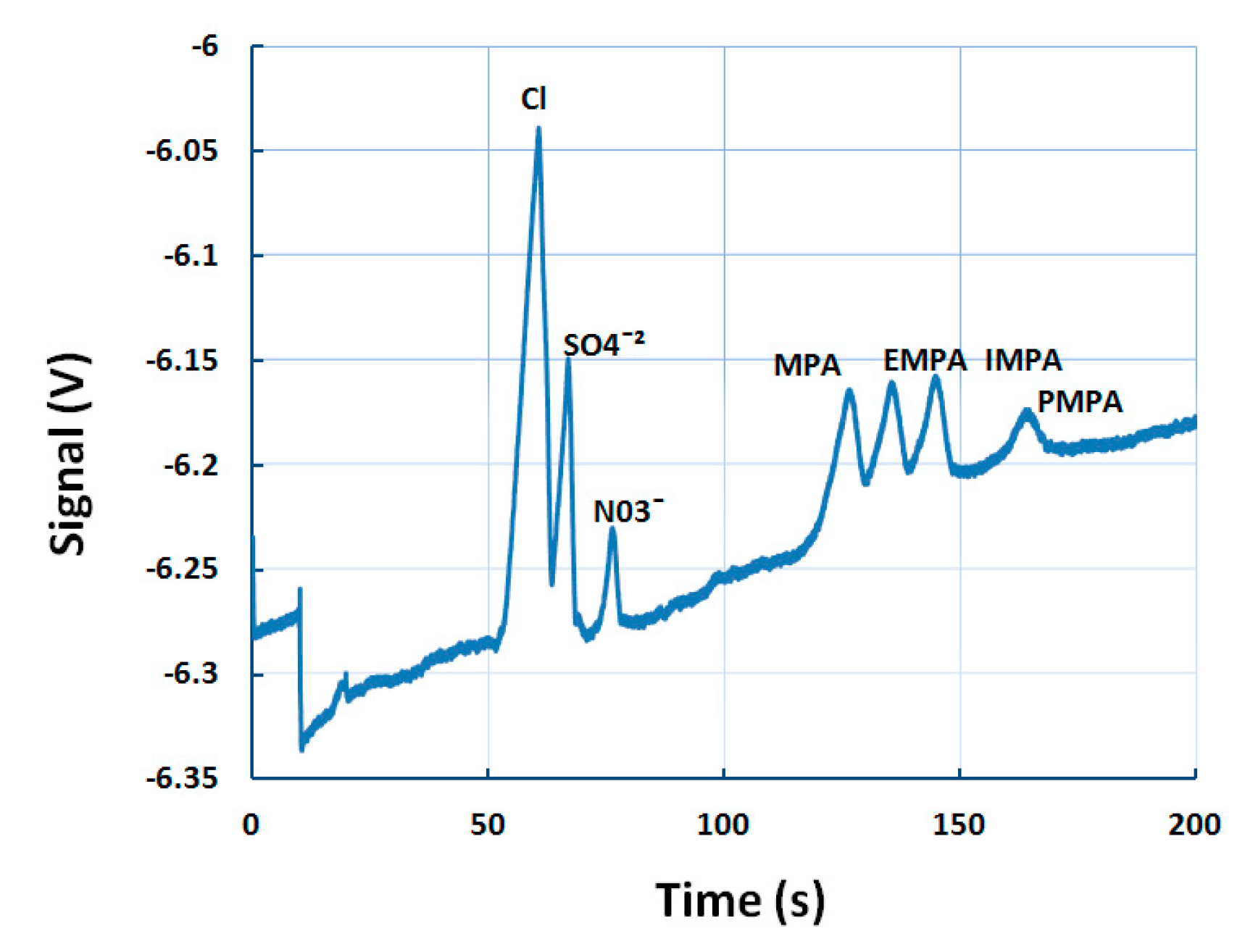
5.1. Designing Background Electrolyte for MCE-C4D
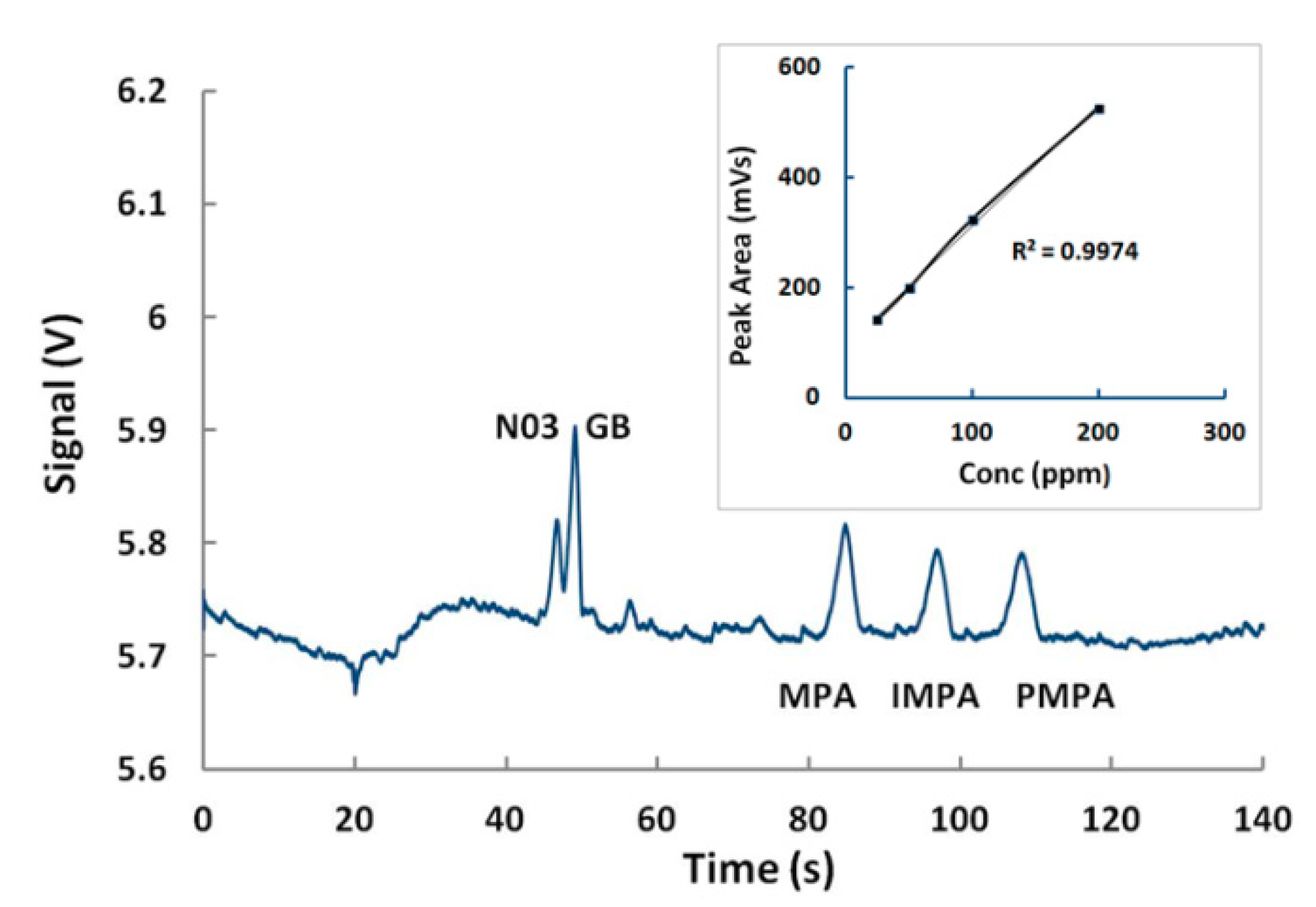
5.2. Reproducibility, Linearity and Limit of Detection
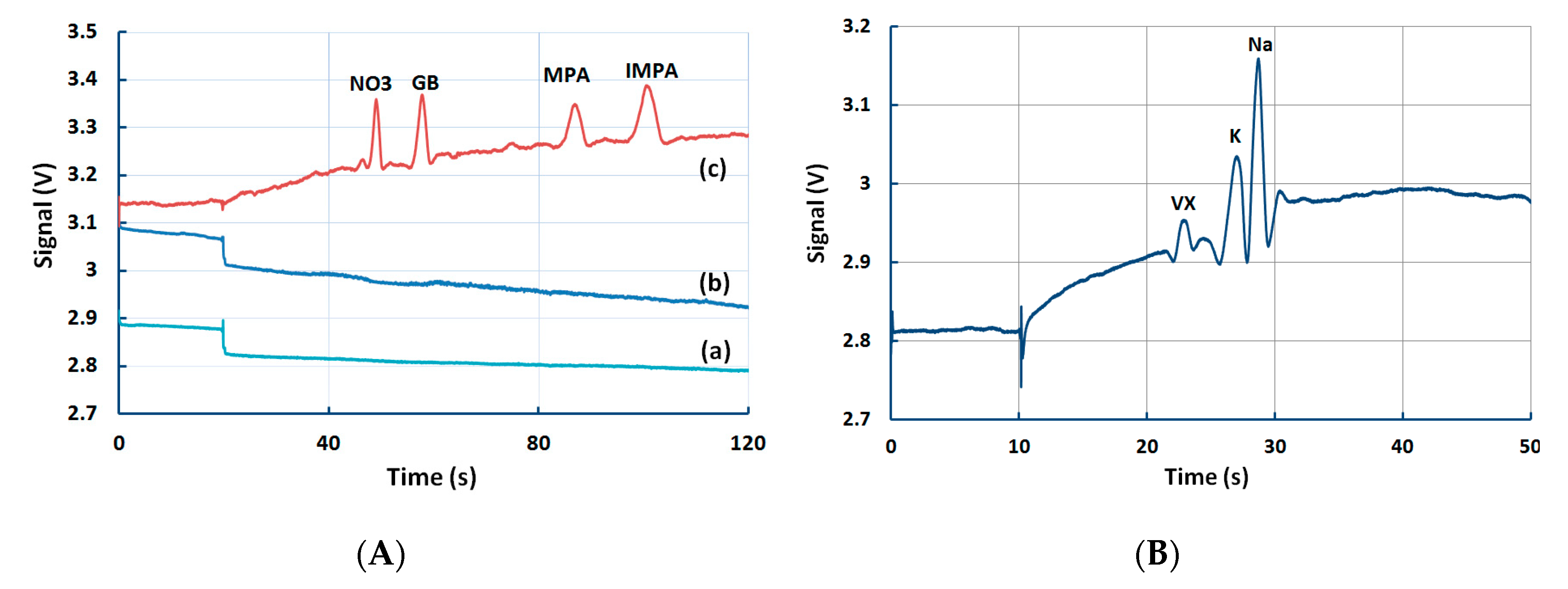
5.3. Detection of GB and VX in Environmental Water Samples
5.4. Detection of Multiple Chemical Warfare Agents (CWAs)
5.5. Detection of Ricin
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Daw, R.; Finkelstein, J. Lab on a chip. Nature 2006, 442, 367–367. [Google Scholar] [CrossRef]
- Petkovic, K.; Metcalfe, G.; Chen, H.; Gao, Y.; Best, M.; Lester, D.; Zhu, Y. Rapid detection of Hendra virus antibodies: an integrated device with nanoparticle assay and chaotic micromixing. Lab Chip 2017, 17, 169–177. [Google Scholar] [CrossRef]
- Varghese, S.S.; Zhu, Y.; Davis, T.J.; Trowell, S.C. FRET for lab-on-a-chip devices-current trends and future prospects. Lab Chip 2010, 10, 1355–1364. [Google Scholar] [CrossRef]
- Yap, L.W.; Chen, H.; Gao, Y.; Petkovic, K.; Liang, Y.; Si, K.J.; Wang, H.; Zhu, Y.; Tang, Z.; Cheng, W. Bifunctional plasmonic-magnetic particles for an enhanced microfluidic SERS immunoassay. Nanoscale 2017, 9, 7822–7829. [Google Scholar] [CrossRef]
- Chin, C.D.; Linder, V.; Sia, S.K. Lab-on-a-chip devices for global health: Past studies and future opportunities. Lab Chip 2007, 7, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Madhivanan, P.; Krupp, K.; Hardin, J.; Karat, C.; Klausner, J.D.; Reingold, A.L. Simple and inexpensive point-of-care tests improve diagnosis of vaginal infections in resource constrained settings. Trop. Med. Int. Health 2009, 14, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Von Lode, P. Point-of-care immunotesting: approaching the analytical performance of central laboratory methods. Clin. Biochem. 2005, 38, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Barthelmebs, L.; Calas-Blanchard, C.; Istamboulie, G.; Marty, J.-L.; Noguer, T. Bio-Farms for Nutraceuticals; Springer: Berlin, Germany, 2010; pp. 293–307. [Google Scholar]
- Rodriguez-Mozaz, S.; de Alda, M.J.L.; Marco, M.P.; Barcelo, D. Biosensors for environmental monitoring: A global perspective. Talanta 2005, 65, 291–297. [Google Scholar] [CrossRef]
- Blanco, G.A.; Nai, Y.H.; Hilder, E.F.; Shellie, R.A.; Dicinoski, G.W.; Haddad, P.R.; Breadmore, M.C. Identification of inorganic improvised explosive devices using sequential injection capillary electrophoresis and contactless conductivity detection. Anal. Chem. 2011, 83, 9068–9075. [Google Scholar] [CrossRef] [PubMed]
- Gaudry, A.J.; Guijt, R.M.; Macka, M.; Hutchinson, J.P.; Johns, C.; Hilder, E.F.; Dicinoski, G.W.; Nesterenko, P.N.; Haddad, P.R.; Breadmore, M.C. On-line simultaneous and rapid separation of anions and cations from a single sample using dual-capillary sequential injection-capillary electrophoresis. Anal. Chim. Acta 2013, 781, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Seiman, A.; Makarotseva, N.; Vaher, M.; Kaljurand, M. The detection of nerve agent degradation products in different soil fractions using capillary electrophoresis with contactless conductivity detection. Chem. Ecol. 2010, 26, 145–155. [Google Scholar] [CrossRef]
- Kientz, C.E. Chromatography and mass spectrometry of chemical warfare agents, toxins and related compounds: state of the art and future prospects. J. Chromatogr. A 1998, 814, 1–23. [Google Scholar] [CrossRef]
- John, H.; Worek, F.; Thiermann, H. LC-MS-based procedures for monitoring of toxic organophosphorus compounds and verification of pesticide and nerve agent poisoning. Anal. Bioanal. Chem. 2008, 391, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Pumera, M. Analysis of nerve agents using capillary electrophoresis and laboratory-on-a-chip technology. J. Chromatogr. A 2006, 1113, 5–13. [Google Scholar] [CrossRef]
- Aleksenko, S.S.; Gareil, P.; Timerbaev, A.R. Analysis of degradation products of chemical warfare agents using capillary electrophoresis. Analyst 2011, 136, 4103–4118. [Google Scholar] [CrossRef]
- Makarotseva, N.; Seiman, A.; Vaher, M.; Kaljurand, M. Analysis of the degradation products of chemical warfare agents using a portable capillary electrophoresis instrument with various sample injection devices. Proc. Chem. 2010, 2, 20–25. [Google Scholar] [CrossRef]
- Liu, K.; Fan, Z.H. Thermoplastic microfluidic devices and their applications in protein and DNA analysis. Analyst 2011, 136, 1288–1297. [Google Scholar] [CrossRef]
- Berthold, A.; Laugere, F.; Schellevis, H.; de Boer, C.R.; Laros, M.; Guijt, R.M.; Sarro, P.M.; Vellekoop, M.J. Fabrication of a glass-implemented microcapillary electrophoresis device with integrated contactless conductivity detection. Electrophoresis 2002, 23, 3511–3519. [Google Scholar] [CrossRef]
- Bharadwaj, R.; Santiago, J.G.; Mohammadi, B. Fabrication of a glass-implemented microcapillary electrophoresis device with integrated contactless conductivity detection. Electrophoresis 2002, 23, 2729–2744. [Google Scholar] [CrossRef]
- Breadmore, M.C.; Shallan, A.I.; Rabanes, H.R.; Gstoettenmayr, D.; Abdul Keyon, A.S.; Gaspar, A.; Dawod, M.; Quirino, J.P. Recent advances in enhancing the sensitivity of electrophoresis and electrochromatography in capillaries and microchips (2010–2012). Electrophoresis 2013, 34, 29–54. [Google Scholar] [CrossRef] [PubMed]
- Dolnik, V.; Liu, S.R.; Jovanovich, S. Capillary electrophoresis on microchip. Electrophoresis 2000, 21, 41–54. [Google Scholar] [CrossRef]
- Kappes, T.; Galliker, B.; Schwarz, M.A.; Hauser, P.C. Portable capillary electrophoresis instrument with amperometric, potentiometric and conductometric detection. Trac-Trend. Anal. Chem. 2001, 20, 133–139. [Google Scholar] [CrossRef]
- Kim, M.S.; Cho, S.I.; Lee, K.-N.; Kim, Y.K. Fabrication of microchip electrophoresis devices and effects of channel surface properties on separation efficiency. Sensors Actuat. B 2005, 107, 818–824. [Google Scholar] [CrossRef]
- Shang, F.J.; Guihen, E.; Glennon, J.D. Recent advances in miniaturisation-the role of microchip electrophoresis in clinical analysis. Electrophoresis 2012, 33, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Nassar, A.-E.F.; Lucas, S.V.; Hoffland, L.D. Determination of Chemical Warfare Agent Degradation Products at Low-Part-per-Billion Levels in Aqueous Samples and Sub-Part-per-Million Levels in Soils Using Capillary Electrophoresis. Anal. Chem. 1999, 71, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Mercier, J.-P.; Morin, P.; Dreux, M.; Tambute, A. Capillary electrophoresis analysis of chemical warfare agent breakdown products I. Counterelectroosmotic separation of alkylphosphonic acids and their monoester derivatives. J. Chromatogr. A 1996, 741, 279–285. [Google Scholar] [CrossRef]
- Rodin, I.; Stavrianidi, A.; Smirnov, R.; Braun, A.; Shpigun, O.; Rybalchenko, I. New Techniques for Nerve Agent Oxidation Products Determination in Environmental Water by High-Performance Liquid Chromatography-Mass Spectrometry (HPLC-MS) and Capillary Electrophoresis (CE) with Direct Ultraviolet (UV) Detection. Environ. Forensic. 2013, 14, 87–96. [Google Scholar] [CrossRef]
- Wang, J.; Pumera, M.; Collins, G.E.; Mulchandani, A. Measurements of chemical warfare agent degradation products using an electrophoresis microchip with contactless conductivity detector. Anal. Chem. 2002, 74, 6121–6125. [Google Scholar] [CrossRef]
- Wang, J.; Chen, G.; Muck, A.; Chatrathi, M.P.; Mulchandani, A.; Chen, W. Microchip enzymatic assay of organophosphate nerve agents. Anal. Chim. Acta 2004, 505, 183–187. [Google Scholar] [CrossRef]
- Wang, J. Microchip devices for detecting terrorist weapons. Anal. Chim. Acta 2004, 507, 3–10. [Google Scholar] [CrossRef]
- Wang, J.; Zima, J.; Lawrence, N.S.; Chatrathi, M.P.; Mulchandani, A.; Collins, G.E. Microchip capillary electrophoresis with electrochemical detection of thiol-containing degradation products of V-type nerve agents. Anal. Chem. 2004, 76, 4721–4726. [Google Scholar] [CrossRef]
- Heleg-Shabtai, V.; Gratziany, N.; Liron, Z. Separation and detection of VX and its methylphosphonic acid degradation products on a microchip using indirect laser-induced fluorescence. Electrophoresis 2006, 27, 1996–2001. [Google Scholar] [CrossRef]
- Tan, H.Y.; Nguyen, N.T.; Loke, W.K.; Tan, Y.T. Microfluidic chip with optical sensor for rapid detection of nerve agent Sarin in water samples. In Proceedings of the SPIE 6416, Biomedical Applications of Micro- and Nanoengineering III, 64160M, Adelaide, Australia, 14 December 2006. [Google Scholar]
- Petkovic-Duran, K.; Swallow, A.; Sexton, B.A.; Glenn, F.; Zhu, Y. Identification of chemical warfare agents using a portable microchip-based detection device. In Proceedings of the SPIE 8204, Smart Nano-Micro Materials and Devices, 82041L, Melbourne, Australia, 23 December 2011. [Google Scholar]
- Rosso, T.E.; Bossle, P.C. Capillary ion electrophoresis screening of nerve agent degradation products in environmental samples using conductivity detection. J. Chromatogr. A 1998, 824, 125–134. [Google Scholar] [CrossRef]
- Zemann, A.J.; Schnell, E.; Volgger, D.; Bonn, G.K. Contactless conductivity detection for capillary electrophoresis. Anal. Chem. 1998, 70, 563–567. [Google Scholar] [CrossRef]
- Brito-Neto, J.G.A.; da Silva, J.A.F.; Blanes, L.; do Lago, C.L. Understanding capacitively coupled contactless conductivity detection in capillary and microchip electrophoresis. Part 1. Fundamentals. Electroanalysis 2005, 17, 1198–1206. [Google Scholar] [CrossRef]
- Pumera, M. Contactless conductivity detection for microfluidics: Designs and applications. Talanta 2007, 74, 358–364. [Google Scholar] [CrossRef]
- Ding, Y.S.; Garcia, C.D.; Rogers, K.R. Poly (dimethylsiloxane) microchip electrophoresis with contactless conductivity detection for measurement of chemical warfare agent degradation products. Anal. Lett. 2008, 41, 335–350. [Google Scholar] [CrossRef]
- Nicolau Dan, V.; Metcalfe, G. Biomedical Applications of Micro-and Nanoengineering IV and Complex Systems. In Proceedings of the SPIE, Melbourne, Australia, 9–12 December 2008. [Google Scholar]
- Kuban, P.; Seiman, A.; Makarotseva, N.; Vaher, M.; Kaljurand, M. In situ determination of nerve agents in various matrices by portable capillary electropherograph with contactless conductivity detection. J. Chromatogr. A 2011, 1218, 2618–2625. [Google Scholar] [CrossRef]
- Fintschenko, Y.; Choi, W.Y.; Cummings, E.B.; Frechet, J.M.J.; Fruetel, J.A.; Hilder, E.F.; Mair, D.A.; Shepodd, T.J.; Svec, F. An answer in the palm of your hand: microfluidics for analytical applications. In Proceedings of the SPIE 4982, Microfluidics, Biomems, and Medical Microsystems, San Jose, CA, USA, 27–29 January 2003. [Google Scholar]
- Saiz, J.; Mai, T.D.; Hauser, P.C.; Garcia-Ruiz, C. Determination of nitrogen mustard degradation products in water samples using a portable capillary electrophoresis instrument. Electrophoresis 2013, 34, 2078–2084. [Google Scholar] [CrossRef]
- Da Costa, E.T.; Neves, C.A.; Hotta, G.M.; Vidal, D.T.R.; Barros, M.F.; Ayon, A.A.; Garcia, C.D.; do Lago, C.L. Unmanned platform for long-range remote analysis of volatile compounds in air samples. Electrophoresis 2012, 33, 2650–2659. [Google Scholar] [CrossRef]
- Ansari, K.; Ying, J.Y.S.; Hauser, P.C.; de Rooij, N.F.; Rodriguez, I. A portable lab-on-a-chip instrument based on MCE with dual top-bottom capacitive coupled contactless conductivity detector in replaceable cell cartridge. Electrophoresis 2013, 34, 1390–1399. [Google Scholar] [CrossRef]
- Munro, N.B.; Talmage, S.S.; Griffin, G.D.; Waters, L.C.; Watson, A.P.; King, J.F.; Hauschild, V. The sources, fate, and toxicity of chemical warfare agent degradation products. Environ. Health Perspect. 1999, 107, 933–974. [Google Scholar] [CrossRef]
- Pumera, M.; Wang, J.; Opekar, F.; Jelinek, I.; Feldman, J.; Lowe, H.; Hardt, S. Contactless conductivity detector for microchip capillary electrophoresis. Anal. Chem. 2002, 74, 1968–1971. [Google Scholar] [CrossRef]
- Wang, J.; Pumera, M. Dual conductivity/amperometric detection system for microchip capillary electrophoresis. Anal. Chem. 2002, 74, 5919–5923. [Google Scholar] [CrossRef]
- Tanyanyiwa, J.; Hauser, P.C. High-voltage capacitively coupled contactless conductivity detection for microchip capillary electrophoresis. Anal. Chem. 2002, 74, 6378–6382. [Google Scholar] [CrossRef]
- Nassar, A.E.F.; Lucas, S.V.; Jones, W.R.; Hoffland, L.D. Separation of chemical warfare agent degradation products by the reversal of electroosmotic flow in capillary electrophoresis. Anal. Chem. 1998, 70, 1085–1091. [Google Scholar] [CrossRef]
- Lucy, C.A. Factors affecting selectivity of inorganic anions in capillary electrophoresis. J. Chromatogr. A 1999, 850, 319–337. [Google Scholar] [CrossRef]
- Na, D.H.; Cho, C.K.; Youn, Y.S.; Choi, Y.; Lee, K.R.; Yoo, S.D.; Lee, K.C. Capillary electrophoresis to characterize ricin and its subunits with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Toxicon 2004, 43, 329–335. [Google Scholar] [CrossRef]
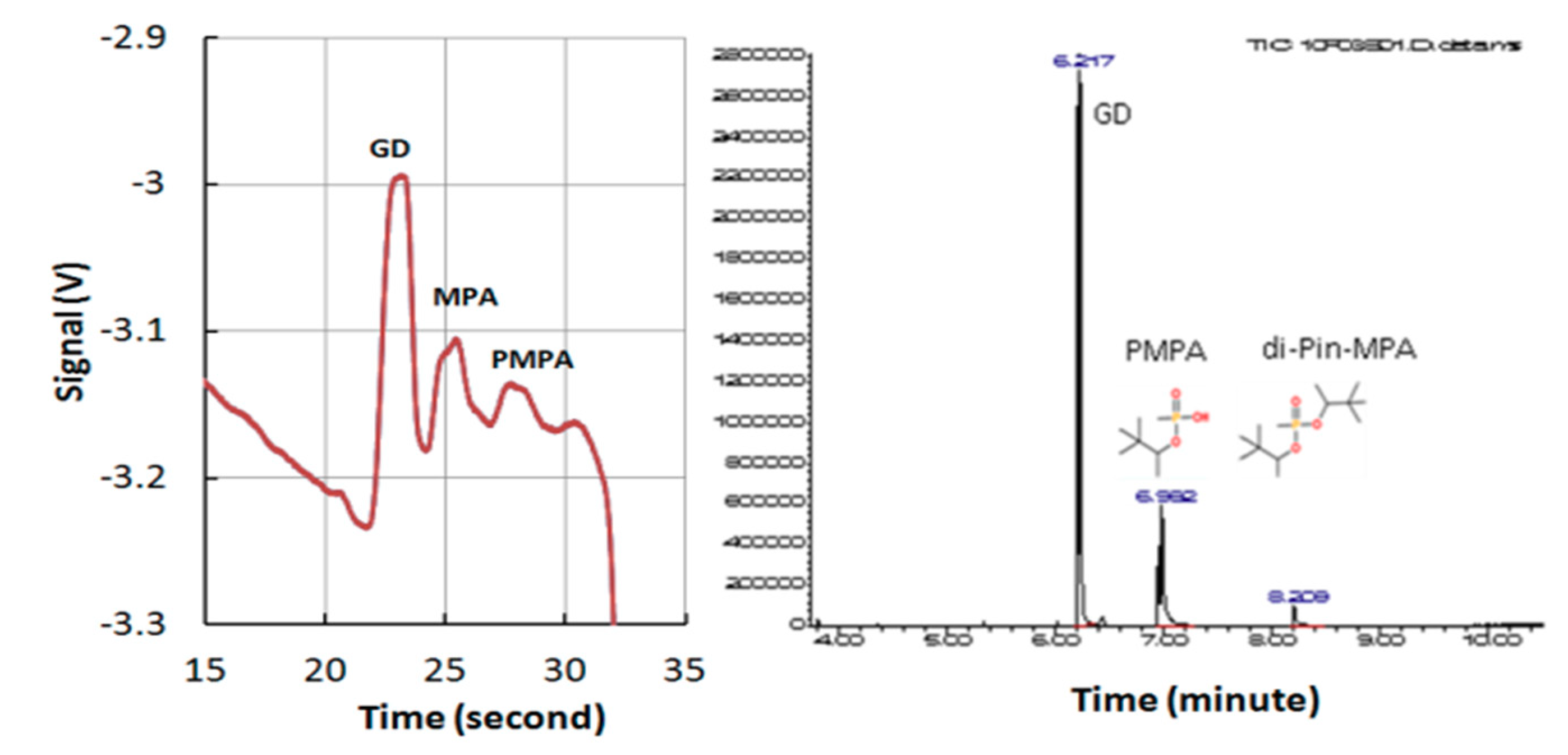
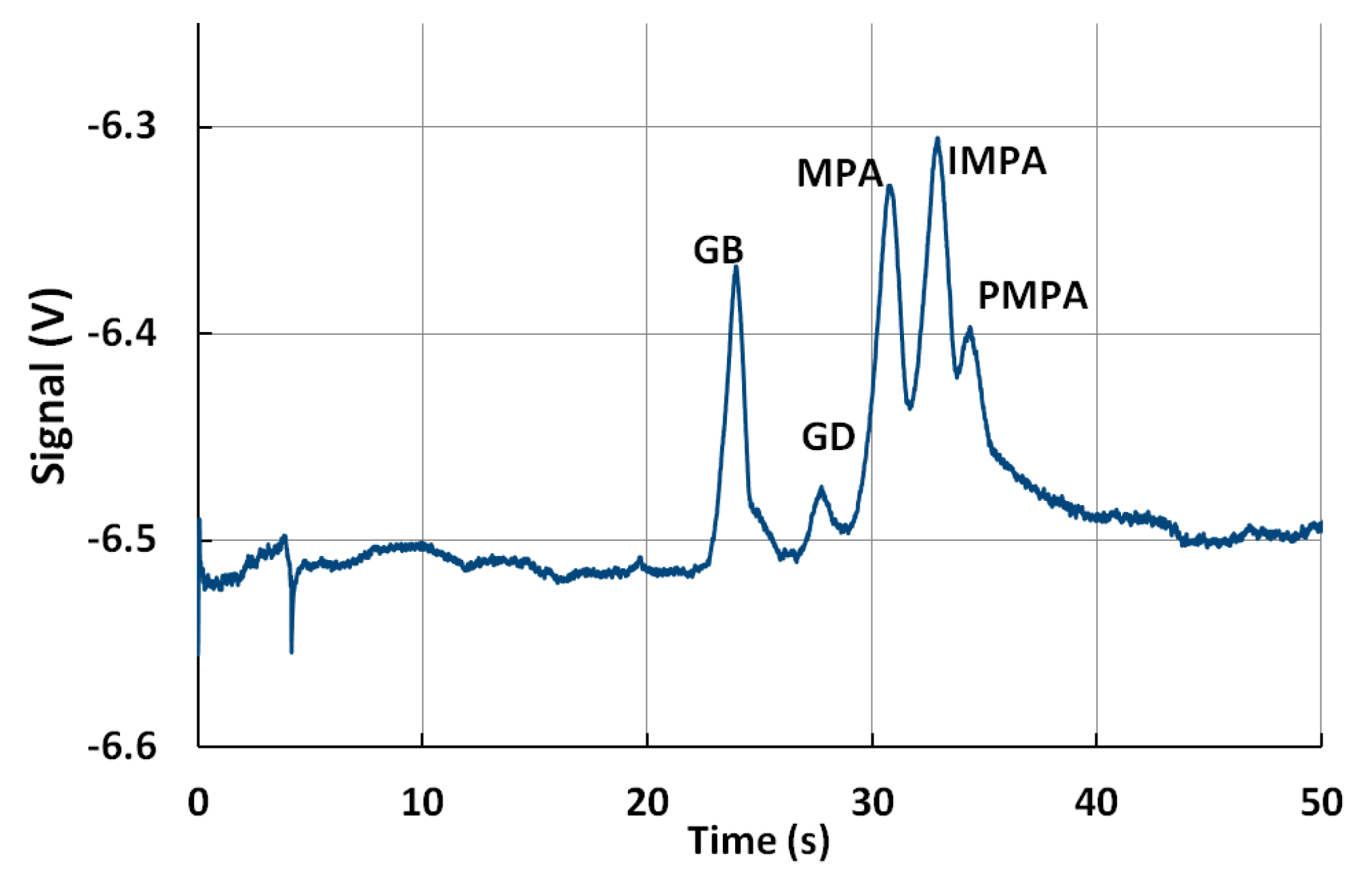
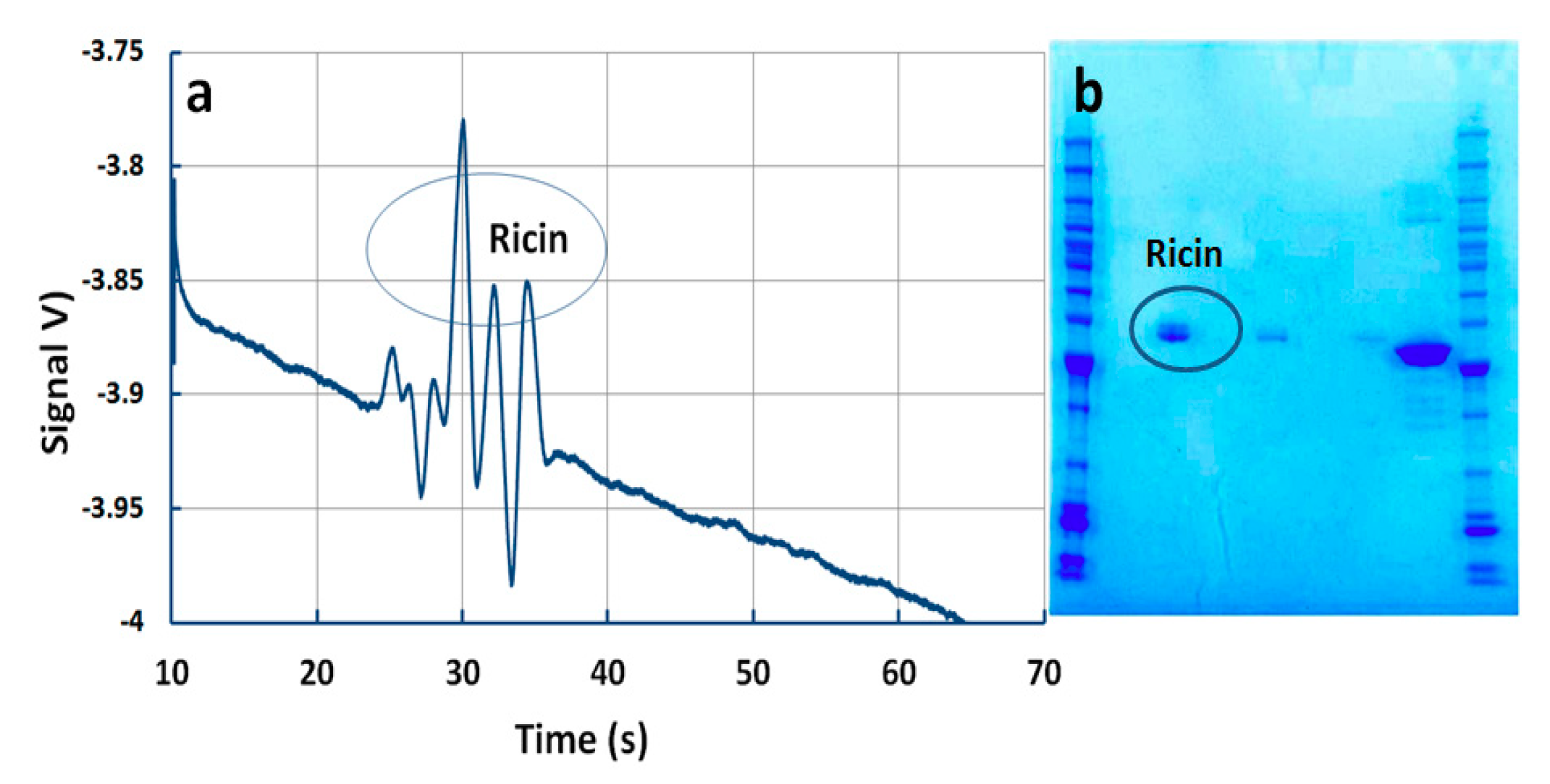
| Analyte | Median Relative Migration Time (s) | Relative Migration Time RSD % (n = 120) | Linearity Correlation | Limit of Detection (ppm) |
|---|---|---|---|---|
| MPA | 1.77 | 0.5 | 0.9898 | 1.73 |
| EMPA | 1.93 | 1.1 | 0.9775 | 3.73 |
| IMPA | 2.12 | 0.9 | 0.9775 | 3.73 |
| PMPA | 2.26 | 2.6 | 0.9924 | 4.13 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petkovic, K.; Swallow, A.; Stewart, R.; Gao, Y.; Li, S.; Glenn, F.; Gotama, J.; Dell’Olio, M.; Best, M.; Doward, J.; et al. An Integrated Portable Multiplex Microchip Device for Fingerprinting Chemical Warfare Agents. Micromachines 2019, 10, 617. https://doi.org/10.3390/mi10090617
Petkovic K, Swallow A, Stewart R, Gao Y, Li S, Glenn F, Gotama J, Dell’Olio M, Best M, Doward J, et al. An Integrated Portable Multiplex Microchip Device for Fingerprinting Chemical Warfare Agents. Micromachines. 2019; 10(9):617. https://doi.org/10.3390/mi10090617
Chicago/Turabian StylePetkovic, Karolina, Anthony Swallow, Robert Stewart, Yuan Gao, Sheng Li, Fiona Glenn, Januar Gotama, Mel Dell’Olio, Michael Best, Justin Doward, and et al. 2019. "An Integrated Portable Multiplex Microchip Device for Fingerprinting Chemical Warfare Agents" Micromachines 10, no. 9: 617. https://doi.org/10.3390/mi10090617
APA StylePetkovic, K., Swallow, A., Stewart, R., Gao, Y., Li, S., Glenn, F., Gotama, J., Dell’Olio, M., Best, M., Doward, J., Ovendon, S., & Zhu, Y. (2019). An Integrated Portable Multiplex Microchip Device for Fingerprinting Chemical Warfare Agents. Micromachines, 10(9), 617. https://doi.org/10.3390/mi10090617







