Tin Dioxide Thin Film with UV-enhanced Acetone Detection in Microwave Frequency Range
Abstract
1. Introduction
2. Materials and Methods
2.1. SnO2 Deposition Technology
2.2. Microwave Measurements
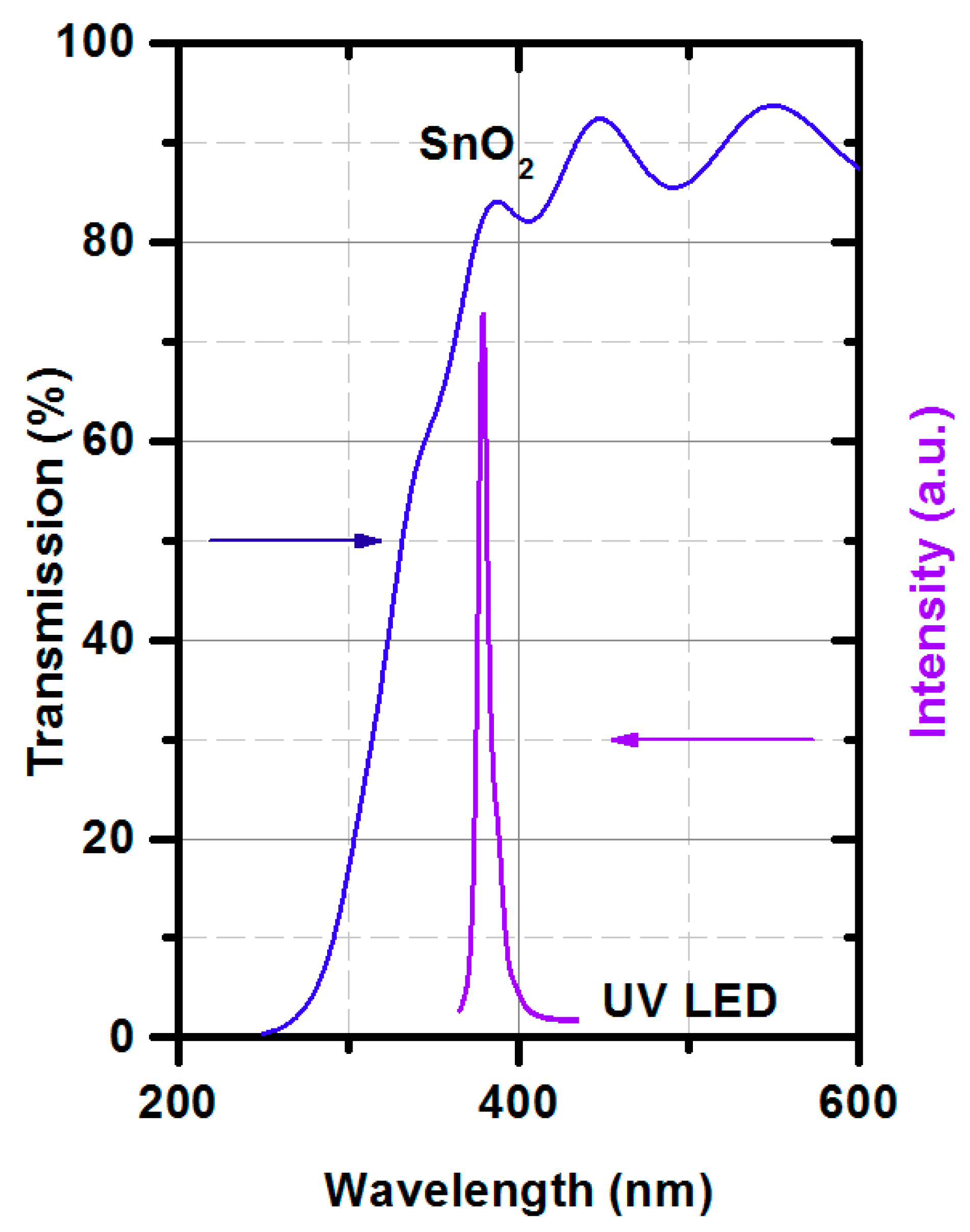
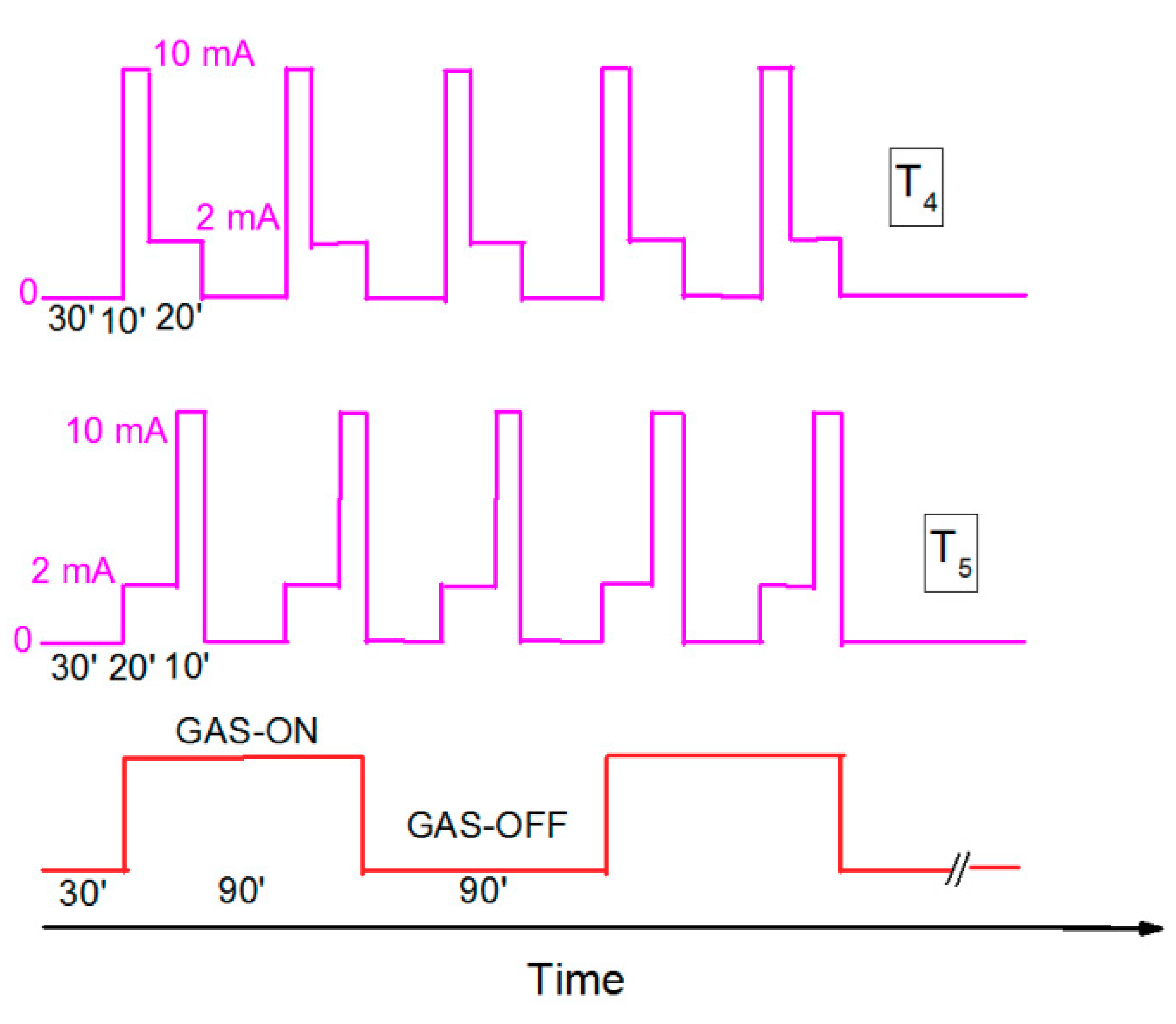
2.3. Gas-sensing Protocol with UV
3. Results and Discussion
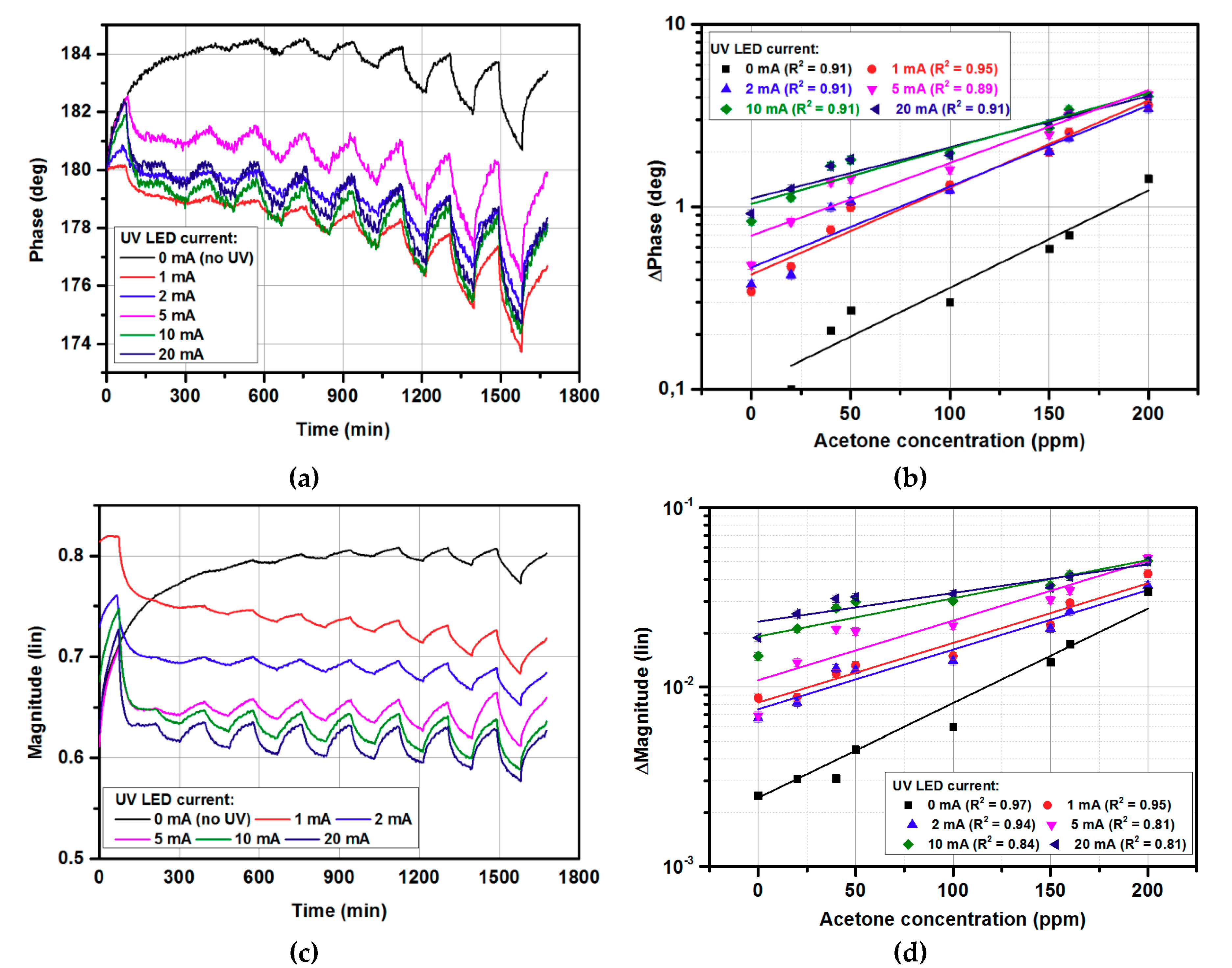
3.1. Gas-sensing Characteristics for Continouse UV Radiation
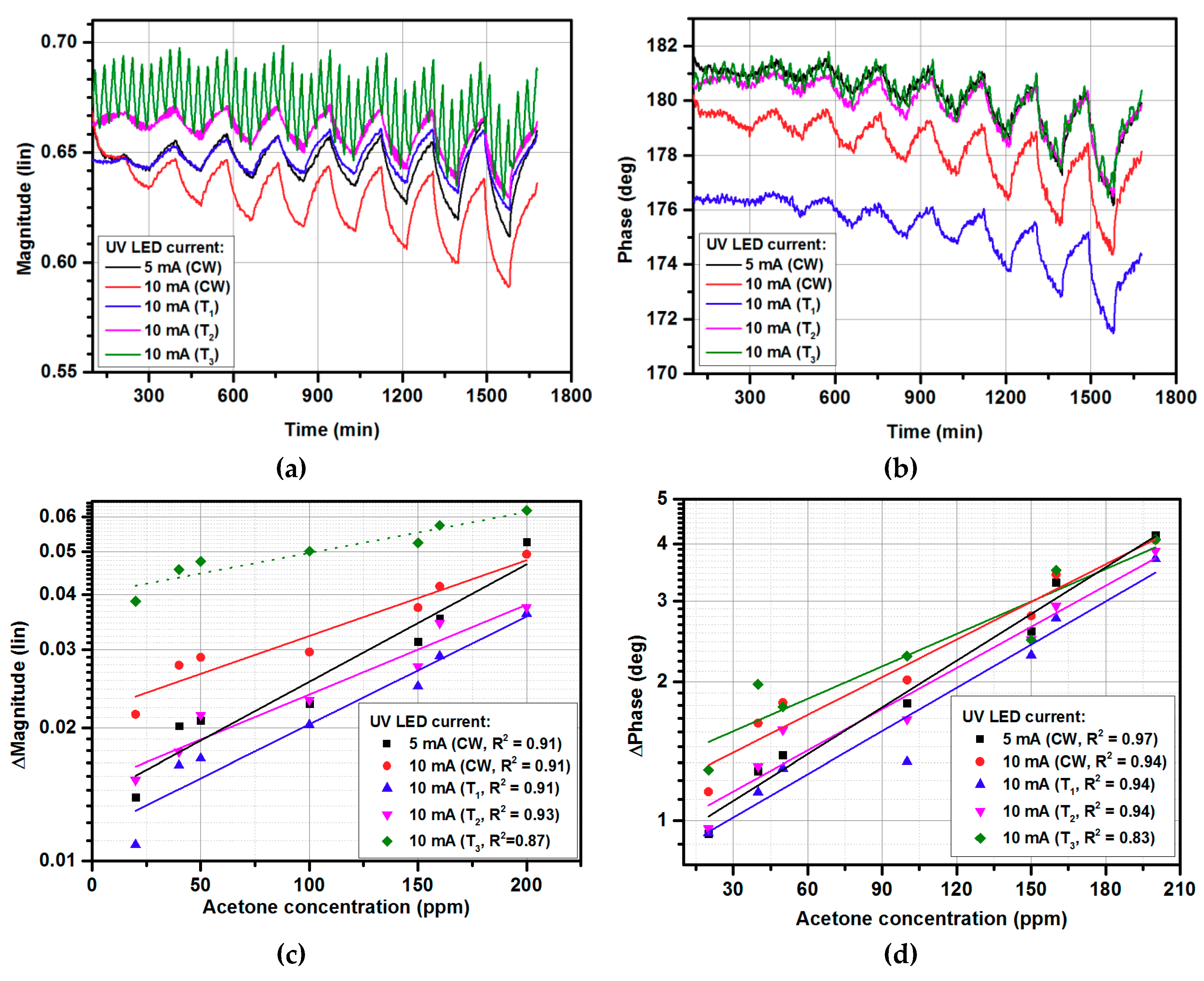
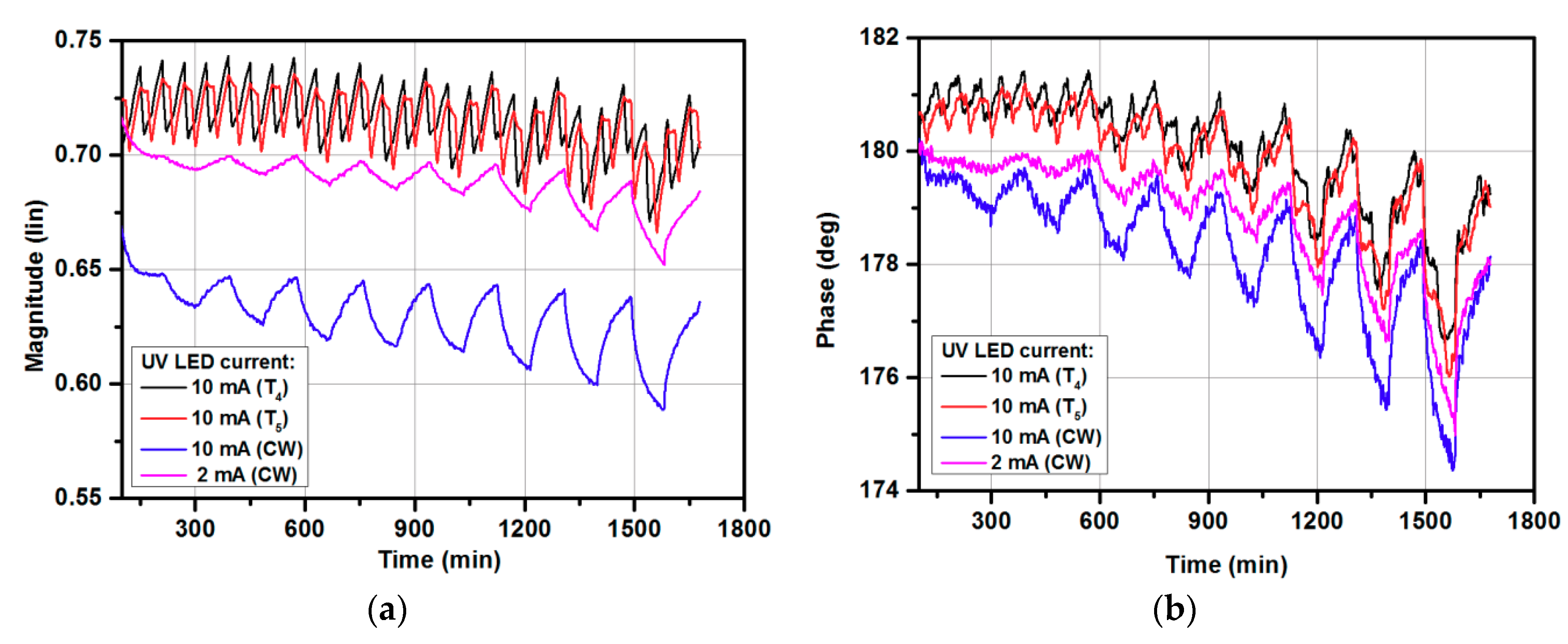
3.2. Gas-sensing Characteristics for Modulated UV Illumination
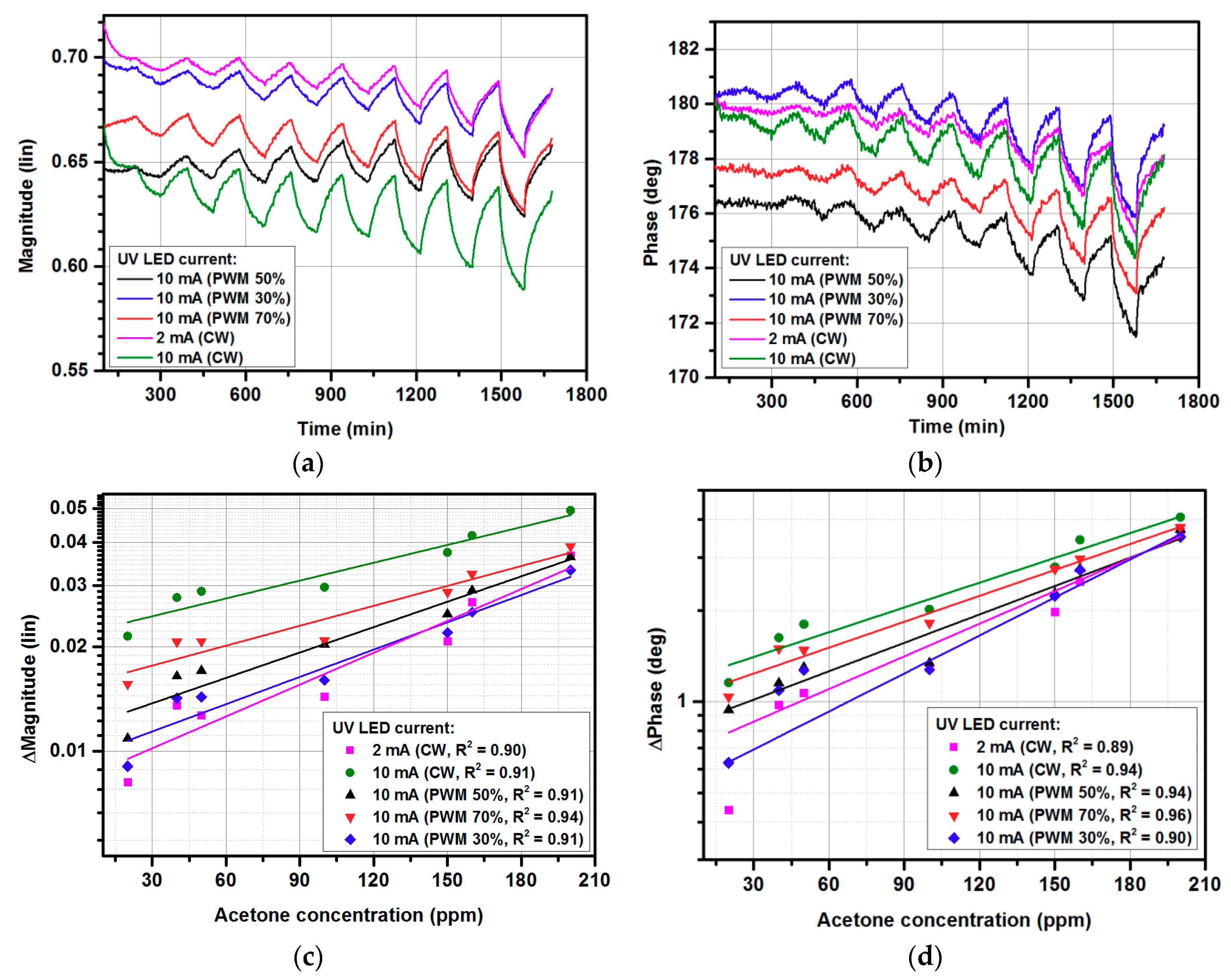
3.3. Gas-sensing Characteristics for Pulse Width Modulated (PWM) UV Illumination
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Crofford, O.B.; Mallard, R.E.; Winton, R.E.; Rogers, N.L.; Jackson, J.C.; Keller, U. Acetone in breath and blood. Trans. Am. Clin. Climatol. Assoc. 1977, 88, 128–139. [Google Scholar]
- Minh, T.D.C.; Blake, D.R.; Galassetti, P.R. The clinical potential of exhaled breath analysis for diabetes mellitus. J. Diabetes Res. 2012, 97, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Amann, A.; Smith, D. Volatile Biomarkers. Non-Invasive Diagnosis in Physiology and Medicine; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Afreen, S.; Zhu, J.-J. Rethinking EBAD: Evolution of smart noninvasive detection of diabetes. TrAC Trends Anal. Chem. 2019, 118, 477–487. [Google Scholar] [CrossRef]
- Rydosz, A. Sensors for Enhanced Detection of Acetone as a Potential Tool for Noninvasive Diabetes Monitoring. Sensors 2018, 18, 2298. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.hmdb.ca/metabolites/HMDB0001659 (accessed on 26 July 2019).
- Available online: http://www.figarosensor.com/products/822pdf.pdf (accessed on 26 July 2019).
- Li, J.; Smeeton, T.M.; Zanola, M.; Barrett, J.; Berryman-Bousquet, V. A compact breath acetone analyzer based on an ultraviolet light emitting diode. Sens. Actuators B 2018, 273, 76–82. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Mondal, S.; Bose, M.; Das, A.K.; Baneerjee, S.; Fas, N.C. Heteroatom doped photoluminescent carbon dots for sensitive detection of acetone in human fluids. Sens. Actuators B 2018, 266, 583–593. [Google Scholar] [CrossRef]
- Chen, Y.; Owyeung, R.E.; Sonkusale, S.R. Combined optical and electronic paper-nose for detection of volatile gases. Anal. Chim. Acta 2018, 1034, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Wu, D.; Wang, Y.; Ouyang, J.; Wang, J.; Liu, T.; Liang, X.; Zhang, C.; Liu, F.; Yan, X.; et al. Gas sniffer (YSZ-based electrochemical gas phase sensor) toward acetone detection. Sens. Actuators B 2019, 278, 1–7. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, K.; Sun, X.; Duan, X.; Zhang, C.; Xu, X. Electrochemical sensor to environmental pollutant of acetone based on Pd-loaded on mesoporous In2O3 architecture. Sens. Actuators B 2019, 290, 217–225. [Google Scholar] [CrossRef]
- Liu, F.; Ma, C.; Hao, X.; Yang, C.h.; Zhu, H.; Liang, X.; Sun, P.; Liu, F.; Chuai, X.; Lu, G. Highly sensitive gas sensor based on stabilized zirconia and CdMoO4 sensing electrode for detection of acetone. Sens. Actuators B 2017, 248, 9–18. [Google Scholar] [CrossRef]
- Yang, F.; Wang, F.; Guo, Z. Characteristics of binary WO3@CuO and ternary WO3@PDA@CuO based on impressive sensing acetone odor. J. Colloid Interface Sci. 2018, 524, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Righettoni, M.; Tricoli, A.; Pratsinis, S.E. Si: WO3 Sensors for highly selective detection of acetone for easy diagnosis of diabetes by breathe analysis. Anal. Chem. 2010, 82, 3581–3587. [Google Scholar] [CrossRef] [PubMed]
- Szkudlarek, A.; Kollbek, K.; Klejna, S.; Rydosz, A. Electronic sensitization of CuO thin films by Cr-doping for enhanced gas sensor response at low detection limit. Mater. Res. Express 2018, 5, 126406. [Google Scholar] [CrossRef]
- Rydosz, A.; Szkudlarek, A.; Ziabka, M.; Domanski, K.; Maziarz, W.; Pisarkiewicz, T. Performance of Si-Doped WO3 Thin Films for Acetone Sensing Prepared by Glancing Angle DC Magnetron Sputtering. IEEE Sens. J. 2016, 16, 1004–1012. [Google Scholar] [CrossRef]
- Hu, J.; Yang, J.; Wang, W.; Xue, Y.; Sun, Y.; Li PLian, K.; Zhang, W.; Chen, L.; Shi, J.; Chen, Y. Synthesis and gas sensing properties of NiO/SnO2 herarchical structures toward ppb-level acetone detection. Mater. Res. Bull. 2018, 102, 294–303. [Google Scholar] [CrossRef]
- Wang, X.-F.; Ma, W.; Jiang, F.; Cao, E.-S.; Sun, K.-M.; Cheng, L.; Song, X.-Z. Prussian Blue analogue derived prorous NiFe2O4 nanocubes for low-concentration acetone sensing at low working temperature. Chem. Eng. J. 2018, 338, 504–512. [Google Scholar] [CrossRef]
- Asgari, M.; Saboor, F.H.; Mortazavi, Y.; Khodadadi, A.K. SnO2 decorated SiO2 chemical sensors: Enhanced sensing performance toward ethanol and acetone. Mater. Sci. Semicond. Process. 2018, 68, 87–96. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, J.; Yu, X.; Zhang, W.; Zhang, X. Determination of acetone in human breath by gas chromatography-mass spectrometry and solid-phase microextraction with on-fiber derivatization. J. Chromatogr. B 2004, 810, 269–275. [Google Scholar] [CrossRef]
- Pauling, L.; Robinson, A.B.; Teranishi, R.; Cary, P. Quantitative analysis of urine vapor and breath by gas-liquid partition chromatography. Proc. Natl. Acad. Sci. USA 1971, 68, 2374–2376. [Google Scholar] [CrossRef]
- Righettoni, M.; Schmid, A.; Amann, A.; Pratsinis, S.E. Correlations between blood glucose and breath components from portable gas sensors and PTR-TOF-MS. Breath Res. 2013, 7, 037110. [Google Scholar] [CrossRef]
- Saidi, T.; Zaim, O.; Moufid, M.; Bari, N.E.; Ionescu, R.; Bouchikhi, B. Exhaled breath analysis using electronic nose and gas chromatography-mass spectrometry for non-invasive diagnosis of chronic kidney disease, diabetes mellitus and healthy subjects. Sens. Actuators B 2018, 257, 178–188. [Google Scholar] [CrossRef]
- Szymanska, E.; Tinnevelt, G.H.; Brodrick, E.; Williams, M.; Davies, A.N.; van Manen, H.-J.; Buydens, L.M.C. Increasing conclusiveness of clinical breath analysis by improved baseline correction of multi capillary column - ion mobility spectrometry (MCC-IMS) data. J. Pharm. Biomed. Anal. 2016, 127, 170175. [Google Scholar] [CrossRef] [PubMed]
- Rydosz, A.; Maciak, E.; Wincza, K.; Gruszczynski, S. Microwave-based sensors with phthalocyanine films for acetone, ethanol and methanol detection. Sens. Actuators B 2016, 237, 876–886. [Google Scholar] [CrossRef]
- Staszek, K.; Rydosz, A.; Maciak, E.; Wincza, K.; Gruszczynski, S. Six-port microwave system for volatile organic compounds detection. Sens. Actuators B 2017, 245, 882–894. [Google Scholar] [CrossRef]
- Rydosz, A. The use of copper oxide thin tilms in gas-sensing applications. Coatings 2018, 8, 425. [Google Scholar] [CrossRef]
- Rydosz, A.; Maciak, E.; Wincza, K.; Slawomir, G. Microwave-based sensors for exhaled acetone and ethanol detection. In Proceedings of the 2018 International Conference on Electromagnetics in Advanced Applications (ICEAA), Cartagena de Indias, Colombia, 10–14 September 2018. [Google Scholar] [CrossRef]
- Rydosz, A.; Brudnik, A.; Staszek, K. Metal Oxide Thin Films Prepared by Magnetron Sputtering Technology for Volatile Organic Compound Detection in the Microwave Frequency Range. Materials 2019, 12, 877. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Newport, D.; Le Calve, S. Development of a Toluene Detector Based on Deep UV Absorption Spectrophotometry Using Glass and Aluminum Capillary Tube Gas Cells with a LED Source. Micromachines 2019, 10, 193. [Google Scholar] [CrossRef] [PubMed]
- Rydosz, A.; Maziarz, W.; Pisarkiewicz, T.; Domanski, K.; Grabiec, P. A gas micropreconcentrator for low level acetone measurements. Microelectron. Reliab. 2012, 52, 2640–2646. [Google Scholar] [CrossRef]
- Rydosz, A. Micropreconcentrators in silicon-glass technology for the detection of diabetes biomarkers. J. Microelectron. Electron. Compon. Mater. 2014, 44, 126–136. [Google Scholar]
- Rydosz, A.; Maziarz, W.; Pisarkiewicz, T.; Bartch de Torres, H.; Mueller, J. Amicropreconcentrator design using low temperature cofired ceramics technology for acetone detection applications. IEEE Sens. J. 2013, 13, 1889–1896. [Google Scholar] [CrossRef]
- Bastatas, L.D.; Wagle, P.; Echeverria, E.; Austin, A.J.; McIlroy, D.N. The Effect of UV Illumination on the room temperature detection of vaporized ammonium nitrate by a ZnO coated nanospring-based sensors. Materials 2019, 12, 302. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Ho, H.P. Light-activated metal oxide gas sensors: A review. Micromachines 2017, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Staszek, K.; Szkudlarek, A.; Kawa, M.; Rydosz, A. Microwave system with sensor utilizing GO-based gas-sensitive layer and its application to acetone detection. Sens. Actuators B 2019, 297, 126699. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rydosz, A.; Staszek, K.; Brudnik, A.; Gruszczynski, S. Tin Dioxide Thin Film with UV-enhanced Acetone Detection in Microwave Frequency Range. Micromachines 2019, 10, 574. https://doi.org/10.3390/mi10090574
Rydosz A, Staszek K, Brudnik A, Gruszczynski S. Tin Dioxide Thin Film with UV-enhanced Acetone Detection in Microwave Frequency Range. Micromachines. 2019; 10(9):574. https://doi.org/10.3390/mi10090574
Chicago/Turabian StyleRydosz, Artur, Kamil Staszek, Andrzej Brudnik, and Slawomir Gruszczynski. 2019. "Tin Dioxide Thin Film with UV-enhanced Acetone Detection in Microwave Frequency Range" Micromachines 10, no. 9: 574. https://doi.org/10.3390/mi10090574
APA StyleRydosz, A., Staszek, K., Brudnik, A., & Gruszczynski, S. (2019). Tin Dioxide Thin Film with UV-enhanced Acetone Detection in Microwave Frequency Range. Micromachines, 10(9), 574. https://doi.org/10.3390/mi10090574





