Label-Free Electrochemical Detection of S. mutans Exploiting Commercially Fabricated Printed Circuit Board Sensing Electrodes
Abstract
1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Preparation of Bacterial Cultures
2.3. Thiolation of Anti-Streptococcus mutans Primary Antibody
2.4. Design of Printed Circuit Board Sensing Electrodes for S. Mutans Detection
2.5. Sensor Fabrication
2.6. Experimental Setup and Electrochemical Measurements
3. Results and Discussion
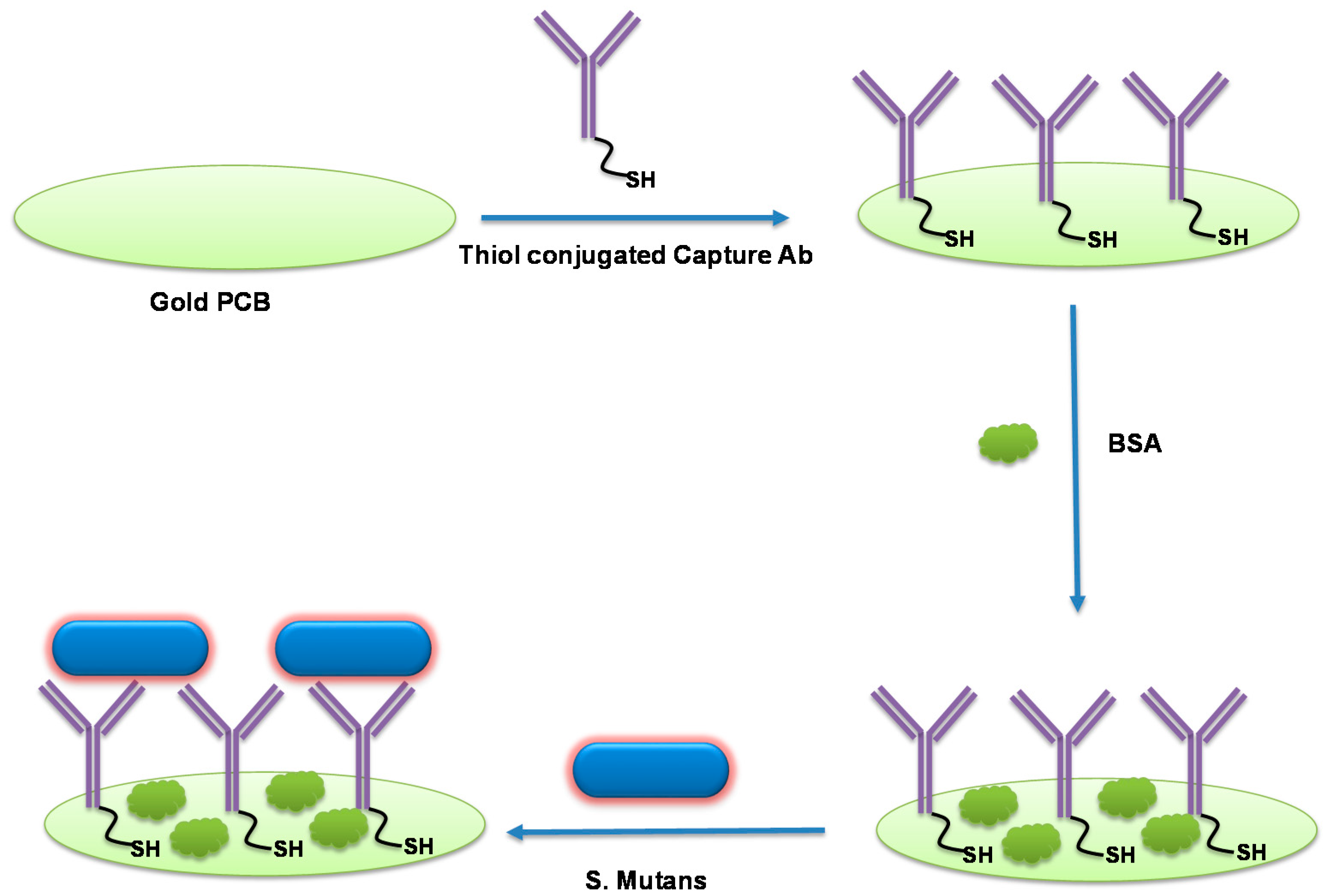
3.1. Description of Label-Free S. mutans Detection Scheme
3.2. Preparation of S. mutans Cultures for Electrochemical Detection
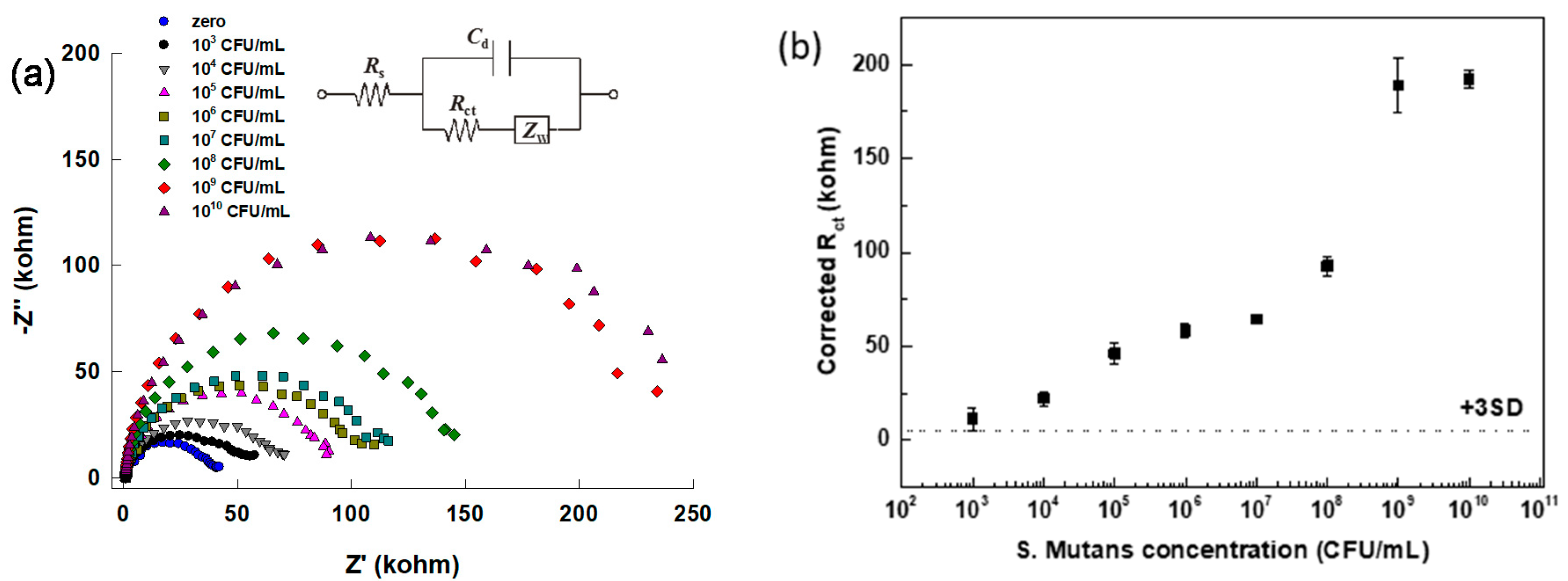
3.3. Quantification of S. mutans Detection via Impedimetric Measurement
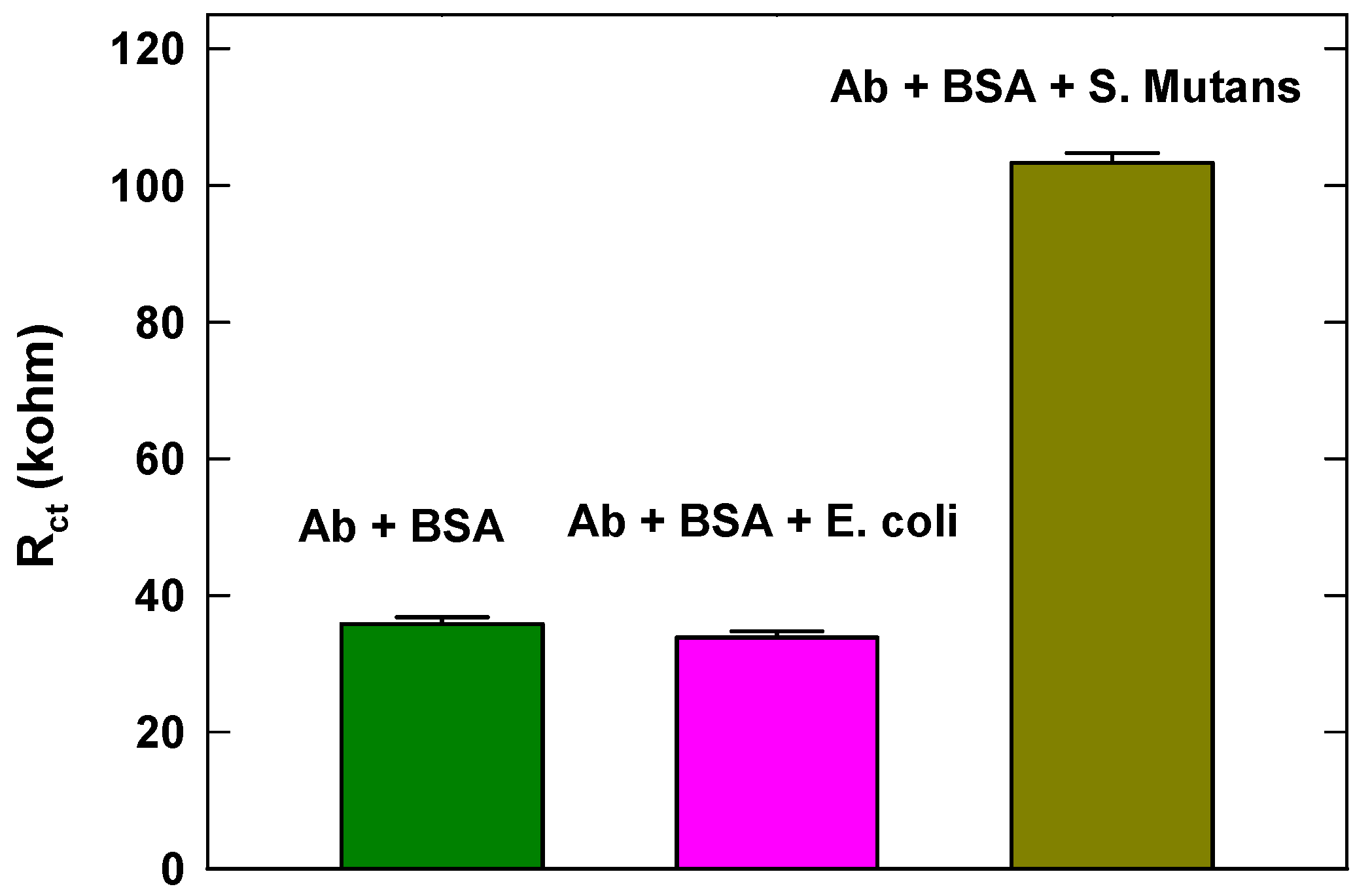
3.4. Specificity Study of the Developed Sensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abbasian, F.; Ghafar-Zadeh, E.; Magierowski, S. Microbiological Sensing Technologies: A Review. Bioengineering 2018, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Templier, V.; Livache, T.; Boisset, S.; Maurin, M.; Slimani, S.; Mathey, R.; Roupioz, Y. Biochips for Direct Detection and Identification of Bacteria in Blood Culture–Like Conditions. Sci. Rep. 2017, 7, 9457. [Google Scholar] [CrossRef] [PubMed]
- Altintas, Z.; Akgun, M.; Kokturk, G.; Uludag, Y. A fully automated microfluidic-based electrochemical sensor for real-time bacteria detection. Biosens. Bioelectron. 2018, 100, 541–548. [Google Scholar] [CrossRef]
- Moschou, D.; Tserepi, A. The lab-on-PCB approach: Tackling the μTAS commercial upscaling bottleneck. Lab Chip 2017, 17, 1388–1405. [Google Scholar] [CrossRef]
- Lebrun-Harris, L.A.; Canto, M.T.; Vodicka, P. Preventive oral health care use and oral health status among US children: 2016 National Survey of Children’s Health. J. Am. Dent. Assoc. 2019, 150, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.C.; Kawachi, I.; Souza, J.G.; Campos, F.L.; Chalub, L.L.; Antunes, J.L. Is reduced dentition with and without dental prosthesis associated with oral health-related quality of life? A cross-sectional study. Health Qual. Life Outcomes 2019, 17, 79. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Akram, M.S.; Lowe, C.R. Paper-based microfluidic point-of-care diagnostic devices. Lab Chip 2013, 13, 2210–2251. [Google Scholar] [CrossRef]
- Croser, D. Oral health waits another seven UN years. Br. Dent. J. 2018, 225, 927–929. [Google Scholar] [CrossRef]
- Gray-Burrows, K.A.; Owen, J.; Day, P.F. Learning from good practice: A review of current oral health promotion materials for parents of young children. Br. Dent. J. 2017, 222, 937–943. [Google Scholar] [CrossRef]
- Fan, C.; Wang, W.; Xu, T.; Zheng, S. Risk factors of early childhood caries among children in Beijing: A case-control study. BMC Oral Health 2016, 16, 206. [Google Scholar] [CrossRef]
- Hemadi, A.S.; Huang, R.; Zhou, Y.; Zou, J. Salivary proteins and microbiota as biomarkers for early childhood caries risk assessment. Int. J. Oral Sci. 2017, 9, e1. [Google Scholar] [CrossRef] [PubMed]
- Philip, N.; Suneja, B.; Walsh, L. Beyond Streptococcus mutans: Clinical implications of the evolving dental caries aetiological paradigms and its associated microbiome. Br. Dent. J. 2018, 224, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.-M.; Klein, C.; Schwindt, D.; Von Ohle, C. Metabolic activity of Streptococcus mutans biofilms and gene expression during exposure to xylitol and sucrose. Int. J. Oral Sci. 2014, 6, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, J.C.; Homer, K.A.; Beighton, D. Analysis of Streptococcus mutans Proteins Modulated by Culture under Acidic Conditions. Appl. Environ. Microbiol. 2002, 68, 2382–2390. [Google Scholar] [CrossRef] [PubMed]
- Oho, T.; Yamashita, Y.; Shimazaki, Y.; Kushiyama, M.; Koga, T. Simple and rapid detection of Streptococcus mutans and Streptococcus sobrinus in human saliva by polymerase chain reaction. Oral Microbiol. Immunol. 2000, 15, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Yan, Q.; Zhang, B.; Tian, X.; Wang, C.; Yu, Z.; Cui, J.; Guo, D.; Ma, X.C.; James, T.D. Ratiometric fluorescent probe for sensing Streptococcus mutans glucosyltransferase, a key factor in the formation of dental caries. Chem. Commun. 2019, 55, 3548–3551. [Google Scholar] [CrossRef] [PubMed]
- Nomura, R.; Nakano, K.; Nemoto, H.; Fujita, K.; Inagaki, S.; Takahashi, T.; Taniguchi, K.; Takeda, M.; Yoshioka, H.; Amano, A.; et al. Isolation and characterization of Streptococcus mutans in heart valve and dental plaque specimens from a patient with infective endocarditis. J. Med. Microbiol. 2006, 55, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Tam, K.; Kinsinger, N.; Ayala, P.; Qi, F.; Shi, W.; Myung, N. Real-Time Monitoring of Streptococcus mutans Biofilm Formation Using a Quartz Crystal Microbalance. Caries Res. 2007, 41, 474–483. [Google Scholar] [CrossRef]
- Kishen, A.; John, M.; Lim, C.; Asundi, A. A fiber optic biosensor (FOBS) to monitor mutans streptococci in human saliva. Biosens. Bioelectron. 2003, 18, 1371–1378. [Google Scholar] [CrossRef]
- Liu, B.H.; Li, K.L.; Kang, K.L.; Huang, W.K.; Liao, J.D. In situ biosensing of the nanomechanical property and electrochemical spectroscopy of Streptococcus mutans-containing biofilms. J. Phys. D Appl. Phys. 2013, 46, 275401. [Google Scholar] [CrossRef]
- Wang, X.; Mei, Z.; Wang, Y.; Tang, L. Gold nanorod biochip functionalization by antibody thiolation. Talanta 2015, 136, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dutta, G.; Lillehoj, P.B. Wash-free, label-free immunoassay for rapid electrochemical detection of PfHRP2 in whole blood samples. Sci. Rep. 2018, 8, 17129. [Google Scholar] [CrossRef] [PubMed]
- Jolly, P.; Rainbow, J.; Regoutz, A.; Estrela, P.; Moschou, D. A PNA-based Lab-on-PCB diagnostic platform for rapid and high sensitivity DNA quantification. Biosens. Bioelectron. 2019, 123, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Ghoreishizadeh, S.S.; Moschou, D.; McBay, D.; Gonalez-Solino, C.; Dutta, G.; Lorenzo, M.D.; Soltan, A. Towards self-powered and autonomous wearable glucose sensor. In Proceedings of the 2018 25th IEEE International Conference on Electronics, Circuits and Systems (ICECS), Bordeaux, France, 9–12 December 2018. [Google Scholar]
- Dutta, G.; Regoutz, A.; Moschou, D. Commercially Fabricated Printed Circuit Board Sensing Electrodes for Biomarker Electrochemical Detection: The Importance of Electrode Surface Characteristics in Sensor Performance. Proceedings 2018, 2, 741. [Google Scholar] [CrossRef]
- Dutta, G.; Park, S.; Singh, A.; Seo, J.; Kim, S.; Yang, H. Low-Interference Washing-Free Electrochemical Immunosensor Using Glycerol-3-phosphate Dehydrogenase as an Enzyme Label. Anal. Chem. 2015, 87, 3574–3578. [Google Scholar] [CrossRef]
- Dutta, G.; Nagarajan, S.; Lapidus, L.J.; Lillehoj, P.B. Enzyme-free electrochemical immunosensor based on methylene blue and the electro-oxidation of hydrazine on Pt nanoparticles. Biosens. Bioelectron. 2017, 92, 372–377. [Google Scholar] [CrossRef]
- Dutta, G.; Kim, S.; Park, S.; Yang, H. Washing-Free Heterogeneous Immunosensor Using Proximity-Dependent Electron Mediation between an Enzyme Label and an Electrode. Anal. Chem. 2014, 86, 4589–4595. [Google Scholar] [CrossRef]
- Dutta, G.; Lillehoj, P.B. An ultrasensitive enzyme-free electrochemical immunosensor based on redox cycling amplification using methylene blue. Analyst 2017, 142, 3492–3499. [Google Scholar] [CrossRef]
- Ibau, C.; Arshad, M.M.; Gopinath, S.C.; Nuzaihan, M.; Fathil, M.F.; Estrela, P. Gold interdigitated triple-microelectrodes for label-free prognosticative aptasensing of prostate cancer biomarker in serum. Biosens. Bioelectron. 2019, 136, 118–127. [Google Scholar] [CrossRef]
- Zia, A.I.; Syaifudin, A.M.; Mukhopadhyay, S.C.; Yu, P.L.; Al-Bahadly, I.H.; Gooneratne, C.P.; Kosel, J.; Liao, T.S. Electrochemical impedance spectroscopy based MEMS sensors for phthalates detection in water and juices. J. Phys. Conf. Ser. 2013, 439, 012026. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutta, G.; Jallow, A.A.; Paul, D.; Moschou, D. Label-Free Electrochemical Detection of S. mutans Exploiting Commercially Fabricated Printed Circuit Board Sensing Electrodes. Micromachines 2019, 10, 575. https://doi.org/10.3390/mi10090575
Dutta G, Jallow AA, Paul D, Moschou D. Label-Free Electrochemical Detection of S. mutans Exploiting Commercially Fabricated Printed Circuit Board Sensing Electrodes. Micromachines. 2019; 10(9):575. https://doi.org/10.3390/mi10090575
Chicago/Turabian StyleDutta, Gorachand, Abdoulie A. Jallow, Debjani Paul, and Despina Moschou. 2019. "Label-Free Electrochemical Detection of S. mutans Exploiting Commercially Fabricated Printed Circuit Board Sensing Electrodes" Micromachines 10, no. 9: 575. https://doi.org/10.3390/mi10090575
APA StyleDutta, G., Jallow, A. A., Paul, D., & Moschou, D. (2019). Label-Free Electrochemical Detection of S. mutans Exploiting Commercially Fabricated Printed Circuit Board Sensing Electrodes. Micromachines, 10(9), 575. https://doi.org/10.3390/mi10090575





