Rapid Prototyping of Soft Lithography Masters for Microfluidic Devices Using Dry Film Photoresist in a Non-Cleanroom Setting
Abstract
1. Introduction
2. Experimental Methods
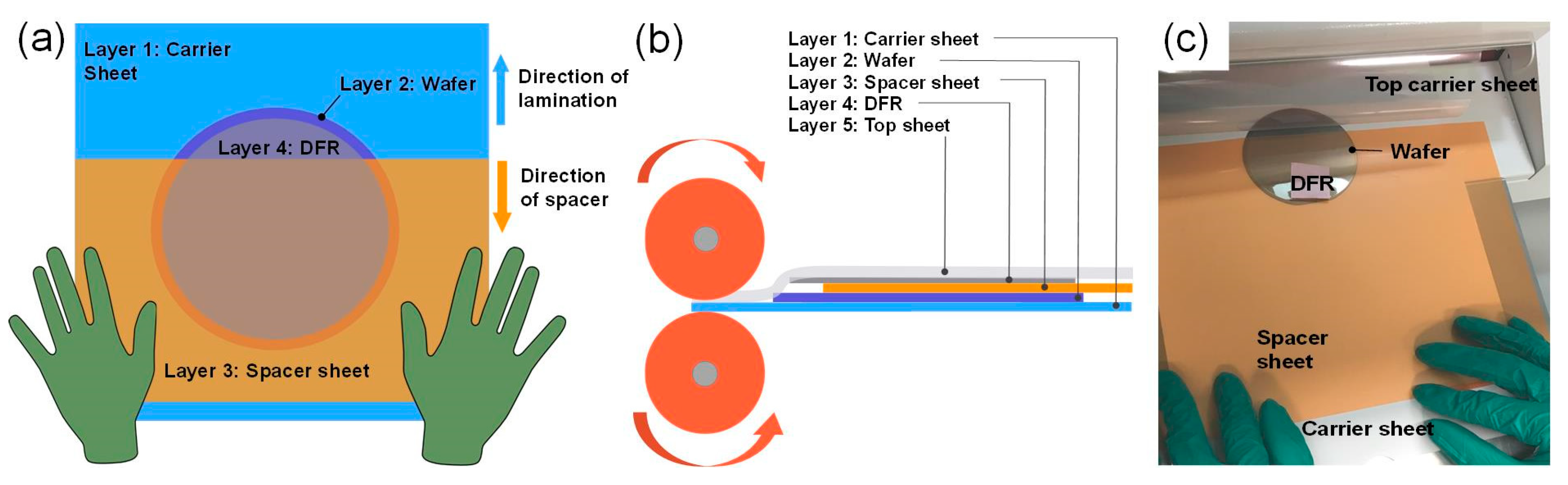
2.1. Photolithography Fabrication Process
2.2. Channel Fabrication by Soft-Lithography
2.3. Imaging and Analysis
3. Results and Discussion
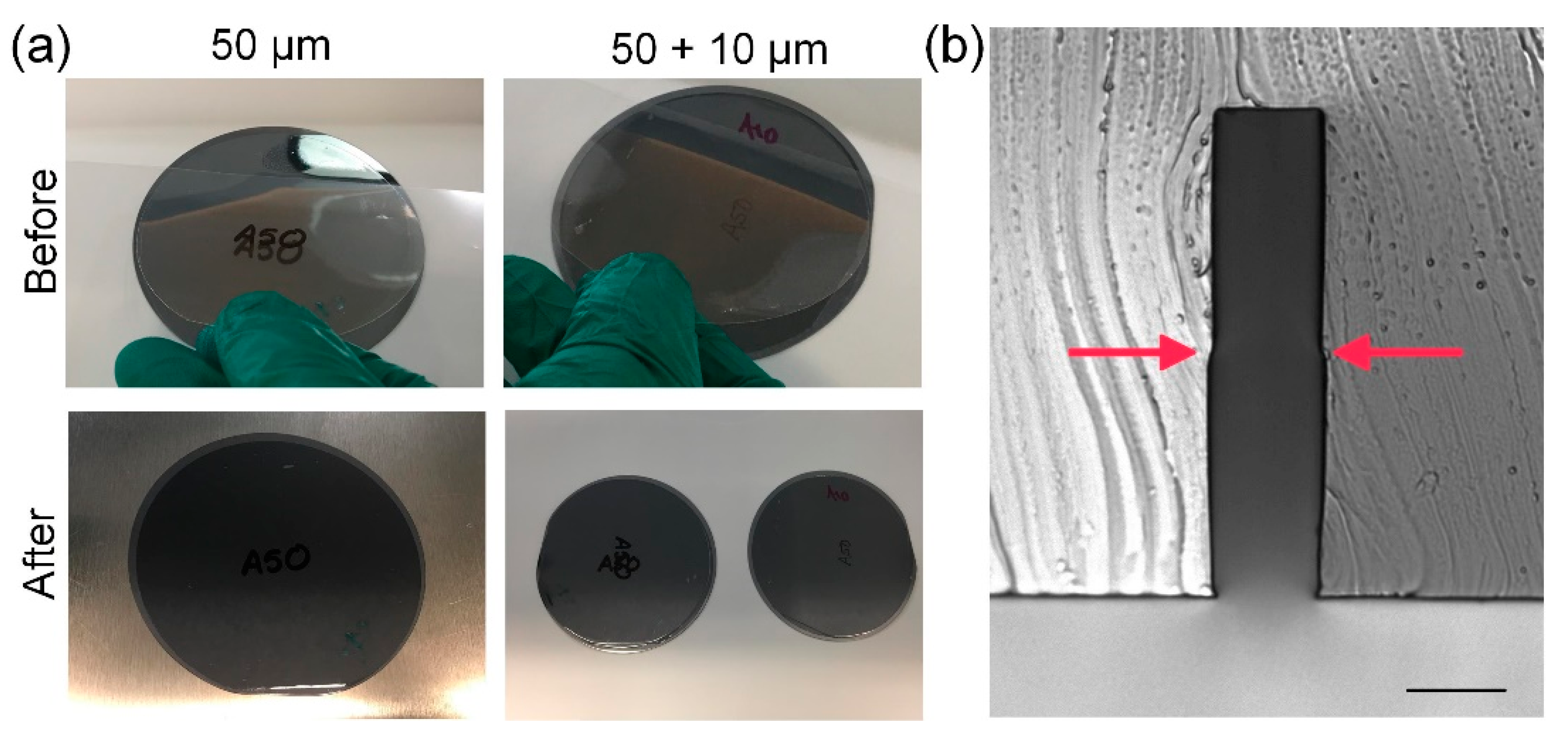
3.1. Microfluidic Fabrication in a Non-Cleanroom Setting
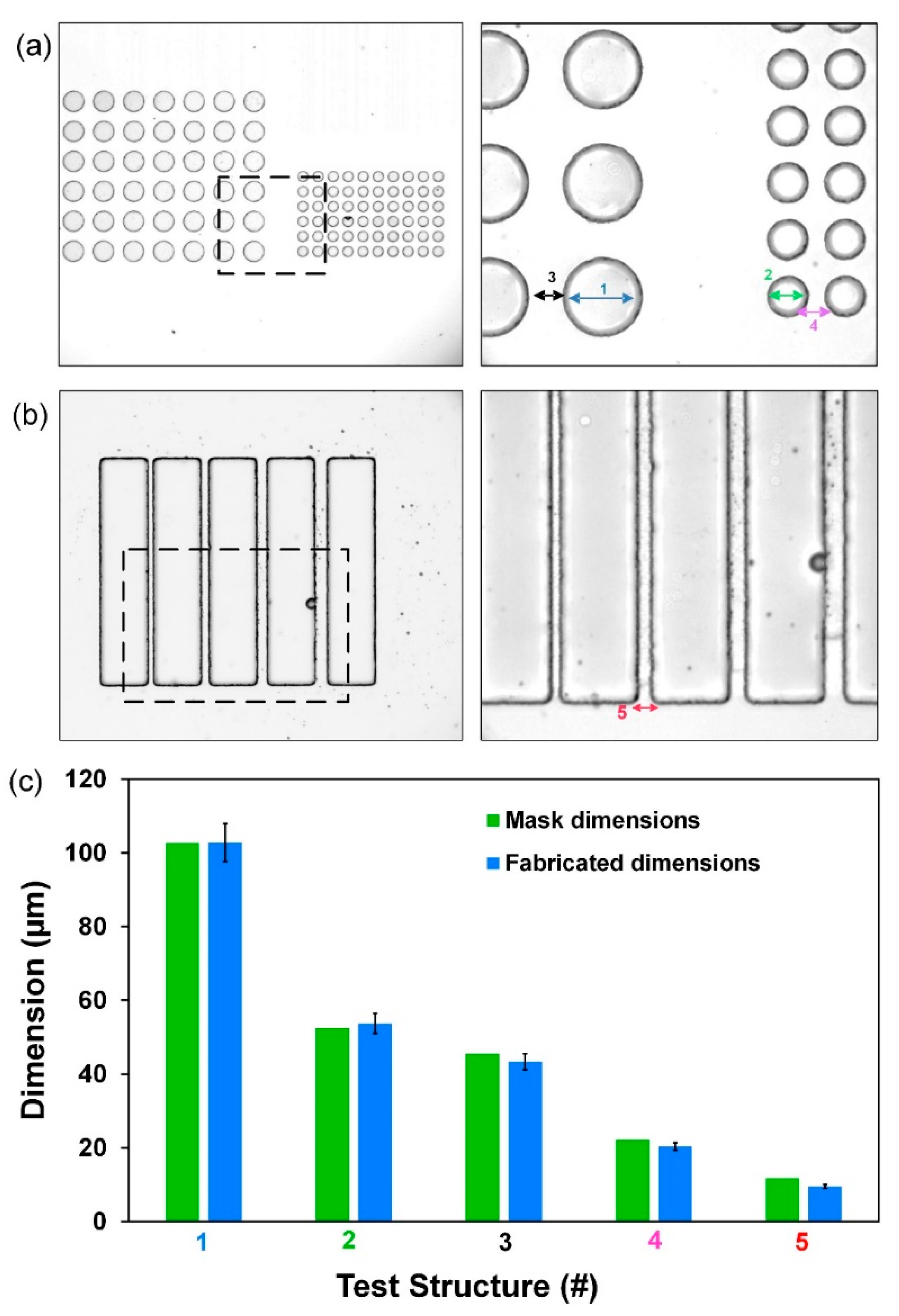
3.2. Photoresist Performance and Characterization
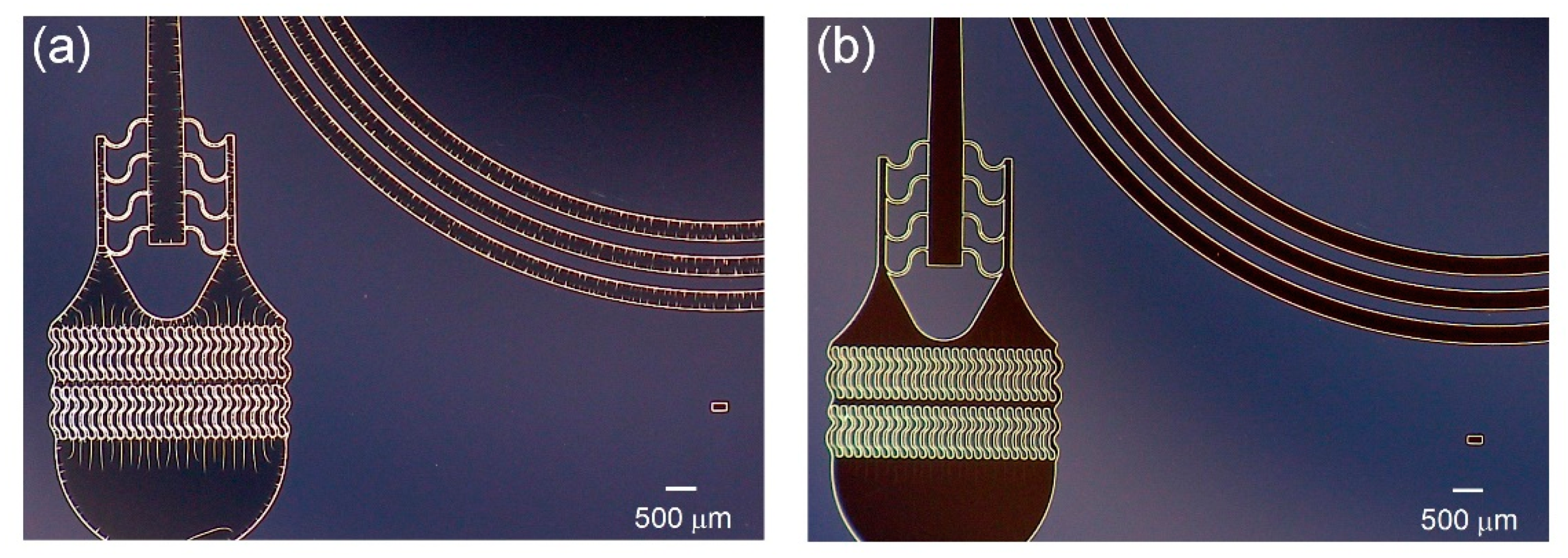
3.3. Comparison with Conventional Photoresist
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jin, J.Y.; Yoo, S.; Bae, J.S.; Kim, Y.K. Deep wet etching of borosilicate glass and fused silica with dehydrated AZ4330 and a Cr/Au mask. J. Micromech. Microeng. 2014, 24, 015003. [Google Scholar] [CrossRef]
- Iliescu, C.; Taylor, H.; Avram, M.; Miao, J.; Franssila, S. A practical guide for the fabrication of microfluidic devices using glass and silicon. Biomicrofluidics 2012, 6, 16505–16550516. [Google Scholar] [CrossRef] [PubMed]
- Guckenberger, D.J.; De Groot, T.E.; Wan, A.M.D.; Beebe, D.J.; Young, E.W.K. Micromilling: A method for ultra-rapid prototyping of plastic microfluidic devices. Lab Chip 2015, 15, 2364–2378. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Zhang, R.H.; Aue-u-lan, Y.; Chang, G.E. Micromachining microchannels on cyclic olefin copolymer (COC) substrates with the taguchi method. Micromachines 2017, 8, 264. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Pan, C.W.; Lee, W.C.; Li, K.M. An experimental study of micromilling parameters to manufacture microchannels on a PMMA substrate. Int. J. Adv. Manuf. Technol. 2014, 71, 1623–1630. [Google Scholar] [CrossRef]
- Attia, U.M.; Marson, S.; Alcock, J.R. Micro-injection moulding of polymer microfluidic devices. Microfluid. Nanofluid. 2009, 7, 1–28. [Google Scholar] [CrossRef]
- Yang, S.; Devoe, D.L. Microfluidic device fabrication by thermoplastic hot-embossing. In Microfluidic Diagnostics; Humana Press: Totowa, NJ, USA, 2013; Volume 49, pp. 115–123. [Google Scholar]
- Darvishi, S.; Cubaud, T.; Longtin, J.P. Ultrafast laser machining of tapered microchannels in glass and PDMS. Opt. Lasers Eng. 2012, 210–214. [Google Scholar] [CrossRef]
- Bishop, G.W.; Satterwhite-warden, J.E.; Kadimisetty, K.; Piqué, A.; Auyeung, R.C.Y.; Kim, H.; Amin, R.; Knowlton, S.; Hart, A.; Yenilmez, B. 3D-printed bioanalytical devices. Nanotechnology 2016, 8, 022001. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Bickham, B.P.; Woolley, A.T.; Nordin, G.P. Custom 3D printer and resin for 18 μm × 20 μm microfluidic flow channels. Lab Chip 2017, 17, 2899–2909. [Google Scholar] [CrossRef] [PubMed]
- Browne, W.; Rust, M.J.; Jung, W.; Lee, S.H.; Ahn, C.H. A rapid prototyping method for polymer microfluidics with fixed aspect ratio and 3D tapered channels. Lab Chip 2009, 9, 2941. [Google Scholar] [CrossRef] [PubMed]
- Sia, S.K.; Whitesides, G.M. Microfluidic devices fabricated in poly(dimethylsiloxane) for biological studies. Electrophoresis 2003, 24, 3563–3576. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, K.; Ahn, B.; Xu, J.; Xu, L.; Oh, K.W. A new fabrication process for uniform SU-8 thick photoresist structures by simultaneously removing edge bead and air bubbles. J. Micromech. Microeng. 2011, 21, 125006. [Google Scholar] [CrossRef]
- Amato, L.; Keller, S.S.; Heiskanen, A.; Dimaki, M.; Emnéus, J.; Boisen, A.; Tenje, M. Fabrication of high-aspect ratio SU-8 micropillar arrays. Microelectron. Eng. 2012, 98, 483–487. [Google Scholar] [CrossRef]
- Tao, S.L.; Popat, K.; Desai, T.A. Off-wafer fabrication and surface modification of asymmetric 3D SU-8 microparticles. Nat. Protoc. 2007, 6, 3153–3158. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.; Nawrocki, D.; Hurditch, R.; Johnson, D. Improving the process capability of SU-8. Microsyst. Technol. 2003, 10, 1–6. [Google Scholar] [CrossRef]
- Hsieh, Y.Z.; Tsai, Y.C.; Yang, S.J.; Lee, H.T.; Jen, H.P. Fabrication of a flexible and disposable microreactor using a dry film photoresist. J. Chinese Chem. Soc. 2006, 53, 683–688. [Google Scholar]
- Vulto, P.; Glade, N.; Altomare, L.; Bablet, J.; Del Tin, L.; Medoro, G.; Chartier, I.; Manaresi, N.; Tartagni, M.; Guerrieri, R. Microfluidic channel fabrication in dry film resist for production and prototyping of hybrid chips. Lab Chip 2005, 5, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, P.; Luo, Z.; Ren, Y.; Liao, M.; Feng, L.; Li, Y.; He, L.J. Rapid fabrication of microfluidic chips based on the simplest LED lithography. Micromech. Microeng. 2015, 25, 055020. [Google Scholar] [CrossRef]
- McDonald, J.C.; Whitesides, G.M. Poly(dimethylsiloxane) as a material for fabricating microfluidic devices. Acc. Chem. Res. 2002, 35, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Kulasinghe, A.; Bogseth, A.; O’Byrne, K.; Punyadeera, C.; Papautsky, I. Isolation of circulating tumor cells in non-small-cell-lung-cancer patients using a multi-flow microfluidic channel. Microsyst. Nanoeng. 2019, 5, 8. [Google Scholar] [CrossRef]
- Kulasinghe, A.; Zhou, J.; Kenny, L.; Papautsky, I.; Punyadeera, C. Capture of circulating tumour cell clusters using straight microfluidic chips. Cancers 2019, 11, 89. [Google Scholar] [CrossRef] [PubMed]
- Gervais, T.; El-Ali, J.F.; Gunther, A.; Jensen, K.F. Flow-induced deformation of shallow microfluidic channels. Lab Chip 2006, 6, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Papautsky, I. Size-based microfluidic multimodal microparticle sorter. Lab Chip 2015, 15, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Karabacak, N.M.; Spuhler, P.S.; Fachin, F.; Lim, E.J.; Pai, V.; Ozkumur, E.; Martel, J.M.; Kojic, N.; Smith, K.; Chen, P.I.; et al. Microfluidic, marker-free isolation of circulating tumor cells from blood samples. Nat. Protoc. 2014, 9, 694–710. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.A.; Bright, L.K.; Aspinwall, C.A. Photolithographic fabrication of microapertures with well-defined, three-dimensional geometries for suspended lipid membrane studies. Anal. Chem. 2013, 85, 9078–9086. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Major, G.H.; Lunt, B.M.; Kaykhaii, M.; Linford, M.R. Polyallylamine as an Adhesion Promoter for SU-8 Photoresist. Microsc. Microanal. 2016, 22, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Nemani, K.V.; Moodie, K.L.; Brennick, J.B.; Su, A.; Gimi, B. In vitro and in vivo evaluation of SU-8 biocompatibility. Mater. Sci. Eng. C 2013, 33, 4453–4459. [Google Scholar] [CrossRef] [PubMed]
- Walther, F.; Davydovskaya, P.; Zürcher, S.; Kaiser, M.; Herberg, H.; Gigler, A.M.; Stark, R.W. Stability of the hydrophilic behavior of oxygen plasma activated SU-8. J. Micromech. Microeng. 2007, 17, 524–531. [Google Scholar] [CrossRef]
- Rasmussen, K.H.; Keller, S.S.; Jensen, F.; Jorgensen, A.M.; Hansen, O. SU-8 etching in inductively coupled oxygen plasma. Microelectron. Eng. 2013, 112, 35–40. [Google Scholar] [CrossRef]
- Cooper, R.; Harrison, A. The exposure to and health effects of antimony. Indian J. Occup. Environ. Med. 2009, 13, 3–10. [Google Scholar] [CrossRef] [PubMed]
- DJ Microlaminates. Available online: https://djmicrolaminates.com/ (accessed on 4 Feburary 2019).
- Mata, A.; Fleischman, A.J.; Roy, S.J. Fabrication of multi-layer SU-8 Microstructures. Micromech. Microeng. 2005, 16, 276–284. [Google Scholar] [CrossRef]


| Thickness (μm) | Soft Bake at 65 °C (min) | PEB at 95 °C (min) | Exposure Energy (mJ/cm2) | Exposure Time (s) | Development Time (min) |
|---|---|---|---|---|---|
| 10 | 2 to 3 | 3 to 5 | 169 | 14 | 4.5 |
| 20 | 2 to 3 | 3 to 5 | 223 | 21 | 5 |
| 25 | 3 to 5 | 3 to 5 | 255 | 24 | 6 |
| 50 | 5 to 10 | 5 to 10 | 350 | 33 | 20 |
| 75 | 5 to 10 | 5 to 10 | 425 | 40 | 25 |
| 100 | 10 | 10–12 | 650 | 60 | 35 |
| Key Features for Master Fabrication | ADEX Process | SU-8 Process |
|---|---|---|
| Processing in a non-cleanroom environment | Yes | No |
| Good adhesion to silicon wafer | Yes | Yes |
| No need for descumming plasma treatment | Yes | No * |
| Uniform thickness without edge bead | Yes | No |
| Flexibility of height | Low | High |
| Uniformity over entire wafer | High | Low |
| Process cleanliness | Yes | No ** |
| Need to siliconize wafer post development ** | No | Yes *** |
| Low-cost setup | Yes | No |
| Level of expertise needed for rapid prototyping | Low | High |
| Developer and film toxicity | Low | High |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukherjee, P.; Nebuloni, F.; Gao, H.; Zhou, J.; Papautsky, I. Rapid Prototyping of Soft Lithography Masters for Microfluidic Devices Using Dry Film Photoresist in a Non-Cleanroom Setting. Micromachines 2019, 10, 192. https://doi.org/10.3390/mi10030192
Mukherjee P, Nebuloni F, Gao H, Zhou J, Papautsky I. Rapid Prototyping of Soft Lithography Masters for Microfluidic Devices Using Dry Film Photoresist in a Non-Cleanroom Setting. Micromachines. 2019; 10(3):192. https://doi.org/10.3390/mi10030192
Chicago/Turabian StyleMukherjee, Prithviraj, Federico Nebuloni, Hua Gao, Jian Zhou, and Ian Papautsky. 2019. "Rapid Prototyping of Soft Lithography Masters for Microfluidic Devices Using Dry Film Photoresist in a Non-Cleanroom Setting" Micromachines 10, no. 3: 192. https://doi.org/10.3390/mi10030192
APA StyleMukherjee, P., Nebuloni, F., Gao, H., Zhou, J., & Papautsky, I. (2019). Rapid Prototyping of Soft Lithography Masters for Microfluidic Devices Using Dry Film Photoresist in a Non-Cleanroom Setting. Micromachines, 10(3), 192. https://doi.org/10.3390/mi10030192






