Wire Electrodes Embedded in Artificial Conduit for Long-term Monitoring of the Peripheral Nerve Signal
Abstract
1. Introduction
2. Materials and Methods
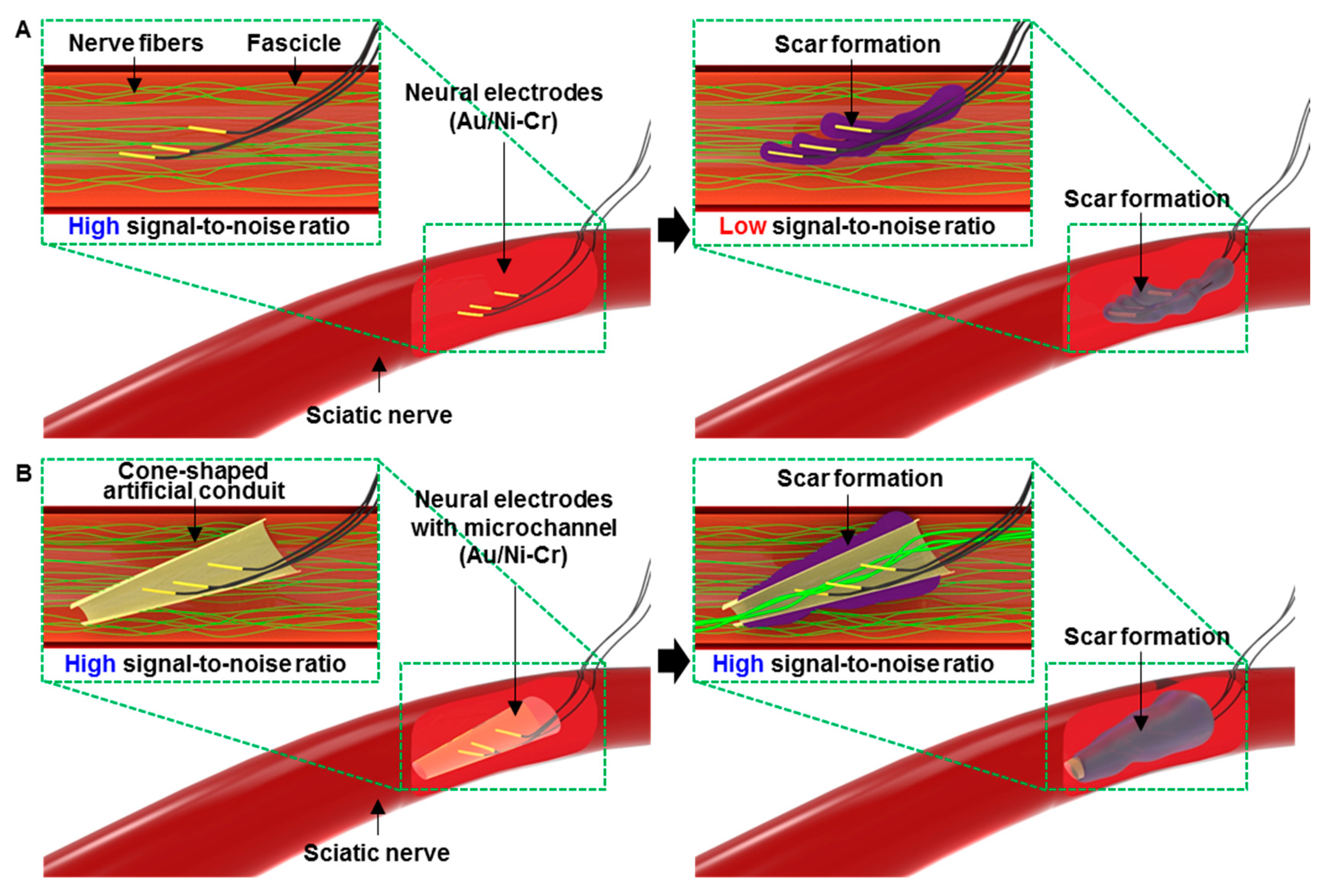
2.1. Overall Design of the Wire Electrodes with an Artificial Conduit
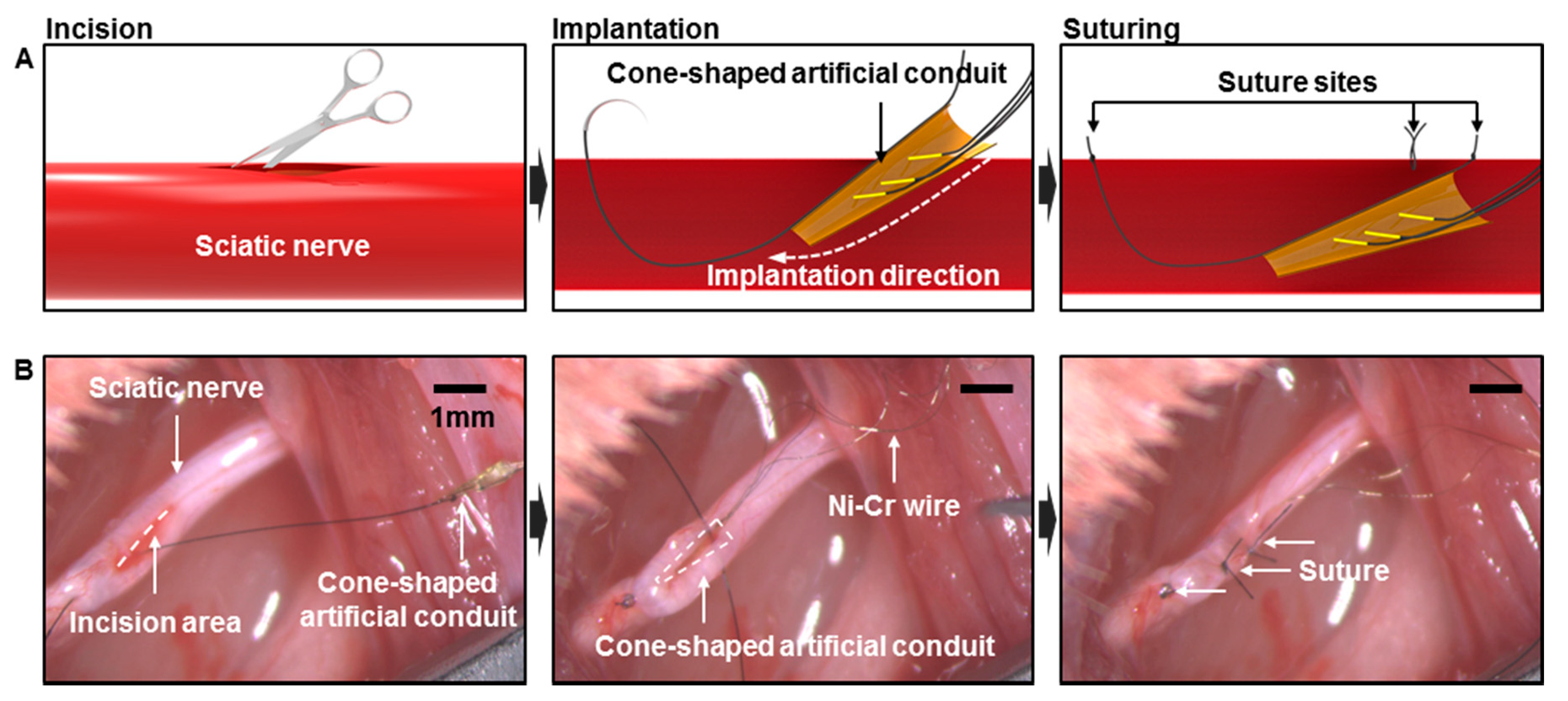
2.2. Fabrication of Wire Electrodes with Artificial Conduit
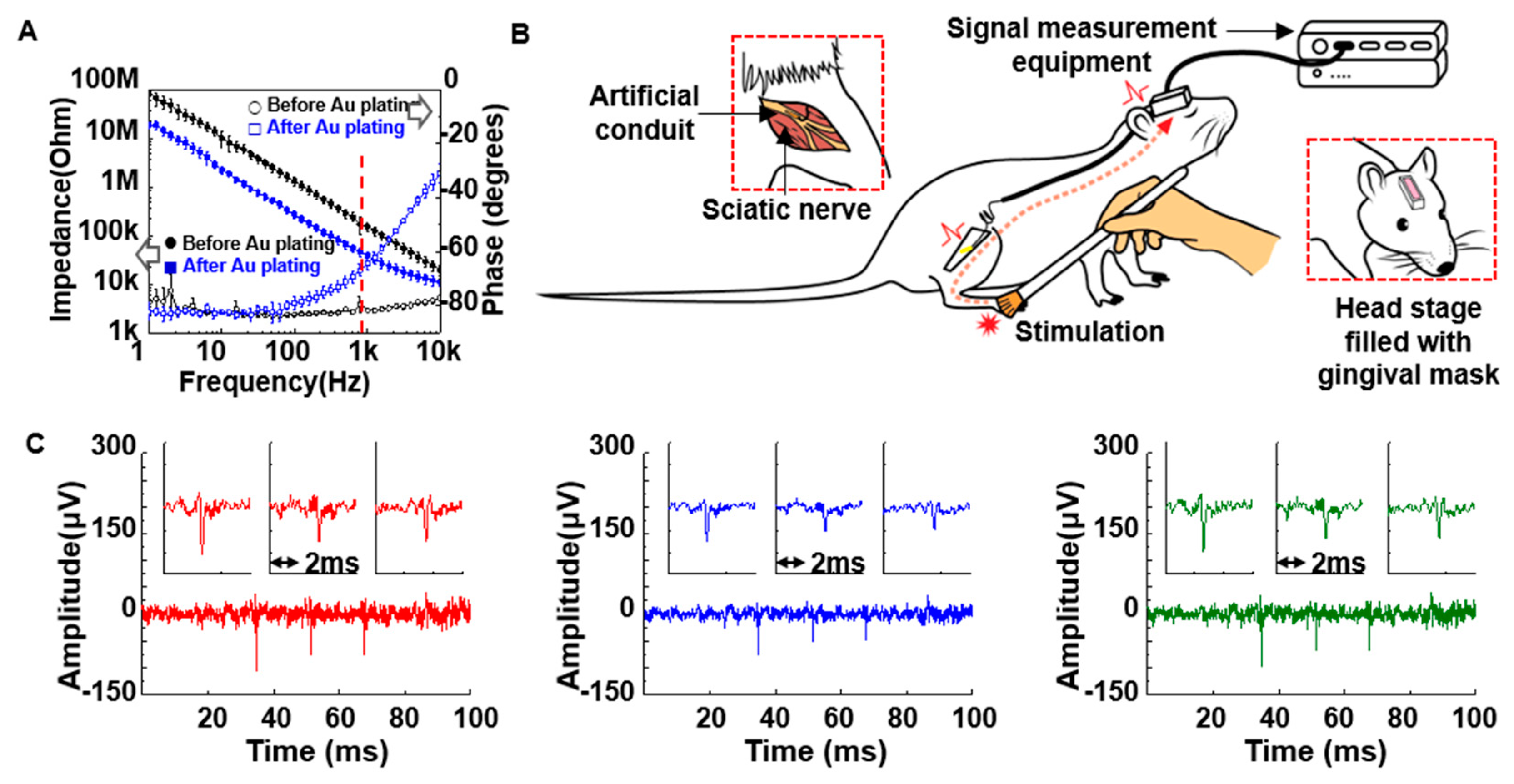
2.3. Electrical Characterizations
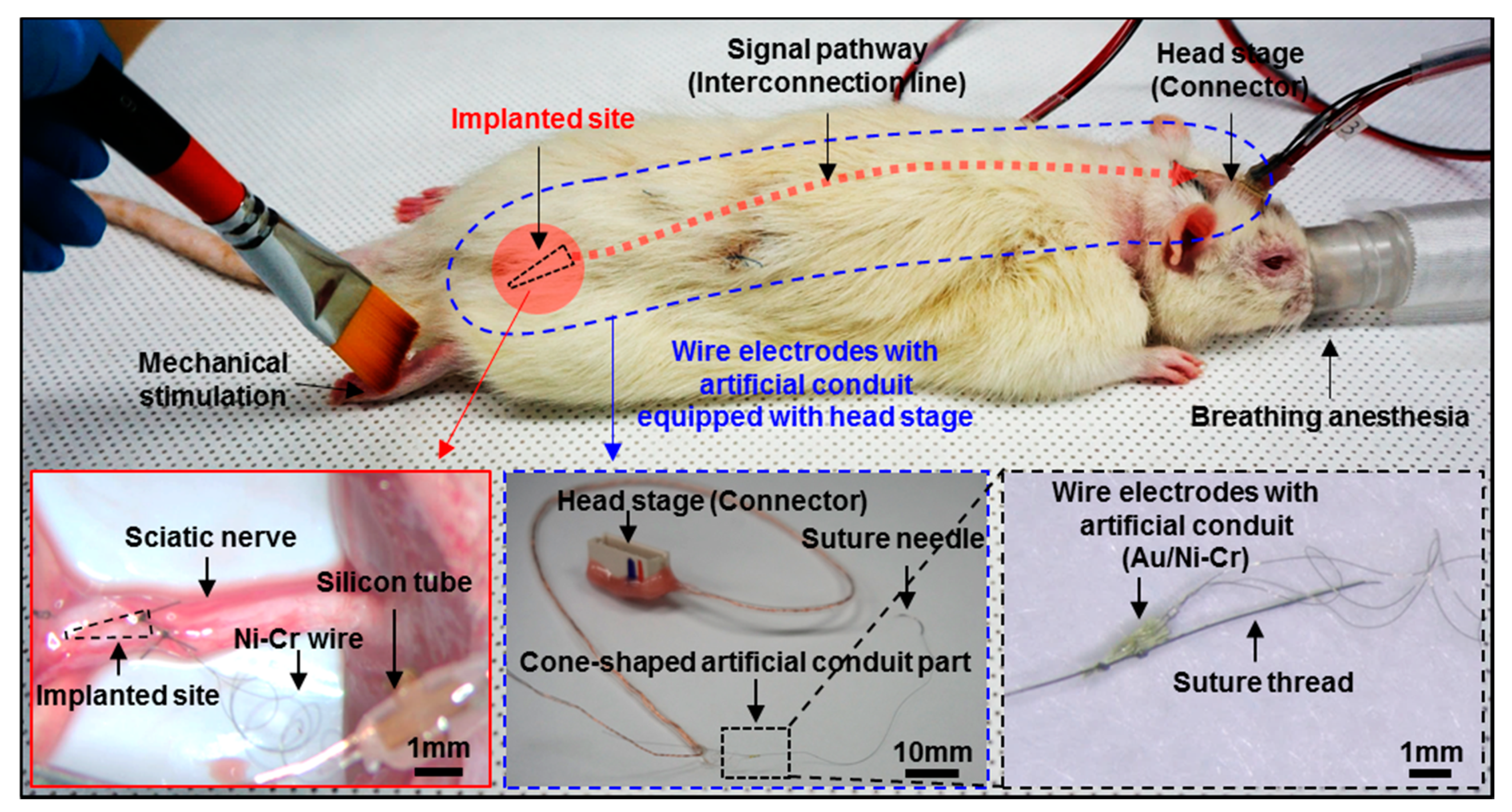
2.4. In vivo Implantation
2.5. Measurement Set-up
2.6. Immunohistochemistry
3. Results and Discussion
3.1. Structural Design and Intrinsic Properties of the Wire Electrodes with the Artificial Conduit
3.2. In vivo Preparation and Long-Term Measurement for Peripheral Neural Signals
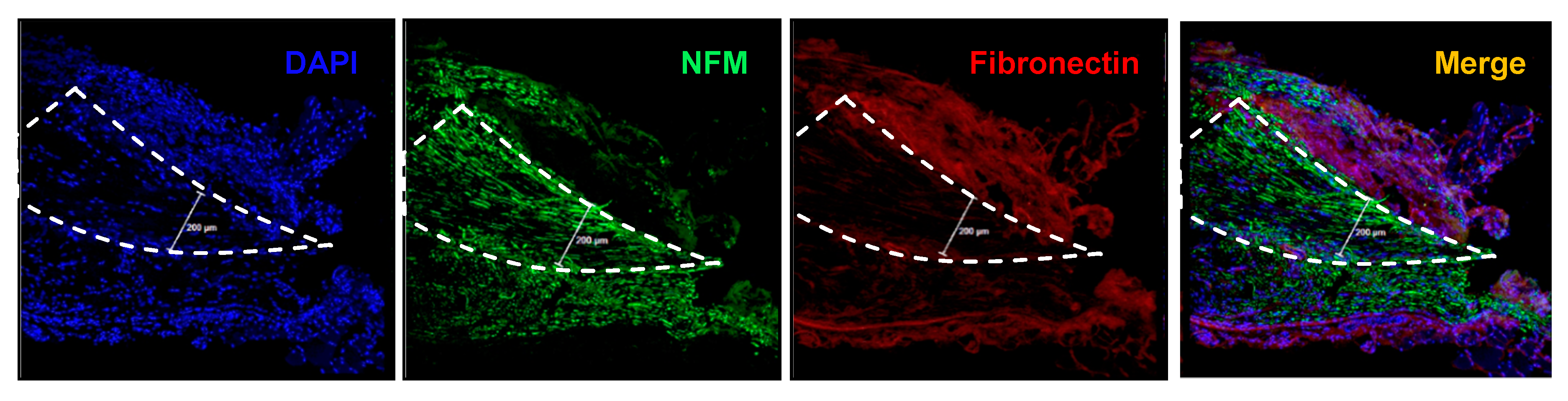
3.3. Immunohistochemistry
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Navarro, X.; Krueger, T.B.; Lago, N.; Micera, S.; Stieglitz, T.; Dario, P. A Critical review of interfaces with the peripheral nervous system for the control of neuroprostheses and hybrid bionic systems. J. Peripher. Nerv. Syst. 2005, 10, 229–258. [Google Scholar] [CrossRef] [PubMed]
- Sebelius, F.C.; Rosen, B.N.; Lundborg, G.N. Refined myoelectric control in below-elbow amputees using artificial neural networks and a data glove. J. Hand Surg. Am. 2005, 30, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Lowery, M.M.; Englehart, K.B.; Huang, H.; Li, G.; Hargrove, L.; Dewald, J.P.; Kuiken, T.A. Decoding a new neural machine interface for control of artificial limbs. J. Neurophysiol. 2007, 98, 2974–2982. [Google Scholar] [CrossRef] [PubMed]
- Lago, N.; Ceballos, D.; Rodriguez, F.J.; Stieglitz, T.; Navarro, X. Long term assessment of axonal regeneration through polyimide regenerative electrodes to interface the peripheral nerve. Biomaterials 2005, 26, 2021–2031. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, A.; Schuettler, M.; Lago, N.; Doerge, T.; Koch, K.P.; Navarro, X.; Hoffmann, K.P.; Stieglitz, T. Design, in vitro and in vivo assessment of a multi-channel sieve electrode with integrated multiplexer. J. Neural Eng. 2006, 3, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Boretius, T.; Badia, J.; Pascual-Font, A.P.; Schuettler, M.; Navarro, X.; Yoshida, K.; Stieglitz, T. A transverse intrafascicular multichannel electrode (TIME) to interface with the peripheral nerve. Biosens. Bioelectron. 2010, 26, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Li, L.J.; Zhang, J.; Zhang, F.; Lineaweaver, W.C.; Chen, T.Y.; Chen, Z.W. Longitudinal Intrafascicular Electrodes in Collection and Analysis of Sensory Signals of the Peripheral Nerve in a Feline Model. Microsurgery 2005, 25, 561–565. [Google Scholar] [CrossRef]
- Lago, N.; Yoshida, K.; Koch, K.P.; Navarro, X. Assessment of Biocompatibility of Chronically Implanted Polyimide and Platinum Intrafascicular Electrodes. IEEE Trans. Biomed. Eng. 2007, 54, 281–290. [Google Scholar] [CrossRef]
- Burnett, M.G.; Zager, E.L. Pathophysiology of peripheral nerve injury: A brief review. Neurosurg. Focus 2004, 16, E1. [Google Scholar] [CrossRef]
- Lefurge, T.; Goodall, E.; Horch, K.; Stensaas, L.; Schoenberg, A. Chronically implanted intrafascicular recording electrodes. Ann. Biomed. Eng. 1991, 19, 197–207. [Google Scholar] [CrossRef]
- Polikov, V.S.; Block, M.L.; Fellous, J.M.; Hong, J.S.; Reichert, W.M. In vitro model of glial scarring around neuroelectrodes chronically implanted in the CNS. Biomaterials 2006, 27, 5368–5376. [Google Scholar] [CrossRef] [PubMed]
- Szarowski, D.H.; Andersen, M.D.; Retterer, S.; Spence, A.J.; Isaacson, M.; Craighead, H.G.; Turner, J.N.; Shain, W. Brain responses to micro-machined silicon devices. Brain Res. 2003, 983, 23–35. [Google Scholar] [CrossRef]
- Turner, J.N.; Shain, W.; Szarowski, D.H.; Andersen, M.; Martins, S.; Isaacson, M.; Craighead, H. Cerebral astrocyte response to micromachined silicon implants. Exp. Neurol. 1999, 156, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Byun, D.; Cho, S.J.; Lee, B.H.; Min, J.; Lee, J.H.; Kim, S. Recording nerve signals in canine sciatic nerves with a flexible penetrating microelectrode array. J. Neural Eng. 2017, 14, 046023. [Google Scholar] [CrossRef] [PubMed]
- Bartels, J.; Andreasen, D.; Ehirim, P.; Mao, H.; Seibert, S.; Wright, E.J.; Kennedy, P. Neurotrophic electrode: Method of assembly and implantation into human motor speech cortex. J. Neurosci. Methods 2008, 174, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.R. The cone electrode: A long-term electrode that records from neurites grown onto its recording surface. J. Neurosci. Methods 1989, 29, 181–193. [Google Scholar] [CrossRef]
- Chew, D.J.; Zhu, L.; Delivopoulos, E.; Minev, I.R.; Musick, K.M.; Mosse, C.A.; Craggs, M.; Donaldson, N.; Lacour, S.P.; McMahon, S.B.; et al. A microchannel neuroprosthesis for bladder control after spinal cord injury in rat. Sci. Transl. Med. 2013, 5, 210ra155. [Google Scholar] [CrossRef]
- Delivopoulos, E.; Chew, D.J.; Minev, I.R.; Fawcett, J.W.; Lacour, S.P. Concurrent recordings of bladder afferents from multiple nerves using a microfabricated PDMS microchannel electrode array. Lab Chip 2012, 12, 2540–2551. [Google Scholar] [CrossRef]
- Minev, I.R.; Chew, D.J.; Delivopoulos, E.; Fawcett, J.W.; Lacour, S.P. High sensitivity recording of afferent nerve activity using ultra-compliant microchannel electrodes: An acute in vivo validation. J. Neural Eng. 2012, 9, 026005. [Google Scholar] [CrossRef]
- Kim, B.J.; Kuo, J.T.; Hara, S.A.; Lee, C.D.; Yu, L.; Gutierrez, C.A.; Hoang, T.Q.; Pikov, V.; Meng, E. 3D Parylene sheath neural probe for chronic recordings. J. Neural Eng. 2013, 10, 045002. [Google Scholar] [CrossRef]
- Kuo, J.T.; Kim, B.J.; Hara, S.A.; Lee, C.D.; Gutierrez, C.A.; Hoang, T.Q.; Meng, E. Novel flexible Parylene neural probe with 3D sheath structure for enhancing tissue integration. Lab Chip 2013, 13, 554–561. [Google Scholar] [CrossRef]
- Lee, C.D.; Hara, S.A.; Yu, L.; Kuo, J.T.; Kim, B.J.; Hoang, T.; Pikov, V.; Meng, E. Matrigel coatings for Parylene sheath neural probes. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Messer, R.L.; Lucas, L.C. Cytotoxicity of nickel-chromium alloys: Bulk alloys compared to multiple ion salt solutions. Dent. Mater. 2000, 16, 207–212. [Google Scholar] [CrossRef]
- Wataha, J.C. Biocompatibility of dental casting alloys: A review. J. Prosthet. Dent. 2000, 83, 223–234. [Google Scholar] [CrossRef]
- Cogan, S.F. Neural Stimulation and Recording Electrodes. Annu. Rev. Biomed. Eng. 2008, 10, 275–309. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Jung, W.; Kim, O.; Chu, J.-U.; Youn, I.; Kim, K.; Oh, S.R.; Park, J.W.; Kim, J. 64-Channel Double-Layered Sieve Electrode with Increased Porosity for Improved Axon Regeneration and High Spatial Resolution. In Proceedings of the 2016 6th IEEE International Conference on Biomedical Robotics and Biomechatronics (BioRob), Singapore, 26–29 June 2016; pp. 1148–1153. [Google Scholar]
- Choi, S.; Han, S.I.; Jung, D.; Hwang, H.J.; Lim, C.; Bae, S.; Park, O.K.; Tschabrunn, C.M.; Lee, M.; Bae, S.Y.; et al. Highly Conductive, Stretchable and Biocompatible Ag–Au core–sheath Nanowire Composite for Wearable and Implantable Bioelectronics. Nat. Nanotechnol. 2018, 13, 1048. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Thow, X.Y.; Wang, H.; Lee, S.; Voges, K.; Thakor, N.V.; Yen, S.-C.; Lee, C. A Highly Selective 3D Spiked Ultraflexible Neural (SUN) Interface for Decoding Peripheral Nerve Sensory Information. Adv. Healthc. Mater. 2018, 1700987. [Google Scholar]
- Peltonen, S.; Alanne, M.; Peltonen, J. Barriers of the peripheral nerve. Tissue Barriers 2013, 1, e24956. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, W.; Jung, S.; Kim, O.; Park, H.; Choi, W.; Son, D.; Chung, S.; Kim, J. Wire Electrodes Embedded in Artificial Conduit for Long-term Monitoring of the Peripheral Nerve Signal. Micromachines 2019, 10, 184. https://doi.org/10.3390/mi10030184
Jung W, Jung S, Kim O, Park H, Choi W, Son D, Chung S, Kim J. Wire Electrodes Embedded in Artificial Conduit for Long-term Monitoring of the Peripheral Nerve Signal. Micromachines. 2019; 10(3):184. https://doi.org/10.3390/mi10030184
Chicago/Turabian StyleJung, Woohyun, Sunyoung Jung, Ockchul Kim, HyungDal Park, Wonsuk Choi, Donghee Son, Seok Chung, and Jinseok Kim. 2019. "Wire Electrodes Embedded in Artificial Conduit for Long-term Monitoring of the Peripheral Nerve Signal" Micromachines 10, no. 3: 184. https://doi.org/10.3390/mi10030184
APA StyleJung, W., Jung, S., Kim, O., Park, H., Choi, W., Son, D., Chung, S., & Kim, J. (2019). Wire Electrodes Embedded in Artificial Conduit for Long-term Monitoring of the Peripheral Nerve Signal. Micromachines, 10(3), 184. https://doi.org/10.3390/mi10030184




