Development of a New Laparoscopic Detection System for Gastric Cancer Using Near-Infrared Light-Emitting Clips with Glass Phosphor
Abstract
1. Introduction
2. Materials and Methods
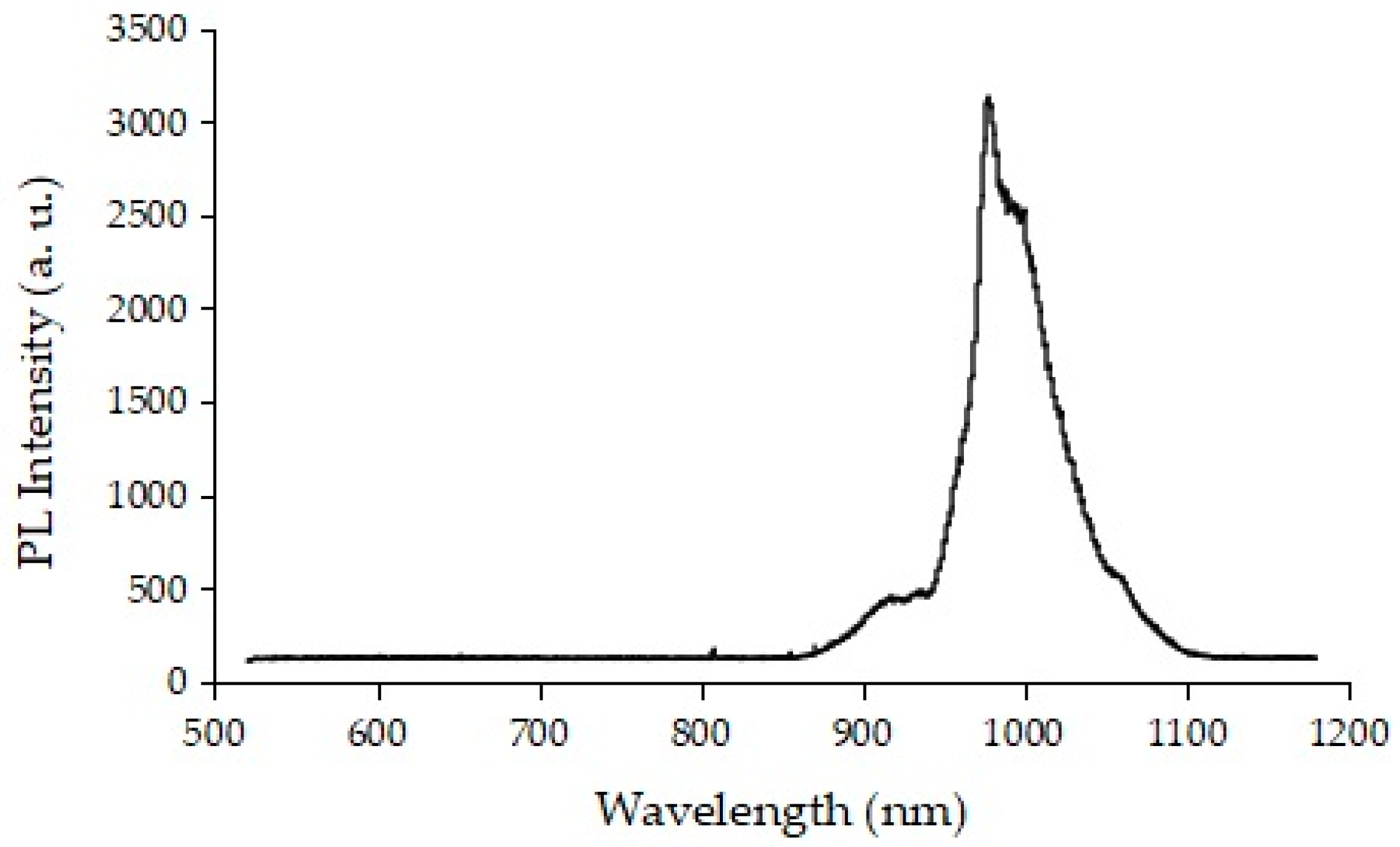
2.1. Development of Glass Phosphor
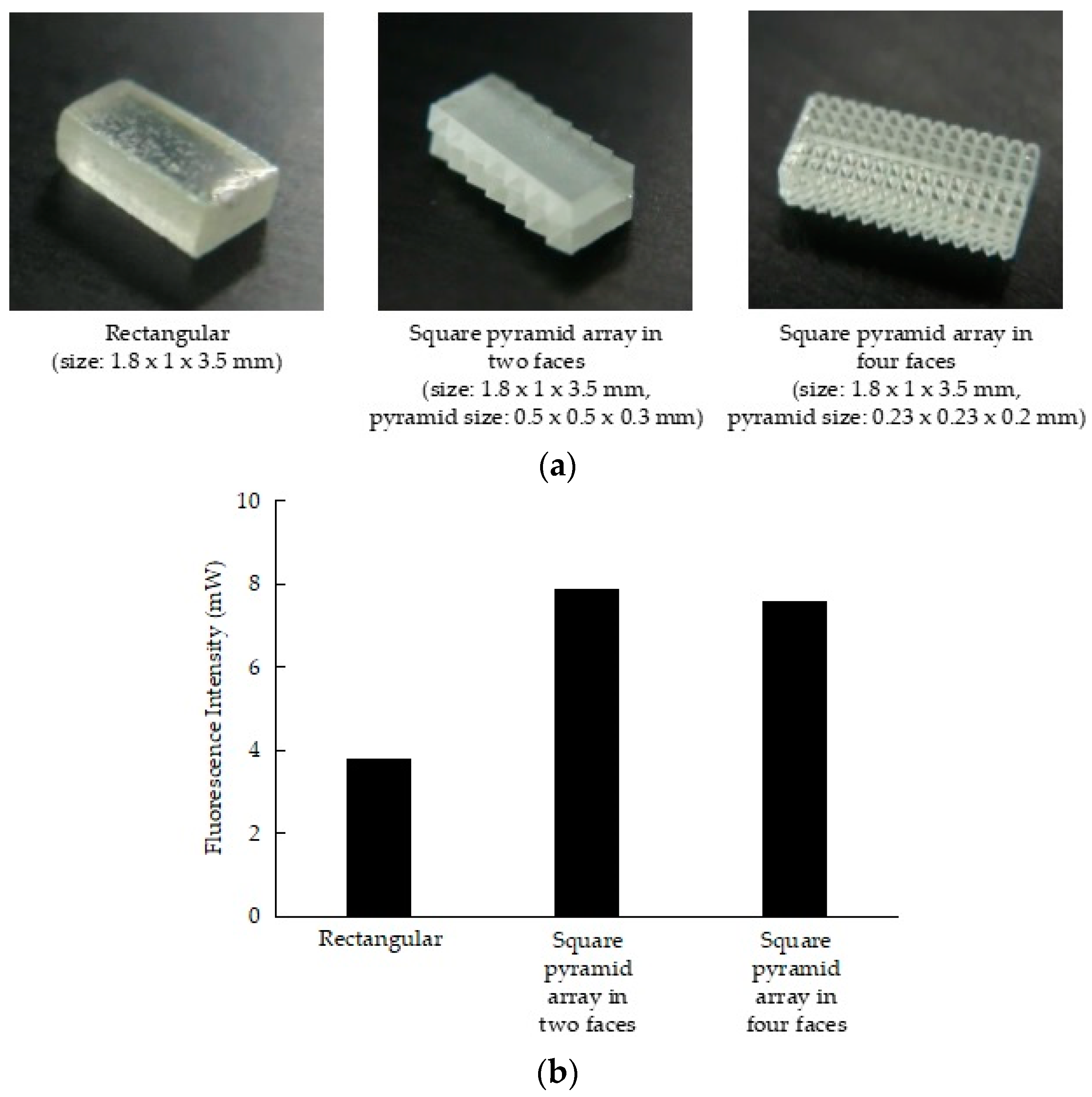
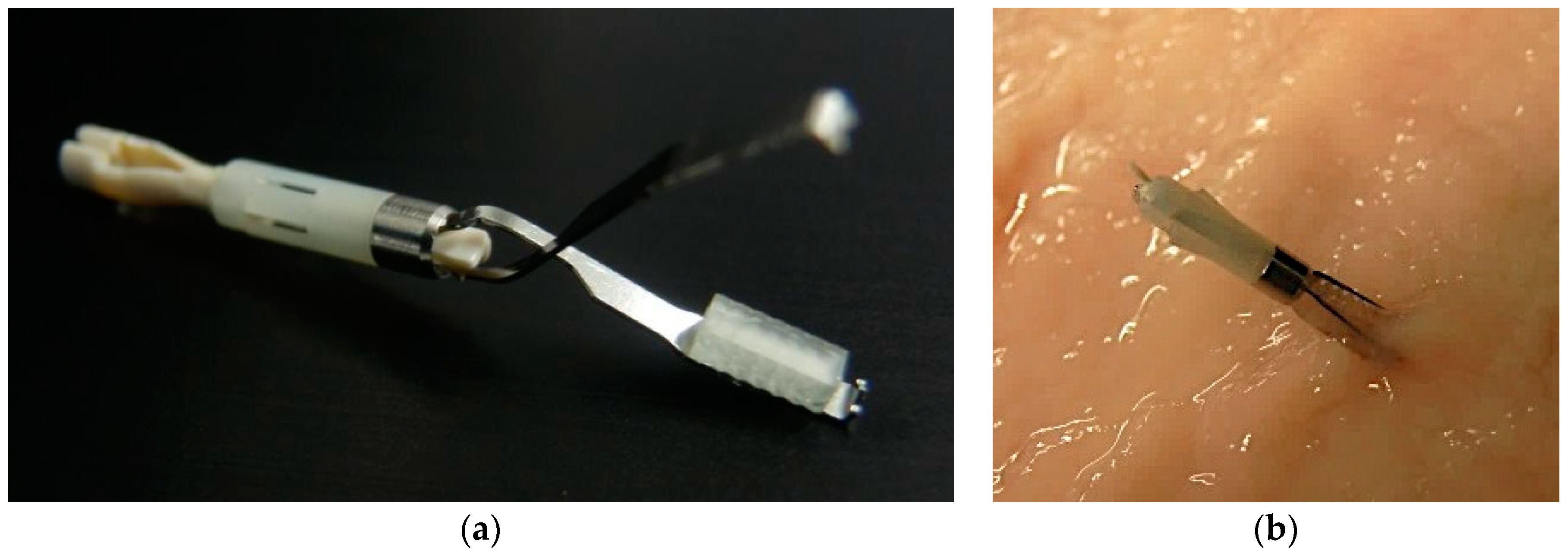
2.2. Development of the Fluorescent Clip with Glass Phosphor
2.3. Development of the Laparoscopic Fluorescent Detection System
2.4. Experimental Procedures
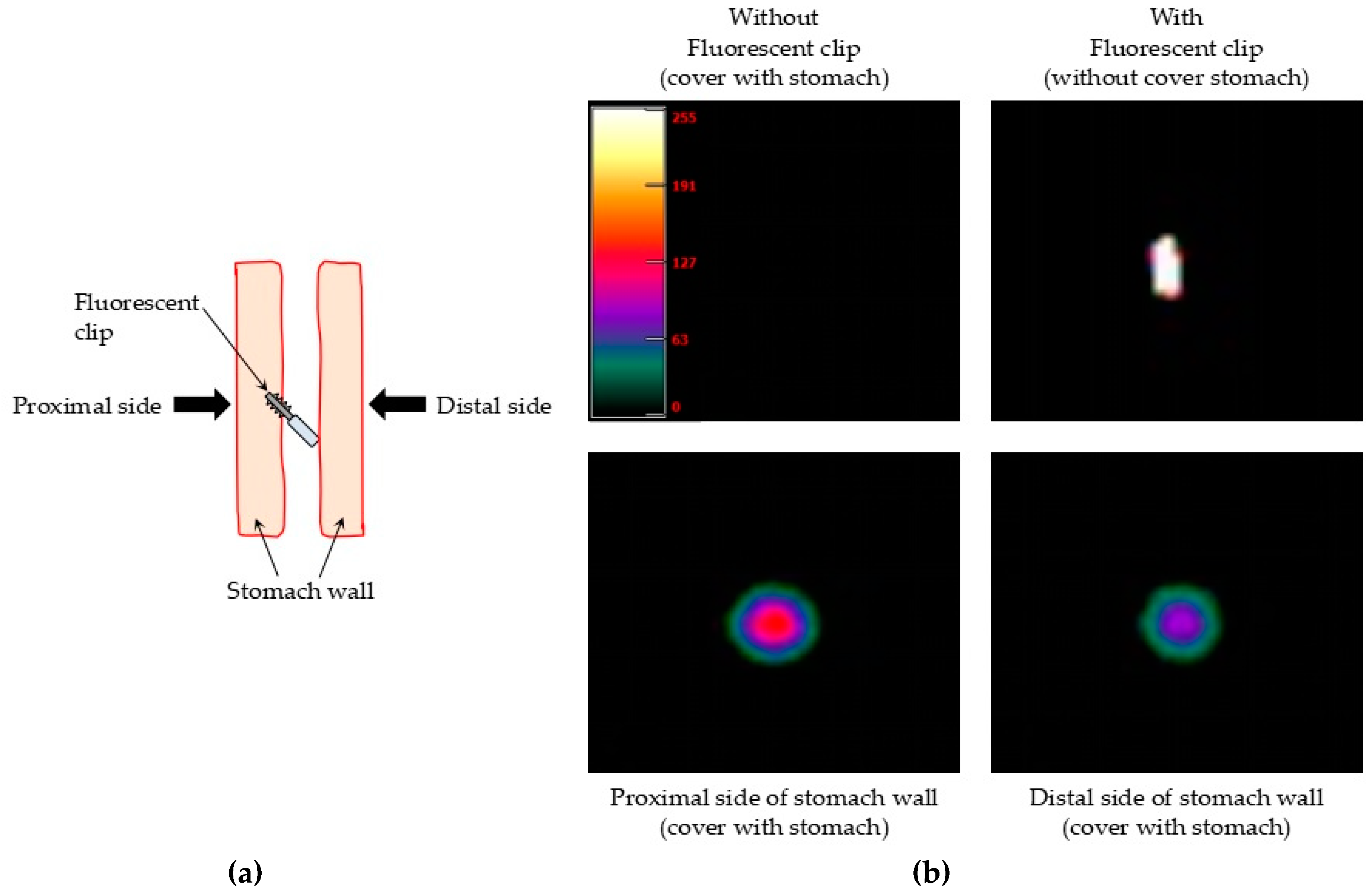
2.4.1. Ex Vivo Detection of the Fluorescent Clips in Pig Stomach
2.4.2. Ex Vivo Detection of the Fluorescent Clips in Human Stomach
2.4.3. Evaluation of the Cytotoxicity of Glass Phosphor
3. Results
3.1. Ex Vivo Detection of the Fluorescent Clips in Pig Stomach
3.2. Ex Vivo Detection of the Fluorescent Clips in Human Stomach
3.3. Cytotoxicity of the Glass Phosphor
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikishit, R.; Eser, S.; Matters, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer Incidence and mortality worldwide: Sources, methods and major patterns in Globocan. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Nagini, S. Carcinoma of the stomach: A review of epidemiology, pathogenesis, molecular genetics and chemoprevention. World J. Gastrointest. Oncol. 2012, 4, 156–169. [Google Scholar] [CrossRef]
- Kitano, S.; Shiraishi, N.; Uyama, I.; Sugihara, K.; Tanigawa, N. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann. Surg. 2007, 245, 68–72. [Google Scholar] [CrossRef]
- Kim, H.H.; Hyung, W.J.; Cho, G.S.; Kim, M.C.; Han, S.U.; Kim, W.; Ryu, S.W.; Lee, H.J.; Song, K.Y. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: An interim report-a phase III multicenter, prospective, randomized trial (KLASS trial). Ann. Surg. 2010, 251, 417–420. [Google Scholar] [CrossRef]
- Katai, H.; Sasako, M.; Fukuda, H.; Nakamura, K.; Hiki, N.; Saka, M.; Yamaue, H.; Yoshikawa, T.; Kojima, K. Safety and feasibility of laparoscopy-assisted distal gastrectomy with suprapancreatic nodal dissection for clinical stage I gastric cancer: A multicenter phase II trial (JCOG 0703). Gastric Cancer 2010, 13, 238–244. [Google Scholar] [CrossRef]
- Kitamura, K.; Takahashi, T.; Yamaguchi, T.; Yamane, T.; Hagiwara, A.; Oyama, T. Identification, by activated carbon injection, of cancer lesion during laparoscopic surgery. Lancet 1994, 343, 789. [Google Scholar] [CrossRef]
- Tokuhara, T.; Nakata, E.; Tenjo, T.; Kawai, I.; Satoi, S.; Inoue, K.; Araki, M.; Ueda, H.; Higashi, C. A novel option for preoperative endoscopic marking with India ink in totally laparoscopic distal gastrectomy for gastric cancer: A useful technique considering the morphological characteristics of the stomach. Mol. Clin. Oncol. 2017, 6, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.R.; Parrish, J.A. The optics of human skin. J. Investig. Dermatol. 1981, 77, 13–19. [Google Scholar] [CrossRef]
- Kakiuchi, T.; Takahara, T.; Kasugai, Y.; Arita, K.; Yoshida, N.; Karube, K.; Suguro, M.; Matsuo, K.; Nakanishi, H.; Kiyono, T.; et al. Modeling mesothelioma utilizing human mesothelial cells reveals involvement of phospholipase-C beta 4 in YAP-active mesothelioma cell proliferation. Carcinogenesis 2016, bgw084. [Google Scholar] [CrossRef] [PubMed]
- Kawakatsu, S.; Ohashi, M.; Hiki, N.; Nunobe, S.; Nagino, M.; Sano, T. Use of endoscopy to determine the resection margin during laparoscopic gastrectomy for cancer. BJS 2017, 104, 1829–1836. [Google Scholar] [CrossRef]
- Chung, J.W.; Seo, K.W.; Jung, K.; Park, M.I.; Kim, S.E.; Park, S.J.; Lee, S.H.; Shin, Y.M. A promising method for tumor localization during total laparoscopic distal gastrectomy: Preoperative endoscopic clipping based on negative biopsy and selective intraoperative radiography findings. J. Gastric Cancer. 2017, 17, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Fuchi, S.; Sakano, A.; Takeda, Y. Wideband Infrared Emission from Yb3+ and Nd3+ Doped Bi2O3-B2O3 Glass Phosphor for an Optical Coherence Tomography Light Source. Jpn. J. Appl. Phys. 2008, 47, 7932–7935. [Google Scholar] [CrossRef]
- Fuchi, S.; Sakano, A.; Mizutani, R.; Takeda, Y. High Power and High Resolution Near-Infrared Light Source for Optical Coherence Tomography Using Glass Phosphor and Light Emitting Diode. Appl. Phys. Express 2009, 2, 032102. [Google Scholar] [CrossRef]
- Sakurai, H.; Kondo, T.; Suzuki, A.; Kitano, T.; Mori, M.; Iwaya, M.; Takeuchi, T.; Kamiyama, S.; Akasaki, I. Fabrication of high-efficiency LED using moth-eye structure. Proc. SPIE 2011, 7939. [Google Scholar] [CrossRef]
- Chan, L.; Morse, D.; Gordon, M. Moth eye-inspired anti-reflective surfaces for improved IR optical systems & visible LEDs fabricated with colloidal lithography and etching. Bioinspir. Biomim. 2018, 13, 041001. [Google Scholar] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inada, S.A.; Nakanishi, H.; Oda, M.; Mori, K.; Ito, A.; Hasegawa, J.; Misawa, K.; Fuchi, S. Development of a New Laparoscopic Detection System for Gastric Cancer Using Near-Infrared Light-Emitting Clips with Glass Phosphor. Micromachines 2019, 10, 81. https://doi.org/10.3390/mi10020081
Inada SA, Nakanishi H, Oda M, Mori K, Ito A, Hasegawa J, Misawa K, Fuchi S. Development of a New Laparoscopic Detection System for Gastric Cancer Using Near-Infrared Light-Emitting Clips with Glass Phosphor. Micromachines. 2019; 10(2):81. https://doi.org/10.3390/mi10020081
Chicago/Turabian StyleInada, Shunko A., Hayao Nakanishi, Masahiro Oda, Kensaku Mori, Akihiro Ito, Junichi Hasegawa, Kazunari Misawa, and Shingo Fuchi. 2019. "Development of a New Laparoscopic Detection System for Gastric Cancer Using Near-Infrared Light-Emitting Clips with Glass Phosphor" Micromachines 10, no. 2: 81. https://doi.org/10.3390/mi10020081
APA StyleInada, S. A., Nakanishi, H., Oda, M., Mori, K., Ito, A., Hasegawa, J., Misawa, K., & Fuchi, S. (2019). Development of a New Laparoscopic Detection System for Gastric Cancer Using Near-Infrared Light-Emitting Clips with Glass Phosphor. Micromachines, 10(2), 81. https://doi.org/10.3390/mi10020081




