Fabrication of TiO2-Nanotube-Array-Based Supercapacitors
Abstract
1. Introduction
2. Experimental Details
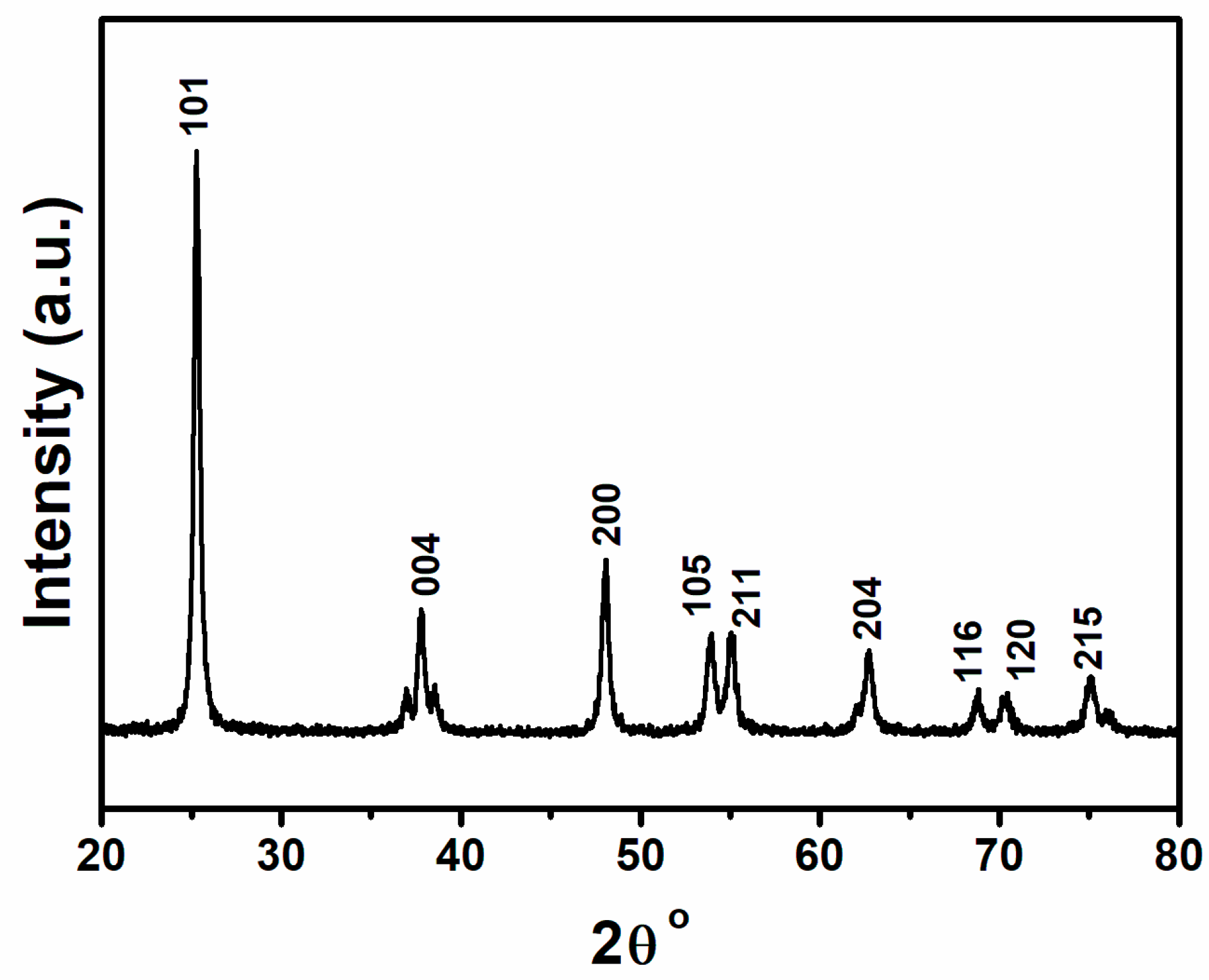
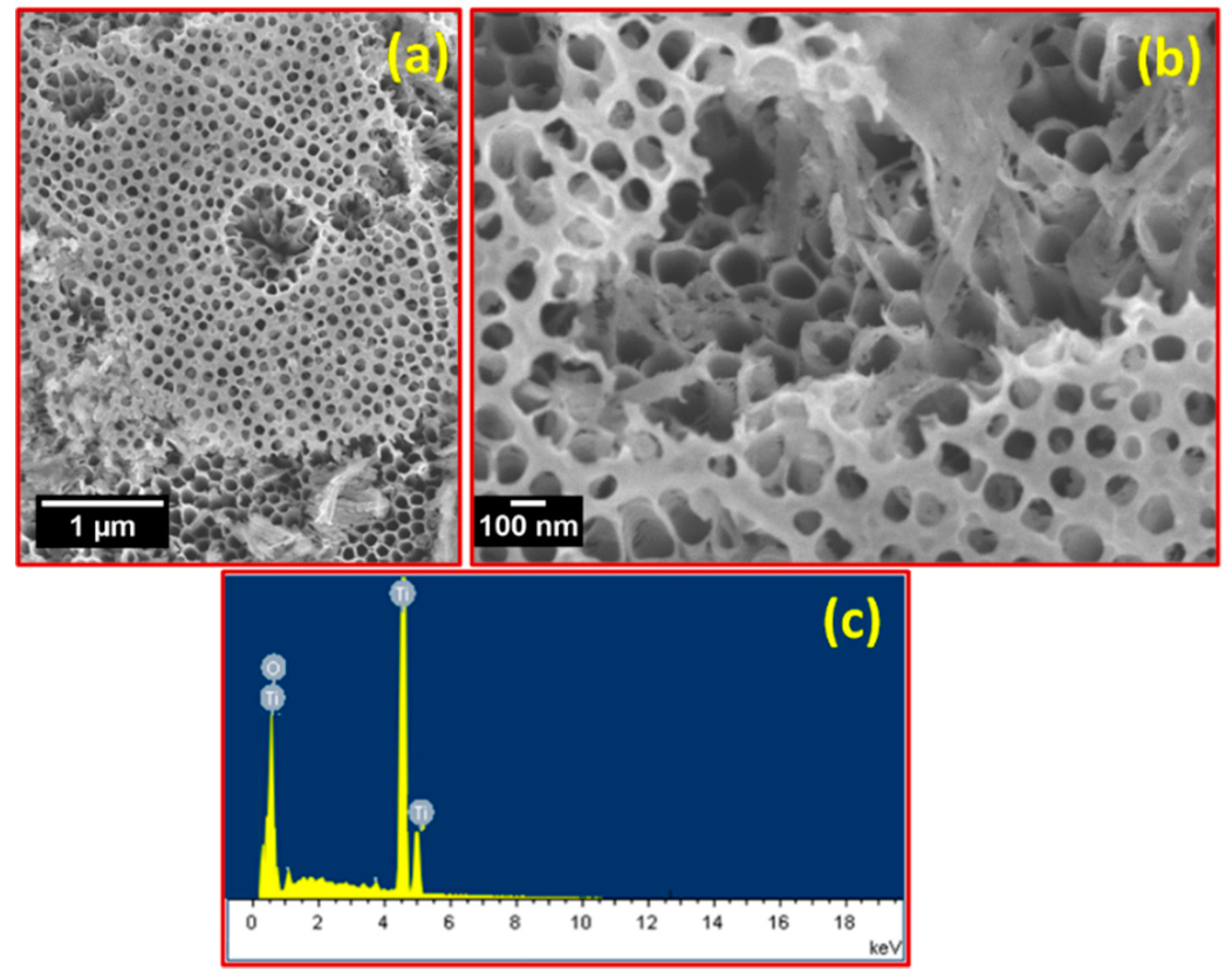
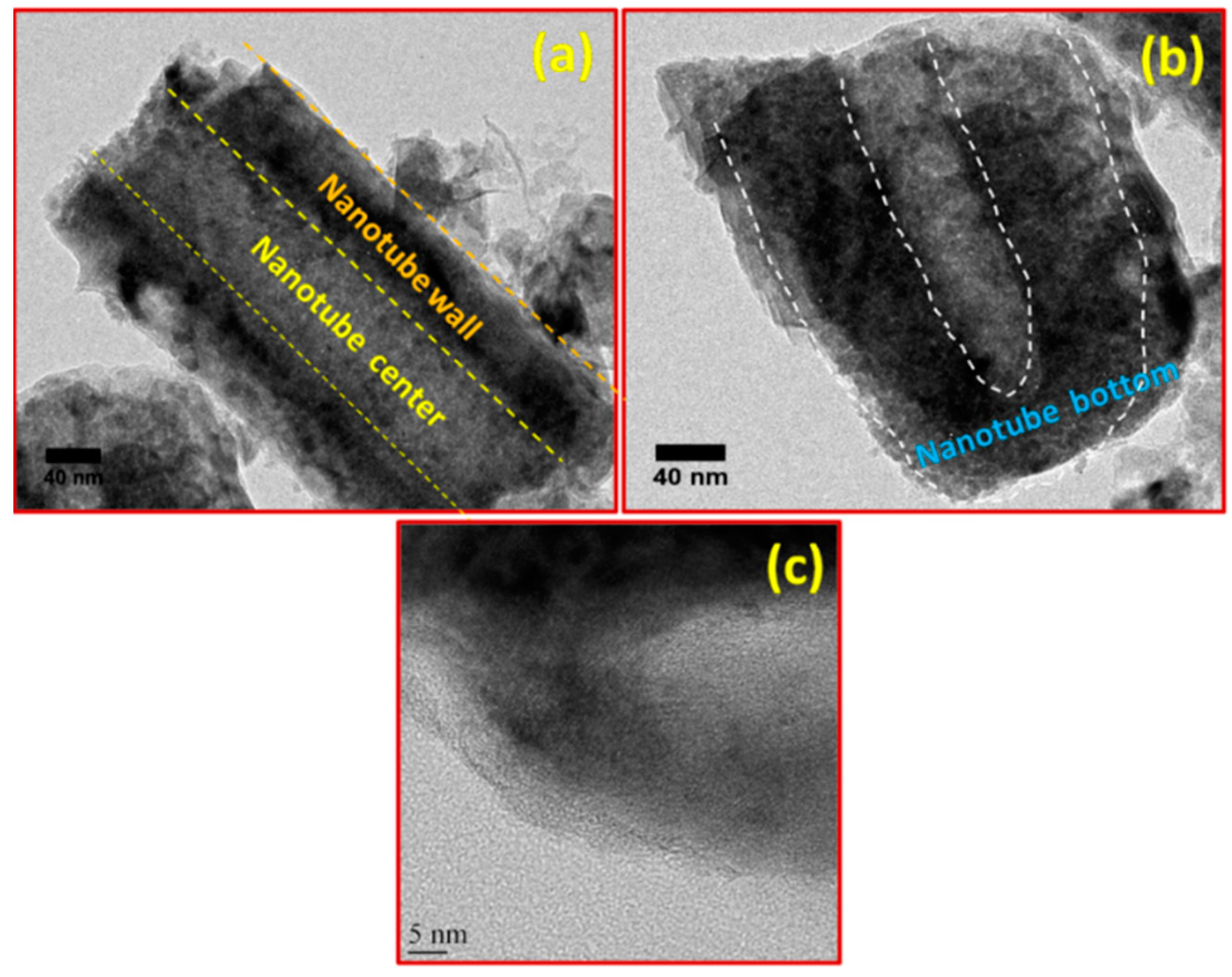
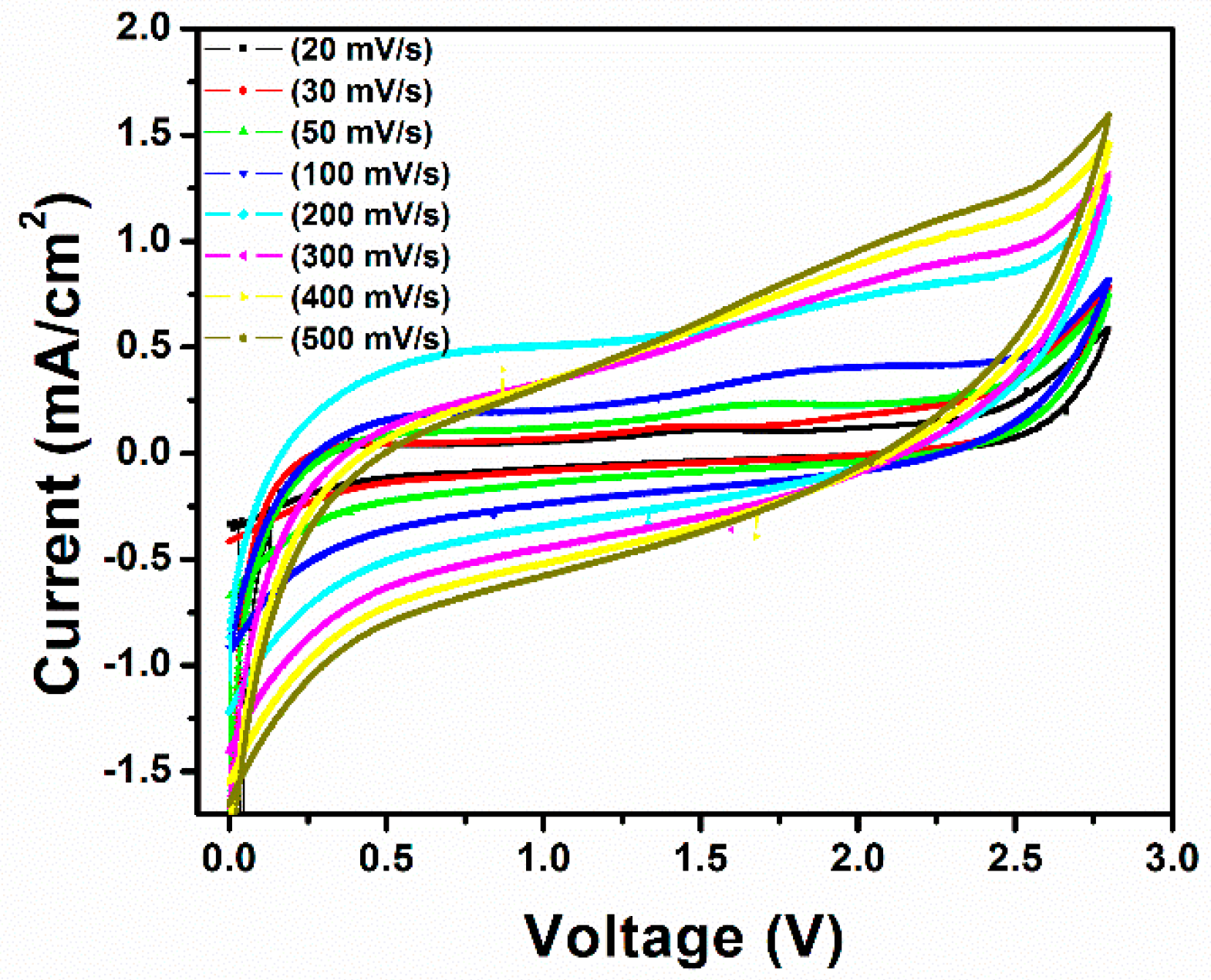
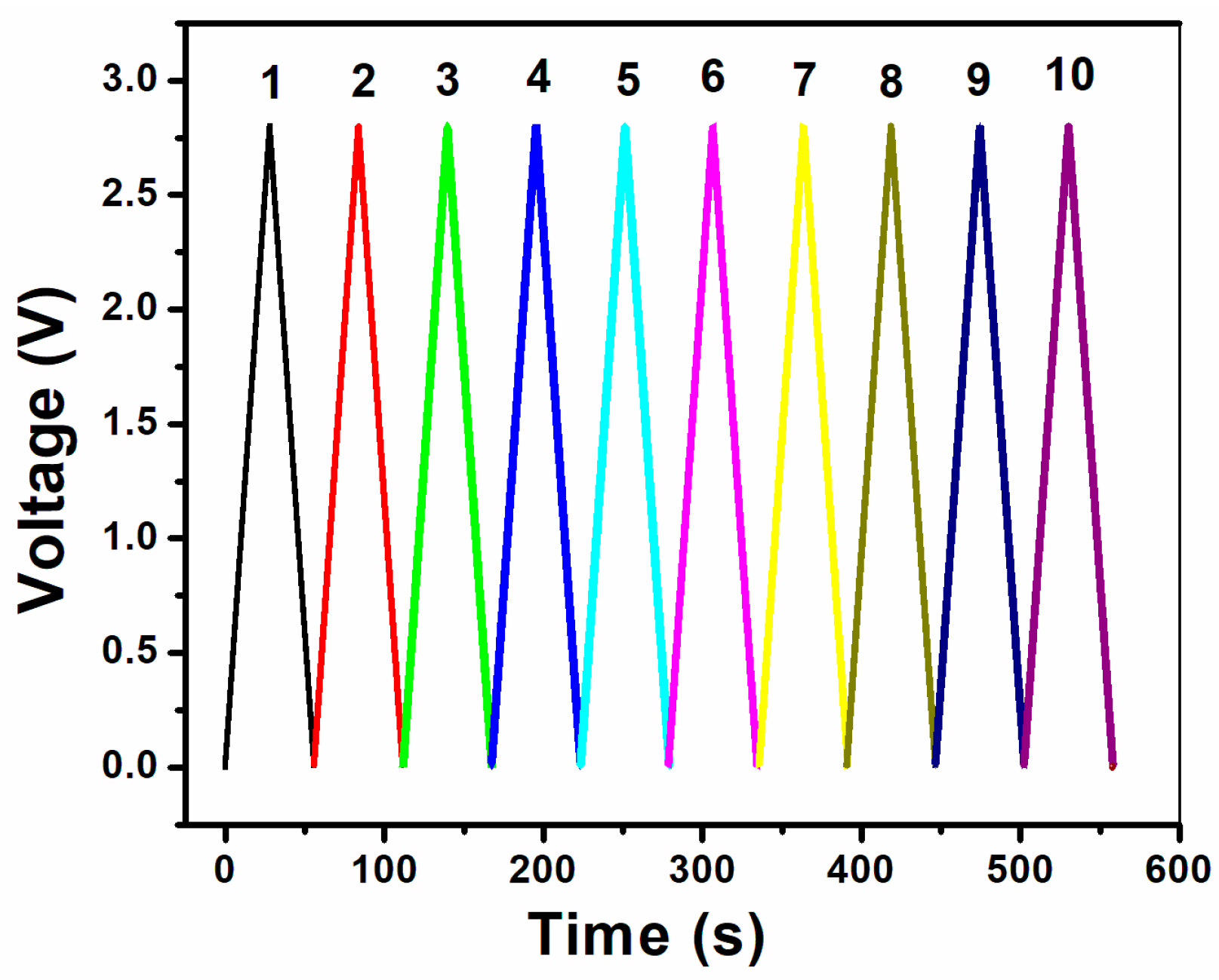
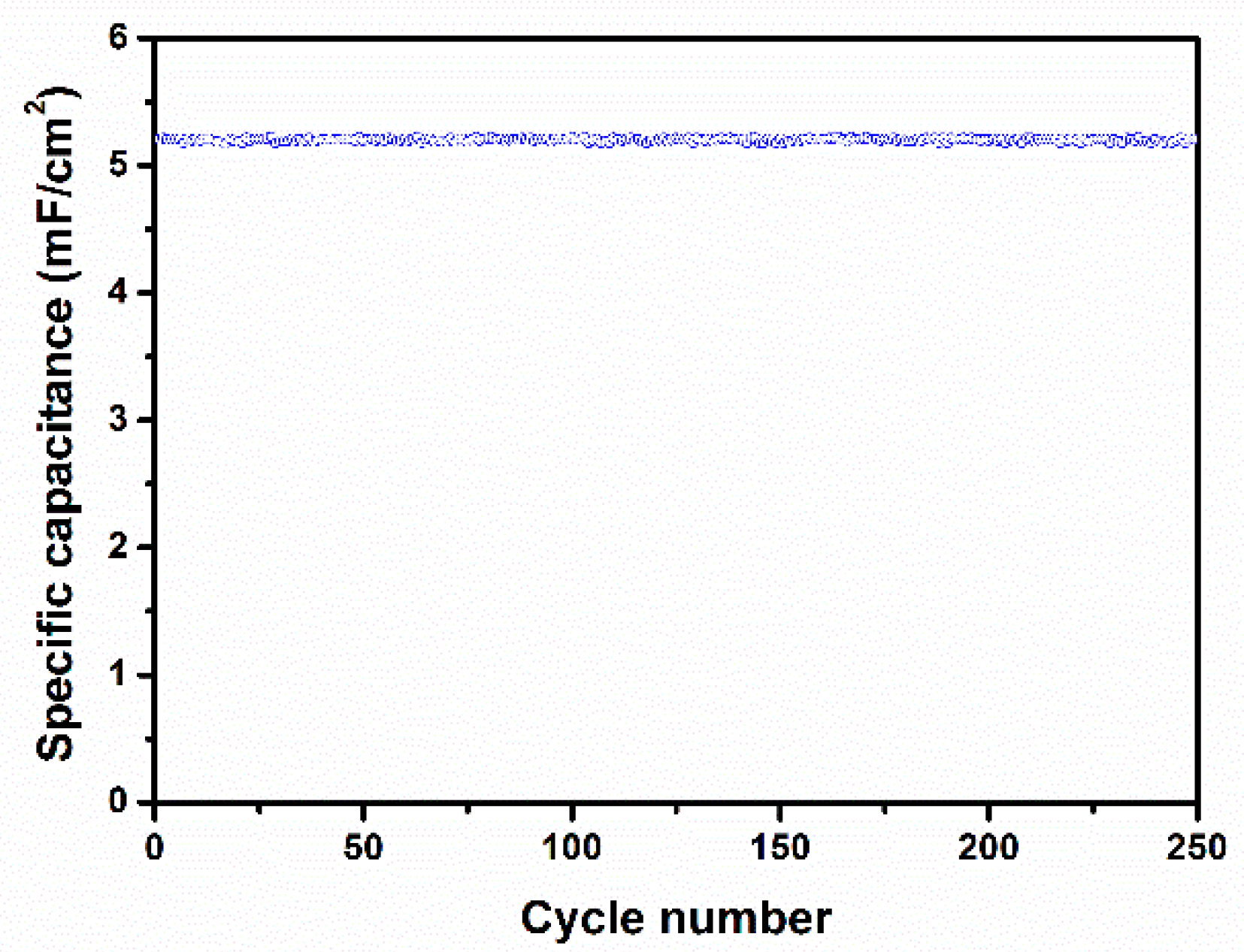
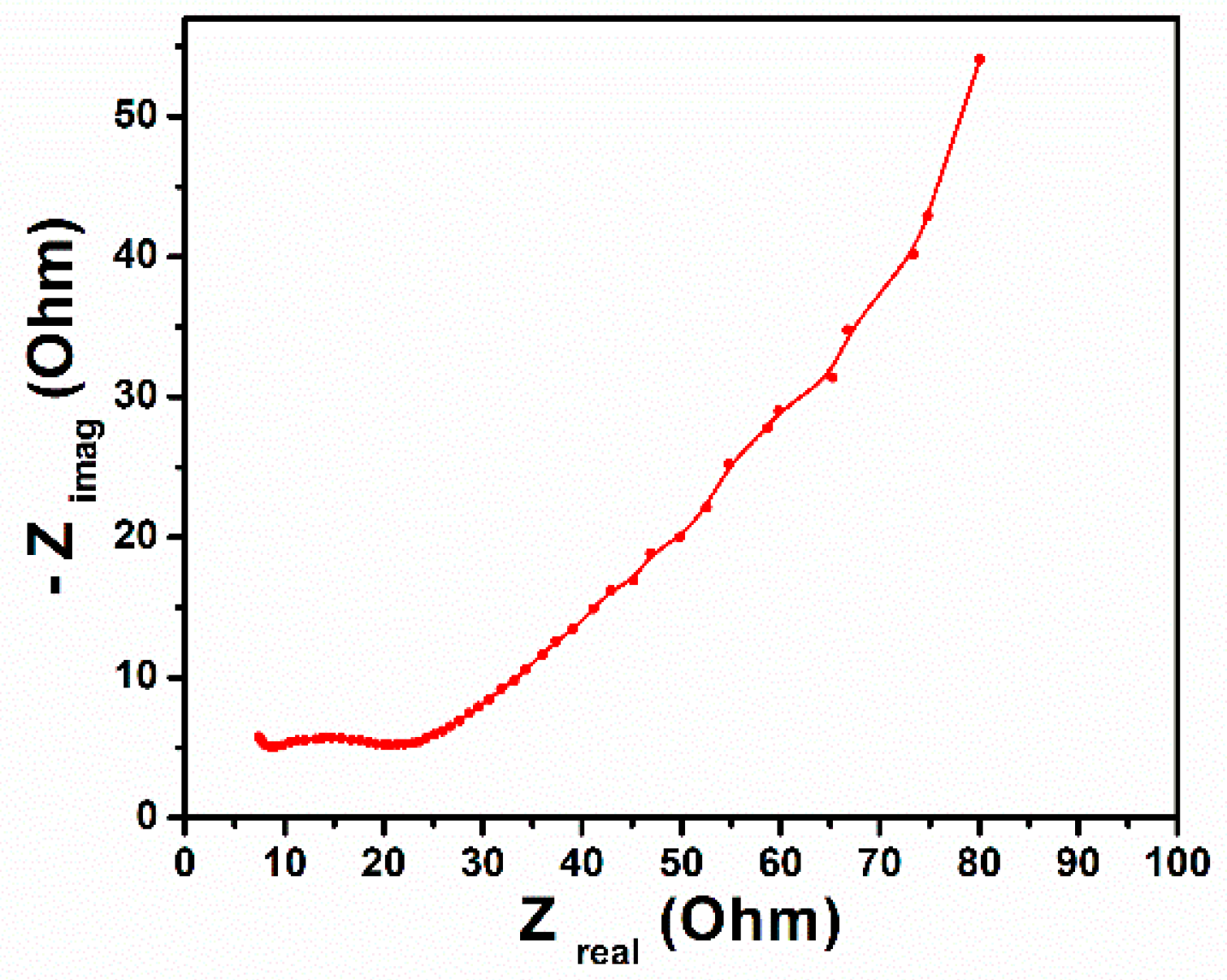
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, D.W.; Rhee, K.Y.; Park, S.J. Synthesis of activated carbon nanotube/copper oxide composites and their electrochemical performance. J. Alloys Comp. 2012, 530, 6–10. [Google Scholar] [CrossRef]
- Cheng, J.P.; Chen, X.; Wu, J.S.; Liu, F.; Zhang, X.B.; Dravid, V.P. Porous cobalt oxides with tunable hierarchical morphologies for supercapacitor electrodes. Cryst. Eng. Comm. 2012, 14, 6702–6709. [Google Scholar] [CrossRef]
- Wang, Z.; Maa, C.; Wang, H.; Liu, Z.; Hao, Z. Facilely synthesized Fe2O3–graphene nanocomposite as novel electrode materials for supercapacitors with high performance. J. Alloys Comp. 2013, 552, 486–491. [Google Scholar] [CrossRef]
- Lei, Z.; Christov, N.; Zhao, X.S. Intercalation of mesoporous carbon spheres between reduced graphene oxide sheets for preparing high-rate supercapacitor electrodes. Energy Environ. Sci. 2011, 4, 1866–1873. [Google Scholar] [CrossRef]
- Xia, X.; Hao, Q.; Lei, W.; Wang, W.; Sun, D.; Wang, X. Nanostructured ternary composites of graphene/Fe2O3/polyaniline for high-performance supercapacitors. J. Mater. Chem. 2012, 22, 16844–16850. [Google Scholar] [CrossRef]
- Liu, W.W.; Yan, X.B.; Lang, J.W.; Peng, C.; Xue, Q. Flexible and conductive nanocomposite electrode based on graphene sheets and cotton cloth for supercapacitor. J. Mater. Chem. 2012, 22, 17245–17253. [Google Scholar] [CrossRef]
- Yang, X.H.; Wang, Y.G.; Xiong, H.M.; Xia, Y.Y. Interfacial synthesis of porous MnO2 and its application in electrochemical capacitor. Electrochim. Acta 2007, 53, 752–757. [Google Scholar] [CrossRef]
- Yang, W.; Gao, Z.; Wang, J.; Wang, B.; Liu, Q.; Li, Z.; Mann, T.; Yang, P.; Zhang, M.; Liu, L. Synthesis of reduced graphene nanosheet/urchin-like manganese dioxide composite and high performance as supercapacitor electrode. Electrochim. Acta 2012, 69, 112–119. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, C.X.; Liu, J.; Chen, T.; Yang, H.; Li, C.M. CeO2 nanoparticles/graphene nanocomposite-based high performance supercapacitor. Dalton Trans. 2011, 40, 6388–6391. [Google Scholar] [CrossRef]
- Lu, X.H.; Zheng, D.Z.; Zhai, T.; Liu, Z.Q.; Huang, Y.Y.; Xie, S.L.; Tong, T.Y.X. Facile synthesis of large-area manganese oxide nanorod arrays as a high-performance electrochemical supercapacitor. Energy Environ. Sci. 2011, 4, 2915–2921. [Google Scholar] [CrossRef]
- Xia, X.H.; Tu, J.P.; Mai, Y.J.; Wang, X.L.; Gu, C.D.; Zhao, X.B. Self-supported hydrothermal synthesized hollow Co3O4 nanowire arrays with high supercapacitor capacitance. J. Mater. Chem. 2011, 21, 9319–9325. [Google Scholar] [CrossRef]
- Feng, D.; Lv, Y.Y.; Wu, Z.X.; Dou, Y.Q.; Han, L.; Sun, Z.K.; Xia, Y.Y.; Zheng, G.F.; Zhao, D.Y. Free-standing mesoporous carbon thin films with highly ordered pore architectures for nanodevices. J. Am. Chem. Soc. 2011, 133, 15148–15156. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yang, L.; Li, C.; Yan, C.; Lee, P.S.; Ma, J. High–rate electrochemical capacitors from highly graphitic carbon–tipped manganese oxide/mesoporous carbon/manganese oxide hybrid nanowires. Energy Environ. Sci. 2011, 4, 1813–1819. [Google Scholar] [CrossRef]
- Guo, S.J.; Dong, S.J.; Wang, E.K. Constructing carbon-nanotube/metal hybrid nanostructures using homogeneous TiO2 as a spacer. Small 2008, 4, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhao, T.; Ma, J.; Yan, C.Y.; Li, C.Z. Ultrafine manganese dioxide nanowire network for high-performance supercapacitors. Chem. Commun. 2011, 47, 1264–1266. [Google Scholar] [CrossRef] [PubMed]
- Ghicov, A.; Albu, S.P.; Hahn, R.; Kim, D.; Stergiopoulos, T.; Kunze, J. TiO2 nanotubes in dye-sensitized solar cells: Critical factors for the conversion efficiency. Chem. Asian J. 2009, 4, 520–525. [Google Scholar] [CrossRef]
- Wang, G.; Liu, Z.Y.; Wu, J.N.; Lu, Q. Preparation and electrochemical capacitance behavior of TiO2-B nanotubes for hybrid supercapacitor. Mater. Lett. 2012, 71, 120–122. [Google Scholar] [CrossRef]
- Salari, M.; Aboutalebi, S.H.; Chidembo, A.T.; Nevirkovets, I.P.; Konstantinov, K.; Li, H.K.u. Enhancement of the electrochemical capacitance of TiO2 nanotube arrays through controlled phase transformation of anatase to rutile. Phys. Chem. Chem. Phys. 2012, 14, 4770–4779. [Google Scholar] [CrossRef]
- Lu, X.H.; Wang, G.M.; Zhai, T.; Yu, M.H.; Gan, J.Y.; Tong, Y.X.; Li, Y. Hydrogenated TiO2 nanotube arrays for supercapacitors. Nano Lett. 2012, 12, 1690. [Google Scholar] [CrossRef]
- Wu, H.; Li, D.; Zhu, X.; Yang, C.; Liu, D.; Chen, X.; Song, Y.; Lu, L. High-performance and renewable supercapacitors based on TiO2 nanotube array electrodes treated by an electrochemical doping approach. Electrochim. Acta 2014, 116, 129–136. [Google Scholar] [CrossRef]
- So, S.; Lee, K.; Schmuki, P. Ultrafast growth of highly ordered anodic TiO2 nanotubes in lactic acid electrolytes. J. Am. Chem. Soc. 2012, 134, 11316–11318. [Google Scholar] [CrossRef] [PubMed]
- Ryu, W.H.; Nam, D.H.; Ko, Y.S.; Kim, R.H.; Kwon, H.S. Electrochemical performance of a smooth and highly ordered TiO2 nanotube electrode for Li-ion batteries. Electrochim. Acta 2012, 61, 19–24. [Google Scholar] [CrossRef]
- Brumbarov, J.; Kunze-Liebhäuser, J. Silicon on conductive self-organized TiO2 nanotubes–A high capacity anode material for Li-ion batteries. J. Power Sources 2014, 258, 129–133. [Google Scholar] [CrossRef]
- Liu, K.K.; Hu, Z.L.; Xue, R.; Zhang, J.R.; Zhu, Z.J. Electropolymerization of high stable poly(3, 4-ethylenedioxythiophene) in ionic liquids and its potential applications in electrochemical capacitor. J. Power Sources 2008, 179, 858–862. [Google Scholar] [CrossRef]
- Salari, M.; Aboutalebi, S.H.; Konstantinov, K.; Liu, H.K. A highly ordered titania nanotube array as a supercapacitor electrode. Phys. Chem. Chem. Phys. 2011, 13, 5038–5041. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, F.; Pervez, S.A.; Aljaafari, A.; Alshoaibi, A.; Abuhimd, H.; Oh, J.; Koo, B.H. Fabrication of TiO2-Nanotube-Array-Based Supercapacitors. Micromachines 2019, 10, 742. https://doi.org/10.3390/mi10110742
Ahmed F, Pervez SA, Aljaafari A, Alshoaibi A, Abuhimd H, Oh J, Koo BH. Fabrication of TiO2-Nanotube-Array-Based Supercapacitors. Micromachines. 2019; 10(11):742. https://doi.org/10.3390/mi10110742
Chicago/Turabian StyleAhmed, Faheem, Syed A. Pervez, Abdullah Aljaafari, Adil Alshoaibi, Hatem Abuhimd, JooHyeon Oh, and Bon Heun Koo. 2019. "Fabrication of TiO2-Nanotube-Array-Based Supercapacitors" Micromachines 10, no. 11: 742. https://doi.org/10.3390/mi10110742
APA StyleAhmed, F., Pervez, S. A., Aljaafari, A., Alshoaibi, A., Abuhimd, H., Oh, J., & Koo, B. H. (2019). Fabrication of TiO2-Nanotube-Array-Based Supercapacitors. Micromachines, 10(11), 742. https://doi.org/10.3390/mi10110742







