Integration of Hierarchical Micro-/Nanostructures in a Microfluidic Chip for Efficient and Selective Isolation of Rare Tumor Cells
Abstract
:1. Introduction
2. Method and Materials
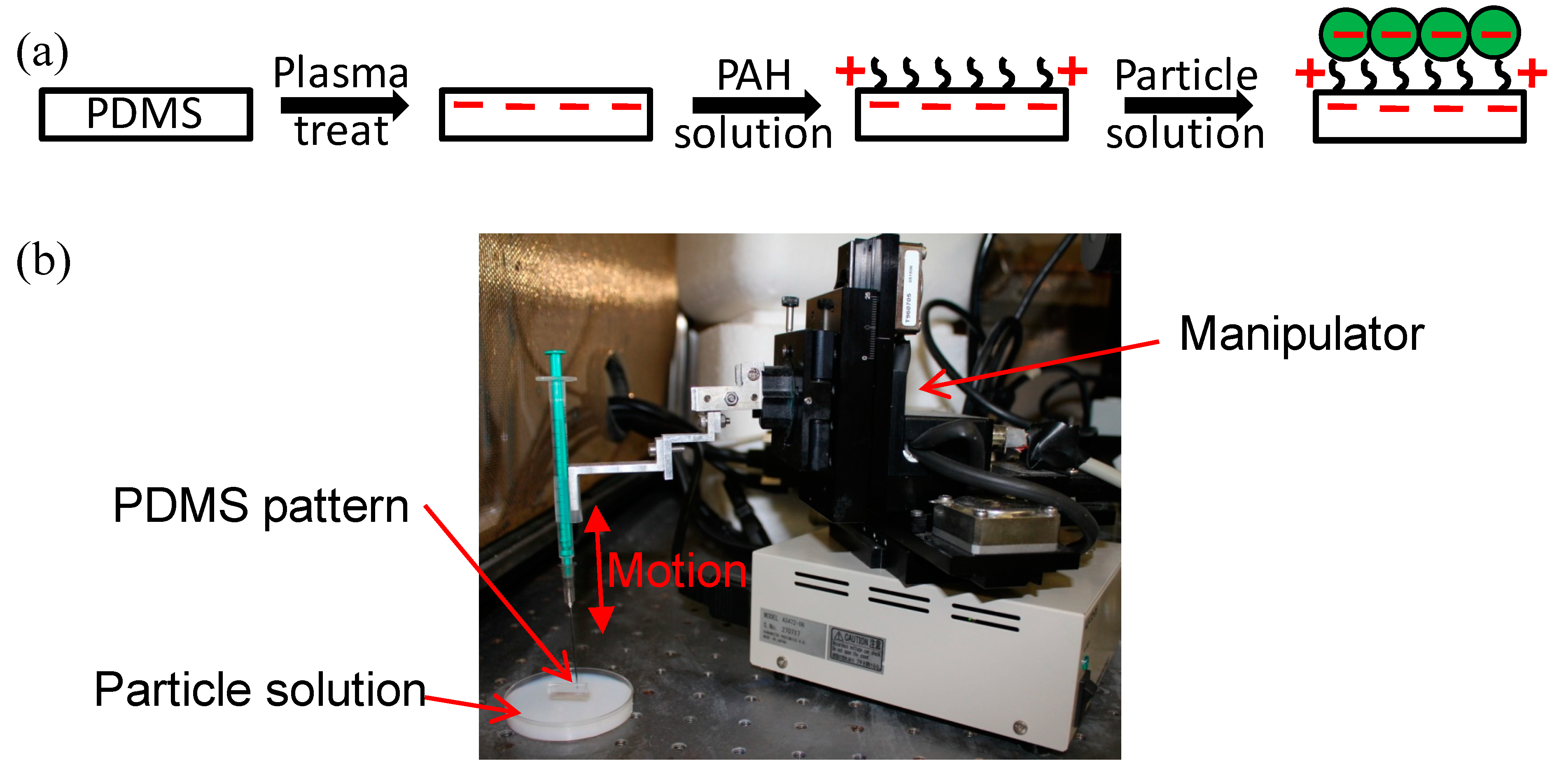
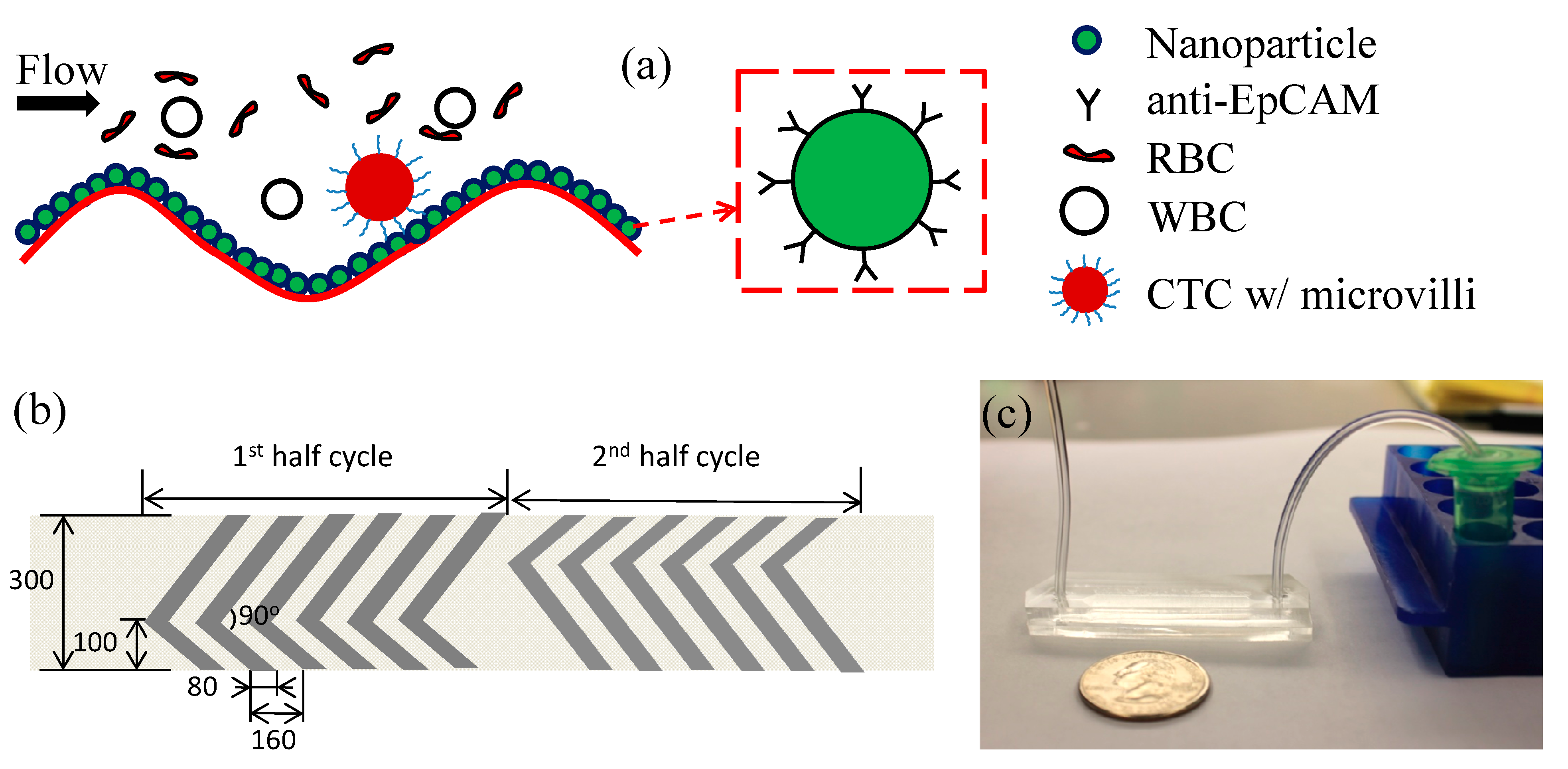
2.1. Fabrication and Surface Functionalization of the Hierarchical CTC Chip
2.2. Preparation of Cell Samplesand Cell Capture
3. Results and Discussions
3.1. Working Mechanism
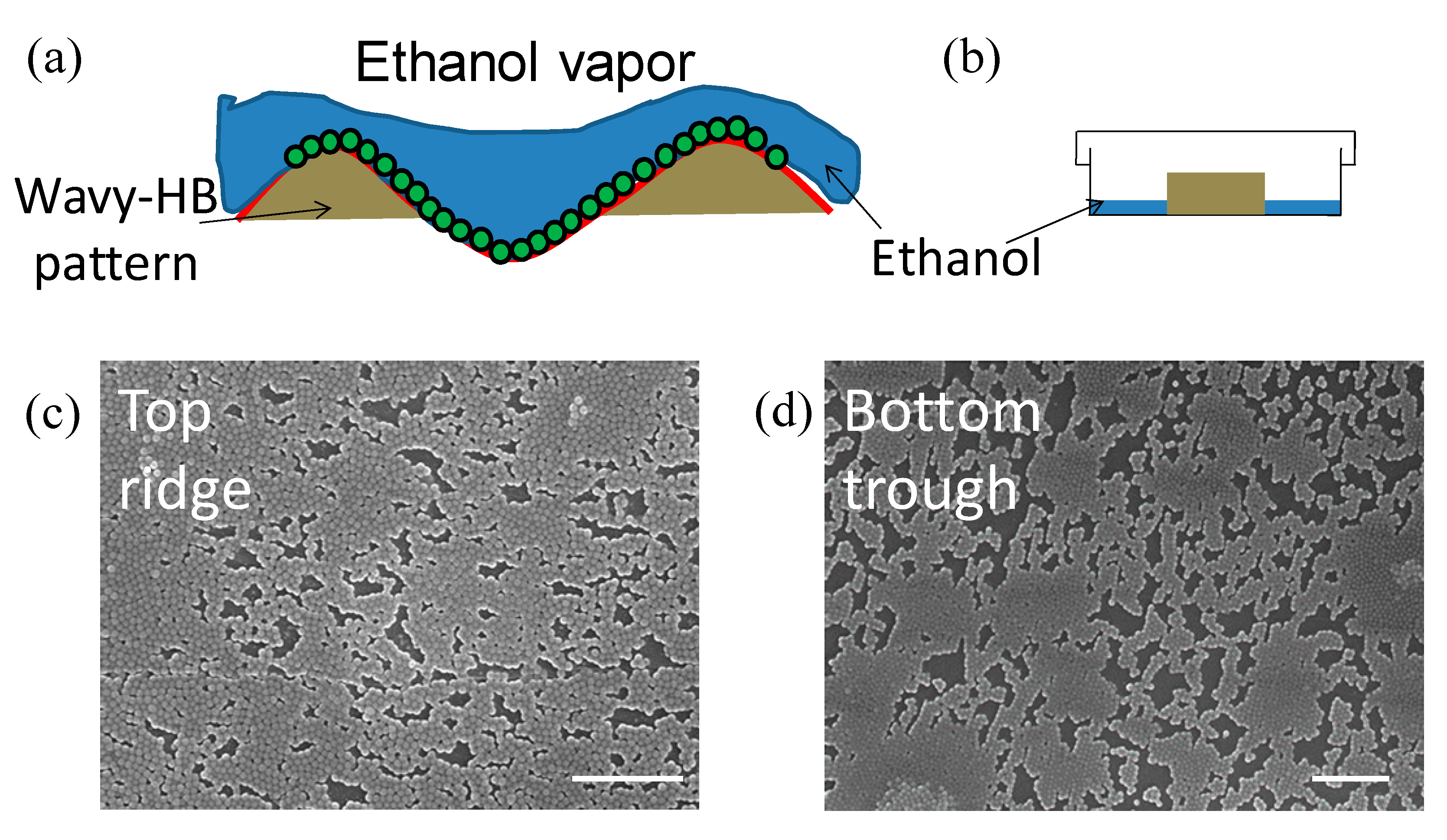
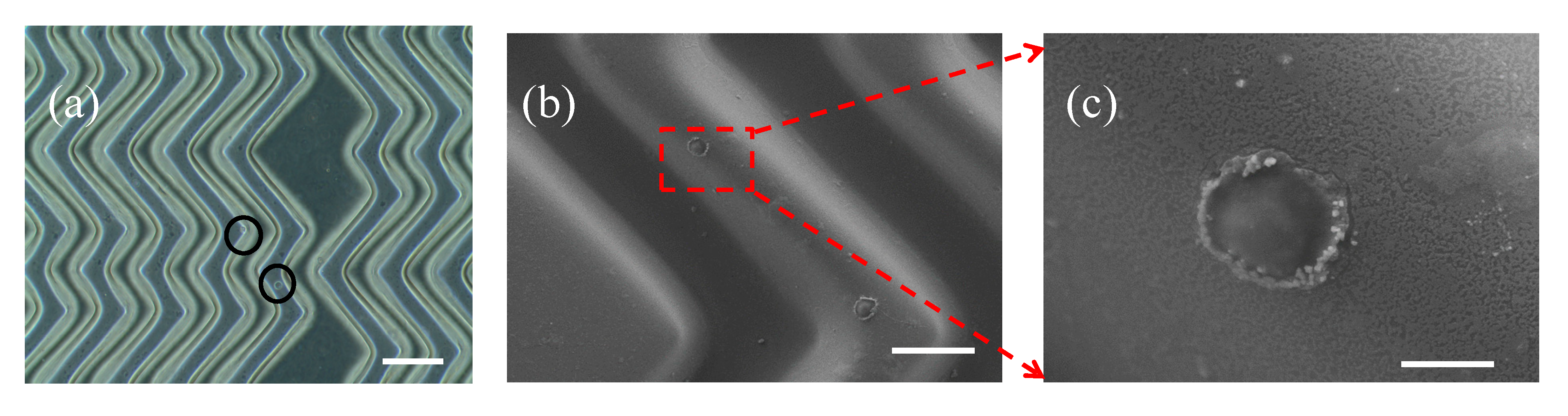
3.2. Characterization of Hierarchical Micro/nanostructures
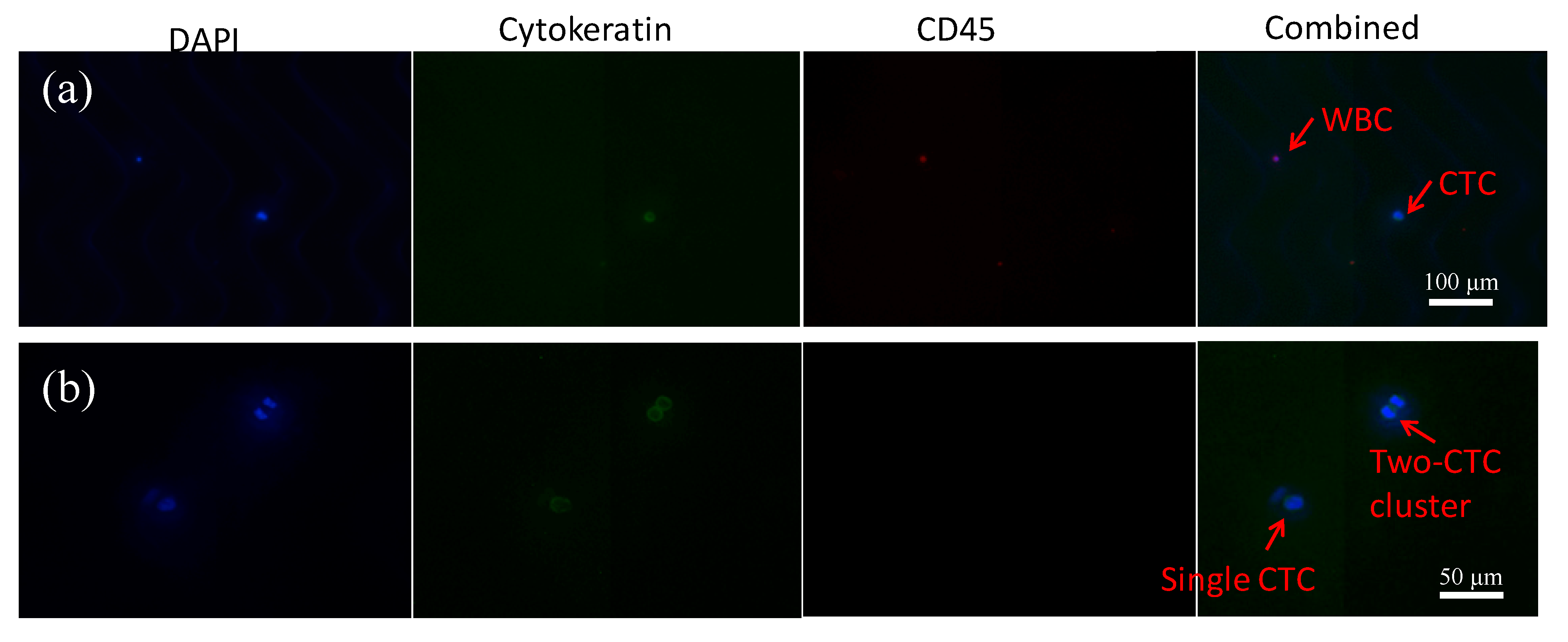
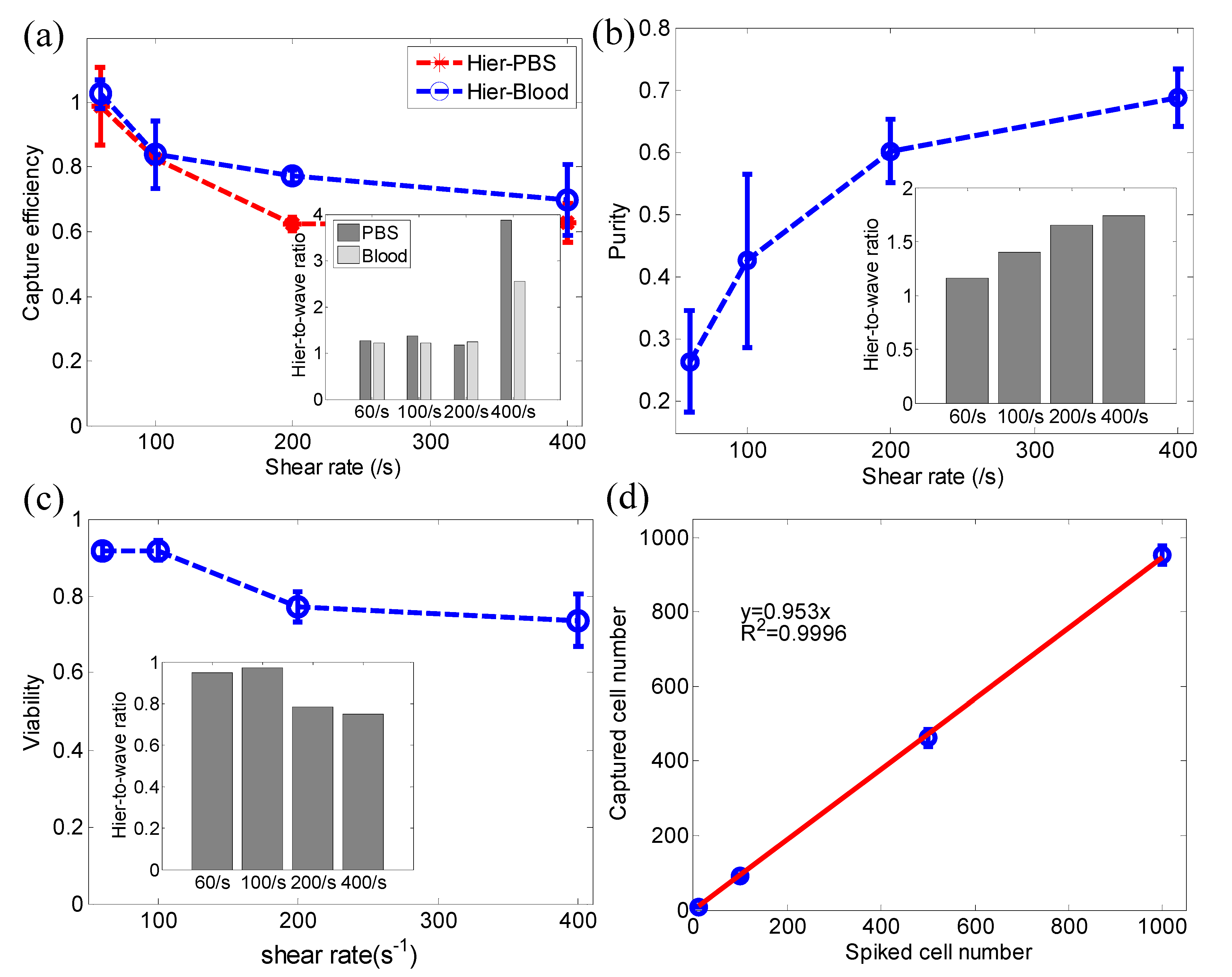
3.3. Cancer Cell Capture
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Albertini, J.J.; Lyman, G.H.; Cox, C.; Yeatman, T.; Balducci, L.; Ku, N.N.; Shivers, S.; Berman, C.; Wells, K.; Rapaport, D.; et al. Lymphatic mapping and sentinel node biopsy in the patient with breast cancer. Jama 1996, 276, 1818–1822. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Jia, X.Y.; de Bono, J.S.; Fleisher, M.; Pienta, K.J.; Raghavan, D.; Heller, G. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: A reanalysis of IMMC38 trial data. Lancet Oncol. 2009, 10, 233–239. [Google Scholar] [CrossRef]
- Heneghan, H.M.; Miller, N.; Lowery, A.J.; Sweeney, K.J.; Newell, J.; Kerin, M.J. Circulating microRNAs as Novel Minimally Invasive Biomarkers for Breast Cancer. Ann. Surg. 2010, 251, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, J.; Skog, J.; Nordstrand, A.; Baranov, V.; Mincheva-Nilsson, L.; Breakefield, X.O.; Widmark, A. Prostate cancer-derived urine exosomes: A novel approach to biomarkers for prostate cancer. Br. J. Cancer 2009, 100, 1603–1607. [Google Scholar] [CrossRef]
- Diamandis, E.P.; Yousef, G.M. Human tissue kallikreins: A family of new cancer biomarkers. Clin. Chem. 2002, 48, 1198–1205. [Google Scholar]
- Ilie, M.; Hofman, V.; Long-Mira, E.; Selva, E.; Vignaud, J.M.; Padovani, B.; Mouroux, J.; Marquette, C.H.; Hofman, P. “Sentinel” circulating tumor cells allow early diagnosis of lung cancer in patients with chronic obstructive pulmonary disease. PLoS ONE 2014, 9, e111597. [Google Scholar] [CrossRef]
- De Bono, J.S.; Scher, H.I.; Montgomery, R.B.; Parker, C.; Miller, M.C.; Tissing, H.; Doyle, G.V.; Terstappen, L.W.W.M.; Pienta, K.J.; Raghavan, D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2008, 14, 6302–6309. [Google Scholar] [CrossRef]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H.L.; et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef]
- Bhagat, A.A.S.; Hou, H.W.; Li, L.D.; Lim, C.T.; Han, J.Y. Pinched flow coupled shear-modulated inertial microfluidics for high-throughput rare blood cell separation. Lab Chip 2011, 11, 1870–1878. [Google Scholar] [CrossRef]
- Sollier, E.; Go, D.E.; Che, J.; Gossett, D.R.; O’Byrne, S.; Weaver, W.M.; Kummer, N.; Rettig, M.; Goldman, J.; Nickols, N.; et al. Size-selective collection of circulating tumor cells using Vortex technology. Lab Chip 2014, 14, 63–77. [Google Scholar] [CrossRef]
- Moon, H.S.; Kwon, K.; Kim, S.I.; Han, H.; Sohn, J.; Lee, S.; Jung, H.I. Continuous separation of breast cancer cells from blood samples using multi-orifice flow fractionation (MOFF) and dielectrophoresis (DEP). Lab Chip 2011, 11, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Pethig, R.; Menachery, A.; Pells, S.; De Sousa, P. Dielectrophoresis: A review of applications for stem cell research. Biomed. Res. Int. 2010, 2010, 182581. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.H.; Mao, W.B.; Byler, R.; Patel, K.; Henegar, C.; Alexeev, A.; Sulchek, T. Stiffness dependent separation of cells in a microfluidic device. PLoS ONE 2013, 8, e75901. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.H.; Crawford, K.; Turbyfield, C.; Lam, W.; Alexeev, A.; Sulchek, T. Microfluidic cellular enrichment and separation through differences in viscoelastic deformation. Lab Chip 2015, 15, 532–540. [Google Scholar] [CrossRef]
- Nagrath, S.; Sequist, L.V.; Maheswaran, S.; Bell, D.W.; Irimia, D.; Ulkus, L.; Smith, M.R.; Kwak, E.L.; Digumarthy, S.; Muzikansky, A.; et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007, 450, 1235. [Google Scholar] [CrossRef]
- Stott, S.L.; Hsu, C.H.; Tsukrov, D.I.; Yu, M.; Miyamoto, D.T.; Waltman, B.A.; Rothenberg, S.M.; Shah, A.M.; Smas, M.E.; Korir, G.K.; et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc. Natl. Acad. Sci. USA 2010, 107, 18392–18397. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Phillips, J.A.; Yan, J.L.; Li, Q.G.; Fan, Z.H.; Tan, W.H. Aptamer-Based Microfluidic Device for Enrichment, Sorting, and Detection of Multiple Cancer Cells. Anal. Chem. 2009, 81, 7436–7442. [Google Scholar] [CrossRef] [Green Version]
- Hyun, K.A.; Lee, T.Y.; Jung, H.I. Negative enrichment of circulating tumor cells using a geometrically activated surface interaction chip. Anal. Chem. 2013, 85, 4439–4445. [Google Scholar] [CrossRef]
- Casavant, B.P.; Mosher, R.; Warrick, J.W.; Maccoux, L.J.; Berry, S.M.F.; Becker, J.T.; Chen, V.; Lang, J.M.; McNeel, D.G.; Beebe, D.J. A negative selection methodology using a microfluidic platform for the isolation and enumeration of circulating tumor cells. Methods 2013, 64, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Sheng, W.A.; Ogunwobi, O.O.; Chen, T.; Zhang, J.L.; George, T.J.; Liu, C.; Fan, Z.H. Capture, release and culture of circulating tumor cells from pancreatic cancer patients using an enhanced mixing chip. Lab Chip 2014, 14, 89–98. [Google Scholar] [CrossRef]
- Zang, F.; Gerasopoulos, K.; Brown, A.D.; Culver, J.N.; Ghodssi, R. Capillary microfluidics-assembled virus-like particle bio-nano-receptor interfaces for label-free biosensing. ACS Appl. Mater. Interfaces 2017, 9, 8471−8479. [Google Scholar] [CrossRef] [PubMed]
- Stroock, A.D.; Dertinger, S.K.W.; Ajdari, A.; Mezic, I.; Stone, H.A.; Whitesides, G.M. Chaotic mixer for microchannels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Ye, K.; Gao, J.; Wu, Y.F.; Guo, J.H.; Hui, K.M.; Kang, Y.J. Isolation and elution of Hep3B circulating tumor cells using a dual-functional herringbone chip. Microfluid. Nanofluid. 2014, 16, 605–612. [Google Scholar] [CrossRef]
- Wang, S.; Sohrabi, S.; Xu, J.; Yang, J.; Liu, Y. Geometry design of herringbone structures for cancer cell capture in a microfluidic device. Microfluid. Nanofluid. 2016, 20, 148. [Google Scholar] [CrossRef]
- Wang, S.T.; Liu, K.; Liu, J.A.; Yu, Z.T.F.; Xu, X.W.; Zhao, L.B.; Lee, T.; Lee, E.K.; Reiss, J.; Lee, Y.K.; et al. Highly efficient capture of circulating tumor cells by using nanostructured silicon substrates with integrated chaotic micromixers. Angew. Chem. Int. Ed. 2011, 50, 3084–3088. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.T.; Zhao, L.B.; Shen, Q.L.; Garcia, M.A.; Wu, D.X.; Hou, S.; Song, M.; Xu, X.C.; OuYang, W.H.; OuYang, W.W.L.; et al. NanoVelcro Chip for CTC enumeration in prostate cancer patients. Methods 2013, 64, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Hou, S.; Zhao, L.B.; Shen, Q.L.; Yu, J.H.; Ng, C.; Kong, X.J.; Wu, D.X.; Song, M.; Shi, X.H.; Xu, X.C.; et al. Polymer Nanofiber-Embedded Microchips for Detection, Isolation, and Molecular Analysis of Single Circulating Melanoma Cells. Angew. Chem. Int. Ed. 2013, 52, 3379–3383. [Google Scholar] [CrossRef] [Green Version]
- Ke, Z.; Lin, M.; Chen, J.F.; Choi, J.S.; Zhang, Y.; Fong, A.; Liang, A.J.; Chen, S.F.; Li, Q.; Fang, W.; et al. Programming thermoresponsiveness of NanoVelcro substrates enables effective purification of circulating tumor cells in lung cancer patients. ACS Nano 2015, 9, 62–70. [Google Scholar] [CrossRef]
- He, R.; Wang, S.; Andrews, G.; Shi, W.; Liu, Y. Generation of customizable micro-wavy pattern through grayscale direct image lithography. Sci. Rep. 2016, 6, 21621. [Google Scholar] [CrossRef]
- Wang, S.Q.; Wan, Y.; Liu, Y.L. Effects of nanopillar array diameter and spacing on cancer cell capture and cell behaviors. Nanoscale 2014, 6, 12482–12489. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.Q.; Thomas, A.; Lee, E.; Yang, S.; Cheng, X.H.; Liu, Y.L. Highly efficient and selective isolation of rare tumor cells using a microfluidic chip with wavy-herringbone micro-patterned surfaces. Analyst 2016, 141, 2228–2237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Lin, P.C.; Yang, S. Mechanically switchable wetting on wrinkled elastomers with dual-scale roughness. Soft Matter 2009, 5, 1011–1018. [Google Scholar] [CrossRef]
- Kumnorkaew, P.; Gilchrist, J.F. Effect of nanoparticle concentration on the convective deposition of binary suspensions. Langmuir 2009, 25, 6070–6075. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.J. Particle convection in an evaporating colloidal droplet. Langmuir 2002, 18, 60–67. [Google Scholar] [CrossRef]
- Wan, Y.; Tan, J.F.; Asghar, W.; Kim, Y.T.; Liu, Y.L.; Iqbal, S.M. Velocity Effect on Aptamer-Based Circulating Tumor Cell Isolation in Microfluidic Devices. J. Phys. Chem. B 2011, 115, 13891–13896. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, X.L.; Su, B.; Li, J.; Jiang, L.; Han, D.; Wang, S.T. Aptamer-Mediated Efficient Capture and Release of T Lymphocytes on Nanostructured Surfaces. Adv. Mater. 2011, 23, 4376–4380. [Google Scholar] [CrossRef]
- Chen, W.Q.; Weng, S.N.; Zhang, F.; Allen, S.; Li, X.; Bao, L.W.; Lam, R.H.W.; Macoska, J.A.; Merajver, S.D.; Fu, J.P. Nanoroughened Surfaces for Efficient Capture of Circulating Tumor Cells without Using Capture Antibodies. ACS Nano 2013, 7, 566–575. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Cho, Y.; Cheng, X.; Yang, S.; Liu, Y.; Liu, Y. Integration of Hierarchical Micro-/Nanostructures in a Microfluidic Chip for Efficient and Selective Isolation of Rare Tumor Cells. Micromachines 2019, 10, 698. https://doi.org/10.3390/mi10100698
Wang S, Cho Y, Cheng X, Yang S, Liu Y, Liu Y. Integration of Hierarchical Micro-/Nanostructures in a Microfluidic Chip for Efficient and Selective Isolation of Rare Tumor Cells. Micromachines. 2019; 10(10):698. https://doi.org/10.3390/mi10100698
Chicago/Turabian StyleWang, Shunqiang, Younghyun Cho, Xuanhong Cheng, Shu Yang, Yi Liu, and Yaling Liu. 2019. "Integration of Hierarchical Micro-/Nanostructures in a Microfluidic Chip for Efficient and Selective Isolation of Rare Tumor Cells" Micromachines 10, no. 10: 698. https://doi.org/10.3390/mi10100698
APA StyleWang, S., Cho, Y., Cheng, X., Yang, S., Liu, Y., & Liu, Y. (2019). Integration of Hierarchical Micro-/Nanostructures in a Microfluidic Chip for Efficient and Selective Isolation of Rare Tumor Cells. Micromachines, 10(10), 698. https://doi.org/10.3390/mi10100698




