A Novel Button-Type Micro Direct Methanol Fuel Cell with Graphene Diffusion Layer
Abstract
1. Introduction
2. Experimental
3. Results and Discussion
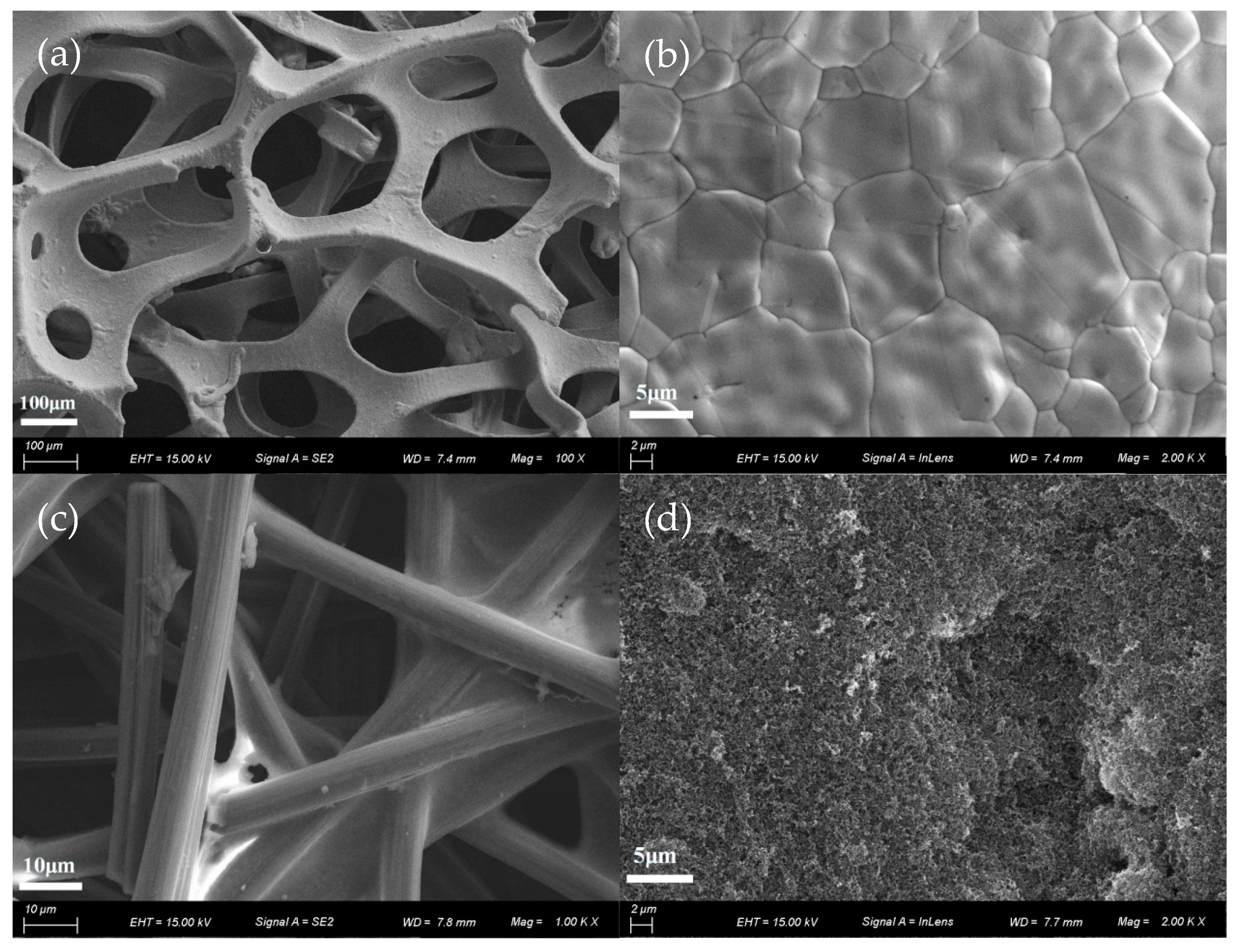
3.1. Physical Characterization of GDL
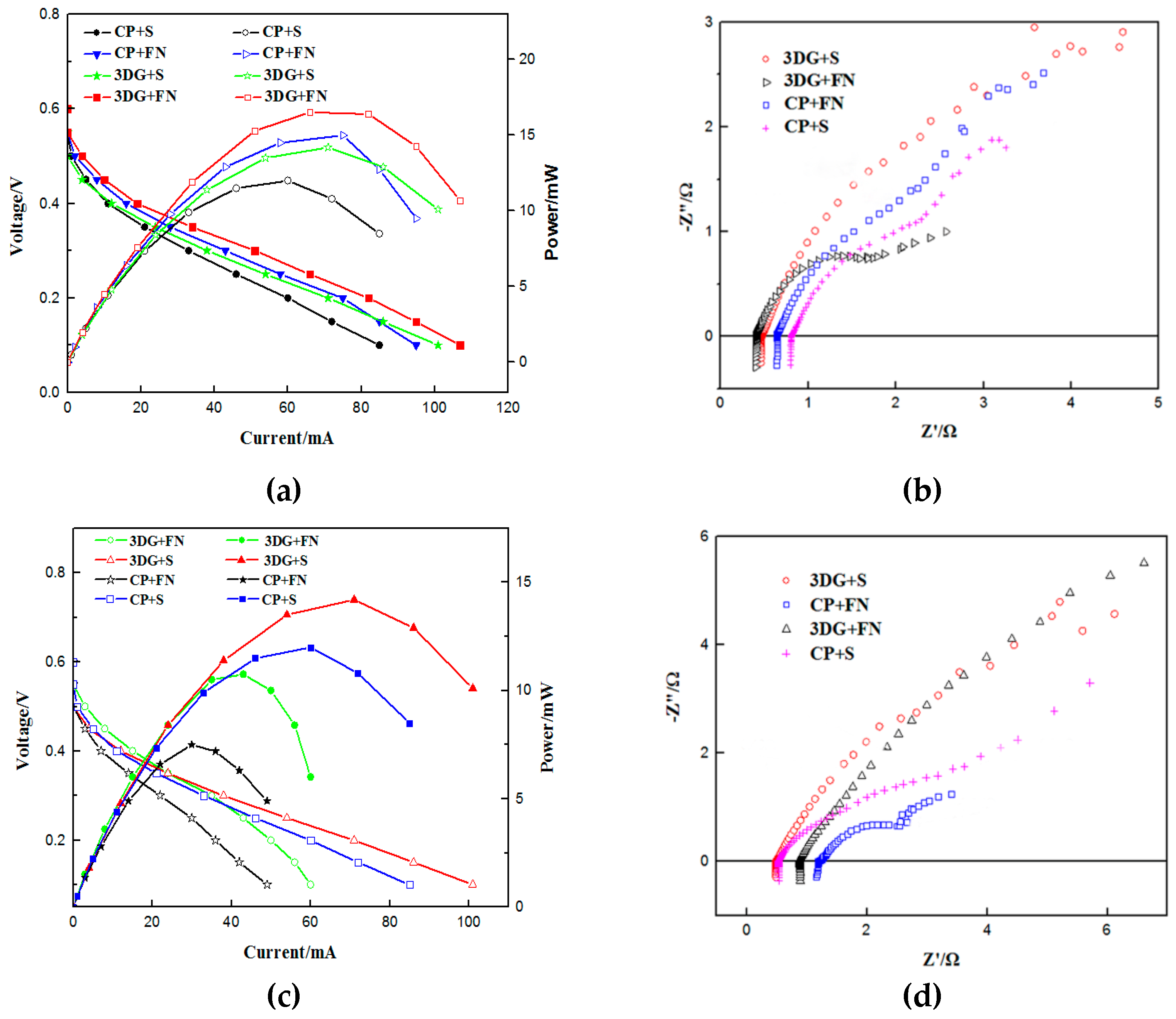
3.2. Cell Performance
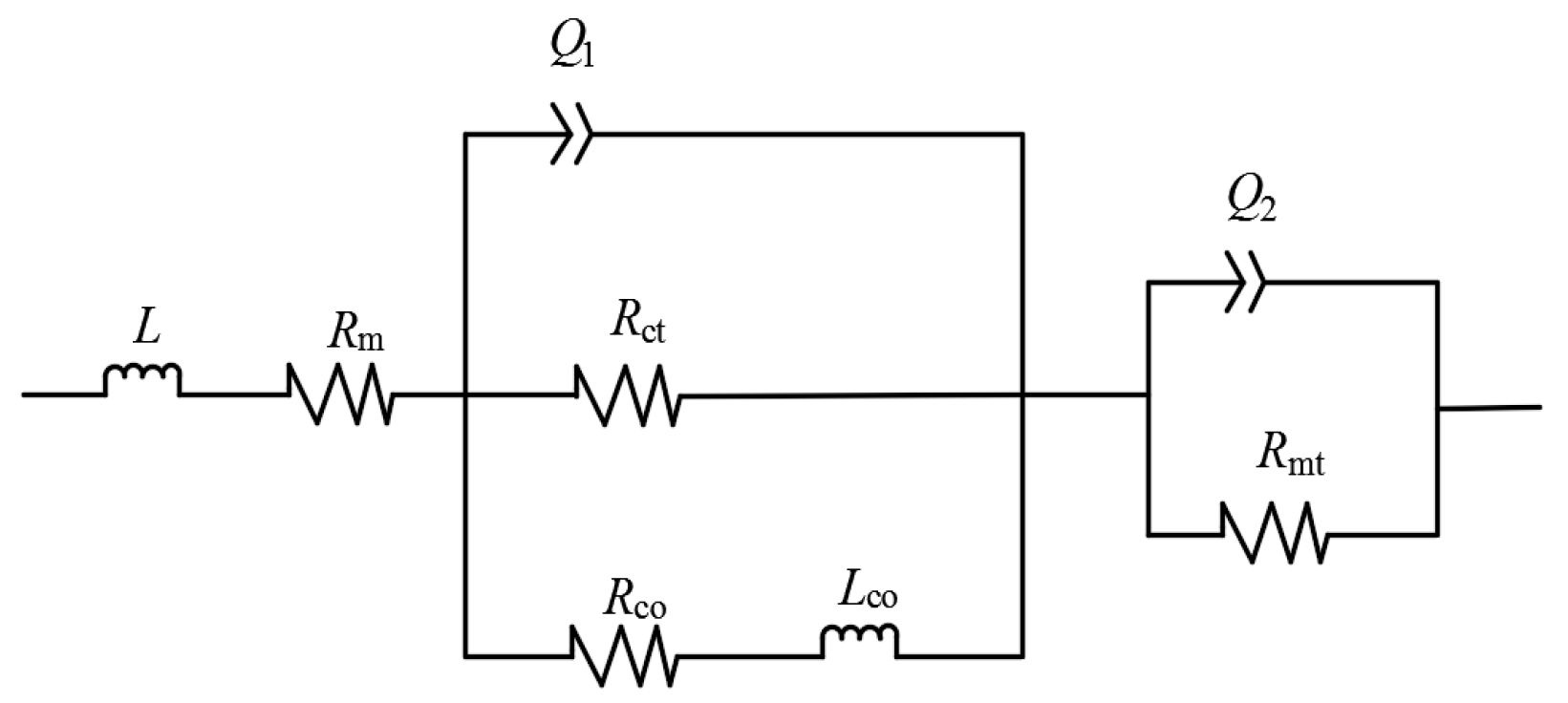
3.3. Equivalent Circuit Fitting
4. Anode Methanol Diffusion Model
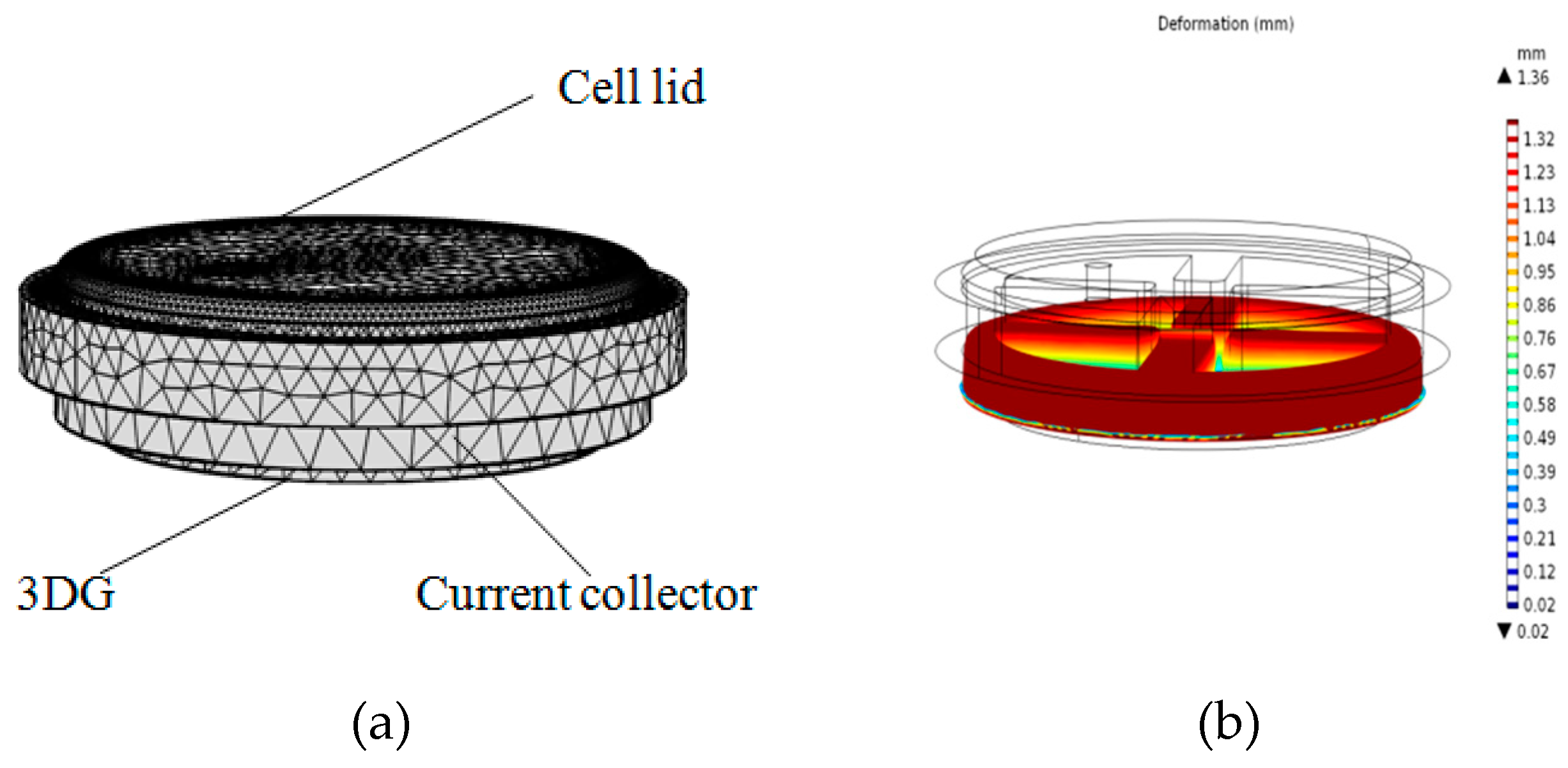
4.1. Deformation Analysis
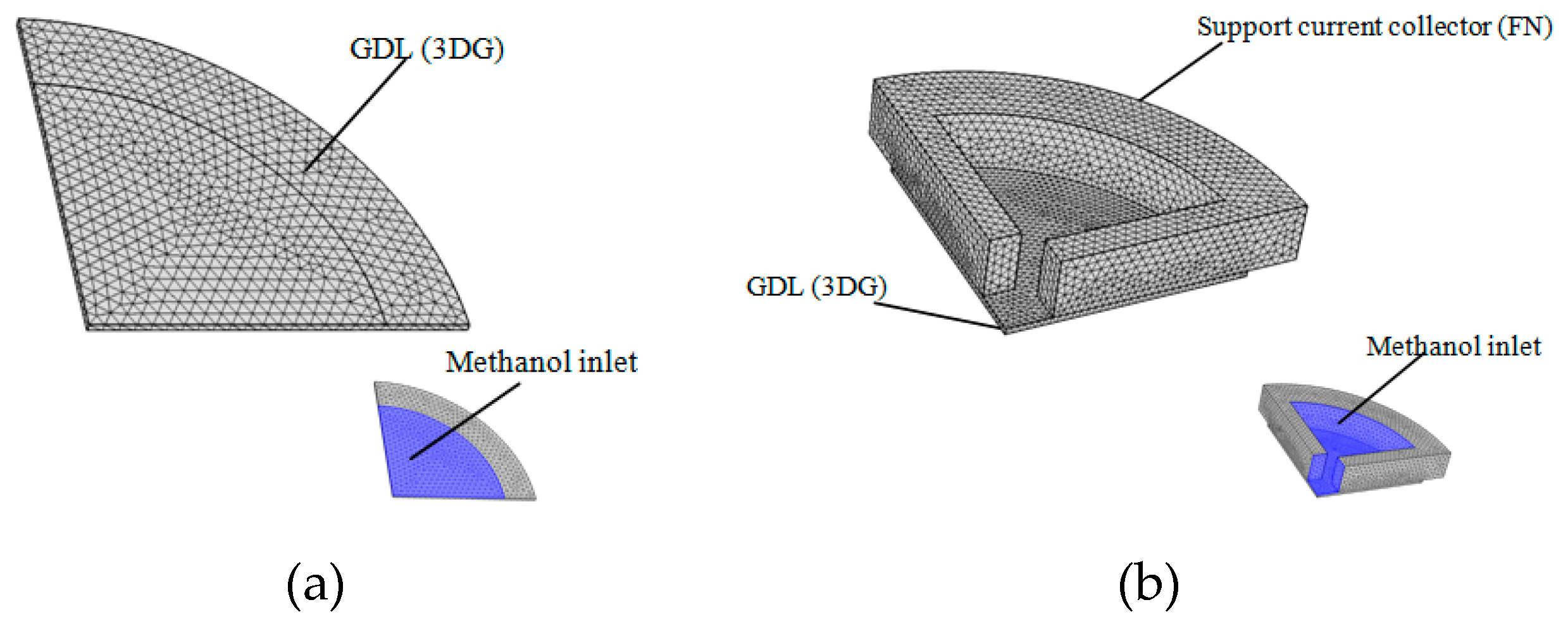
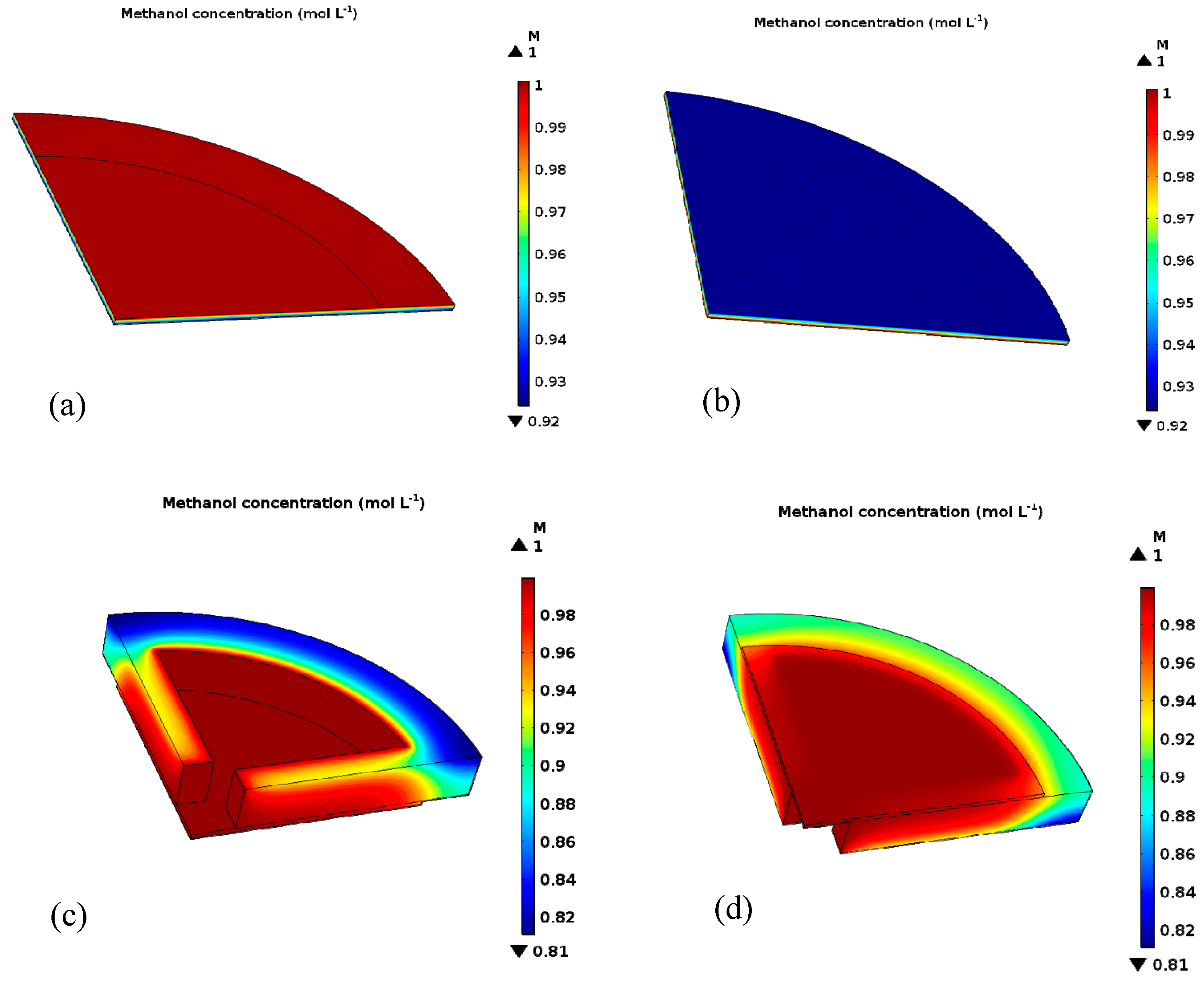
4.2. Methanol Concentration Diffusion Model
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mallick, R.K.; Thombre, S.B. Performance of passive DMFC with expanded metal mesh current collectors. Electrochim. Acta 2005, 7, 427–430. [Google Scholar] [CrossRef]
- Kamarudin, S.K.; Daud, W.R.W.; Ho, S.L.; Hasran, U.A. Overview on the challenges and developments of micro-direct methanol fuel cells (DMFC). J. Power Sources 2007, 163, 743–754. [Google Scholar] [CrossRef]
- Lian, K.Y.; Yang, C.M. Sensor-less adaptive fuel concentration control for direct methanol fuel cells under varying load. J. Power Sources 2013, 231, 239–245. [Google Scholar] [CrossRef]
- Taymaz, F.A.; Benli, M. Application of response surface methodology to optimize and investigate the effects of operating conditions on the performance of DMFC. Energy 2011, 36, 1155–1160. [Google Scholar] [CrossRef]
- Sudaroli, B.M.; Kolaran, A.K. Experimental study on the effect of membrane thickness and PTFE (polytetrafluoroethylene) loading on methanol crossover in direct methanol fuel cell. Energy 2016, 98, 204–214. [Google Scholar] [CrossRef]
- Li, X.; Faghri, A. Local entropy generation analysis on passive high-concentration DMFCs (direct methanol fuel cell) with different cell structures. Energy 2011, 36, 403–414. [Google Scholar] [CrossRef]
- Young, H.S.; Hyung, J.K.; Woong, K.J.; Byeong, H.K. Development of active breathing micro PEM fuel cell. Int. J. Precis. Eng. Manuf. 2014, 1, 101–106. [Google Scholar]
- Takahiro, S.M.; Toshiyuki, M. Design and fabrication of pumpless small direct methanol fuel cells for portable applications. J. Power Sources 2004, 137, 277–283. [Google Scholar]
- Min, K.B.; Tanaka, S.J.; Esashi, M.S. Silicon-based micro-polymer electrolyte fuel cells. In Proceedings of the The Sixteenth Annual International Conference on Micro Electro Mechanical Systems, Kyoto, Japan, 23 January 2003; pp. 379–382. [Google Scholar]
- Yeom, J.; Mozsgai, G.Z.; Flachsbart, B.R. Microfabrication and characterization of a silicon-based millimeter scale, PEM fuel cell operating with hydrogen, methanol, or formic acid. Sens. Actuator B Chem. 2005, 107, 882–891. [Google Scholar] [CrossRef]
- Guo, Z.; Faghri, A. Development of Planar Air Breathing Direct Methanol Fuel Cell Stacks. J. Power Sources 2006, 160, 1183–1194. [Google Scholar] [CrossRef]
- Stamm, J.; Varzi, A.; Latz, A. Modeling nucleation and growth of zinc oxide during discharge of primary zinc-air batteries. J. Power Sources 2017, 360, 136–149. [Google Scholar] [CrossRef]
- Cheng, C.-K.; Yeh, T.-K.; Tsai, M.-C.; Chou, H.-Y.; Wu, H.-C.; Hsieh, C.-K. The hybrid nanostructure of vertically aligned cobalt sulfide nanoneedles on three-dimensional graph decorated nickel foam for high performance methanol oxidation. Surf. Coat. Technol. 2017, 320, 536–541. [Google Scholar]
- Zhu, Y.L.; Liu, C.; Liang, J.S.; Wang, L.D. Investigation of the effects of compression pressure on direct methanol fuel cell. J. Power Sources 2011, 196, 264–269. [Google Scholar] [CrossRef]
- Liu, X.W.; Fang, S.; Ma, Z.Z.; Zhang, Y.F. Structure design and implementation of the passive μ-DMFC. Micromachines 2015, 6, 230–238. [Google Scholar] [CrossRef]
- Wang, X.H.; Zhou, Y.A.; Liu, L.T. Capillary-based water removal cathode for an air-breathing micro direct methanol fuel cell. Microsyst. Technol. 2014, 6, 1093–1101. [Google Scholar] [CrossRef]
- Munjewar, S.S.; Thombre, S.B. Effect of current collector roughness on performance of passive direct methanol fuel cell. Renew. Energy 2019, 138, 272–283. [Google Scholar] [CrossRef]
- Jin, B.D.; Wang, S.B.; Xie, X.F.; Guo, J.W.; Qi, L.; Wang, J.H. AC resistance spectrum of direct methanol fuel cell with low cathode platinum load catalyst. J. Tsinghua Univ. Nat. Sci. Ed. 2008, 12, 2107–2110. [Google Scholar]
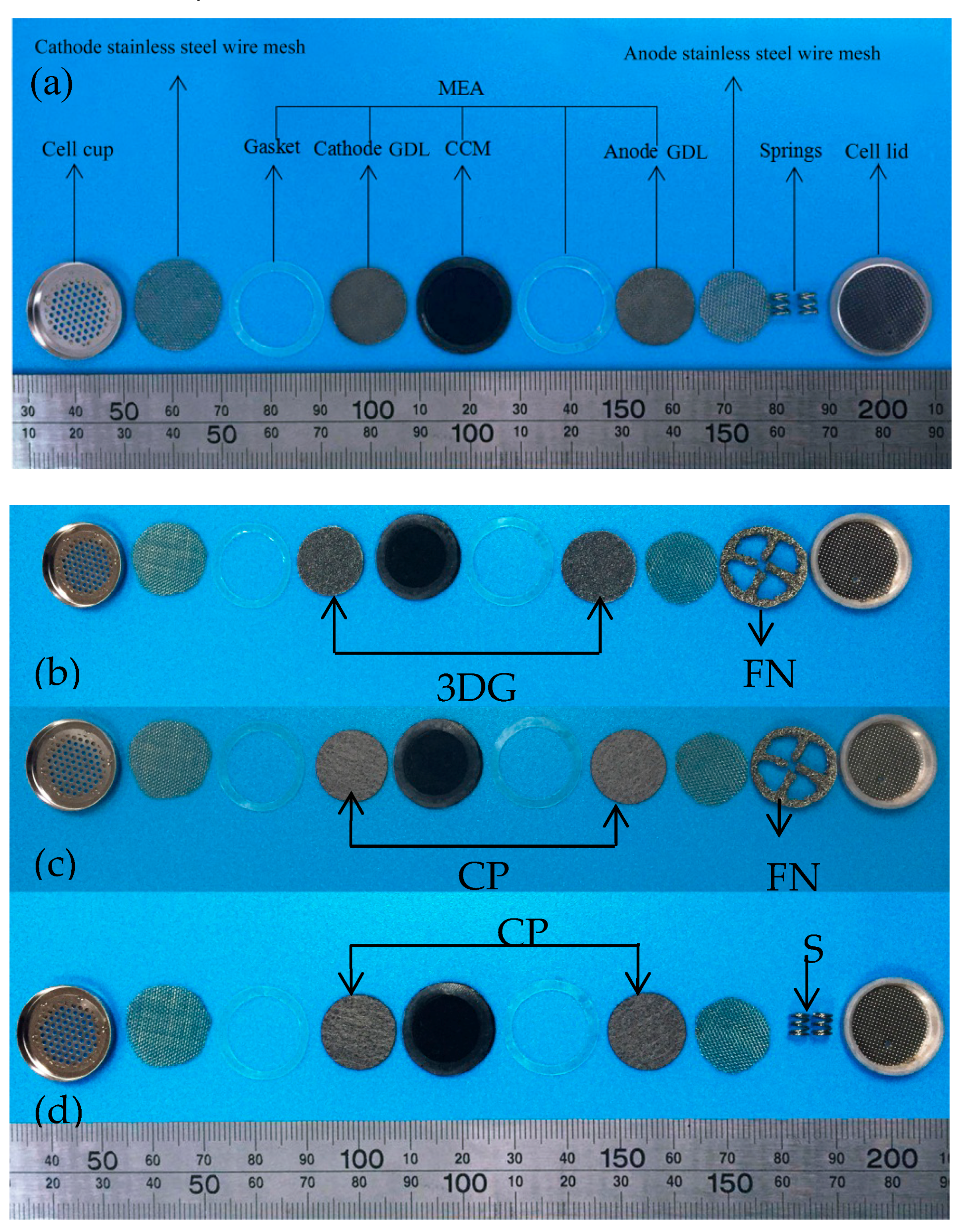
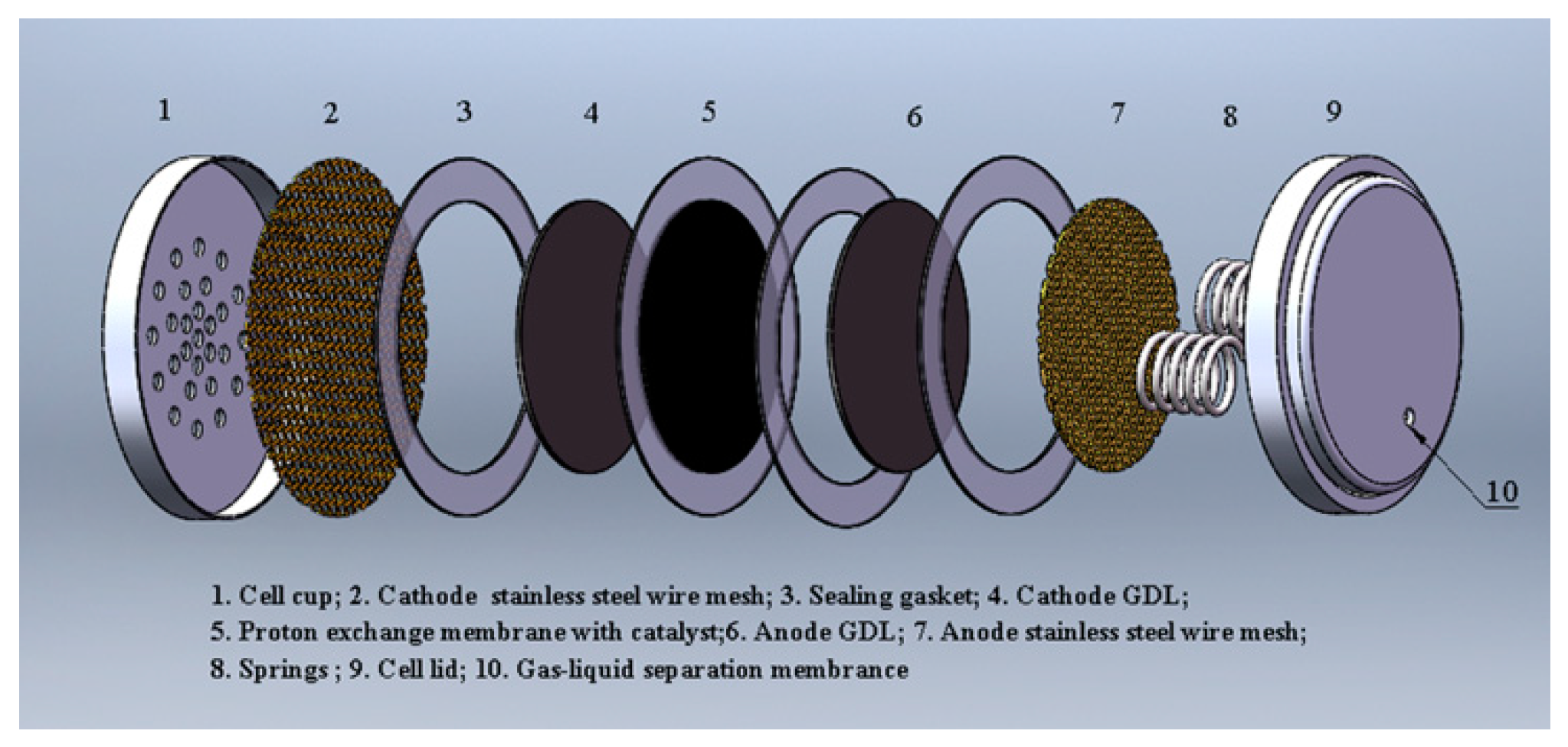
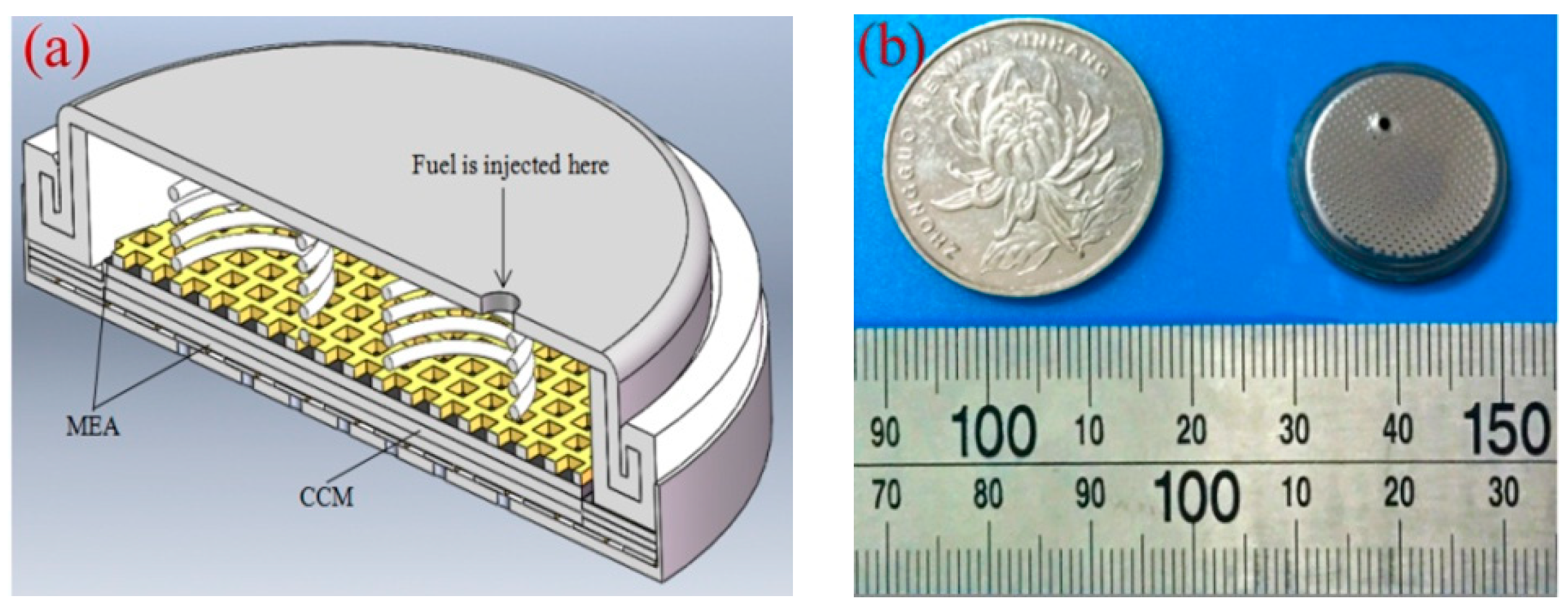
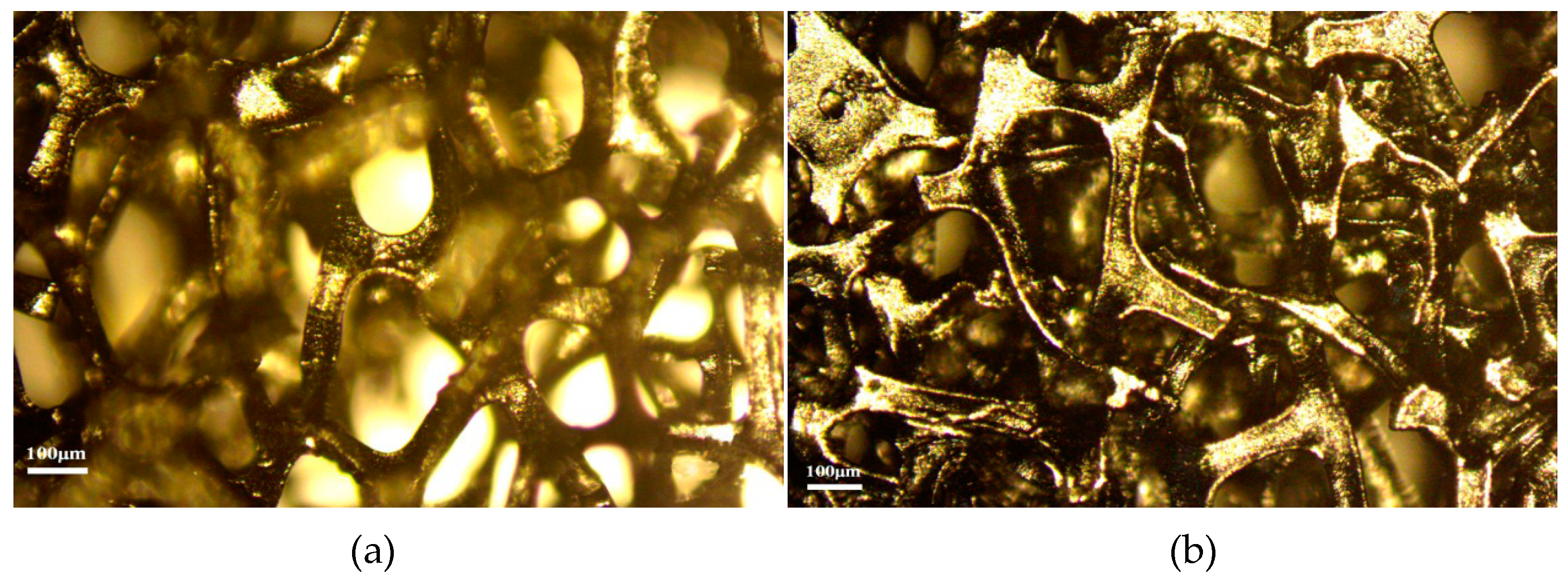
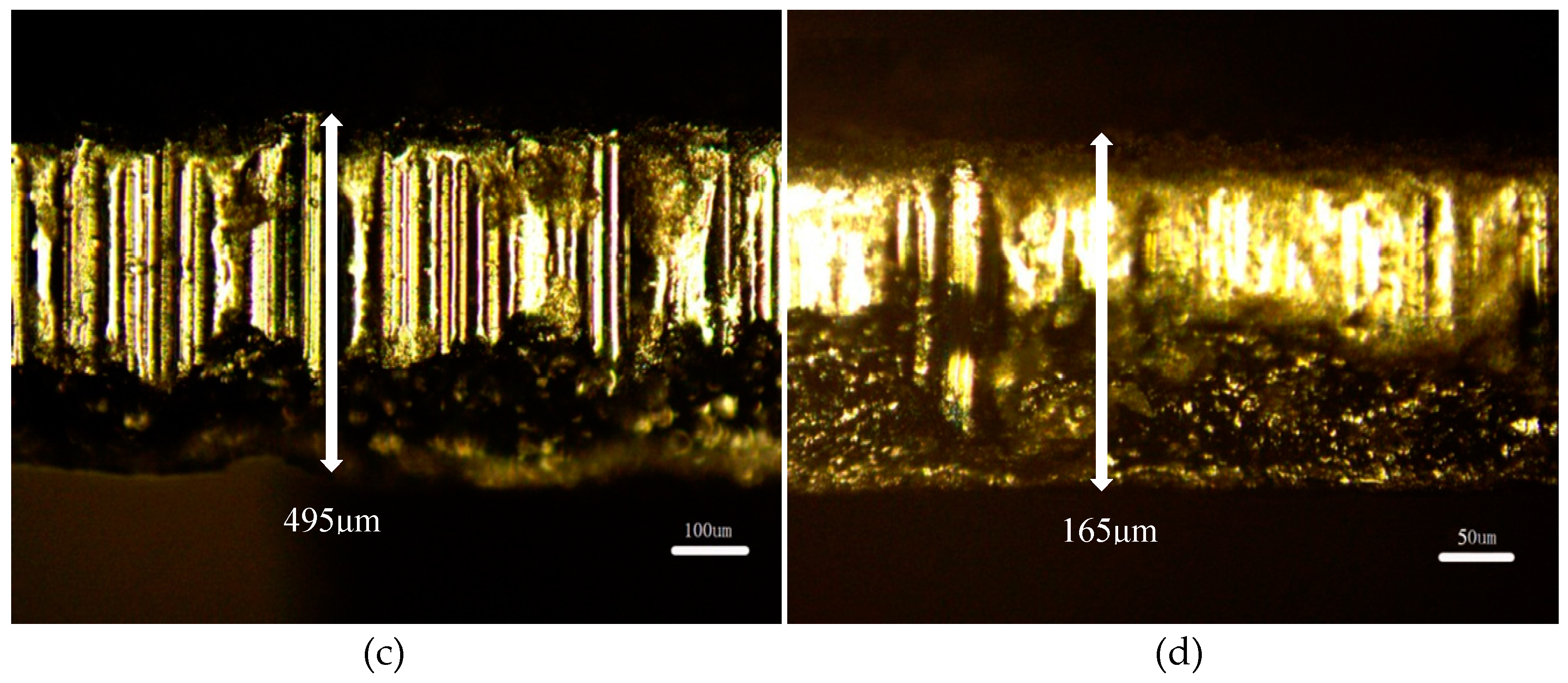
| Cell Type | GDL | CC | Thickness or Specificationof CC (mm) | Porosity of GDL | Thickness of GDL (mm) |
|---|---|---|---|---|---|
| 3DG + S | 3DG | Spring | 0.5 × 4 × 5 | 0.9 | 0.5 |
| 3DG + FN | 3DG | FN | 4 | 0.9 | 0.5 |
| CP + S | CP | Spring | 0.5 × 4 × 5 | 0.78 | 0.28 |
| CP + FN | CP | FN | 4 | 0.78 | 0.28 |
| Type | Area of μDMFC | Maximum Current Density | Maximum Power Density | Power Density per Unit Volume |
|---|---|---|---|---|
| μDMFC packaged by bolts [16] | 4.84 cm2 | 30 mA cm−2 | 2.35 mW cm−2 | 2.94 mW cm−3 |
| DMFC packaged by bolts [17] | 72.25 cm2 | 40 mA cm−2 | 19.7 mW cm−2 | 5.57 mW cm−3 |
| B-μDMFC | 2.96 cm2 | 34 mA cm−2 | 4.78 mW cm−2 | 11.85 mW cm−3 |
| Type of μDMFC | Rm | Rct | Rco | Rmt | Maximum Power Density |
|---|---|---|---|---|---|
| 3DG + S | 0.45 Ω | 1.82 Ω | 3.52 Ω | 3.6 Ω | 4.78 mW cm−2 |
| CP + S | 0.54 Ω | 3.80 Ω | 0.61 Ω | 6.2 Ω | 4.20 mW cm−2 |
| CP + FN | 1.15 Ω | 0.67 Ω | 0.27 Ω | 2.01 Ω | 2.53 mW cm−2 |
| 3DG + FN | 0.86 Ω | 0.15 Ω | 0.38 Ω | 11.6 Ω | 3.62 mW cm−2 |
| Parameters | Units | Values |
|---|---|---|
| Operating temperature T | K | 300 |
| Thickness of FN | mm | 2.4 |
| Thickness of 3DG | mm | 0.16 |
| Porosity of FN | - | 0.59 |
| Porosity of 3DG | - | 0.7 |
| Diffusion coefficient of methanol in water | m2 s−1 | 10−5.4163−999.778/T |
| Methanol penetration rate | mol cm−2 s−1 | 2 × 10−7 |
| Discharge current I | mA cm−2 | 34.12 |
| Methanol consumption flux | mol cm−2 s−1 | 2.6045 × 10−7 |
| Methanol inlet concentration | mol L−1 | 1 |
| F | C mol−1 | 96,485 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Gao, L.; Li, J. A Novel Button-Type Micro Direct Methanol Fuel Cell with Graphene Diffusion Layer. Micromachines 2019, 10, 658. https://doi.org/10.3390/mi10100658
Zhu Y, Gao L, Li J. A Novel Button-Type Micro Direct Methanol Fuel Cell with Graphene Diffusion Layer. Micromachines. 2019; 10(10):658. https://doi.org/10.3390/mi10100658
Chicago/Turabian StyleZhu, Yingli, Lei Gao, and Jianyu Li. 2019. "A Novel Button-Type Micro Direct Methanol Fuel Cell with Graphene Diffusion Layer" Micromachines 10, no. 10: 658. https://doi.org/10.3390/mi10100658
APA StyleZhu, Y., Gao, L., & Li, J. (2019). A Novel Button-Type Micro Direct Methanol Fuel Cell with Graphene Diffusion Layer. Micromachines, 10(10), 658. https://doi.org/10.3390/mi10100658





