Improvements of Microcontact Printing for Micropatterned Cell Growth by Contrast Enhancement
Abstract
1. Introduction
2. Materials and Methods
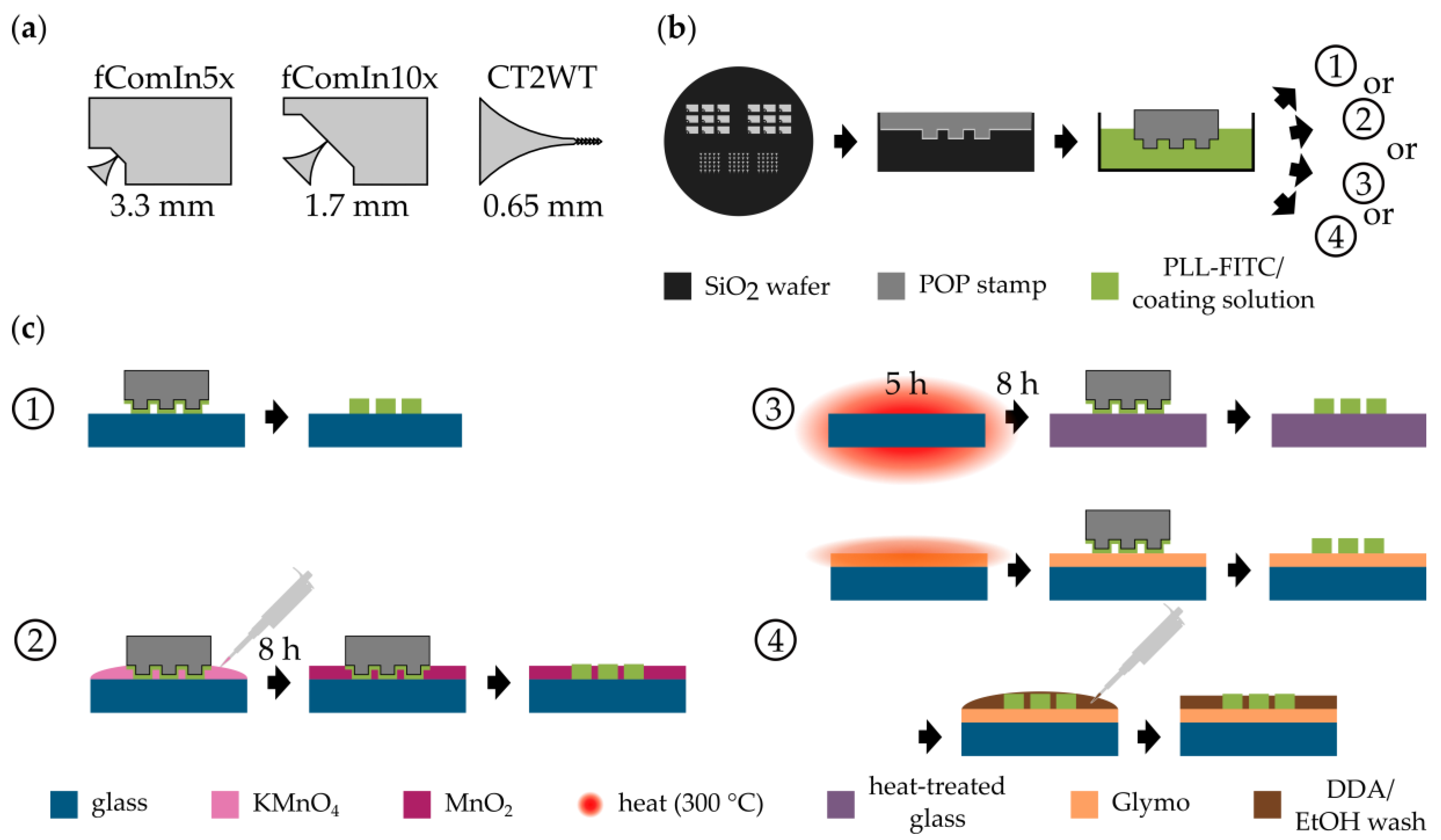
2.1. Stamp Fabrication
2.2. Chemical Vapor Deposition (CVD) of Silanes
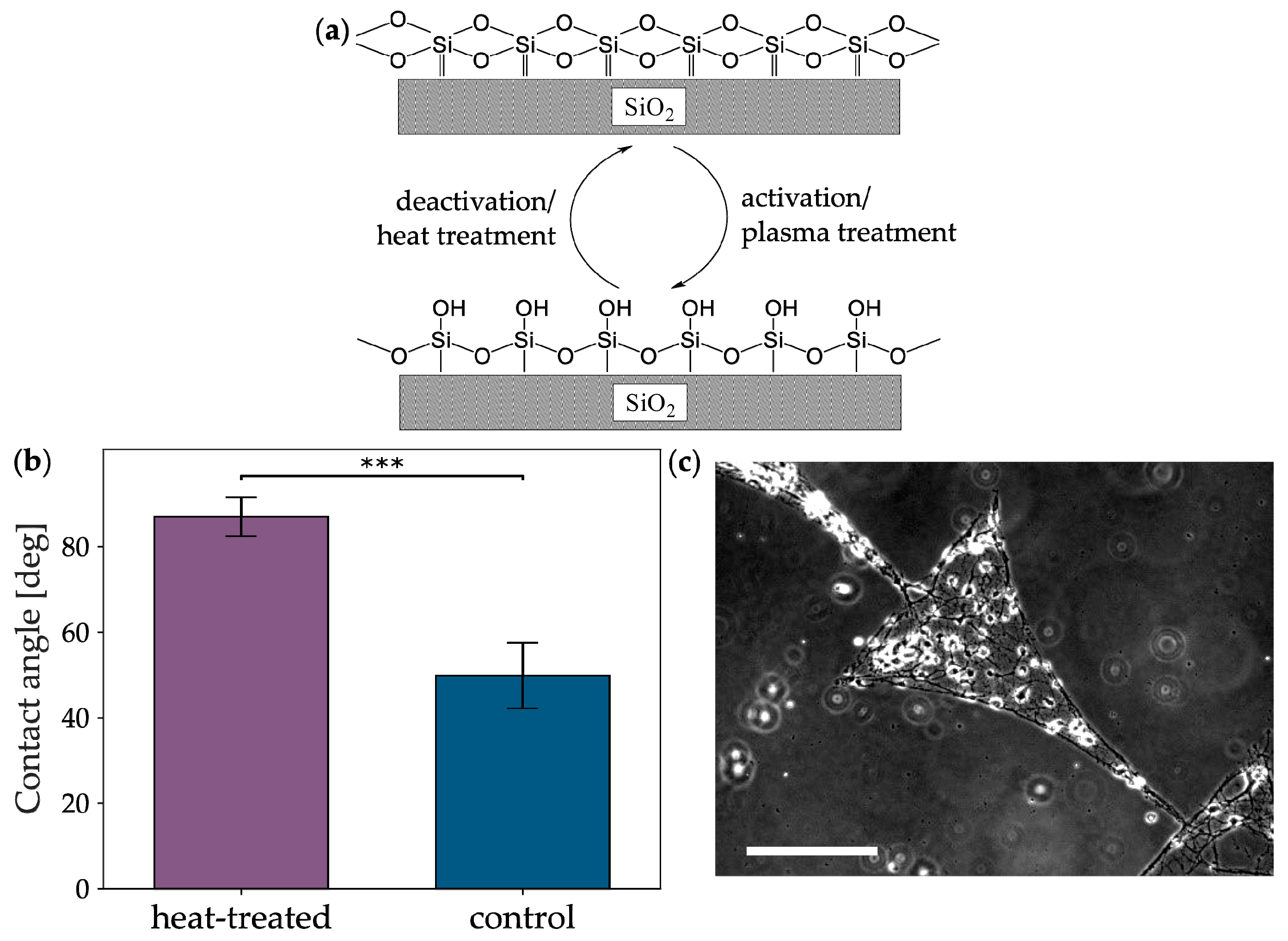
2.3. Heat Treatment
2.4. Microcontact Printing
2.5. Capillary Deposition of MnO2
2.6. Contact Angle Measurements
2.7. Light Microscopy
2.8. Cell Culture
2.9. Live-Dead Stainings
3. Results and Discussion
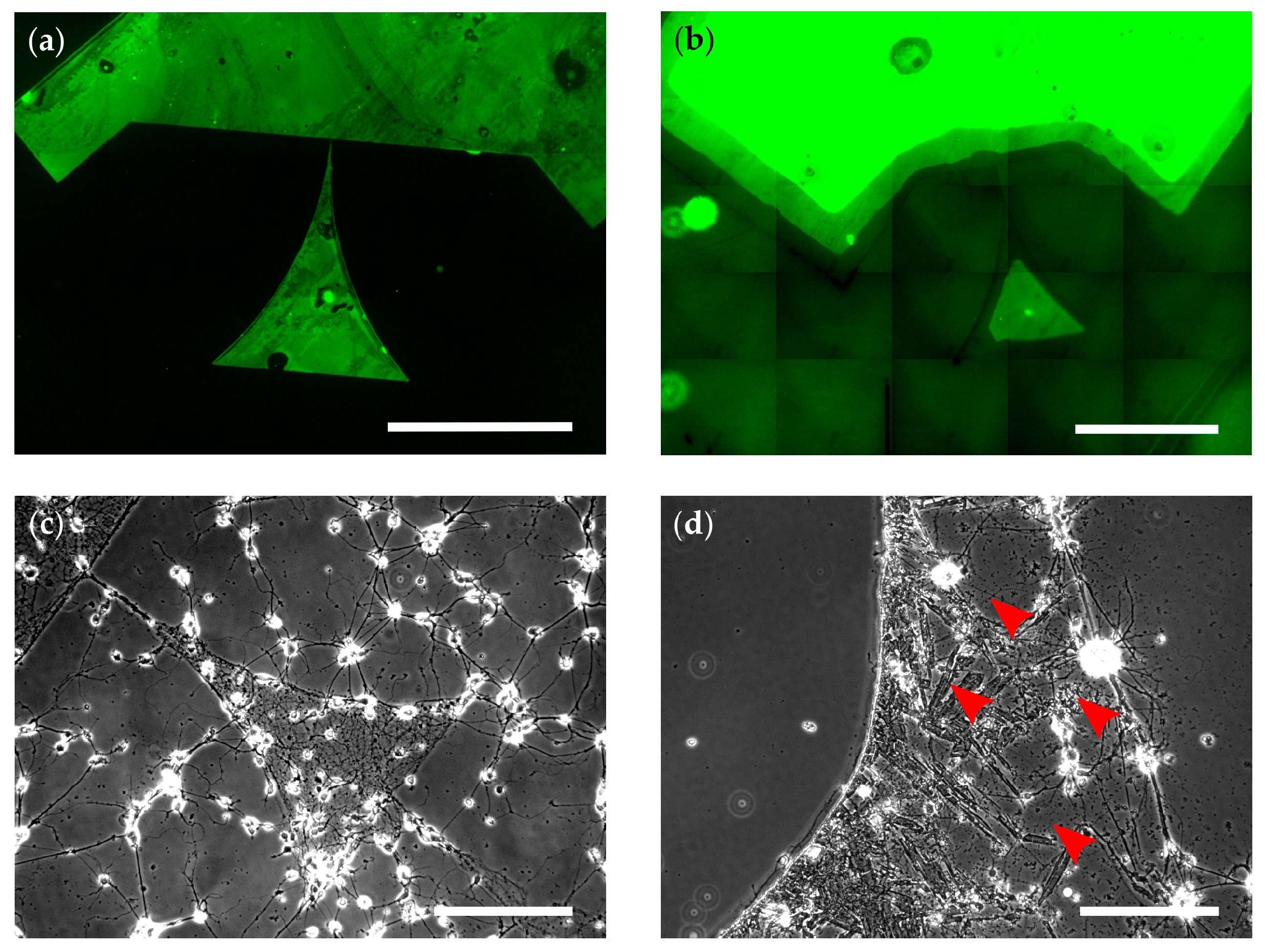
3.1. Background Toxification via Water-Insoluble MnO2
3.2. Heat-Induced Background Hydrophobization of Glass
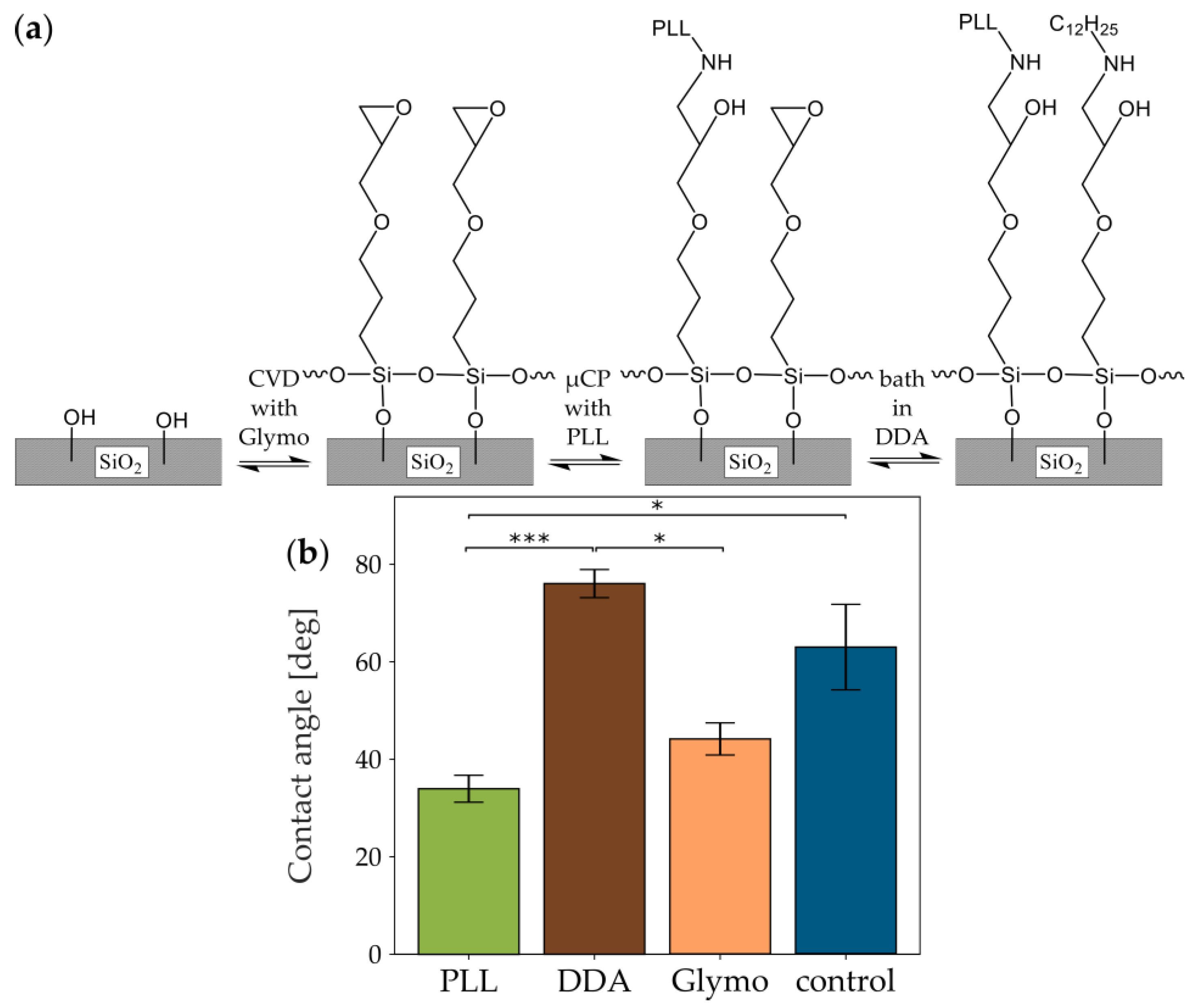
3.3. Glymo Silanization for Covalent Bonding of Amino Acids on a Cell-Repellent Background
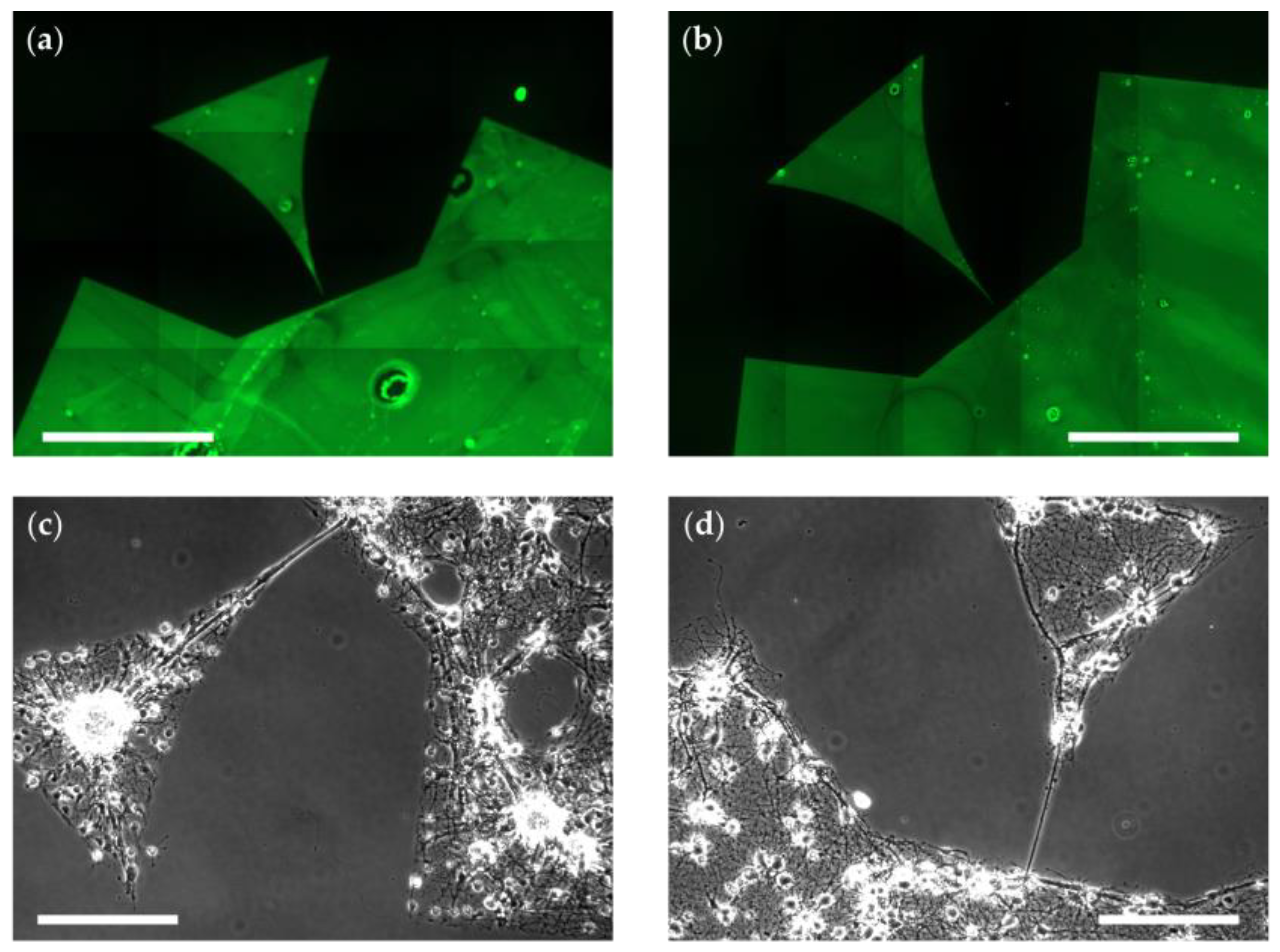
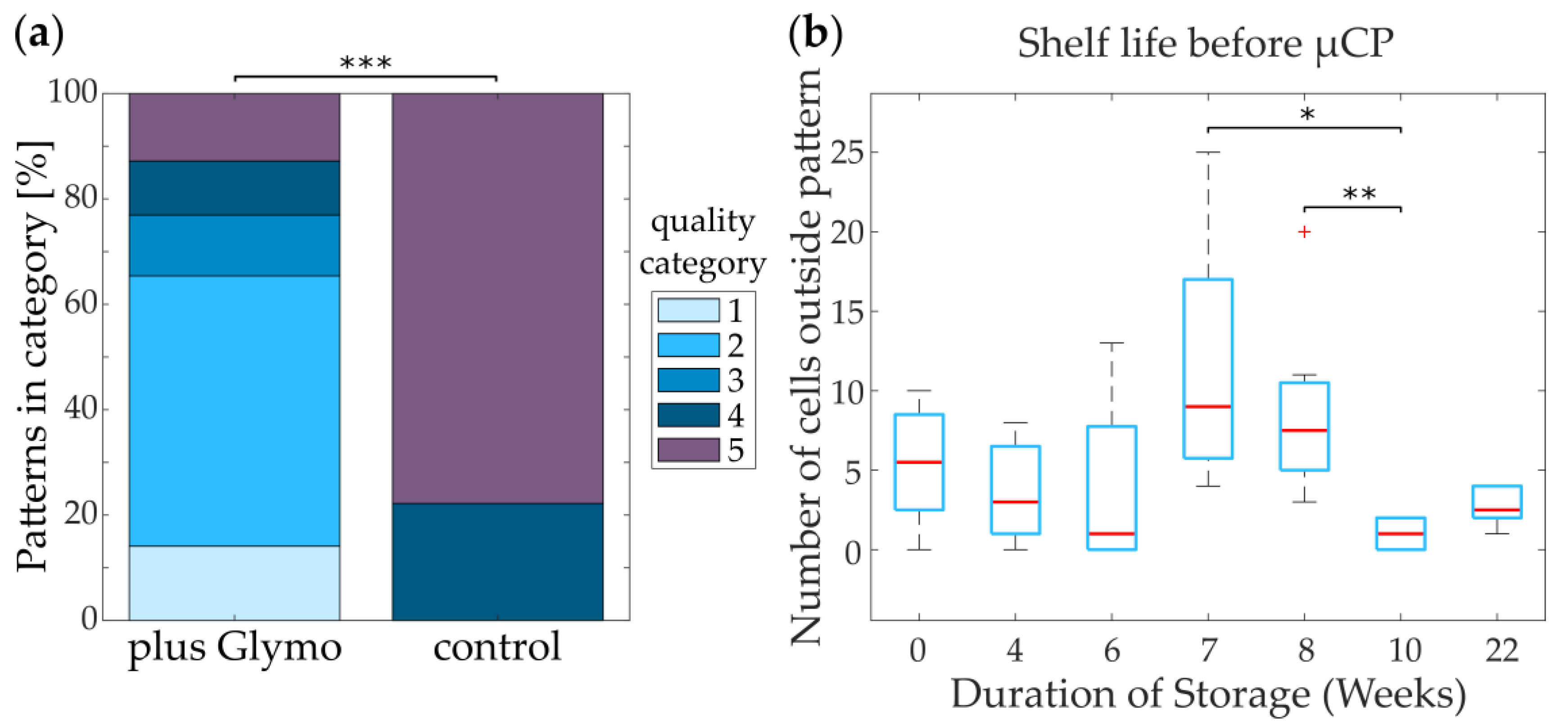
3.3.1. Glymo-Functionalized Glass Improves the Contrast between Pattern and Background
3.3.2. Glymo Is Long-Term Stable on Glass
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nam, Y.; Branch, D.W.; Wheeler, B.C. Epoxy-silane linking of biomolecules is simple and effective for patterning neuronal cultures. Biosens. Bioelectron. 2006, 22, 589–597. [Google Scholar] [CrossRef] [PubMed]
- James, C.D.; Spence, A.J.H.; Dowell-Mesfin, N.M.; Hussain, R.J.; Smith, K.L.; Craighead, H.G.; Isaacson, M.S.; Shain, W.; Turner, J.N. Extracellular recordings from patterned neuronal networks using planar microelectrode arrays. IEEE Trans. Biomed. Eng. 2004, 51, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Jungblut, M.; Knoll, W.; Thielemann, C.; Pottek, M. Triangular neuronal networks on microelectrode arrays: An approach to improve the properties of low-density networks for extracellular recording. Biomed. Microdevices 2009, 11, 1269–1278. [Google Scholar] [CrossRef][Green Version]
- Albers, J.; Offenhäusser, A. Signal propagation between neuronal populations controlled by micropatterning. Front. Bioeng. Biotechnol. 2016, 4, 46. [Google Scholar] [CrossRef] [PubMed]
- Feinerman, O.; Rotem, A.; Moses, E. Reliable neuronal logic devices from patterned hippocampal cultures. Nat. Phys. 2008, 4, 967–973. [Google Scholar] [CrossRef]
- Feinerman, O.; Segal, M.; Moses, E. Signal propagation along unidimensional neuronal networks. J. Neurophysiol. 2005, 94, 3406–3416. [Google Scholar] [CrossRef]
- Chen, H.-C.I.; Wolf, J.; Smith, D. Multichannel activity propagation across an engineered axon network. J. Neural Eng. 2017, 14, 026016. [Google Scholar] [CrossRef]
- Forró, C.; Thompson-Steckel, G.; Weaver, S.; Weydert, S.; Ihle, S.; Dermutz, H.; Aebersold, M.J.; Pilz, R.; Demkó, L.; Vörös, J. Modular microstruture design to build neuronal networks of defined functional connectivity. Biosens. Bioelectron. 2018, 122, 75–87. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front. Pharmacol. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Renault, R.; Sukenik, N.; Descroix, S.; Malaquin, L.; Viovy, J.L.; Peyrin, J.M.; Bottani, S.; Monceau, P.; Moses, E.; Vignes, M. Combining microfluidics, optogenetics and calcium imaging to study neuronal communication in vitro. PLoS ONE 2015, 10, e0120680. [Google Scholar] [CrossRef]
- Taylor, A.M.; Blurton-Jones, M.; Rhee, S.W.; Cribbs, D.H.; Cotman, C.W.; Jeon, N.L. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat. Methods 2005, 2, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Peyrin, J.-M.; Deleglise, B.; Saias, L.; Vignes, M.; Gougis, P.; Magnifico, S.; Betuing, S.; Pietri, M.; Caboche, J.; Vanhoutte, P.; et al. Axon diodes for the reconstruction of oriented neuronal networks in microfluidic chambers. Lab Chip 2011, 11, 3663. [Google Scholar] [CrossRef]
- Renault, R.; Durand, J.-B.; Viovy, J.-L.; Villard, C. Asymmetric axonal edge guidance: A new paradigm for building oriented neuronal networks. Lab Chip 2016, 16, 2188–2191. [Google Scholar] [CrossRef] [PubMed]
- Millet, L.J.; Gillette, M.U. New perspectives on neuronal development via microfluidic environments. Trends Neurosci. 2012, 35, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Faid, K.; Voicu, R.; Bani-Yaghoub, M.; Tremblay, R.; Mealing, G.; Py, C.; Barjovanu, R. Rapid fabrication and chemical patterning of polymer microstructures and their applications as a platform for cell cultures. Biomed. Microdevices 2005, 7, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Haq, F.; Anandan, V.; Keith, C.; Zhang, G. Neurite development in PC12 cells cultured on nanopillars and nanopores with sizes comparable with filopodia. Int. J. Nanomed. 2007, 2, 107. [Google Scholar] [CrossRef]
- Rajnicek, A.; Britland, S.; McCaig, C. Contact guidance of CNS neurites on grooved quartz: Influence of groove dimensions, neuronal age and cell type. J. Cell Sci. 1997, 110, 2905–2913. [Google Scholar] [PubMed]
- Simitzi, C.; Ranella, A.; Stratakis, E. Controlling the morphology and outgrowth of nerve and neuroglial cells: The effect of surface topography. Acta Biomater. 2017, 51, 21–52. [Google Scholar] [CrossRef]
- Aebersold, M.J.; Dermutz, H.; Forró, C.; Weydert, S.; Thompson-Steckel, G.; Vörös, J.; Demkó, L. “Brains on a chip”: Towards engineered neural networks. TrAC Trends Anal. Chem. 2016, 78, 60–69. [Google Scholar] [CrossRef]
- Wu, C.; Zhu, X.; Man, T.; Chung, P.S.; Teitell, M.A.; Chiou, P.-Y. Lift-off cell lithography for cell patterning with clean background. Lab Chip 2018, 18, 3074–3078. [Google Scholar] [CrossRef]
- Mrksich, M.; Dike, L.E.; Tien, J.; Ingber, D.E.; Whitesides, G.M. Using microcontact printing to pattern the attachment of mammalian cells to Self-Assembled Monolayers of Alkanethiolates on Transparent Films of Gold and Silver. Exp. Cell. Res. 1997, 235, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhu, M.; Zhang, W.; Zhen, X.; Pei, Z.; Xue, Q.; Zhi, C.; Shi, P. Ultrathin MXene-micropattern-based field-effect transistor for probing neural activity. Adv. Mater. 2016, 28, 3333–3339. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef] [PubMed]
- Offenhäusser, A.; Bocker-Meffert, S.; Decker, T.; Helpenstein, R.; Gasteier, P.; Groll, J.; Moller, M.; Reska, A.; Schafer, S.; Schulte, P.; et al. Microcontact printing of proteins for neuronal cell guidance. Soft Matter. 2007, 3, 290–298. [Google Scholar] [CrossRef]
- Markov, A.; Maybeck, V.; Wolf, N.; Mayer, D.; Offenhäusser, A.; Wördenweber, R. Engineering of neuron growth and enhancing cell-chip communication via mixed SAMs. ACS Appl. Mater. Interfaces 2018, 10, 18507–18514. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Matsumura, R.; Takaoki, H.; Katsurabayashi, S.; Hirano-Iwata, A.; Niwano, M. Unidirectional signal propagation in primary neurons micropatterned at a single-cell resolution. Appl. Phys. Lett. 2016, 109, 043703. [Google Scholar] [CrossRef]
- Martinez-Rivas, A.; González-Quijano, G.K.; Proa-Coronado, S.; Séverac, C.; Dague, E. Methods of micropatterning and manipulation of cells for biomedical applications. Micromachines 2017, 8, 347. [Google Scholar] [CrossRef]
- Chang, J.C.; Brewer, G.J.; Wheeler, B.C. Modulation of neural network activity by patterning. Biosens. Bioelectron. 2001, 16, 527–533. [Google Scholar] [CrossRef]
- Charrier, A.; Martinez, D.; Monette, R.; Comas, T.; Movileanu, R.; Py, C.; Denhoff, M.; Krantis, A.; Mealing, G. Cell placement and guidance on substrates for neurochip interfaces. Biotechnol. Bioeng. 2010, 105, 368–373. [Google Scholar] [CrossRef]
- Fricke, R.; Zentis, P.D.; Rajappa, L.T.; Hofmann, B.; Banzet, M.; Offenhäusser, A.; Meffert, S.H. Axon guidance of rat cortical neurons by microcontact printed gradients. Biomaterials 2011, 32, 2070–2076. [Google Scholar] [CrossRef]
- Roth, S.; Bugnicourt, G.; Bisbal, M.; Gory-Fauré, S.; Brocard, J.; Villard, C. Neuronal architectures with axo-dendritic polarity above silicon nanowires. Small 2012, 8, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Mourzina, Y.; Kaliaguine, D.; Schulte, P.; Offenhäusser, A. Patterning chemical stimulation of reconstructed neuronal networks. Anal. Chim. Acta 2006, 575, 281–289. [Google Scholar] [CrossRef]
- Wheeler, B.C.; Brewer, G.J. Designing neural networks in culture. Proc. IEEE 2010, 98, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Schwaab, D.; Zentis, P.; Winter, S.; Meffert, S.; Offenhäusser, A.; Mayer, D. Generation of protein nanogradients by microcontact printing. Jpn. J. Appl. Phys. 2013, 52, 05DA19. [Google Scholar] [CrossRef]
- GhoshMoulick, R.; Vu, X.T.; Gilles, S.; Mayer, D.; Offenhäusser, A.; Ingebrandt, S. Impedimetric detection of covalently attached biomolecules on field-effect transistors. Phys. Status Solidi Appl. Mater. Sci. 2009, 206, 417–425. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Method 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Brewer, G.J.; Torricelli, J.R.; Eve, E.K.; Price, P.J. Optimized survival of hippocampal neurons in B27-supplemented neurobasal, a new serum-free medium combination. J. Neurosci. Res. 1993, 35, 567–576. [Google Scholar] [CrossRef]
- Li, Y.; Wong, C.; Xiong, J.; Hodgson, P.; Wen, C. Cytotoxicity of titanium and titanium alloying elements. J. Dent. Res. 2010, 89, 493–497. [Google Scholar] [CrossRef]
- Karmakar, A.; Zhang, Q.; Zhang, Y. Neurotoxicity of nanoscale materials. J. Food Drug Anal. 2014, 22, 147–160. [Google Scholar] [CrossRef]
- Dobson, A.W.; Erikson, K.M.; Aschner, M. Manganese neurotoxicity. Ann. N. Y. Acad. Sci. 2004, 1012, 115–128. [Google Scholar] [CrossRef]
- Hanawa, T. Metal ion release from metal implants. Mater. Sci. Eng. C 2004, 24, 745–752. [Google Scholar] [CrossRef]
- Ross, A.M.; Jiang, Z.; Bastmeyer, M.; Lahann, J. Physical aspects of cell culture substrates: Topography, roughness, and elasticity. Small 2012, 8, 336–355. [Google Scholar] [CrossRef] [PubMed]
- Gurav, J.L.; Jung, I.K.; Park, H.H.; Kang, E.S.; Nadargi, D.Y. Silica aerogel: Synthesis and applications. J. Nanomater. 2010, 2010, 23. [Google Scholar] [CrossRef]
- Branch, D.W.; Corey, J.M.; Weyhenmeyer, J.A.; Brewer, G.J.; Wheeler, B.C. Microstamp patterns of biomolecules for high-resolution neuronal networks. Med. Biol. Eng. Comput. 1998, 36, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Markov, A.; Wolf, N.; Yuan, X.; Mayer, D.; Maybeck, V.; Offenhäusser, A.; Wördenweber, R. Controlled engineering of oxide surfaces for bioelectronics applications using organic mixed monolayers. ACS Appl. Mater. Interfaces 2017, 9, 29265–29272. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hondrich, T.J.J.; Deußen, O.; Grannemann, C.; Brinkmann, D.; Offenhäusser, A. Improvements of Microcontact Printing for Micropatterned Cell Growth by Contrast Enhancement. Micromachines 2019, 10, 659. https://doi.org/10.3390/mi10100659
Hondrich TJJ, Deußen O, Grannemann C, Brinkmann D, Offenhäusser A. Improvements of Microcontact Printing for Micropatterned Cell Growth by Contrast Enhancement. Micromachines. 2019; 10(10):659. https://doi.org/10.3390/mi10100659
Chicago/Turabian StyleHondrich, Timm J. J., Oliver Deußen, Caroline Grannemann, Dominik Brinkmann, and Andreas Offenhäusser. 2019. "Improvements of Microcontact Printing for Micropatterned Cell Growth by Contrast Enhancement" Micromachines 10, no. 10: 659. https://doi.org/10.3390/mi10100659
APA StyleHondrich, T. J. J., Deußen, O., Grannemann, C., Brinkmann, D., & Offenhäusser, A. (2019). Improvements of Microcontact Printing for Micropatterned Cell Growth by Contrast Enhancement. Micromachines, 10(10), 659. https://doi.org/10.3390/mi10100659




