Continuous-Flow Separation and Efficient Concentration of Foodborne Bacteria from Large Volume Using Nickel Nanowire Bridge in Microfluidic Chip
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
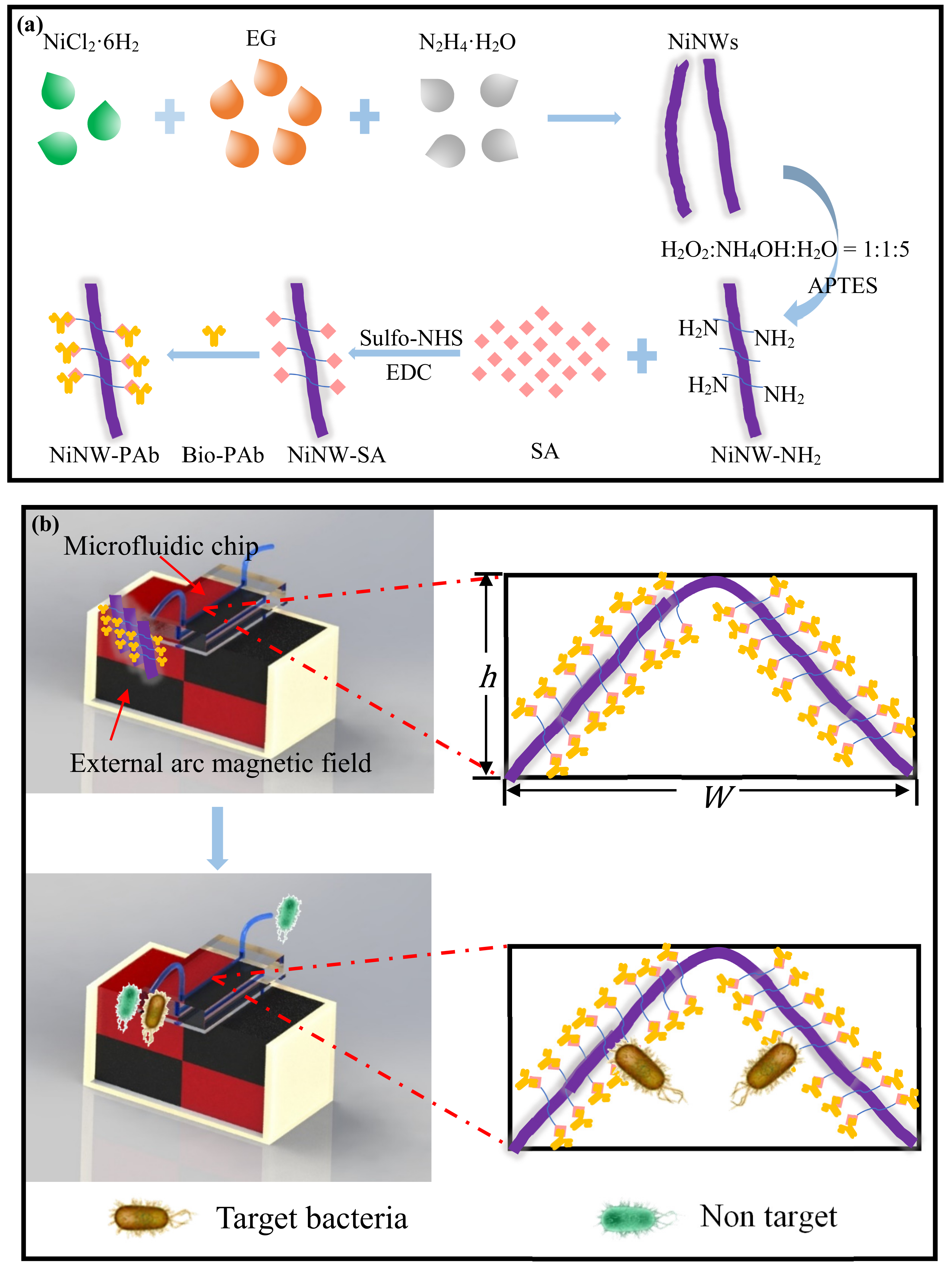
2.2. Fabrication of the Microfluidic Chip
2.3. Synthesis of the Nickel Nanowires
2.4. Functionalization of the Nickel Nanowires
2.5. Culture and Enumeration of the Target Bacteria
2.6. Separation and Concentration of the Target Bacteria
3. Results
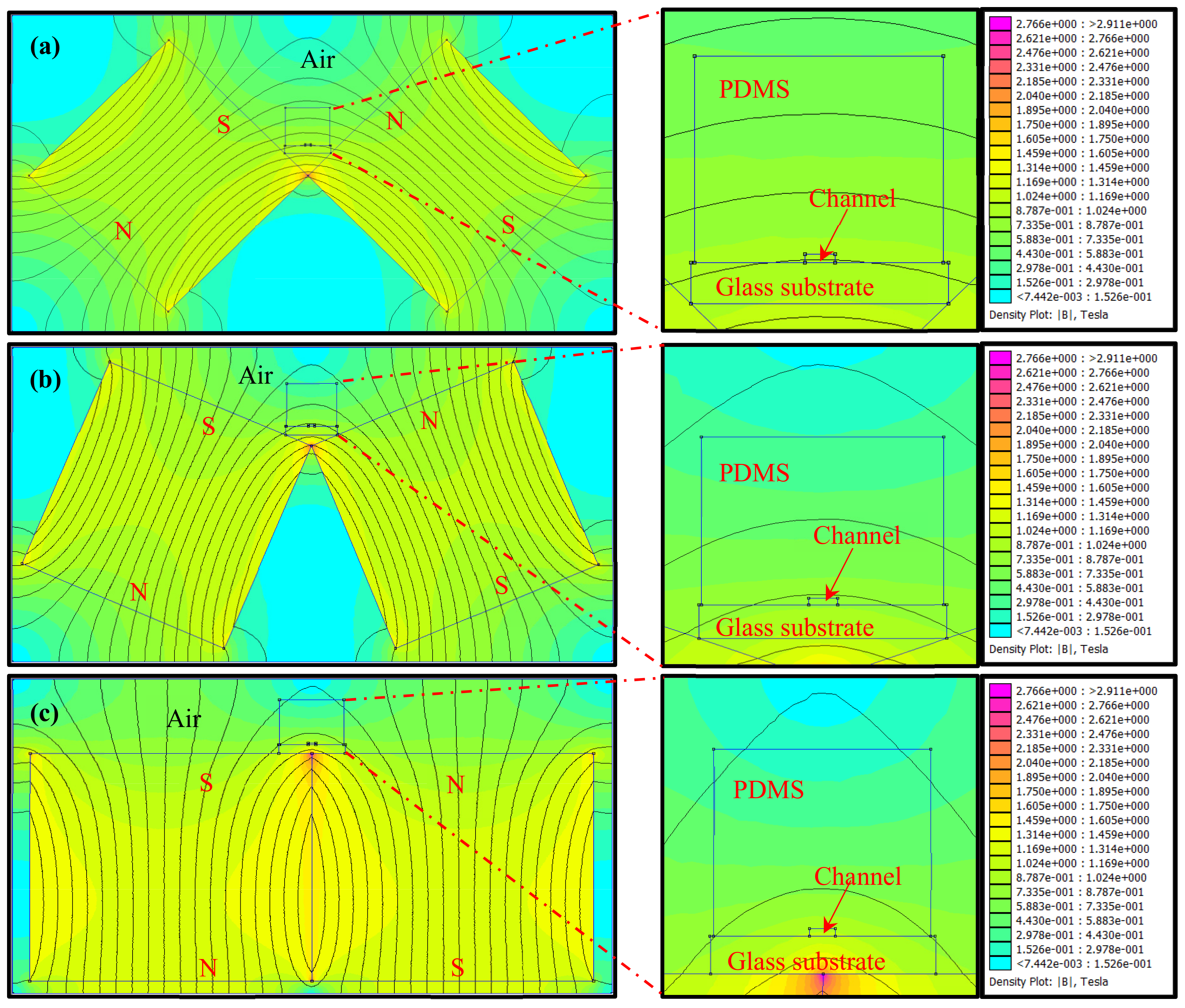
3.1. Simulation of the Magnetic Field
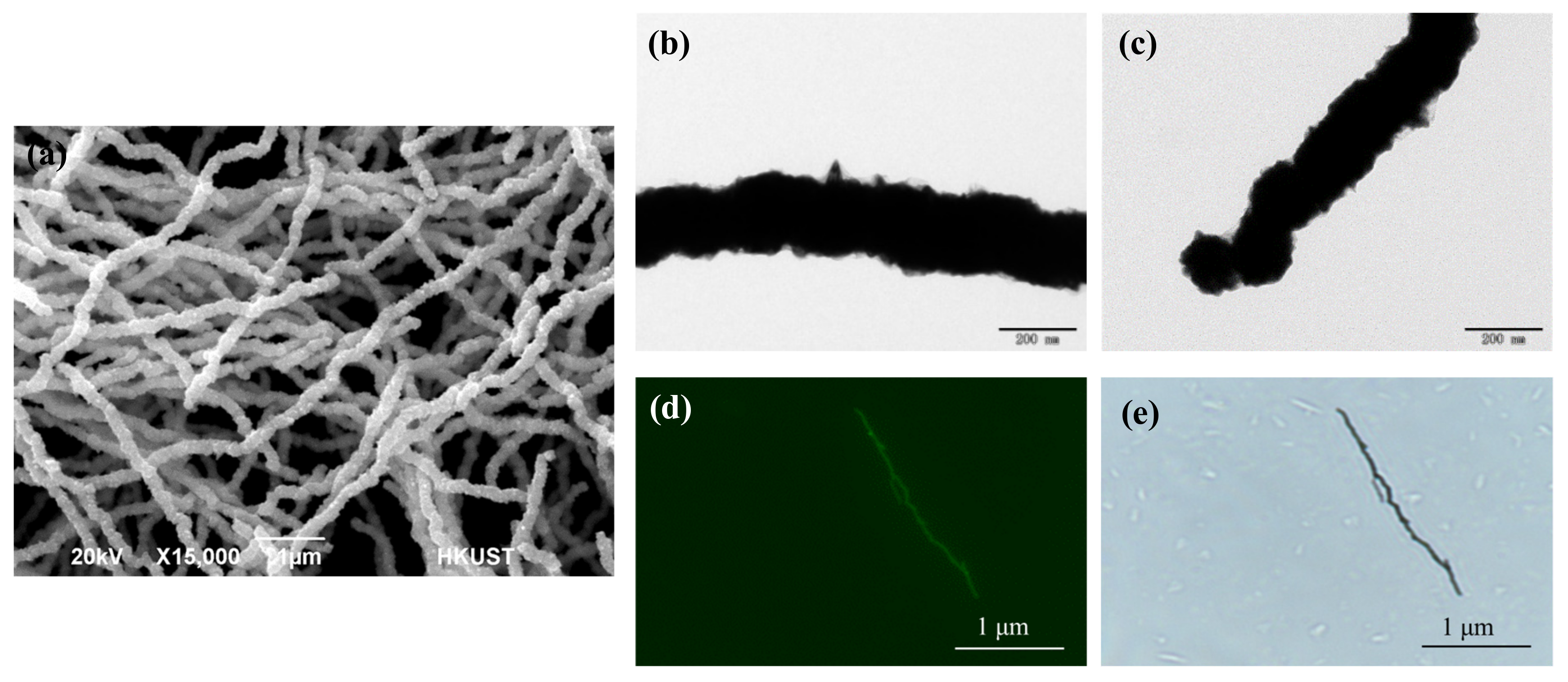
3.2. Characterization of the Nickel Nanowires
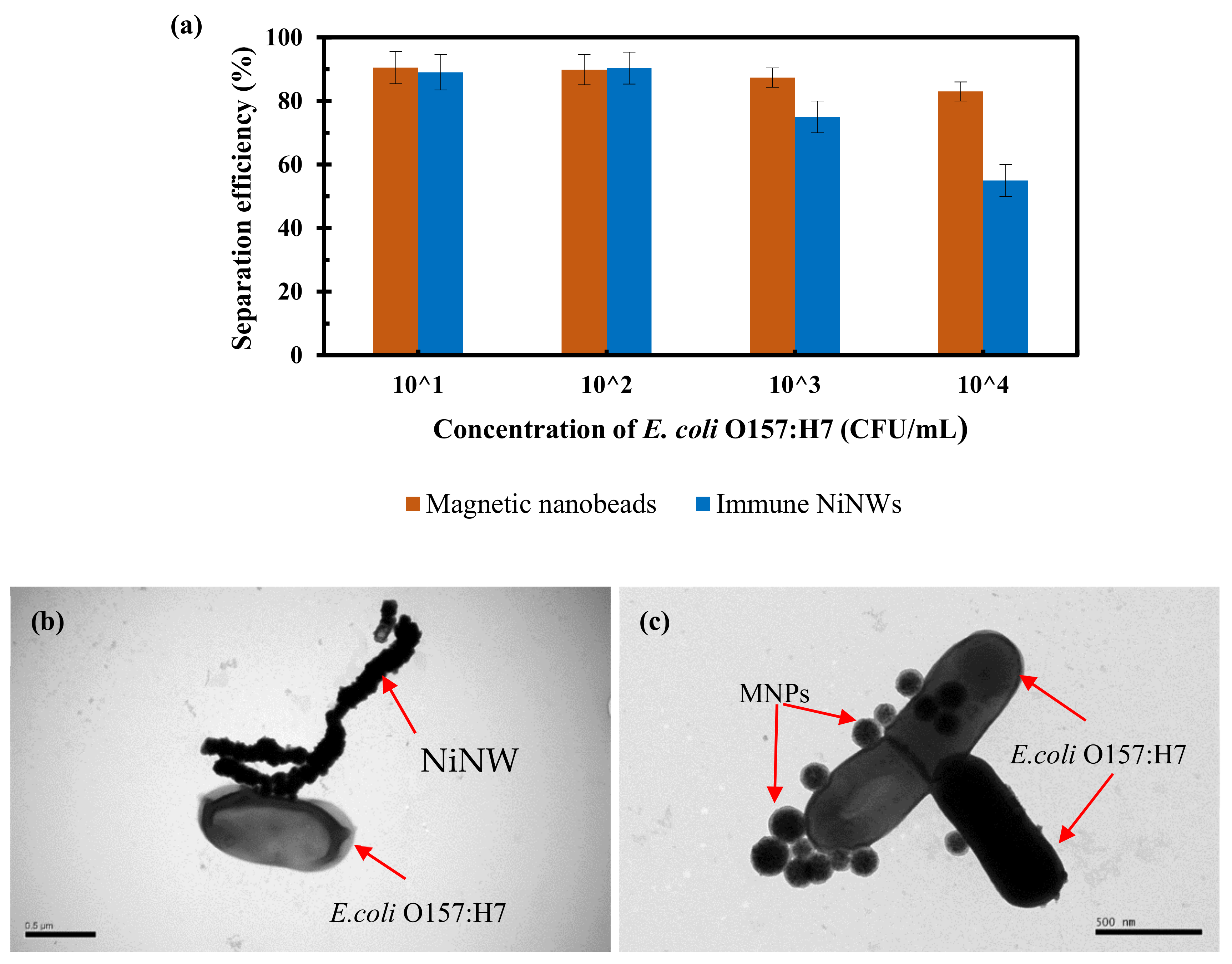
3.3. Comparison of the NiNWs and MNPs for Conventional Magnetic Separation of Bacteria
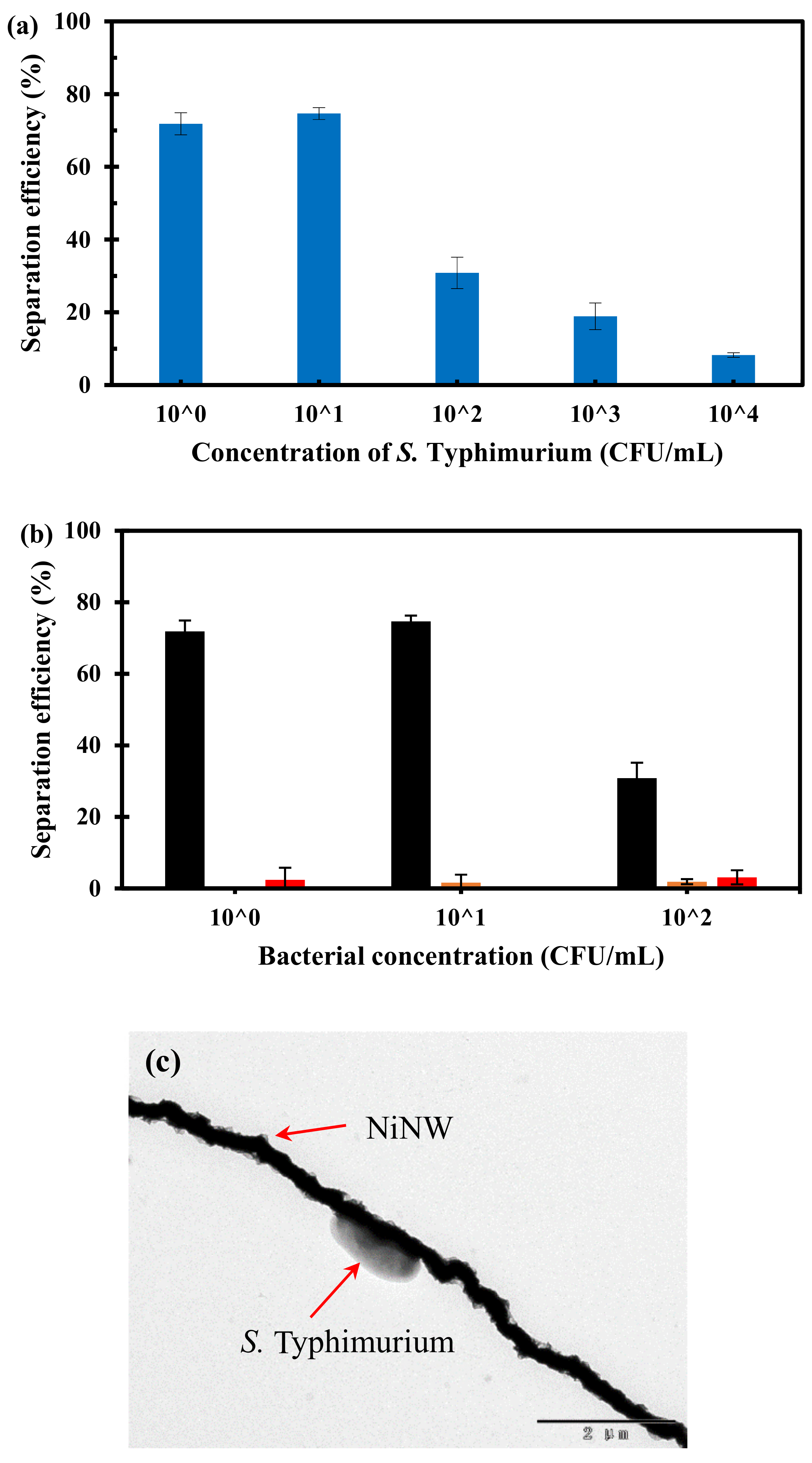
3.4. Separation of the Pure Bacteria from Large Volume in Microfluidic Chip
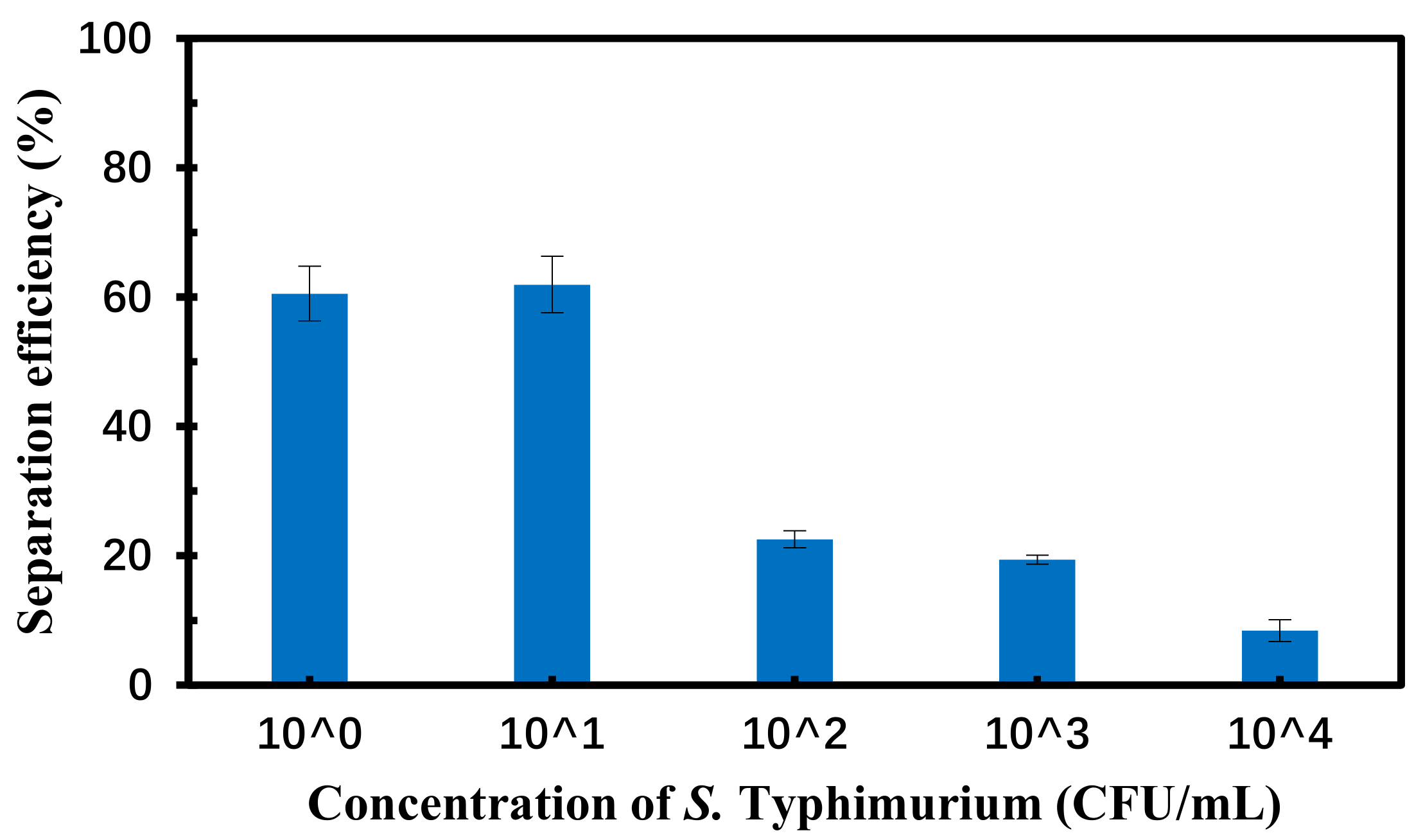
3.5. Separation of the Target Bacteria in the Spiked Chicken Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guo, M.; Yadav, M.P.; Jin, T.Z. Antimicrobial edible coatings and films from micro-emulsions and their food applications. Int. J. Food Microbiol. 2017, 263, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Malik, R.K.; Panwar, H.; Barui, A.K. Microencapsulation of reuterin to enhance long-term efficacy against food-borne pathogen Listeria monocytogenes. 3 Biotech 2018, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Osei-Tutu, B.; Anto, F. Trends of reported foodborne diseases at the Ridge Hospital, Accra, Ghana: A retrospective review of routine data from 2009–2013. BMC Infect. Dis. 2016, 16, 139. [Google Scholar] [CrossRef] [PubMed]
- Opening Speech at WHO/FAO/AU International Food Safety Conference. Available online: https://www.who.int/dg/speeches/detail/opening-speech-at-who-fao-au-international-food-safety-conference (accessed on 12 February 2019).
- World Health Day 2015: Food Safety. Available online: https://www.who.int/campaigns/world-health-day/2015/event/en/ (accessed on 7 April 2015).
- Singh, S.; Eldin, C.; Kowalczewska, M.; Raoult, D. Axenic culture of fastidious and intracellular bacteria. Trends Microbiol. 2013, 21, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Jiang, M.; Xiong, J.; Li, J.; Zhang, X.; Wei, H.; Yu, J. Exploring a phage-based real-time PCR assay for diagnosing Acinetobacter baumannii bloodstream infections with high sensitivity. Anal. Chimica Acta 2018, 1044, 147–153. [Google Scholar] [CrossRef]
- De la Torre, D.; Astolfi-Ferreira, C.S.; Chacon, R.D.; Piantino Ferreira, A.J. Sensitive SYBR green-real time PCR for the detection and quantitation of Avian Rotavirus A. Vet. Sci. 2019, 6, 2. [Google Scholar] [CrossRef]
- Wang, W.; Li, J.; Dong, C.; Li, Y.; Kou, Q.; Yan, J.; Zhang, L. Ultrasensitive ELISA for the detection of hCG based on assembled gold nanoparticles induced by functional polyamidoamine dendrimers. Anal. Chimica Acta 2018, 1042, 116–124. [Google Scholar] [CrossRef]
- Zai, J.; Yi, K.; Xie, L.; Zhu, J.; Feng, X.; Li, Y. Dual monoclonal antibody-based sandwich ELISA for detection of in vitro packaged Ebola virus. Diagn. Pathol. 2018, 13, 96. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Kaur, A.; Jain, S.; Sabharwal, P.K.; Singh, H. Polymer functionalized magnetic nanoconstructs for immunomagnetic separation of analytes. RSC Adv. 2016, 6, 66505–66515. [Google Scholar] [CrossRef]
- Gourikutty, S.B.N.; Chang, C.P.; Puiu, P.D. Microfluidic immunomagnetic cell separation from whole blood. Journal of chromatography. J.Chromatogr. B 2016, 1011, 77–88. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, Y.; Lei, C.; Luo, J.; Xie, S.; Pu, H. Magnetic impedance biosensor: A review. Biosens. Bioelectron. 2017, 90, 418–435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tian, J.; Li, K.; Tian, H.; Xu, W. Label-free visual biosensor based on cascade amplification for the detection of Salmonella. Anal. Chimica Acta 2019, 1075, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Qi, P.; Zhang, D. DNA-templated fluorescent silver nanoclusters for sensitive detection of pathogenic bacteria based on MNP-DNAzyme-AChE complex. Sens. Actuators B Chem. 2018, 276, 42–47. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, K.; Shi, Y.; Huang, Y.; Wang, X. Efficient removal of Escherichia coli from ballast water using a combined high-gradient magnetic separation-ultraviolet photocatalysis (HGMS-UV/TiO2) system. Water Air Soil Pollut. 2018, 229, 243. [Google Scholar]
- Jeong, A.; Lim, H.B. Magnetophoretic separation ICP-MS immunoassay using Cs-doped multicore magnetic nanoparticles for the determination of salmonella typhimurium. Talanta 2018, 178, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kwon, D.; Chung, B.; Jung, G.Y.; Au, A.; Folch, A.; Jeon, S. Ultrarapid detection of pathogenic bacteria using a 3D immunomagnetic flow assay. Anal. Chem. 2014, 86, 6683–6688. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, M.; Guyodo, Y.; Guyot, F.; Gatel, C.; Menguy, N.; Chebbi, I.; Haye, B.; Durand-Dubief, M.; Alphandery, E.; Brayner, R.; et al. Magnetic-field induced rotation of magnetosome chains in silicified magnetotactic bacteria. Sci. Rep. 2018, 8, 7699. [Google Scholar]
- Li, K.; Feng, J.; Kwak, J.; Yang, J.; Gordon, R.G. Pure and conformal CVD nickel and nickel monosilicide in high-aspect-ratio structures analyzed by atom probe tomography. J. Appl. Phys. 2017, 121, 175301. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Yue, H.Y.; Yu, Z.M.; Huang, S.; Gao, X.; Wang, B.; Song, S.S.; Guan, E.H.; Wang, W.Q.; Zhang, H.J. A novel 3D porous graphene foam prepared by chemical vapor deposition using nickel nanoparticles: Electrochemical determination of levodopa in the presence of uric acid. Microchem. J. 2019, 147, 163–169. [Google Scholar] [CrossRef]
- Pourfarzad, H.; Shabani-Nooshabadi, M.; Ganjali, M.R.; Kashani, H. Synthesis of Ni–Co-Fe layered double hydroxide and Fe2O3/Graphene nanocomposites as actively materials for high electrochemical performance supercapacitors. Electrochimica Acta 2019, 317, 83–92. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Zhang, X.; Zhou, C.; Munagala, S.P.; Tian, Y.; Ren, J.; Jiang, K. Graphene platelet (GPL)/nickel (ni) laminate coatings for improved surface properties. Adv. Eng. Mater. 2017, 19, 1600795. [Google Scholar] [CrossRef]
- Huang, Y.; Li, C.; Bai, J.; Sun, W.; Wang, J. Fabrication of Ni nanoparticles loaded on β-cyclodextrin/polymethyl methacrylate composite nanofibers via electrospinning, immersion, and chemical reduction. J. Macromol. Sci. Part B 2015, 54, 231–238. [Google Scholar] [CrossRef]
- Shen, Y.; Wei, Y.; Ma, J.; Li, Q.; Li, J.; Shao, W.; Yan, P.; Huang, G.; Du, X. Tunable microwave absorption properties of nickel-carbon nanofibers prepared by electrospinning. Ceram. Int. 2019, 45, 3313–3324. [Google Scholar] [CrossRef]
- Peng, J.; Peng, Z.; Zhu, Z.; Augustine, R.; Mahmoud, M.M.; Tang, H.; Rao, M.; Zhang, Y.; Li, G.; Jiang, T. Achieving ultra-high electromagnetic wave absorption by anchoring Co0.33Ni0.33Mn0.33Fe2O4 nanoparticles on graphene sheets using microwave-assisted polyol method. Ceram. Int. 2018, 44, 21015–21026. [Google Scholar]
- Kashid, S.B.; Raut, R.W.; Malghe, Y.S. Microwave assisted synthesis of nickel nanostructures by hydrazine reduction route: Effect of solvent and capping agent on morphology and magnetic properties. Mater. Chem. Phys. 2016, 170, 24–31. [Google Scholar] [CrossRef]
- Wei, P.; Li, X.; Li, J.; Bai, J.; Jiang, C.; Liu, L. A facile synthesis of ternary nickel iron sulfide nanospheres as counter electrode in dye-sensitized solar cells. Chem. Eur. J. 2018, 24, 19032–19037. [Google Scholar] [CrossRef]
- Huang, L.; Hou, H.; Liu, B.; Zeinu, K.; Yuan, X.; Zhu, X.; He, X.; Wu, L.; Hu, J.; Yang, J. Phase-controlled solvothermal synthesis and morphology evolution of nickel sulfide and its pseudocapacitance performance. Ceram. Int. 2017, 43, 3080–3088. [Google Scholar] [CrossRef]
- Xia, Z.; Wen, W. Synthesis of nickel nanowires with tunable characteristics. Nanomaterials 2016, 6, 19. [Google Scholar] [CrossRef]
- Xia, Z.; Wu, X.; Peng, G.; Wang, L.; Li, W.; Wen, W. A novel nickel nanowire based magnetorheological material. Smart Mater. Struct. 2017, 26, 054006. [Google Scholar] [CrossRef]
- Jung, S.H.; Hahn, Y.K.; Oh, S.; Kwon, S.; Um, E.; Choi, S.; Kang, J.H. Advection flows-enhanced magnetic separation for high-throughput bacteria separation from undiluted whole blood. Small 2018, 14, 1801731. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.; Ban, C.; Jia, Z.; He, X.; Ma, J.; Pu, X.; Li, W.; Zhi, L. Halbach array assisted assembly of orderly aligned nickel nanowire networks as transparent conductive films. Nanotechnology 2019, 30, 355301. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, X.; Chen, Q.; Wang, L.; Cai, G.; Qi, W.; Xia, Z.; Wen, W.; Lin, J. Continuous-Flow Separation and Efficient Concentration of Foodborne Bacteria from Large Volume Using Nickel Nanowire Bridge in Microfluidic Chip. Micromachines 2019, 10, 644. https://doi.org/10.3390/mi10100644
Huo X, Chen Q, Wang L, Cai G, Qi W, Xia Z, Wen W, Lin J. Continuous-Flow Separation and Efficient Concentration of Foodborne Bacteria from Large Volume Using Nickel Nanowire Bridge in Microfluidic Chip. Micromachines. 2019; 10(10):644. https://doi.org/10.3390/mi10100644
Chicago/Turabian StyleHuo, Xiaoting, Qi Chen, Lei Wang, Gaozhe Cai, Wuzhen Qi, Zengzilu Xia, Weijia Wen, and Jianhan Lin. 2019. "Continuous-Flow Separation and Efficient Concentration of Foodborne Bacteria from Large Volume Using Nickel Nanowire Bridge in Microfluidic Chip" Micromachines 10, no. 10: 644. https://doi.org/10.3390/mi10100644
APA StyleHuo, X., Chen, Q., Wang, L., Cai, G., Qi, W., Xia, Z., Wen, W., & Lin, J. (2019). Continuous-Flow Separation and Efficient Concentration of Foodborne Bacteria from Large Volume Using Nickel Nanowire Bridge in Microfluidic Chip. Micromachines, 10(10), 644. https://doi.org/10.3390/mi10100644






