Preliminary Assessment of Alkaloid Content in Cocoa (Theobroma cacao L.) Hulls for Safe Consumption as a Feed Ingredient
Abstract
1. Introduction
2. Results and Discussion
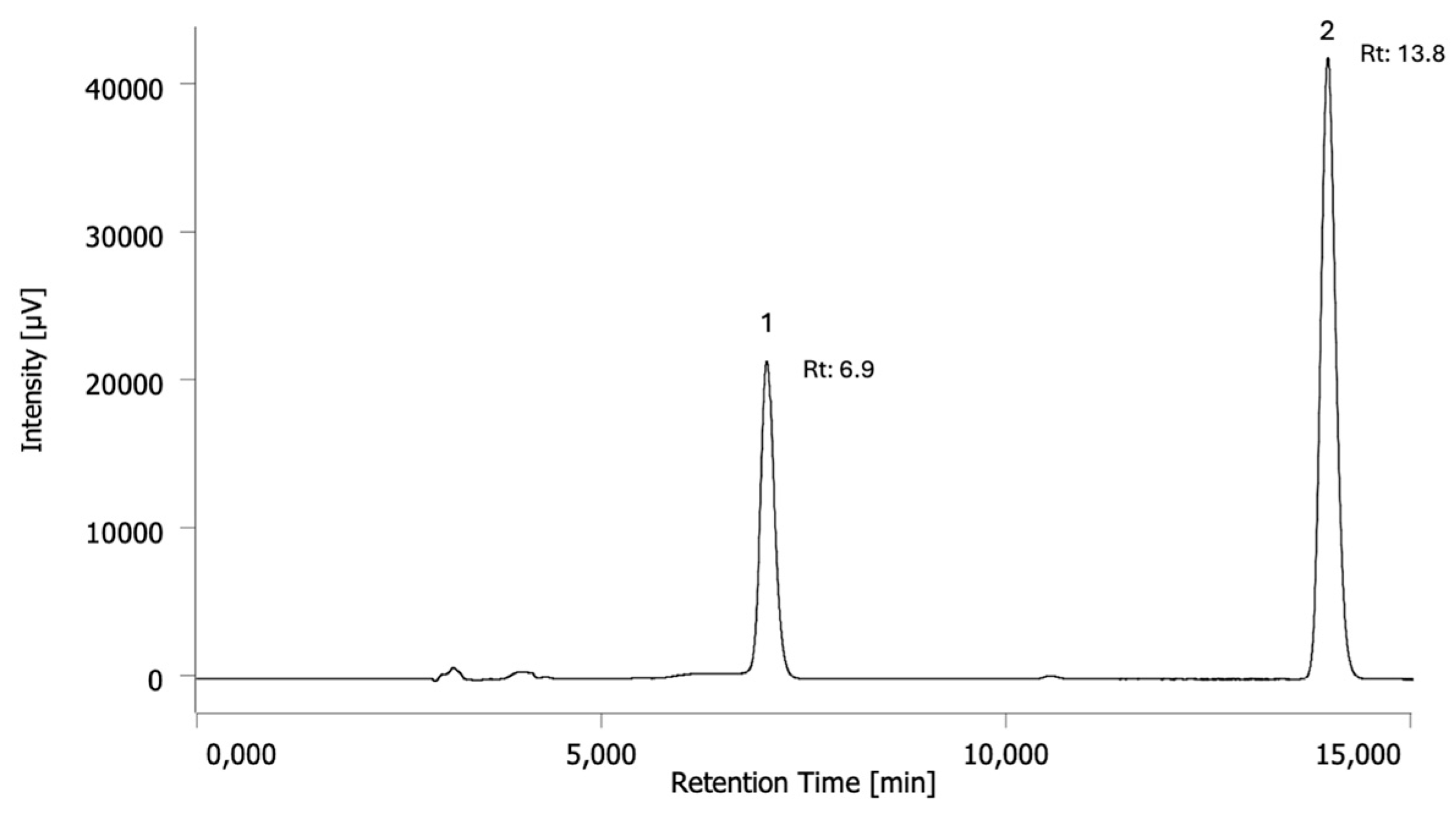
2.1. Development and Validation of the High-Performance Liquid Chromatography (HPLC) Method
2.2. Measurement of Theobromine and Caffeine by HPLC and Safety Considerations
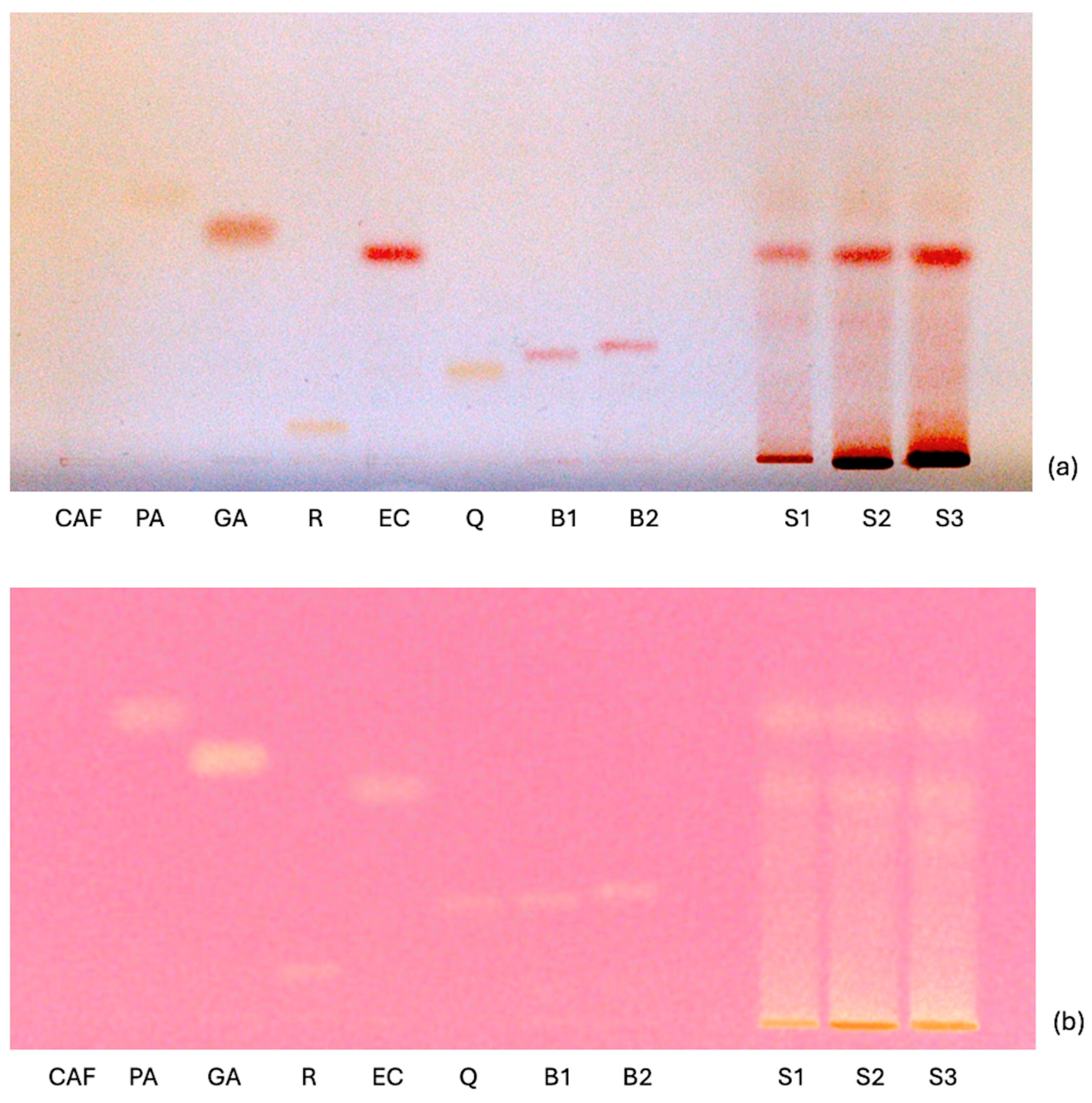
2.3. Screening of Other Constituents of Nutritional Interest by High-Performance Thin-Layer Chromatography (HPTLC)
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Samples, Particle Size Determination, and Extraction Method
4.3. High-Performance Thin-Layer Chromatography (HPTLC)
4.4. High-Performance Liquid Chromatography Coupled with Ultraviolet Detection (HPLC-UV)
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADI | Acceptable daily intake |
| b.w. | Body weight |
| CAF | Caffeine |
| CH | Cocoa hull |
| CSN | Central nervous system |
| CV | Coefficient of variation |
| DPPH | 1,1-diphenyl-2-picryl-hydrazyl free radical |
| EC | Epicatechin |
| EU | European Union |
| EFSA | European Food Safety Authority |
| FDA | Food and Drug Administration |
| HBA | p-hydroxybenzoic acid |
| HPLC | High-Performance Liquid Chromatography |
| HPTLC | High-Performance Thin-Layer Chromatography |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| ML | Maximum level |
| NOAEL | No Observed Adverse Effect Level |
| Rf | Ratio frontis |
| Rt | Retention time |
| SD | Standard deviation |
| TB | Theobromine |
| TC | Theobromine content |
References
- European Commission Directorate-General for Communication. Circular Economy Action Plan—For a Cleaner and More Competitive Europe; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- Hamam, M.; Chinnici, G.; Di Vita, G.; Pappalardo, G.; Pecorino, B.; Maesano, G.; D’Amico, M. Circular Economy Models in Agro-Food Systems: A Review. Sustainability 2021, 13, 3453. [Google Scholar] [CrossRef]
- Chiaraluce, G.; Bentivoglio, D.; Finco, A. Circular Economy for a Sustainable Agri-Food Supply Chain: A Review for Current Trends and Future Pathways. Sustainability 2021, 13, 9294. [Google Scholar] [CrossRef]
- Iqbal, M.W.; Kang, Y. Circular Economy of Food: A Secondary Supply Chain Model on Food Waste Management Incorporating IoT Based Technology. J. Clean. Prod. 2024, 435, 140566. [Google Scholar] [CrossRef]
- De Pascale, A.; Di Vita, G.; Giannetto, C.; Ioppolo, G.; Lanfranchi, M.; Limosani, M.; Szopik-Depczyńska, K. The Circular Economy Implementation at the European Union Level. Past, Present and Future. J. Clean. Prod. 2023, 423, 138658. [Google Scholar] [CrossRef]
- Vásquez, Z.S.; de Carvalho Neto, D.P.; Pereira, G.V.M.; Vandenberghe, L.P.S.; de Oliveira, P.Z.; Tiburcio, P.B.; Rogez, H.L.G.; Góes Neto, A.; Soccol, C.R. Biotechnological Approaches for Cocoa Waste Management: A Review. Waste Manag. 2019, 90, 72–83. [Google Scholar] [CrossRef]
- ICCO—International Cocoa Organization. Quarterly Bulletin of Cocoa Statistics; ICCO: Abidjan, Côte d’Ivoire, 2023; Volume XLIX. [Google Scholar]
- Mendoza-Meneses, C.J.; Feregrino-Pérez, A.A.; Gutiérrez-Antonio, C. Potential Use of Industrial Cocoa Waste in Biofuel Production. J. Chem. 2021, 2021, 3388067. [Google Scholar] [CrossRef]
- Tušek, K.; Valinger, D.; Jurina, T.; Sokač Cvetnić, T.; Gajdoš Kljusurić, J.; Benković, M. Bioactives in Cocoa: Novel Findings, Health Benefits, and Extraction Techniques. Separations 2024, 11, 128. [Google Scholar] [CrossRef]
- Gil, M.; Uribe, D.; Gallego, V.; Bedoya, C.; Arango-Varela, S. Traceability of Polyphenols in Cocoa during the Postharvest and Industrialization Processes and Their Biological Antioxidant Potential. Heliyon 2021, 7, e07738. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Laca, A.; Laca, A.; Díaz, M. Cocoa Bean Shell: A By-Product with High Potential for Nutritional and Biotechnological Applications. Antioxidants 2023, 12, 1028. [Google Scholar] [CrossRef]
- Younes, A.; Li, M.; Karboune, S. Cocoa Bean Shells: A Review into the Chemical Profile, the Bioactivity and the Biotransformation to Enhance Their Potential Applications in Foods. Crit. Rev. Food Sci. Nutr. 2022, 63, 9111–9135. [Google Scholar] [CrossRef]
- Rojo-Poveda, O.; Barbosa-Pereira, L.; Zeppa, G.; Stévigny, C. Cocoa Bean Shell—A By-Product with Nutritional Properties and Biofunctional Potential. Nutrients 2020, 12, 1123. [Google Scholar] [CrossRef]
- Younes, A.; Karboune, S.; Liu, L.; Andreani, E.S.; Dahman, S. Extraction and Characterization of Cocoa Bean Shell Cell Wall Polysaccharides. Polymers 2023, 15, 745. [Google Scholar] [CrossRef] [PubMed]
- Fetriyuna, F.; Djali, M.; Rafi, A.Z.; Nurunnisa, D.A.; Purwestri, R.C. Cocoa Bean Shells: A Potential Chocolate Replacement in Food Production. Int. J. Adv. Sci. Eng. Inf. Technol. 2025, 15, 147–155. [Google Scholar] [CrossRef]
- Tretola, M.; Ottoboni, M.; Di Rosa, A.R.; Giromini, C.; Fusi, E.; Rebucci, R.; Leone, F.; Dell’Orto, V.; Chiofalo, V.; Pinotti, L. Former Food Products Safety Evaluation: Computer Vision as an Innovative Approach for the Packaging Remnants Detection. J. Food Qual. 2017, 2017, 1–6. [Google Scholar] [CrossRef]
- Pinotti, L.; Cheli, F.; Govoni, C.; Rulli, M.C.; Premarajan, P.; Cattaneo, D.M.I.R. The ‘One Nutrition’ Approach: Connecting Crop Production, Animal Nutrition and Human Nutrition. Ital. J. Anim. Sci. 2025, 24, 978–987. [Google Scholar] [CrossRef]
- Cocoa Hulls. Tables of Composition and Nutritional Values of Feed Materials INRA CIRAD AFZ. Available online: https://www.feedtables.com/content/cocoa-hulls (accessed on 23 June 2025).
- Reiche, A.-M.; Tretola, M.; Eggerschwiler, L.; Pinotti, L.; Dohme-Meier, F. Former Food and Cocoa Bean Shells in Early-Lactating Cows on a Herbage-Based Diet: Effects on Ruminal Fermentation and Blood Metabolites. Animal 2025, 19, 101477. [Google Scholar] [CrossRef]
- Besharati, M.; Maggiolino, A.; Palangi, V.; Kaya, A.; Jabbar, M.; Eseceli, H.; De Palo, P.; Lorenzo, J.M. Tannin in Ruminant Nutrition: Review. Molecules 2022, 27, 8273. [Google Scholar] [CrossRef]
- EFSA CONTAM Panel. Theobromine as Undesirable Substances in Animal Feed —Scientific opinion of the Panel on Contaminants in the Food Chain. EFSA J. 2008, 6, 725. [Google Scholar] [CrossRef]
- Braude, R. Toxic Effects in The Feeding of Cocoa Meal to Pigs. Vet. J. 1943, 99, 302–307. [Google Scholar] [CrossRef]
- Organization for Economic Cooperation and Development (OECD). CAFFEINE CAS: 58-08-2. SIDS Initial Assessment Report for SIAM 14; UNEP Publications: Paris, France, 2002. [Google Scholar]
- Commission Regulation (EU) 2022/1104 of 1 July 2022 Amending Regulation (EU) No 68/2013 on the Catalogue of Feed Materials (Text with EEA Relevance). Available online: http://data.europa.eu/eli/reg/2022/1104/oj (accessed on 11 April 2024).
- Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on Additives for Use in Animal Nutrition. Available online: http://data.europa.eu/eli/reg/2003/1831/oj (accessed on 11 April 2024).
- U.S. Food and Drug Administration. Bioanalytical Method Validation Guidance for Industry Biopharmaceutics Bioanalytical Method Validation; Guidance for Industry; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2018. [Google Scholar]
- Stanford, K.; Schwartzkopf-Genswein, K.S.; Meléndez, D.M.; Ngo, S.; Harding, M.; McAllister, T.A.; Schatzmayr, D.; Swift, M.L.; Blakley, B.; Ribeiro, G.O. Effects of Heating, Pelleting, and Feed Matrix on Apparent Concentrations of Cereal Ergot Alkaloids in Relation to Growth Performance and Welfare Parameters of Backgrounding Beef Steers. Toxins 2022, 14, 580. [Google Scholar] [CrossRef]
- Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on Undesirable Substances in Animal Feed. Available online: http://data.europa.eu/eli/dir/2002/32/2019-11-28 (accessed on 11 April 2024).
- EFSA FEEDAP Panel; Guido, R.; Aquilina, G.; Azimonti, G.; Bampidis, V.; Bastos, M.d.L.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G.; et al. Guidance on the Assessment of the Safety of Feed Additives for the Target Species. EFSA J. 2017, 15, e05021. [Google Scholar] [CrossRef]
- Knapp, A.W.; Churchman, A. Cacao Shell and Its Use as an Accessory Fodder. J. Soc. Chem. Ind. 1937, 56, 29–33. [Google Scholar] [CrossRef]
- Aplin, R.D.; Ellenberger, H.B. Effect of Feeding Cocoa Meal to Milking Cows; Vermont Agricultural Experiment Station: Burlington, NJ, USA, 1927. [Google Scholar]
- Curtis, P.E.; Griffiths, J.E. Suspected Chocolate Poisoning of Calves. Vet. Rec. 1972, 90, 313–314. [Google Scholar]
- Welker, T.L.; Overturf, K.; Snyder, S.; Liu, K.; Abernathy, J.; Frost, J.; Barrows, F.T. Effects of Feed Processing Method (Extrusion and Expansion-Compression Pelleting) on Water Quality and Growth of Rainbow Trout in a Commercial Setting. J. Appl. Aquac. 2018, 30, 97–124. [Google Scholar] [CrossRef]
- Day, E.J.; Dilworth, B.C. Toxicity of Jimson Weed Seed and Cocoa Shell Meal to Broilers. Poult. Sci. 1984, 63, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Emiola, I.A.; Ojebiyi, O.O.; Akande, T.O. Performance and Organ Weights of Laying Hens Fed Diets Containing Graded Levels of Sun-Dried Cocoa Bean Shell (CBS). Int. J. Poult. Sci. 2011, 10, 986–989. [Google Scholar] [CrossRef]
- Olubamiwa, O.; Ikyo, S.M.; Adebowale, B.A.; Omojola, A.B.; Hamzat, R.A. Effect of Boiling Time on the Utilization of Cocoa Bean Shell in Laying Hen Feeds. Int. J. Poult. Sci. 2006, 5, 1137–1139. [Google Scholar] [CrossRef]
- Magistrelli, D.; Zanchi, R.; Malagutti, L.; Galassi, G.; Canzi, E.; Rosi, F. Effects of Cocoa Husk Feeding on the Composition of Swine Intestinal Microbiota. J. Agric. Food Chem. 2016, 64, 2046–2052. [Google Scholar] [CrossRef] [PubMed]
- Ayinde, O.E.; Ojo, V.; Adeyina, A.A.; Adesoye, O. Economics of Using Cocoa Bean Shell as Feed Supplement for Rabbits. Pak. J. Nutr. 2010, 9, 195–197. [Google Scholar] [CrossRef]
- Ogunsipe, M.H.; Ibidapo, I.; Oloruntola, O.D.; Agbede, J.O. Growth Performance of Pigs on Dietary Cocoa Bean Shell Meal. Livest. Res. Rural. Dev. 2017, 29, 1–5. [Google Scholar]
- Tiamiyu, L.O.; Okomoda, V.T.; Ogodo, J.U. Growth Performance of Clarias Gariepinus Fed Varying Levels of Sorghum Bicolor Waste Meal. Int. J. Aquac. 2016, 6, 1–7. [Google Scholar] [CrossRef]
- Bamba, Y.; Ouattara, N.; Soro, Y.; Ouattara, A.; Yao, K.; Gourène, G. Evaluation of Production Efficiency of Nile Tilapia (Oreochromis niloticus L.) Fed Diets Containing Crop Residues in Combination with Cocoa Bean Shell and Coconut Oil Cake in Côte d’Ivoire. Livest. Res. Rural. Dev. 2014, 26, 8. [Google Scholar]
- Soeharsono; Amin, M.; Cahyono, A. The Use of Cocoa Bean Waste as a Supplement in Male Bali Cattle Feeding. In Proceedings of the International Seminar on Livestock Production and Veterinary Technology, Denpasar, Indonesia, 10–12 August 2016; Indonesian Center for Animal Research and Development (ICARD): Kemang, Indonesia, 2017. [Google Scholar]
- Campione, A.; Pauselli, M.; Natalello, A.; Valenti, B.; Pomente, C.; Avondo, M.; Luciano, G.; Caccamo, M.; Morbidini, L. Inclusion of Cocoa By-Product in the Diet of Dairy Sheep: Effect on the Fatty Acid Profile of Ruminal Content and on the Composition of Milk and Cheese. Animal 2021, 15, 100243. [Google Scholar] [CrossRef]
- Adeloye, A. Efficiencies of Conversion of Some Lignocellulosic Waste Materials by Goats. Bioresour. Technol. 1992, 40, 167–169. [Google Scholar] [CrossRef]
- Renna, M.; Lussiana, C.; Colonna, L.; Malfatto, V.M.; Mimosi, A.; Cornale, P. Inclusion of Cocoa Bean Shell in the Diet of Dairy Goats: Effects on Milk Production Performance and Milk Fatty Acid Profile. Front. Vet. Sci. 2022, 9, 848452. [Google Scholar] [CrossRef]
- Ale, O.M.; Omotoso, O.B.; Fajemisin, A.N. Silage Characteristics, Nutrient Profiles and in Vitro Digestibility of Differently Ensiled Theobroma Cacao Bean Shell Meals. Acta Fytotech. Zootech. 2023, 26, 1–7. [Google Scholar] [CrossRef]
- Adamafio, N.A. Theobromine Toxicity and Remediation of Cocoa By-Products: An Overview. J. Biol. Sci. 2013, 13, 570–576. [Google Scholar] [CrossRef]
- Odunsi, A.; Onifade, A.; Longe, O. Effect of Alkali or Hot Water Treatment of Cocoa Bean Cake Fed to Broiler Finishers as Partial Replacement for Dietary Groundnut Cake. Arch. Zootec. 1999, 48, 337–342. [Google Scholar]
- Oduro-Mensah, D.; Ocloo, A.; Lowor, S.T.; Bonney, E.Y.; Okine, L.K.N.A.; Adamafio, N.A. Isolation and Characterisation of Theobromine-Degrading Filamentous Fungi. Microbiol. Res. 2018, 206, 16–24. [Google Scholar] [CrossRef]
- Aromolaran, O.; Ogunsakin, F.M. Degradation of Theobromine in Cocoa (Theobroma Cacao) by-Products by Fermentation with Aspergillus Niger. S. Asian J. Res. Microbiol. 2019, 2, 1–6. [Google Scholar] [CrossRef]
- Oduro-Mensah, D.; Ocloo, A.; Lowor, S.T.; Mingle, C.; Okine, L.K.N.A.; Adamafio, N.A. Bio-Detheobromination of Cocoa Pod Husks: Reduction of Ochratoxin A Content without Change in Nutrient Profile. Microb. Cell Fact. 2018, 17, 79. [Google Scholar] [CrossRef]
- Zhu, Y.; Hassan, Y.I.; Lepp, D.; Shao, S.; Zhou, T. Strategies and Methodologies for Developing Microbial Detoxification Systems to Mitigate Mycotoxins. Toxins 2017, 9, 130. [Google Scholar] [CrossRef]
- Kobori, K.; Maruta, Y.; Mineo, S.; Shigematsu, T.; Hirayama, M. Polyphenol-Retaining Decaffeinated Cocoa Powder Obtained by Supercritical Carbon Dioxide Extraction and Its Antioxidant Activity. Foods 2013, 2, 462–477. [Google Scholar] [CrossRef]
- Kraft, S.K.; Kellner, F. Can Blockchain Be a Basis to Ensure Transparency in an Agricultural Supply Chain? Sustainability 2022, 14, 8044. [Google Scholar] [CrossRef]
- Kongor, J.E.; Owusu, M.; Oduro-Yeboah, C. Cocoa Production in the 2020s: Challenges and Solutions. CABI Agric. Biosci. 2024, 5, 102. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA); European Food Safety Authority (EFSA). Scientific Opinion on the Substantiation of a Health Claim Related to Cocoa Flavanols and Maintenance of Normal Endothelium—Dependent Vasodilation Pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA J. 2012, 10, 2809–2829. [Google Scholar] [CrossRef]
- Serra, V.; Salvatori, G.; Pastorelli, G. Dietary Polyphenol Supplementation in Food Producing Animals: Effects on the Quality of Derived Products. Animals 2021, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- ISO 2591-1:1988; Test Sieving—Part 1: Methods Using Test Sieves of Woven Wire Cloth and Perforated Metal Plate. ISO: Geneva, Switzerland, 1988. Available online: https://www.iso.org/standard/7569.html (accessed on 15 May 2025).
- Reich, E.; Schibli, A. High Performance Thin-Layer Chromatography for the Analysis of Medicinal Plants; CIC Edizione Internazionale: Genova, Italy, 2006. [Google Scholar]
- Colombo, F.; Di Lorenzo, C.; Petroni, K.; Silano, M.; Pilu, R.; Falletta, E.; Biella, S.; Restani, P. Pigmented Corn Varieties as Functional Ingredients for Gluten-Free Products. Foods 2021, 10, 1770. [Google Scholar] [CrossRef]

| Linearity | Sensitivity | Recovery | Stability | Precision | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Linear range | R2 | LOD | LOQ | % | Variation% | Intraday | Interday | ||||
| (μg/mL) | (ng/mL) | (ng/g) | (ng/mL) | (ng/g) | 24 h | 20 days | (CV%) | (CV%) | |||
| TB | 10–100 | 0.9820 | 3 | 30 | 10 | 100 | 95 | 1.43 | 7.74 | 3.11 | 6.93 |
| CAF | 10–100 | 0.9973 | 10 | 100 | 40 | 400 | 84 | 2.54 | 9.53 | 2.72 | 6.27 |
| Compound | S1 | S2 | S3 |
|---|---|---|---|
| TB | 4199.3 ± 86.97 a | 4036.65 ± 80.53 a | 5463.44 ± 109.84 b |
| CAF | 349.57 ± 13.19 a | 259.17 ± 6.46 a | 535.51 ± 16.84 b |
| Sieve Openings (µm) | Cumulative Particle Size | ||
|---|---|---|---|
| S1 | S2 | S3 | |
| 4000 | 100 | 100 | 100 |
| 2000 | 85.15 ± 0.09 a | 85.47 ± 0.08 a | 98.89 ± 0.10 b |
| 1000 | 33.93 ± 0.11 a | 33.69 ± 0.30 a | 97.31 ± 0.14 b |
| 800 | 14.69 ± 0.16 a | 14.24 ± 0.13 a | 95.25 ± 0.04 b |
| 630 | 11.63 ± 0.26 a | 11.64 ± 0.06 a | 93.94 ± 0.06 b |
| 400 | 9.40 ± 0.20 a | 9.78 ± 0.01 a | 6.40 ± 0.41 b |
| 250 | 5.72 ± 0.13 a | 6.60 ± 0.07 a | 3.70 ± 0.33 b |
| 125 | 3.33 ± 0.10 | 4.26 ± 0.04 | 0.00 |
| 0 | 0.61 ± 0.09 | 0.71 ± 0.03 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercogliano, F.; Bani, C.; Tretola, M.; Landolfi, C.; Ottoboni, M.; Cheli, F.; Restani, P.; Pinotti, L.; Di Lorenzo, C. Preliminary Assessment of Alkaloid Content in Cocoa (Theobroma cacao L.) Hulls for Safe Consumption as a Feed Ingredient. Toxins 2025, 17, 441. https://doi.org/10.3390/toxins17090441
Mercogliano F, Bani C, Tretola M, Landolfi C, Ottoboni M, Cheli F, Restani P, Pinotti L, Di Lorenzo C. Preliminary Assessment of Alkaloid Content in Cocoa (Theobroma cacao L.) Hulls for Safe Consumption as a Feed Ingredient. Toxins. 2025; 17(9):441. https://doi.org/10.3390/toxins17090441
Chicago/Turabian StyleMercogliano, Francesca, Corinne Bani, Marco Tretola, Carla Landolfi, Matteo Ottoboni, Federica Cheli, Patrizia Restani, Luciano Pinotti, and Chiara Di Lorenzo. 2025. "Preliminary Assessment of Alkaloid Content in Cocoa (Theobroma cacao L.) Hulls for Safe Consumption as a Feed Ingredient" Toxins 17, no. 9: 441. https://doi.org/10.3390/toxins17090441
APA StyleMercogliano, F., Bani, C., Tretola, M., Landolfi, C., Ottoboni, M., Cheli, F., Restani, P., Pinotti, L., & Di Lorenzo, C. (2025). Preliminary Assessment of Alkaloid Content in Cocoa (Theobroma cacao L.) Hulls for Safe Consumption as a Feed Ingredient. Toxins, 17(9), 441. https://doi.org/10.3390/toxins17090441







