Bee Venom: Composition and Anticancer Properties
Abstract
1. Introduction
2. Bee Venom
3. The Composition of Bee Venom
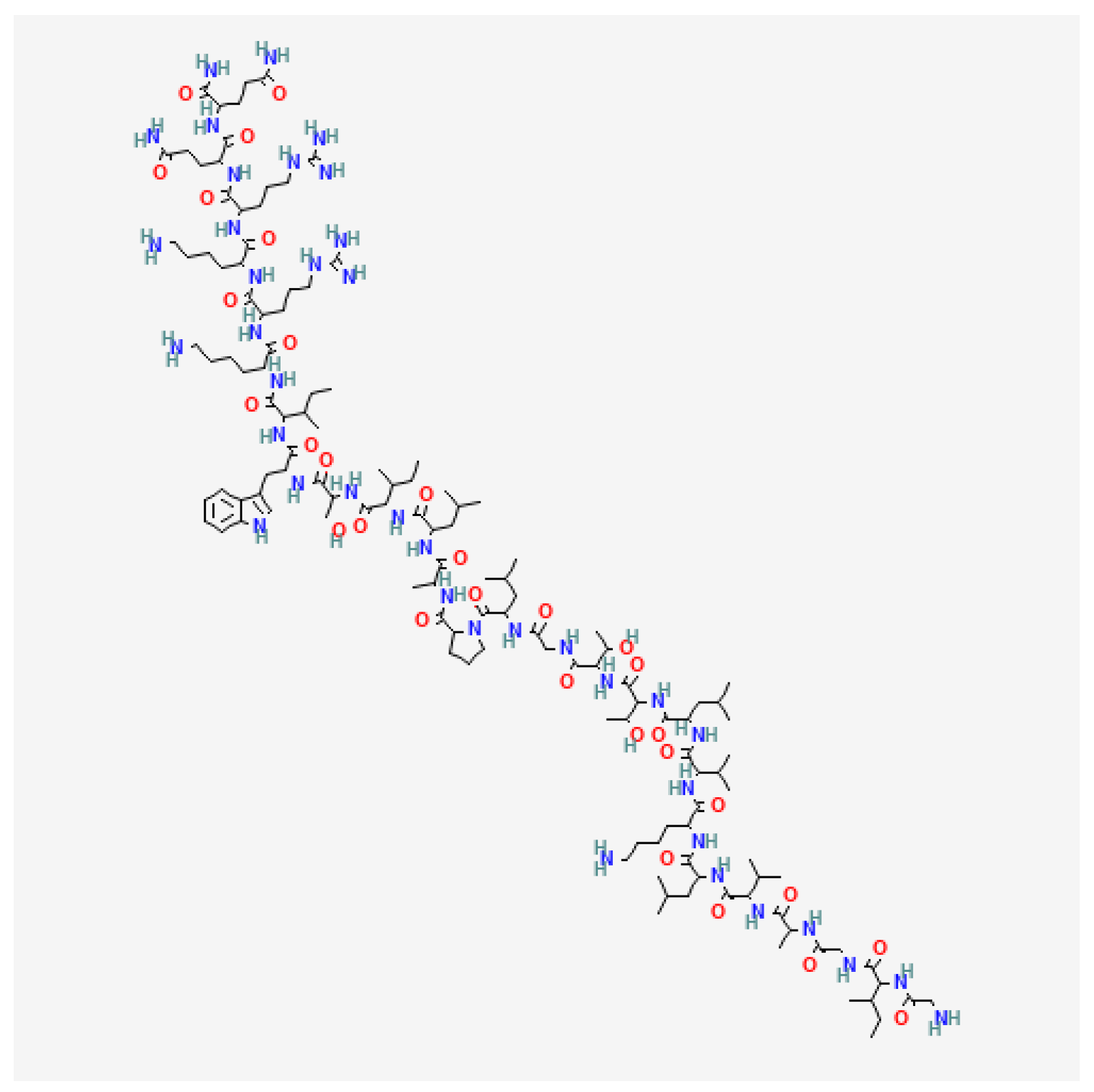
3.1. Melittin
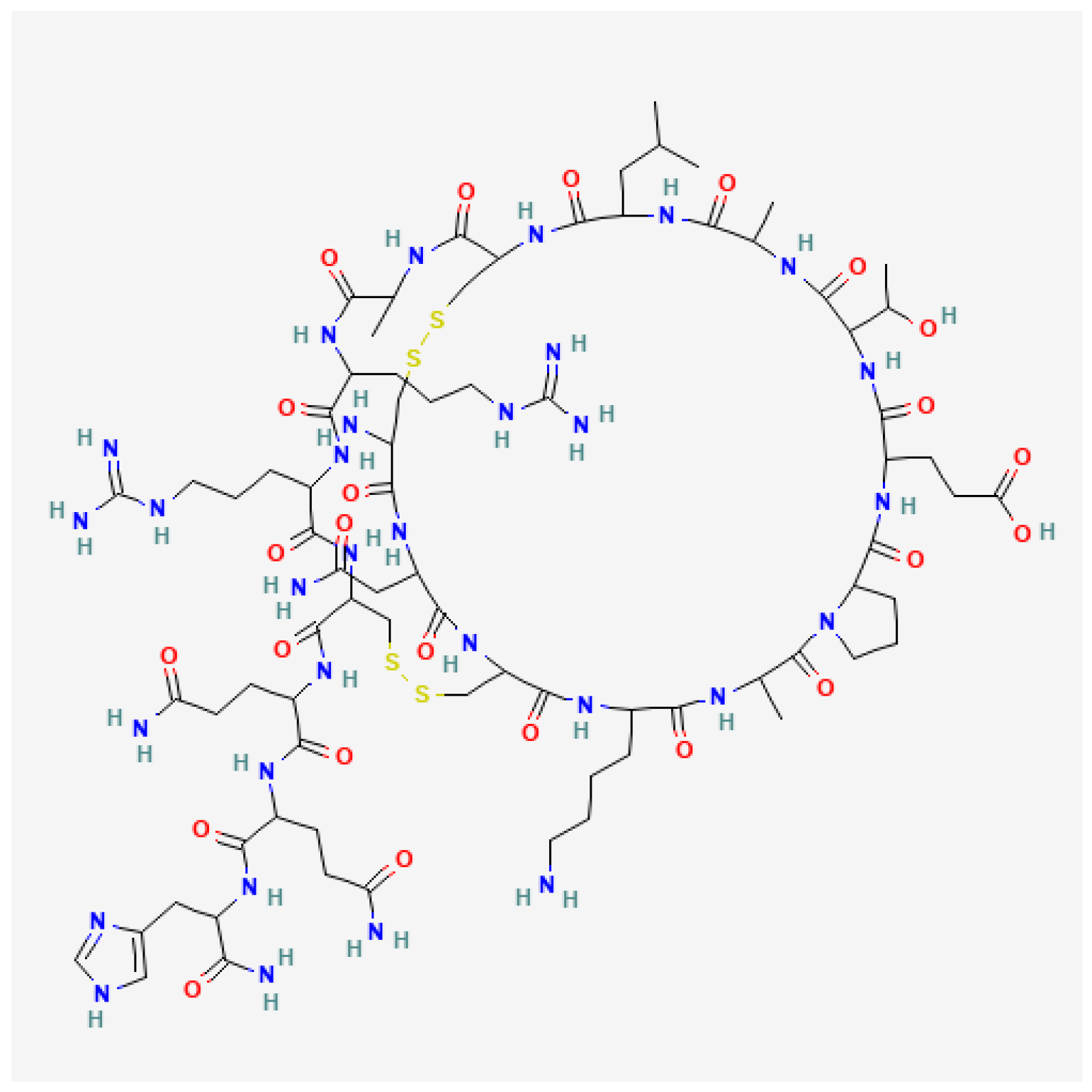
3.2. Apamin
3.3. Mast Cell Degranulating (MCD) Peptide
3.4. Other Peptides of Bee Venom
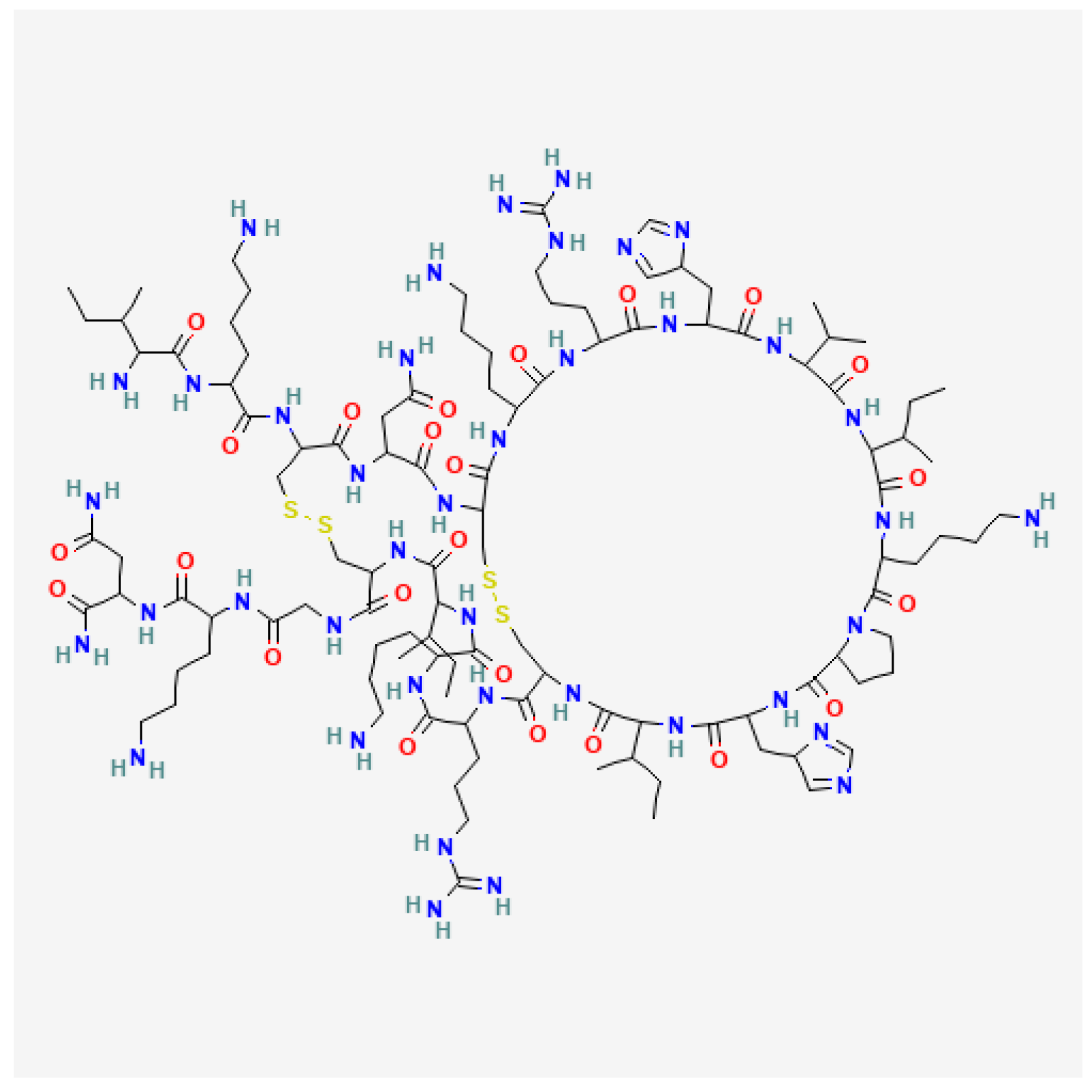
3.5. Phospholipase A2
3.6. Other Enzymes of Bee Venom
4. The Therapeutic Properties of Bee Venom
5. Anticancer Effects of Bee Venom and Its Components
5.1. Anticancer Effects of Bee Venom
5.2. Anticancer Effect of Melittin
5.3. Anticancer Effects of Phospholipase A2
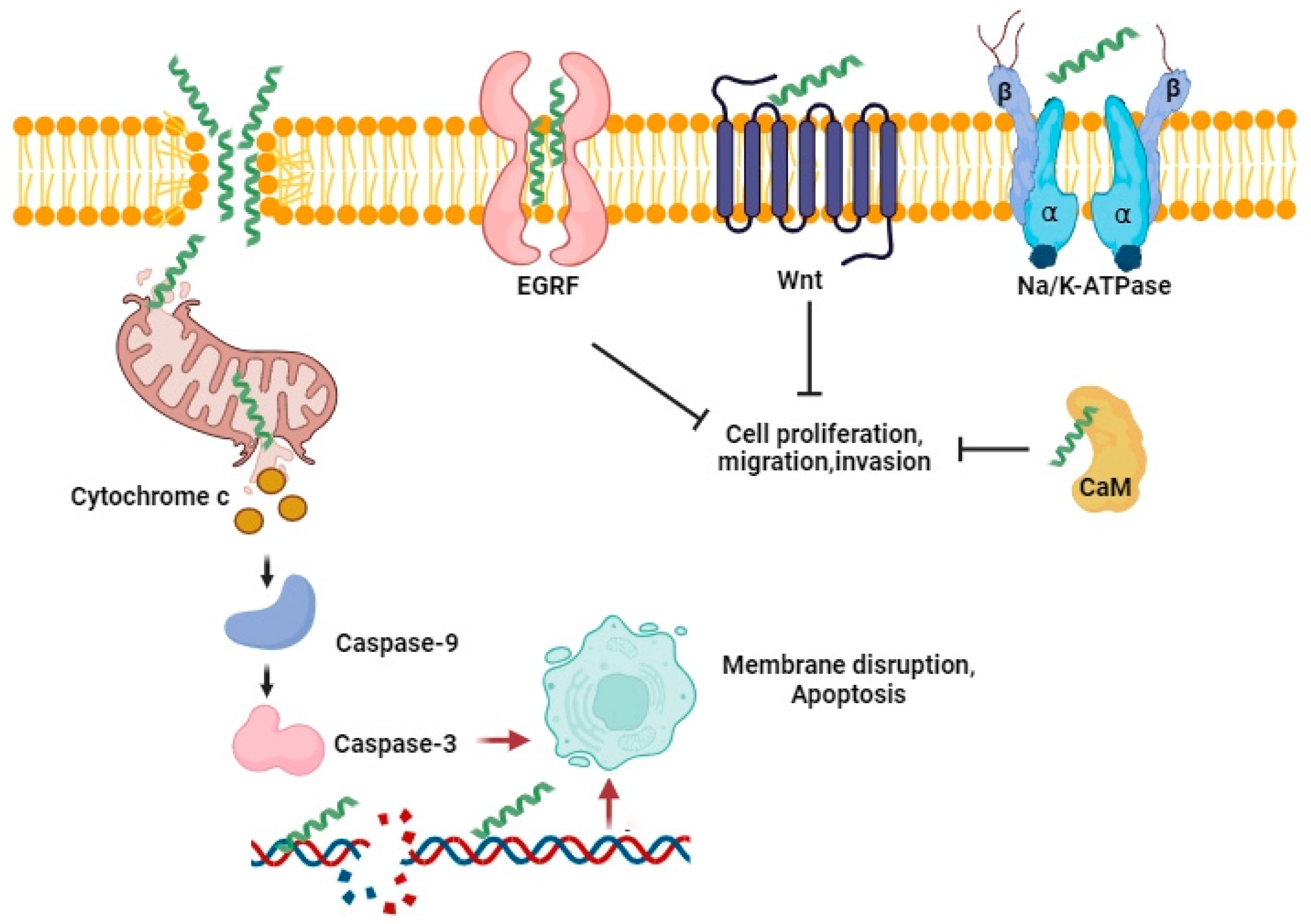
5.4. The Mechanisms of Bee Venom and Melittin Anticancer Activity
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harvey, A. From Demons to Darlings: Drugs from Venoms. Drug Discov. Today 1998, 3, 531–532. [Google Scholar] [CrossRef]
- von Reumont, B.M.; Anderluh, G.; Antunes, A.; Ayvazyan, N.; Beis, D.; Caliskan, F.; Crnković, A.; Damm, M.; Dutertre, S.; Ellgaard, L.; et al. Modern Venomics-Current Insights, Novel Methods, and Future Perspectives in Biological and Applied Animal Venom Research. Gigascience 2022, 11, giac048. [Google Scholar] [CrossRef] [PubMed]
- Sjakste, N.; Gajski, G. A Review on Genotoxic and Genoprotective Effects of Biologically Active Compounds of Animal Origin. Toxins 2023, 15, 165. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A. Strategies for Discovering Drugs from Previously Unexplored Natural Products. Drug Discov. Today 2000, 5, 294–300. [Google Scholar] [CrossRef]
- Harvey, A.L.; Clark, R.L.; Mackay, S.P.; Johnston, B.F. Current Strategies for Drug Discovery through Natural Products. Expert Opin. Drug Discov. 2010, 5, 559–568. [Google Scholar] [CrossRef]
- Parekh, H.S.; Liu, G.; Wei, M.Q. A New Dawn for the Use of Traditional Chinese Medicine in Cancer Therapy. Mol. Cancer 2009, 8, 21. [Google Scholar] [CrossRef]
- Graziose, R.; Lila, M.A.; Raskin, I. Merging Traditional Chinese Medicine with Modern Drug Discovery Technologies to Find Novel Drugs and Functional Foods. Curr. Drug Discov. Technol. 2010, 7, 2–12. [Google Scholar] [CrossRef]
- Sung, S.-H.; Lee, H.-J.; Han, J.-E.; Sung, A.D.-M.; Park, M.; Shin, S.; Jeong, H.I.; Jang, S.; Lee, G. Bee Venom Acupuncture for Neck Pain: A Review of the Korean Literature. Toxins 2023, 15, 129. [Google Scholar] [CrossRef]
- Jia, Z.; Zhu, X.; Zhou, Y.; Wu, J.; Cao, M.; Hu, C.; Yu, L.; Xu, R.; Chen, Z. Polypeptides from Traditional Chinese Medicine: Comprehensive Review of Perspective towards Cancer Management. Int. J. Biol. Macromol. 2024, 260, 129423. [Google Scholar] [CrossRef]
- Amin, A.R.M.R.; Kucuk, O.; Khuri, F.R.; Shin, D.M. Perspectives for Cancer Prevention with Natural Compounds. J. Clin. Oncol. 2009, 27, 2712–2725. [Google Scholar] [CrossRef]
- Herzig, V.; Cristofori-Armstrong, B.; Israel, M.R.; Nixon, S.A.; Vetter, I.; King, G.F. Animal Toxins-Nature’s Evolutionary-Refined Toolkit for Basic Research and Drug Discovery. Biochem. Pharmacol. 2020, 181, 114096. [Google Scholar] [CrossRef]
- Son, D.J.; Lee, J.W.; Lee, Y.H.; Song, H.S.; Lee, C.K.; Hong, J.T. Therapeutic Application of Anti-Arthritis, Pain-Releasing, and Anti-Cancer Effects of Bee Venom and Its Constituent Compounds. Pharmacol. Ther. 2007, 115, 246–270. [Google Scholar] [CrossRef]
- Bordon, K.d.C.F.; Cologna, C.T.; Fornari-Baldo, E.C.; Pinheiro-Júnior, E.L.; Cerni, F.A.; Amorim, F.G.; Anjolette, F.A.P.; Cordeiro, F.A.; Wiezel, G.A.; Cardoso, I.A.; et al. From Animal Poisons and Venoms to Medicines: Achievements, Challenges and Perspectives in Drug Discovery. Front. Pharmacol. 2020, 11, 1132. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Li, C.; Aidoo, O.F.; Fernando, I.; Haddad, M.A.; Pereira, J.A.M.; Blinov, A.; Golik, A.; Câmara, J.S. Unravelling the Potential of Insects for Medicinal Purposes—A Comprehensive Review. Heliyon 2023, 9, e15938. [Google Scholar] [CrossRef] [PubMed]
- Gajski, G.; Garaj-Vrhovac, V. Melittin: A Lytic Peptide with Anticancer Properties. Environ. Toxicol. Pharmacol. 2013, 36, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N. Bee Venom in Cancer Therapy. Cancer Metast. Rev. 2012, 31, 173–194. [Google Scholar] [CrossRef]
- Khalil, A.; Elesawy, B.H.; Ali, T.M.; Ahmed, O.M. Bee Venom: From Venom to Drug. Molecules 2021, 26, 4941. [Google Scholar] [CrossRef]
- Gajski, G.; Garaj-Vrhovac, V. Radioprotective Effects of Honeybee Venom (Apis mellifera) against 915-MHz Microwave Radiation?Induced DNA Damage in Wistar Rat Lymphocytes: In Vitro Study. Int. J. Toxicol. 2009, 28, 88–98. [Google Scholar] [CrossRef]
- Varanda, E.A.; Tavares, D.C. Radioprotection: Mechanisms and radioprotective agents including honeybee venom. J. Venom. Anim. Toxins 1998, 4, 5–21. [Google Scholar] [CrossRef]
- Shafiga, T.; Elmar, B. Radioprotective Action of Venom of Honey Bee Apis mellifera Caucasica. Int. J. Environ. Agric. Biotechnol. 2017, 2, 2288–2292. [Google Scholar] [CrossRef][Green Version]
- Ullah, A.; Aldakheel, F.M.; Anjum, S.I.; Raza, G.; Khan, S.A.; Tlak Gajger, I. Pharmacological Properties and Therapeutic Potential of Honey Bee Venom. Saudi Pharm. J. 2023, 31, 96–109. [Google Scholar] [CrossRef]
- Shi, P.; Xie, S.; Yang, J.; Zhang, Y.; Han, S.; Su, S.; Yao, H. Pharmacological Effects and Mechanisms of Bee Venom and Its Main Components: Recent Progress and Perspective. Front. Pharmacol. 2022, 13, 1001553. [Google Scholar] [CrossRef]
- Nam, K.-W.; Je, K.-H.; Lee, J.H.; Han, H.J.; Lee, H.J.; Kang, S.K.; Mar, W. Inhibition of COX-2 Activity and Proinflammatory Cytokines (TNF-α and IL-1β) Production by Water-Soluble Sub-Fractionated Parts from Bee (Apis mellifera) Venom. Arch. Pharm. Res. 2003, 26, 383–388. [Google Scholar] [CrossRef]
- Lee, J.H.; Kwon, Y.B.; Han, H.J.; Mar, W.C.; Lee, H.J.; Yang, I.S.; Beitz, A.J.; Kang, S.K. Bee Venom Pretreatment Has Both an Antinociceptive and Anti-Inflammatory Effect on Carrageenan-Induced Inflammation. J. Vet. Med. Sci. 2001, 63, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.H.; Huh, J.E.; Lee, J.D.; Choi, D.Y.; Park, D.S. Antinociceptive Effect and the Mechanism of Bee Venom Acupuncture (Apipuncture) on Inflammatory Pain in the Rat Model of Collagen-Induced Arthritis: Mediation by α2-Adrenoceptors. Brain Res. 2006, 1073–1074, 305–310. [Google Scholar] [CrossRef]
- El-Seedi, H.; Abd El-Wahed, A.; Yosri, N.; Musharraf, S.G.; Chen, L.; Moustafa, M.; Zou, X.; Al-Mousawi, S.; Guo, Z.; Khatib, A.; et al. Antimicrobial Properties of Apis mellifera’s Bee Venom. Toxins 2020, 12, 451. [Google Scholar] [CrossRef]
- Nainu, F.; Masyita, A.; Bahar, M.A.; Raihan, M.; Prova, S.R.; Mitra, S.; Emran, T.B.; Simal-Gandara, J. Pharmaceutical Prospects of Bee Products: Special Focus on Anticancer, Antibacterial, Antiviral, and Antiparasitic Properties. Antibiotics 2021, 10, 822. [Google Scholar] [CrossRef]
- Viegas, S.; Ladeira, C.; Costa-Veiga, A.; Perelman, J.; Gajski, G. Forgotten Public Health Impacts of Cancer—An Overview. Arh. Hig. Rada Toksikol. 2017, 68, 287–297. [Google Scholar] [CrossRef]
- Gotay, C.C. Cancer Prevention: Major Initiatives and Looking into the Future. Expert Rev. Pharmacoecon. Outcomes Res. 2010, 10, 143–154. [Google Scholar] [CrossRef]
- Wild, C.; Weiderpass, E.; Stewart, B. World Cancer Report: Cancer Research for Cancer Development; IARC: Lyon, France, 2020; ISBN 9789283204473.
- Stewart, B.W.; Wild, C.; International Agency for Research on Cancer; World Health Organization. World Cancer Report 2014; IARC: Lyon, France, 2014; ISBN 9789283204299.
- Mehta, R.G.; Pezzuto, J.M. Discovery of Cancer Preventive Agents from Natural Products: From Plants to Prevention. Curr. Oncol. Rep. 2002, 4, 478–486. [Google Scholar] [CrossRef]
- Mehta, R.G.; Murillo, G.; Naithani, R.; Peng, X. Cancer Chemoprevention by Natural Products: How Far Have We Come? Pharm. Res. 2010, 27, 950–961. [Google Scholar] [CrossRef]
- Guilford, J.M.; Pezzuto, J.M. Natural Products as Inhibitors of Carcinogenesis. Expert Opin. Investig. Drugs 2008, 17, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Cherniack, P. Bugs as Drugs, Part 1: Insects. The “New” Alternative Medicine for the 21st Century? Altern. Med. Rev. 2010, 15, 124–135. [Google Scholar]
- Cherniack, E. Bugs as Drugs, Part Two: Worms, Leeches, Scorpions, Snails, Ticks, Centipedes, and Spiders. Altern. Med. Rev. 2011, 16, 50–58. [Google Scholar]
- Fratellone, P.M.; Tsimis, F.; Fratellone, G. Apitherapy Products for Medicinal Use. J. Altern. Complement. Med. 2016, 22, 1020–1022. [Google Scholar] [CrossRef]
- Abd El-Aziz, T.M.; Garcia Soares, A.; Stockand, J.D. Snake Venoms in Drug Discovery: Valuable Therapeutic Tools for Life Saving. Toxins 2019, 11, 564. [Google Scholar] [CrossRef]
- Kalita, B.; Saviola, A.J.; Mukherjee, A.K. From Venom to Drugs: A Review and Critical Analysis of Indian Snake Venom Toxins Envisaged as Anticancer Drug Prototypes. Drug Discov. Today 2021, 26, 993–1005. [Google Scholar] [CrossRef]
- Qi, J.; Zulfiker, A.H.M.; Li, C.; Good, D.; Wei, M.Q. The Development of Toad Toxins as Potential Therapeutic Agents. Toxins 2018, 10, 336. [Google Scholar] [CrossRef]
- Saez, N.J.; Senff, S.; Jensen, J.E.; Er, S.Y.; Herzig, V.; Rash, L.D.; King, G.F. Spider-Venom Peptides as Therapeutics. Toxins 2010, 2, 2851–2871. [Google Scholar] [CrossRef] [PubMed]
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in Peptide Drug Discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Lischer, K.; Sitorus, S.; Guslianto, B.; Avila, F.; Khayrani, A.; Sahlan, M. Anti-Breast Cancer Activity on MCF-7 Cells of Melittin from Indonesia’s Apis Cerana: An In Vitro Study. Asian Pacific J. Cancer Prev. 2021, 22, 3913–3920. [Google Scholar] [CrossRef] [PubMed]
- Sangboonruang, S.; Kitidee, K.; Chantawannakul, P.; Tragoolpua, K.; Tragoolpua, Y. Melittin from Apis Florea Venom as a Promising Therapeutic Agent for Skin Cancer Treatment. Antibiotics 2020, 9, 517. [Google Scholar] [CrossRef]
- Salcido, R. Honey, Is Apitherapy an Emergency? Adv. Skin Wound Care 2008, 21, 552. [Google Scholar] [CrossRef] [PubMed]
- Weis, W.A.; Ripari, N.; Conte, F.L.; da Silva Honorio, M.; Sartori, A.A.; Matucci, R.H.; Sforcin, J.M. An Overview about Apitherapy and Its Clinical Applications. Phytomed. Plus 2022, 2, 100239. [Google Scholar] [CrossRef]
- Małek, A.; Strzemski, M.; Kurzepa, J.; Kurzepa, J. Can Bee Venom Be Used as Anticancer Agent in Modern Medicine? Cancers 2023, 15, 3714. [Google Scholar] [CrossRef]
- Mebs, D. Venomous and Poisonous Animals: A Handbook for Biologists, Toxicologists and Toxinologists, Physicians and Pharmacists; CRC Press: Boca Raton, FL, USA, 2002; ISBN 0849312647. [Google Scholar]
- Chen, J.; Lariviere, W.R. The Nociceptive and Anti-Nociceptive Effects of Bee Venom Injection and Therapy: A Double-Edged Sword. Prog. Neurobiol. 2010, 92, 151–183. [Google Scholar] [CrossRef]
- Radić, S. Pčelinji Otrov: Prirodni Antireumatik i Anelgetik, 1st ed.; Graf Form: Split, Croatia, 2005. [Google Scholar]
- Stell, I. Understanding Bee Anatomy: A Full Colour Guide; The Catford Press: London, UK, 2012; ISBN 0957422806. [Google Scholar]
- Roat, T.C.; Nocelli, R.C.F.; da Cruz Landim, C. The Venom Gland of Queens of Apis mellifera (Hymenoptera, Apidae): Morphology and Secretory Cycle. Micron 2006, 37, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.J.; Dils, V.; Billen, J. Morphology of the Dufour Gland within the Honey Bee Sting Gland Complex. Apidologie 2005, 36, 543–546. [Google Scholar] [CrossRef]
- Bridges, A.R.; Owen, M.D. The Morphology of the Honey Bee (Apis mellifera L.) Venom Gland and Reservoir. J. Morphol. 1984, 181, 69–86. [Google Scholar] [CrossRef]
- Przybilla, B.; Ruëff, F. Hymenoptera Venom Allergy. JDDG J. Dtsch. Dermatol. Ges. 2010, 8, 114–129. [Google Scholar] [CrossRef]
- Brown, T.C.; Tankersley, M.S. The Sting of the Honeybee: An Allergic Perspective. Ann. Allergy. Asthma Immunol. 2011, 107, 463–470, quiz 471. [Google Scholar] [CrossRef]
- Temizoz, O.; Celik, Y.; Asil, T.; Balci, K.; Unlu, E.; Yilmaz, A. Stroke Due to Bee Sting. Neurologist 2009, 15, 42–43. [Google Scholar] [CrossRef]
- Brown, T.C. Reactions to Honeybee Stings: An Allergic Prospective. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 365–371. [Google Scholar] [CrossRef]
- Yuan, I.H.; Golden, D.B.K. Wings and Stings: Hymenoptera on Vacation. Ann. Allergy. Asthma Immunol. 2023, 130, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Burzyńska, M.; Piasecka-Kwiatkowska, D. A Review of Honeybee Venom Allergens and Allergenicity. Int. J. Mol. Sci. 2021, 22, 8371. [Google Scholar] [CrossRef] [PubMed]
- Elavarasi, A.; Haq, T.M.; Thahira, T.; Bineesh, C.; Kancharla, L.B. Acute Ischemic Stroke Due to Multiple Bee Stings_A Delayed Complication. Ann. Indian Acad. Neurol. 2020, 23, 135–136. [Google Scholar] [CrossRef] [PubMed]
- Rajendiran, C.; Puvanalingam, A.; Thangam, D.; Ragunanthanan, S.; Ramesh, D.; Venkatesan, S.; Sundar, C. Stroke after Multiple Bee Sting. J. Assoc. Physicians India 2012, 60, 122–124. [Google Scholar] [PubMed]
- Eskridge, E.M.; Elliott, W.B.; Elliott, A.H.; Eskridge, P.B.; Doerr, J.C.; Schneller, N.; Reisman, R.E. Adaptation of the Electrical Stimulation Procedure for the Collection of Vespid Venoms. Toxicon 1981, 19, 893–897. [Google Scholar] [CrossRef]
- Turillazzi, F.; Pieraccini, G.; Turillazzi, S.; Orsi Battaglini, N.; Severino, M. Venom Collection by Electrical Stimulation in the Invasive Species Polistes Dominula Reared Using a Vespiculture Regime. Molecules 2022, 27, 8821. [Google Scholar] [CrossRef]
- Benton, A.W.; Morse, R.A.; Stewart, J.D. Venom Collection from Honey Bees. Science 1963, 142, 228–230. [Google Scholar] [CrossRef]
- Mueller, U.; Reisman, R.; Wypych, J.; Elliott, W.; Steger, R.; Walsh, S.; Arbesman, C. Comparison of Vespid Venoms Collected by Electrostimulation and by Venom Sac Extraction. J. Allergy Clin. Immunol. 1981, 68, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Habermann, E. Bee and Wasp Venoms. Science 1972, 177, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Gauldie, J.; Hanson, J.M.; Shipolini, R.A.; Vernon, C.A. The Structures of Some Peptides from Bee Venom. Eur. J. Biochem. 1978, 83, 405–410. [Google Scholar] [CrossRef]
- Dotimas, E.M.; Hider, R.C. Honeybee Venom. Bee World 1987, 68, 51–70. [Google Scholar] [CrossRef]
- Hider, R.C. Honeybee Venom: A Rich Source of Pharmacologically Active Peptides. Endeavour 1988, 12, 60–65. [Google Scholar] [CrossRef]
- Wehbe, R.; Frangieh, J.; Rima, M.; El Obeid, D.; Sabatier, J.-M.; Fajloun, Z. Bee Venom: Overview of Main Compounds and Bioactivities for Therapeutic Interests. Molecules 2019, 24, 2997. [Google Scholar] [CrossRef]
- Obeidat, M.; Al-khraisat, I.F.; Jaradat, D.M.M.; Ghanim, B.Y.; Abdallah, Q.M.; Arqoub, D.A.; Sabbah, D.; Al-Sanabra, O.M.; Arafat, T.; Qinna, N.A. Mellitin Peptide Quantification in Seasonally Collected Crude Bee Venom and Its Anticancer Effects on Myelogenous K562 Human Leukaemia Cell Line. BMC Complement. Med. Ther. 2023, 23, 132. [Google Scholar] [CrossRef]
- Terwilliger, T.C.; Eisenberg, D. The Structure of Melittin. I. Structure Determination and Partial Refinement. J. Biol. Chem. 1982, 257, 6010–6015. [Google Scholar] [CrossRef]
- Terwilliger, T.C.; Eisenberg, D. The Structure of Melittin. II. Interpretation of the Structure. J. Biol. Chem. 1982, 257, 6016–6022. [Google Scholar] [CrossRef]
- Terwilliger, T.C.; Weissman, L.; Eisenberg, D. The Structure of Melittin in the Form I Crystals and Its Implication for Melittin’s Lytic and Surface Activities. Biophys. J. 1982, 37, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, C.E. The Actions of Melittin on Membranes. Biochim. Biophys. Acta-Rev. Biomembr. 1990, 1031, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Bechinger, B. Structure and Functions of Channel-Forming Peptides: Magainins, Cecropins, Melittin and Alamethicin. J. Membr. Biol. 1997, 156, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Bechinger, B.; Lohner, K. Detergent-like Actions of Linear Amphipathic Cationic Antimicrobial Peptides. Biochim. Biophys. Acta 2006, 1758, 1529–1539. [Google Scholar] [CrossRef] [PubMed]
- Lad, P.J.; Thomas Shier, W. Activation of Microsomal Guanylate Cyclase by a Cytotoxic Polypeptide: Melittin. Biochem. Biophys. Res. Commun. 1979, 89, 315–321. [Google Scholar] [CrossRef]
- Shier, W.T. Activation of High Levels of Endogenous Phospholipase A2 in Cultured Cells. Proc. Natl. Acad. Sci. USA 1979, 76, 195–199. [Google Scholar] [CrossRef]
- Ladokhin, A.S.; White, S.H. Folding of Amphipathic α-Helices on Membranes: Energetics of Helix Formation by Melittin. J. Mol. Biol. 1999, 285, 1363–1369. [Google Scholar] [CrossRef]
- Gajski, G.; Domijan, A.-M.; Žegura, B.; Štern, A.; Gerić, M.; Novak Jovanović, I.; Vrhovac, I.; Madunić, J.; Breljak, D.; Filipič, M.; et al. Melittin Induced Cytogenetic Damage, Oxidative Stress and Changes in Gene Expression in Human Peripheral Blood Lymphocytes. Toxicon 2016, 110, 56–67. [Google Scholar] [CrossRef]
- Stuhlmeier, K.M. Apis mellifera Venom and Melittin Block Neither NF-Kappa B-P50-DNA Interactions nor the Activation of NF-Kappa B, Instead They Activate the Transcription of Proinflammatory Genes and the Release of Reactive Oxygen Intermediates. J. Immunol. 2007, 179, 655–664. [Google Scholar] [CrossRef]
- Watala, C.; Kowalczyk, J.K. Hemolytic Potency and Phospholipase Activity of Some Bee and Wasp Venoms. Comp. Biochem. Physiol. C 1990, 97, 187–194. [Google Scholar] [CrossRef]
- Shaposhnikova, V.V.; Egorova, M.V.; Kudryavtsev, A.A.; Levitman, M.K.; Korystov YuN, K.Y. The Effect of Melittin on Proliferation and Death of Thymocytes. FEBS Lett. 1997, 410, 285–288. [Google Scholar] [CrossRef]
- Maher, S.; McClean, S. Melittin Exhibits Necrotic Cytotoxicity in Gastrointestinal Cells Which Is Attenuated by Cholesterol. Biochem. Pharmacol. 2008, 75, 1104–1114. [Google Scholar] [CrossRef]
- Fletcher, J.E.; Jiang, M.S. Possible Mechanisms of Action of Cobra Snake Venom Cardiotoxins and Bee Venom Melittin. Toxicon 1993, 31, 669–695. [Google Scholar] [CrossRef]
- Raghuraman, H.; Chattopadhyay, A. Melittin: A Membrane-Active Peptide with Diverse Functions. Biosci. Rep. 2007, 27, 189–223. [Google Scholar] [CrossRef]
- Hider, R.C.; Ragnarsson, U. A Proposal for the Structure of Apamin. FEBS Lett. 1980, 111, 189–193. [Google Scholar] [CrossRef]
- Habermann, E. Apamin. Pharmacol. Ther. 1984, 25, 255–270. [Google Scholar] [CrossRef]
- Habermann, E. Neurotoxicity of Apamin and MCD Peptide upon Central Application. Naunyn. Schmiedeberg’s Arch. Pharmacol. 1977, 300, 189–191. [Google Scholar] [CrossRef]
- Strong, P.N. Potassium Channel Toxins. Pharmacol. Ther. 1990, 46, 137–162. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Han, S.M.; Park, K.-K. Therapeutic Effects of Apamin as a Bee Venom Component for Non-Neoplastic Disease. Toxins 2020, 12, 195. [Google Scholar] [CrossRef] [PubMed]
- Ziai, M.R.; Russek, S.; Wang, H.-C.; Beer, B.; Blume, A.J. Mast Cell Degranulating Peptide: A Multi-Functional Neurotoxin. J. Pharm. Pharmacol. 1990, 42, 457–461. [Google Scholar] [CrossRef]
- Dotimas, E.M.; Hamid, K.R.; Hider, R.C.; Ragnarsson, U. Isolation and Structure Analysis of Bee Venom Mast Cell Degranulating Peptide. Biochim. Biophys. Acta-Protein Struct. Mol. Enzymol. 1987, 911, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Buku, A. Mast Cell Degranulating (MCD) Peptide: A Prototypic Peptide in Allergy and Inflammation. Peptides 1999, 20, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Palma, M.S. Insect Venom Peptides. In Handbook of Biologically Active Peptides; Kastin, A., Ed.; Academic Press: New York, NY, USA, 2006; pp. 409–416. [Google Scholar]
- Shkenderov, S.; Koburova, K. Adolapin—A Newly Isolated Analgetic and Anti-Inflammatory Polypeptide from Bee Venom. Toxicon 1982, 20, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Koburova, K.; Michailova, S.; Shkenderov, S. Further Investigation on the Antiinflammatory Properties of Adolapin–Bee Venom Polypeptide. Acta Physiol. Pharmacol. Bulg. 1985, 11, 50–55. [Google Scholar]
- Miroshnikov, A.; Boĭko, V.; Snezhkova, L.; Severin, S.; Shvets, V. [Interaction of Tertiapin, a Neurotoxin from Bee Venom, with Calmodulin]. Bioorg. Khim. 1983, 9, 26–32. [Google Scholar] [PubMed]
- Shipolini, R.A.; Callewaert, G.L.; Cottrell, R.C.; Doonan, S.; Vernon, C.A.; Banks, B.E.C. Phospholipase A from Bee Venom. Eur. J. Biochem. 1971, 20, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Shipolini, R.A.; Doonan, S.; Vernon, C.A. The Disulphide Bridges of Phospholipase A2 from Bee Venom. Eur. J. Biochem. 1974, 48, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Valentin, E.; Lambeau, G. What Can Venom Phospholipases A2 Tell Us about the Functional Diversity of Mammalian Secreted Phospholipases A2? Biochimie 2000, 82, 815–831. [Google Scholar] [CrossRef] [PubMed]
- Six, D.A.; Dennis, E.A. The Expanding Superfamily of Phospholipase A2 Enzymes: Classification and Characterization. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2000, 1488, 1–19. [Google Scholar] [CrossRef]
- Lee, G.; Bae, H. Bee Venom Phospholipase A2: Yesterday’s Enemy Becomes Today’s Friend. Toxins 2016, 8, 48. [Google Scholar] [CrossRef]
- Doery, H.M.; Pearson, J.E. Phospholipase B in Snake Venoms and Bee Venom. Biochem. J. 1964, 92, 599–602. [Google Scholar] [CrossRef]
- Kreil, G. Hyaluronidases—A Group of Neglected Enzymes. Protein Sci. 1995, 4, 1666–1669. [Google Scholar] [CrossRef]
- Karpozilos, A.; Pavlidis, N. The Treatment of Cancer in Greek Antiquity. Eur. J. Cancer 2004, 40, 2033–2040. [Google Scholar] [CrossRef]
- Hellner, M.; Winter, D.; von Georgi, R.; Münstedt, K. Apitherapy: Usage and Experience in German Beekeepers. Evid. Based. Complement. Alternat. Med. 2008, 5, 475–479. [Google Scholar] [CrossRef]
- Šuligoj, M. Origins and Development of Apitherapy and Apitourism. J. Apic. Res. 2021, 60, 369–374. [Google Scholar] [CrossRef]
- Mizrahi, A.; Lensky, Y. (Eds.) Bee Products: Properties, Applications, and Apitherapy; Springer: Boston, MA, USA, 1997; ISBN 978-1-4757-9373-4. [Google Scholar]
- Havas, L.J. Effect of Bee Venom on Colchicine-Induced Tumours. Nature 1950, 166, 567–568. [Google Scholar] [CrossRef]
- Mufson, R.A.; Laskin, J.D.; Fisher, P.B.; Weinstein, I.B. Melittin Shares Certain Cellular Effects with Phorbol Ester Tumour Promoters. Nature 1979, 280, 72–74. [Google Scholar] [CrossRef]
- McDonald, J.A.; Li, F.P.; Mehta Cyrus, R. Cancer Mortality Among Beekeepers. J. Occup. Med. 1979, 21, 811–813. [Google Scholar]
- Chaisakul, J.; Hodgson, W.C.; Kuruppu, S.; Prasongsook, N. Effects of Animal Venoms and Toxins on Hallmarks of Cancer. J. Cancer 2016, 7, 1571–1578. [Google Scholar] [CrossRef]
- Chatterjee, B. Animal Venoms Have Potential to Treat Cancer. Curr. Top. Med. Chem. 2018, 18, 2555–2566. [Google Scholar] [CrossRef]
- Liu, C.-C.; Hao, D.-J.; Zhang, Q.; An, J.; Zhao, J.-J.; Chen, B.; Zhang, L.-L.; Yang, H. Application of Bee Venom and Its Main Constituent Melittin for Cancer Treatment. Cancer Chemother. Pharmacol. 2016, 78, 1113–1130. [Google Scholar] [CrossRef]
- Hait, W.N.; Grais, L.; Benz, C.; Cadman, E.C. Inhibition of Growth of Leukemic Cells by Inhibitors of Calmodulin: Phenothiazines and Melittin. Cancer Chemother. Pharmacol. 1985, 14, 202–205. [Google Scholar] [CrossRef]
- Lee, G.L.; Hait, W.N. Inhibition of Growth of C6 Astrocytoma Cells by Inhibitors of Calmodulin. Life Sci. 1985, 36, 347–354. [Google Scholar] [CrossRef]
- Lazo, J.S.; Hait, W.N.; Kennedy, K.A.; Braun, I.D.; Meandzija, B. Enhanced Bleomycin-Induced DNA Damage and Cytotoxicity with Calmodulin Antagonists. Mol. Pharmacol. 1985, 27, 387–393. [Google Scholar]
- Lazo, J.S.; Chen, D.-L.; Gallicchio, V.S.; Hait, W.N. Increased Lethality of Calmodulin Antagonists and Bleomycin to Human Bone Marrow and Bleomycin-Resistant Malignant Cells. Cancer Res. 1986, 46, 2236–2240. [Google Scholar]
- Hait, W.N.; Lee, G.L. Characteristics of the Cytotoxic Effects of the Phenothiazine Class of Calmodulin Antagonists. Biochem. Pharmacol. 1985, 34, 3973–3978. [Google Scholar] [CrossRef]
- Killion, J.J.; Dunn, J.D. Differential Cytolysis of Murine Spleen, Bone-Marrow and Leukemia Cells by Melittin Reveals Differences in Membrane Topography. Biochem. Biophys. Res. Commun. 1986, 139, 222–227. [Google Scholar] [CrossRef]
- Zhu, H.; Tayeh, I.; Israel, L.; Castagna, M. Different Susceptibility of Lung Cell Lines to Inhibitors of Tumor Promotion and Inducers of Differentiation. J. Biol. Regul. Homeost. Agents 1991, 5, 52–58. [Google Scholar]
- Sharma, S.V. Melittin Resistance: A Counterselection for Ras Transformation. Oncogene 1992, 7, 193–201. [Google Scholar] [PubMed]
- Sharma, S. Melittin-Induced Hyperactivation of Phospholipase A2 Activity and Calcium Influx in Ras-Transformed Cells. Oncogene 1993, 8, 939–947. [Google Scholar]
- Hanada, K.; Kinoshita, E.; Itoh, M.; Hirata, M.; Kajiyama, G.; Sugiyama, M. Human Pancreatic Phospholipase A2 Stimulates the Growth of Human Pancreatic Cancer Cell Line. FEBS Lett. 1995, 373, 85–87. [Google Scholar] [CrossRef][Green Version]
- Arora, A.S.; de Groen, P.C.; Croall, D.E.; Emori, Y.; Gores, G.J. Hepatocellular Carcinoma Cells Resist Necrosis during Anoxia by Preventing Phospholipase-Mediated Calpain Activation. J. Cell. Physiol. 1996, 167, 434–442. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Jiang, X.-R.; Newland, A.C.; Kelsey, S.M. Failure to Activate Cytosolic Phospholipase A2 Causes TNF Resistance in Human Leukemic Cells. J. Immunol. 1998, 160, 5929–5935. [Google Scholar] [CrossRef]
- Saini, S.S.; Chopra, A.K.; Peterson, J.W. Melittin Activates Endogenous Phospholipase D during Cytolysis of Human Monocytic Leukemia Cells. Toxicon 1999, 37, 1605–1619. [Google Scholar] [CrossRef]
- Putz, T.; Ramoner, R.; Gander, H.; Rahm, A.; Bartsch, G.; Thurnher, M. Antitumor Action and Immune Activation through Cooperation of Bee Venom Secretory Phospholipase A2 and Phosphatidylinositol-(3,4)-Bisphosphate. Cancer Immunol. Immunother. 2006, 55, 1374–1383. [Google Scholar] [CrossRef]
- Putz, T.; Ramoner, R.; Gander, H.; Rahm, A.; Bartsch, G.; Bernardo, K.; Ramsay, S.; Thurnher, M. Bee Venom Secretory Phospholipase A2 and Phosphatidylinositol-Homologues Cooperatively Disrupt Membrane Integrity, Abrogate Signal Transduction and Inhibit Proliferation of Renal Cancer Cells. Cancer Immunol. Immunother. 2007, 56, 627–640. [Google Scholar] [CrossRef]
- Moon, D.-O.; Park, S.-Y.; Heo, M.-S.; Kim, K.-C.; Park, C.; Ko, W.S.; Choi, Y.H.; Kim, G.-Y. Key Regulators in Bee Venom-Induced Apoptosis Are Bcl-2 and Caspase-3 in Human Leukemic U937 Cells through Downregulation of ERK and Akt. Int. Immunopharmacol. 2006, 6, 1796–1807. [Google Scholar] [CrossRef]
- Holle, L.; Song, W.; Holle, E.; Wei, Y.; Wagner, T.; Yu, X. A Matrix Metalloproteinase 2 Cleavable Melittin/Avidin Conjugate Specifically Targets Tumor Cells In Vitro and In Vivo. Int. J. Oncol. 2003, 22, 93–98. [Google Scholar] [CrossRef]
- Gawronska, B.; Leuschner, C.; Enright, F.M.; Hansel, W. Effects of a Lytic Peptide Conjugated to β HCG on Ovarian Cancer: Studies In Vitro and In Vivo. Gynecol. Oncol. 2002, 85, 45–52. [Google Scholar] [CrossRef]
- Liu, X.; Chen, D.; Xie, L.; Zhang, R. Effect of Honey Bee Venom on Proliferation of K1735M2 Mouse Melanoma Cells In-Vitro and Growth of Murine B16 Melanomas in-Vivo. J. Pharm. Pharmacol. 2002, 54, 1083–1089. [Google Scholar] [CrossRef]
- Jang, M.-H.; Shin, M.-C.; Lim, S.; Han, S.-M.; Park, H.-J.; Shin, I.; Lee, J.-S.; Kim, K.-A.; Kim, E.-H.; Kim, C.-J. Bee Venom Induces Apoptosis and Inhibits Expression of Cyclooxygenase-2 MRNA in Human Lung Cancer Cell Line NCI-H1299. J. Pharmacol. Sci. 2003, 91, 95–104. [Google Scholar] [CrossRef]
- Chen, Y.-Q.; Zhu, Z.-A.; Hao, Y.-Q.; Dai, K.-R.; Zhang, C. Effect of Melittin on Apoptsis and Necrosis of U2OS Cells. J. Chin. Integr. Med. 2004, 2, 208–209. [Google Scholar] [CrossRef]
- Li, B.; Ling, C.; Zhang, C.; Gu, W.; Li, S.; Huang, X.; Zhang, Y.; Yu, C. The Induced Apoptosis of Recombinant Adenovirus Carrying Melittin Gene for Hepatocellular Carcinoma Cell. Zhonghua Gan Zang Bing Za Zhi 2004, 12, 453–455. [Google Scholar]
- Hu, H.; Chen, D.; Li, Y.; Zhang, X. Effect of Polypeptides in Bee Venom on Growth Inhibition and Apoptosis Induction of the Human Hepatoma Cell Line SMMC-7721 In-Vitro and Balb/c Nude Mice in-Vivo. J. Pharm. Pharmacol. 2006, 58, 83–89. [Google Scholar] [CrossRef]
- Orsolić, N.; Sver, L.; Verstovsek, S.; Terzić, S.; Basić, I. Inhibition of Mammary Carcinoma Cell Proliferation In Vitro and Tumor Growth In Vivo by Bee Venom. Toxicon 2003, 41, 861–870. [Google Scholar] [CrossRef]
- Russell, P.J.; Hewish, D.; Carter, T.; Sterling-Levis, K.; Ow, K.; Hattarki, M.; Doughty, L.; Guthrie, R.; Shapira, D.; Molloy, P.L.; et al. Cytotoxic Properties of Immunoconjugates Containing Melittin-like Peptide 101 against Prostate Cancer: In Vitro and In Vivo Studies. Cancer Immunol. Immunother. 2004, 53, 411–421. [Google Scholar] [CrossRef]
- Li, S.; Ling, C.; Liu, X. Impact of Infection with Recombinant Adenovirus Carrying Melittin Gene on CD54 Expression in HepG2 Cells. Di Yi Jun Yi Da Xue Xue Bao 2003, 23, 300–305. [Google Scholar]
- Ling, C.-Q.; Li, B.; Zhang, C.; Gu, W.; Li, S.-X.; Huang, X.-Q.; Zhang, Y.-N. Anti-Hepatocarcinoma Effect of Recombinant Adenovirus Carrying Melittin Gene. Zhonghua Gan Zang Bing Za Zhi 2004, 12, 741–744. [Google Scholar]
- Pandey, P.; Khan, F.; Khan, M.A.; Kumar, R.; Upadhyay, T.K. An Updated Review Summarizing the Anticancer Efficacy of Melittin from Bee Venom in Several Models of Human Cancers. Nutrients 2023, 15, 3111. [Google Scholar] [CrossRef]
- Zorilă, B.; Necula, G.; Radu, M.; Bacalum, M. Melittin Induces Local Order Changes in Artificial and Biological Membranes as Revealed by Spectral Analysis of Laurdan Fluorescence. Toxins 2020, 12, 705. [Google Scholar] [CrossRef]
- Li, J.; Hanlon, P.; Gasanoff, E.S. Interaction of Bee Venom Melittin, a Potential Anti-Cancer Drug, with Phosphatidylcholine Membrane Enriched with Phosphatidylserine. EC Pharmacol. Toxicol. 2020, 8, 119–129. [Google Scholar]
- Li, M.; Gasanoff, E.S. Cationic Proteins Rich in Lysine Residue Trigger Formation of Non-Bilayer Lipid Phases in Model and Biological Membranes: Biophysical Methods of Study. J. Membr. Biol. 2023, 256, 373–391. [Google Scholar] [CrossRef]
- Sun, L.; Wang, S.; Tian, F.; Zhu, H.; Dai, L. Organizations of Melittin Peptides after Spontaneous Penetration into Cell Membranes. Biophys. J. 2022, 121, 4368–4381. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Shinoda, W. Cooperative Antimicrobial Action of Melittin on Lipid Membranes: A Coarse-Grained Molecular Dynamics Study. Biochim. Biophys. Acta Biomembr. 2022, 1864, 183955. [Google Scholar] [CrossRef]
- Ganpule, S.; Vijaya, A.K.; Sukova, A.; Preta, G. Membrane Cholesterol Content and Lipid Organization Influence Melittin and Pneumolysin Pore-Forming Activity. Toxins 2022, 14, 346. [Google Scholar] [CrossRef]
- Jepson, T.A.; Hall, S.C.; Chung, J.K. Single-Molecule Phospholipase A2 Becomes Processive on Melittin-Induced Membrane Deformations. Biophys. J. 2022, 121, 1417–1423. [Google Scholar] [CrossRef]
- Shorina, E.A.; Dolgova, N.V.; Rubtsov, A.M.; Storey, K.B.; Lopina, O.D. Melittin Induces Both Time-Dependent Aggregation and Inhibition of Na,K-ATPase from Duck Salt Glands However These Two Processes Appear to Occur Independently. Biochim. Biophys. Acta 2004, 1661, 188–195. [Google Scholar] [CrossRef][Green Version]
- Ertilav, K.; Nazıroğlu, M. Honey Bee Venom Melittin Increases the Oxidant Activity of Cisplatin and Kills Human Glioblastoma Cells by Stimulating the TRPM2 Channel. Toxicon 2023, 222, 106993. [Google Scholar] [CrossRef]
- Duffy, C.; Sorolla, A.; Wang, E.; Golden, E.; Woodward, E.; Davern, K.; Ho, D.; Johnstone, E.; Pfleger, K.; Redfern, A.; et al. Honeybee Venom and Melittin Suppress Growth Factor Receptor Activation in HER2-Enriched and Triple-Negative Breast Cancer. npj Precis. Oncol. 2020, 4, 24. [Google Scholar] [CrossRef]
- Xie, X.; Li, Y.; Zhu, H.; Chen, L.; Chen, D.; Lin, S.; Fan, T. Melittin Inhibits Growth of Human Osteosarcoma 143B Cells through Induction of Apoptosis via Suppressing the Wnt/β-Catenin Signaling Pathway. Anticancer. Agents Med. Chem. 2022, 22, 3172–3181. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Meng, Y.-Q.; Qiao, H.; Zhai, K.-R.; Li, Z.-Q.; Wei, S.-L.; Li, B. Melittin Kills A549 Cells by Targeting Mitochondria and Blocking Mitophagy Flux. Redox Rep. 2023, 28, 2284517. [Google Scholar] [CrossRef]
- Gasanoff, E.; Liu, Y.; Li, F.; Hanlon, P.; Garab, G. Bee Venom Melittin Disintegrates the Respiration of Mitochondria in Healthy Cells and Lymphoblasts, and Induces the Formation of Non-Bilayer Structures in Model Inner Mitochondrial Membranes. Int. J. Mol. Sci. 2021, 22, 11122. [Google Scholar] [CrossRef] [PubMed]
- Korovesis, D.; Gaspar, V.P.; Beard, H.A.; Chen, S.; Zahédi, R.P.; Verhelst, S.H.L. Mapping Peptide–Protein Interactions by Amine-Reactive Cleavable Photoaffinity Reagents. ACS Omega 2023, 8, 25487–25495. [Google Scholar] [CrossRef]
- Dürvanger, Z.; Juhász, T.; Liliom, K.; Harmat, V. Structures of Calmodulin-Melittin Complexes Show Multiple Binding Modes Lacking Classical Anchoring Interactions. J. Biol. Chem. 2023, 299, 104596. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, E.; Zhao, Y.; Yang, B. Inhibitory Effect of Melittin on Endonuclease-like Activity of Centrin. J. Inorg. Biochem. 2018, 186, 280–293. [Google Scholar] [CrossRef]
- Shi, W.; Li, C.; Li, M.; Zong, X.; Han, D.; Chen, Y. Antimicrobial Peptide Melittin against Xanthomonas Oryzae Pv. Oryzae, the Bacterial Leaf Blight Pathogen in Rice. Appl. Microbiol. Biotechnol. 2016, 100, 5059–5067. [Google Scholar] [CrossRef]
- Tetikoğlu, S.; Çelik-Uzuner, S. Bee Venom Induces the Interaction between Phosphorylated Histone Variant, H2AX, and the Intracellular Site of Beta-Actin in Liver and Breast Cancer Cells. Chem. Biodivers. 2023, 20, e202300401. [Google Scholar] [CrossRef]
- Uzuner, S.Ç.; Birinci, E.; Tetikoğlu, S.; Birinci, C.; Kolaylı, S. Distinct Epigenetic Reprogramming, Mitochondrial Patterns, Cellular Morphology, and Cytotoxicity after Bee Venom Treatment. Recent Pat. Anticancer Drug Discov. 2021, 16, 377–392. [Google Scholar] [CrossRef]
- Wattenberg, L.W. Chemoprevention of Cancer. Cancer Res. 1985, 45, 1–8. [Google Scholar] [CrossRef]
- Young, D. Natural Agents Examined as Sources for Chemopreventive Therapies. Am. J. Health. Syst. Pharm. 2006, 63, 1681–1682. [Google Scholar] [CrossRef] [PubMed]
- Dennis, T.; Fanous, M.; Mousa, S. Natural Products for Chemopreventive and Adjunctive Therapy in Oncologic Disease. Nutr. Cancer 2009, 61, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Pemberton, R.W. Insects and Other Arthropods Used as Drugs in Korean Traditional Medicine. J. Ethnopharmacol. 1999, 65, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.L. Biological Activity of Bee Propolis in Health and Diseas. Asian Pacific J. Cancer Prev. 2006, 7, 22–31. [Google Scholar]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Alvarez, J.A. Functional Properties of Honey, Propolis, and Royal Jelly. J. Food Sci. 2008, 73, R117–R124. [Google Scholar] [CrossRef]
- Cerrato, P.L. A Therapeutic Bee Sting? RN 1998, 61, 57–59. [Google Scholar]
- Liu, H.; Tong, F. Advances in the Study of Bee Venom and Its Clinical Uses. Zhong Yao Cai 2003, 26, 456–458. [Google Scholar]
- Ransome, H.M. The Sacred Bee in Ancient Times and Folklore; Dover Publications: Mineola, NY, USA, 2004; ISBN 048643494X. [Google Scholar]
- Heinen, T.E.; da Veiga, A.B.G. Arthropod Venoms and Cancer. Toxicon 2011, 57, 497–511. [Google Scholar] [CrossRef]
- Ling, C.-Q.; Li, B.; Zhang, C.; Zhu, D.-Z.; Huang, X.-Q.; Gu, W.; Li, S.-X. Inhibitory Effect of Recombinant Adenovirus Carrying Melittin Gene on Hepatocellular Carcinoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2005, 16, 109–115. [Google Scholar] [CrossRef]
- Leuschner, C.; Hansel, W. Membrane Disrupting Lytic Peptides for Cancer Treatments. Curr. Pharm. Des. 2004, 10, 2299–2310. [Google Scholar] [CrossRef]
- Papo, N.; Shai, Y. Host Defense Peptides as New Weapons in Cancer Treatment. Cell. Mol. Life Sci. 2005, 62, 784–790. [Google Scholar] [CrossRef]
- Giuliani, A.; Pirri, G.; Nicoletto, S. Antimicrobial Peptides: An Overview of a Promising Class of Therapeutics. Open Life Sci. 2007, 2, 1–33. [Google Scholar] [CrossRef]
- Gajski, G.; Čimbora-Zovko, T.; Osmak, M.; Garaj-vrhovac, V. Bee Venom and Melittin Are Cytotoxic against Different Types of Tumor and Non-Tumor Cell Lines In Vitro. Cancer Res. J. 2011, 4, 159–174. [Google Scholar]
- Gajski, G.; Garaj-Vrhovac, V. Bee Venom Induced Cytogenetic Damage and Decreased Cell Viability in Human White Blood Cells after Treatment In Vitro: A Multi-Biomarker Approach. Environ. Toxicol. Pharmacol. 2011, 32, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Garaj-Vrhovac, V.; Gajski, G. Evaluation of the Cytogenetic Status of Human Lymphocytes after Exposure to a High Concentration of Bee Venom In Vitro. Arh. Hig. Rada Toksikol. 2009, 60, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Gajski, G.; Garaj-Vrhovac, V. Genotoxic Potential of Bee Venom (Apis mellifera) on Human Peripheral Blood Lymphocytes In Vitro Using Single Cell Gel Electrophoresis Assay. J. Environ. Sci. Health. A Tox. Hazard. Subst. Environ. Eng. 2008, 43, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Gajski, G.; Domijan, A.-M.; Garaj-Vrhovac, V. Alterations of GSH and MDA Levels and Their Association with Bee Venom-Induced DNA Damage in Human Peripheral Blood Leukocytes. Environ. Mol. Mutagen. 2012, 53, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Gajski, G.; Garaj-Vrhovac, V. Increased Frequency of Sister Chromatid Exchanges and Decrease in Cell Viability and Proliferation Kinetics in Human Peripheral Blood Lymphocytes after In Vitro Exposure to Whole Bee Venom. J. Environ. Sci. Health-Part A Toxic/Hazardous Subst. Environ. Eng. 2010, 45, 1654–1659. [Google Scholar] [CrossRef]
- Gajski, G.; Čimbora-Zovko, T.; Osmak, M.; Garaj-Vrhovac, V. Bee Venom and Melittin Are Cytotoxic against Different Types of Tumor and Non-Tumor Cell Lines In Vitro. In Advancements in Cancer Research; Viktorsson, K., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2012; pp. 163–178. ISBN 978-1-61470-252-8. [Google Scholar]
- Soman, N.R.; Baldwin, S.L.; Hu, G.; Marsh, J.N.; Lanza, G.M.; Heuser, J.E.; Arbeit, J.M.; Wickline, S.A.; Schlesinger, P.H. Molecularly Targeted Nanocarriers Deliver the Cytolytic Peptide Melittin Specifically to Tumor Cells in Mice, Reducing Tumor Growth. J. Clin. Investig. 2009, 119, 2830–2842. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Soman, N.R.; Schlesinger, P.H.; Lanza, G.M.; Wickline, S.A. Cytolytic Peptide Nanoparticles (‘NanoBees’) for Cancer Therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Chatzi Memet, B.; Demirpolat, E.; Yildirim, T.; Aydin, O. Development of Polymer-Based Nanoparticles for the Reduction of Melittin Toxicity. In Mediterranean Conference on Medical and Biological Engineering and Computing; Springer: Cham, Switzerland, 2024; pp. 45–50. ISBN 978-3-031-49068-2. [Google Scholar]
- Zhang, J.; Liu, X.; Xia, Y.; Xu, S.; Liu, X.; Xiao, H.; Wang, X.; Liu, C.; Liu, G. Genetically Engineered Nano-melittin Vesicles for Multimodal Synergetic Cancer Therapy. Bioeng. Transl. Med. 2023, 8, e10482. [Google Scholar] [CrossRef]
- Alizadehnohi, M.; Nabiuni, M.; Nazari, Z.; Safaeinejad, Z.; Irian, S. The Synergistic Cytotoxic Effect of Cisplatin and Honey Bee Venom on Human Ovarian Cancer Cell Line A2780cp. J. Venom Res. 2012, 3, 22–27. [Google Scholar]
- Gajski, G.; Čimbora-Zovko, T.; Rak, S.; Rožman, M.; Osmak, M.; Garaj-Vrhovac, V. Combined Antitumor Effects of Bee Venom and Cisplatin on Human Cervical and Laryngeal Carcinoma Cells and Their Drug Resistant Sublines. J. Appl. Toxicol. 2014, 34, 1332–1341. [Google Scholar] [CrossRef]
- Gajski, G.; Čimbora-Zovko, T.; Rak, S.; Osmak, M.; Garaj-Vrhovac, V. Antitumour Action on Human Glioblastoma A1235 Cells through Cooperation of Bee Venom and Cisplatin. Cytotechnology 2016, 68, 1197–1205. [Google Scholar] [CrossRef]
- Sengul, F.; Vatansev, H.; Ozturk, B. Investigation the Effects of Bee Venom and H-Dental-Derived Mesenchymal Stem Cells on Non-Small Cell Lung Cancer Cells (A549). Mol. Biol. Rep. 2023, 51, 2. [Google Scholar] [CrossRef]
- Duarte, D.; Falcão, S.I.; El Mehdi, I.; Vilas-Boas, M.; Vale, N. Honeybee Venom Synergistically Enhances the Cytotoxic Effect of CNS Drugs in HT-29 Colon and MCF-7 Breast Cancer Cell Lines. Pharmaceutics 2022, 14, 511. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.; Park, S.H.; Kim, T.M.; Jung, Y.Y.; Park, M.H.; Oh, S.H.; Yun, H.S.; Jun, H.O.; Yoo, H.S.; Han, S.-B.; et al. Bee Venom Inhibits Growth of Human Cervical Tumors in Mice. Oncotarget 2015, 6, 7280–7292. [Google Scholar] [CrossRef][Green Version]
- Lee, C.; Bae, S.-J.S.; Joo, H.; Bae, H.; Lee, C.; Bae, S.-J.S.; Joo, H.; Bae, H. Melittin Suppresses Tumor Progression by Regulating Tumor-Associated Macrophages in a Lewis Lung Carcinoma Mouse Model. Oncotarget 2017, 8, 54951–54965. [Google Scholar] [CrossRef] [PubMed]
- El Bakary, N.M.; Alsharkawy, A.Z.; Shouaib, Z.A.; Barakat, E.M.S. Role of Bee Venom and Melittin on Restraining Angiogenesis and Metastasis in γ-Irradiated Solid Ehrlich Carcinoma-Bearing Mice. Integr. Cancer Ther. 2020, 19, 153473542094447. [Google Scholar] [CrossRef]
- El-Beltagy, A.E.-F.B.; Elsyyad, H.I.; Abdelaziz, K.K.; Madany, A.S.; Elghazaly, M.M. Therapeutic Role of Annona Muricata Fruit and Bee Venom Against MNU-Induced Breast Cancer in Pregnant Rats and Its Complications on the Ovaries. Breast Cancer Targets Ther. 2021, 13, 431–445. [Google Scholar] [CrossRef]
- Rocha, M.M.; Dariva, I.; Zornoff, G.C.; De Laurentis, G.S.; Mendes, G.C.; Santana, M.G.; de Miguel, G.C.; Ferreira Junior, R.S.; Sciani, J.M.; Priolli, D.G. A New Therapeutic Approach for Bone Metastasis in Colorectal Cancer: Intratumoral Melittin. J. Venom. Anim. Toxins Incl. Trop. Dis. 2022, 28, e20210067. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-F.; Chen, Z. Melittin Exerts an Antitumor Effect on Non-Small Cell Lung Cancer Cells. Mol. Med. Rep. 2017, 16, 3581–3586. [Google Scholar] [CrossRef]

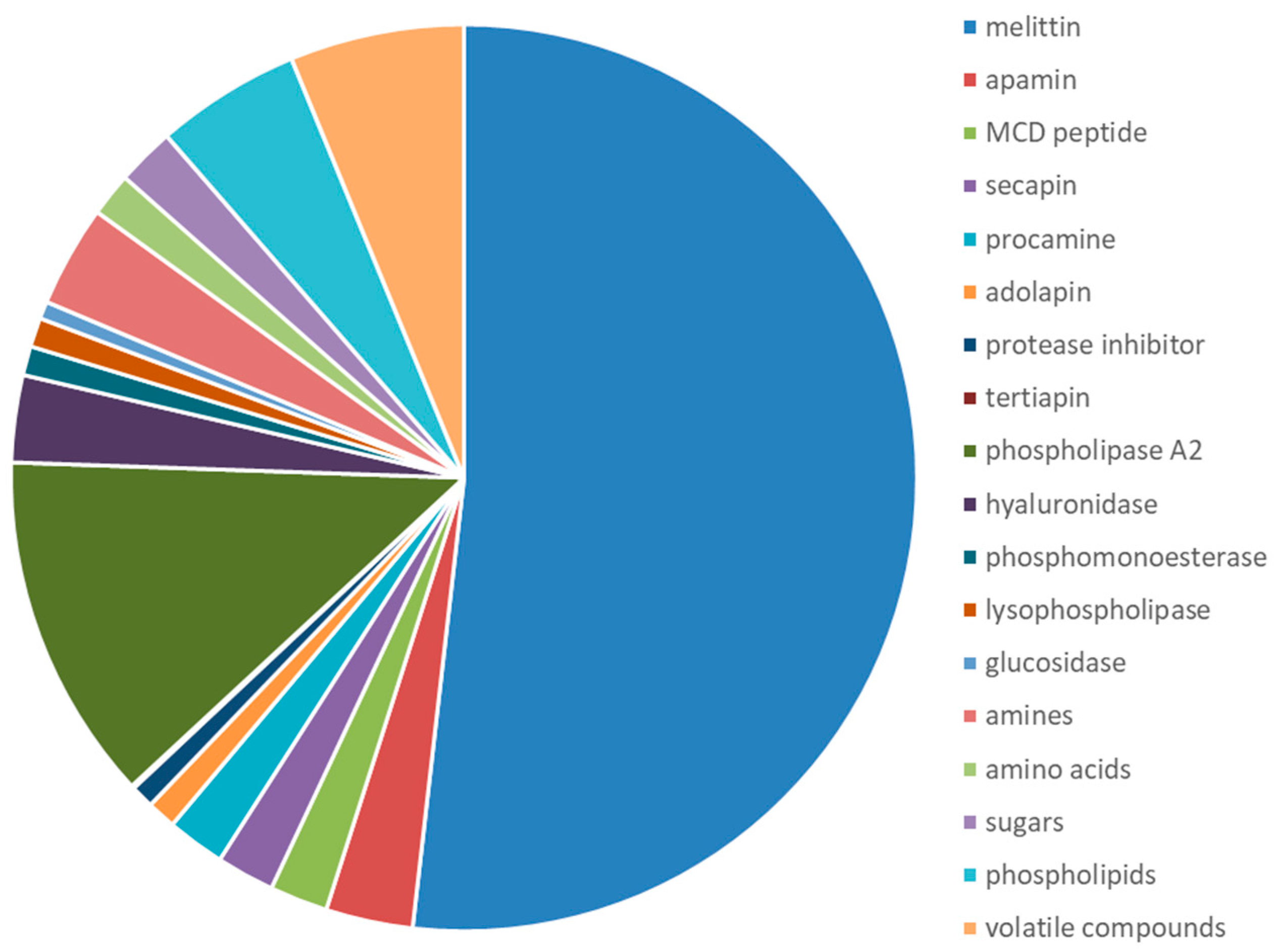
| Class of Molecules | Components | % of Dry BV |
|---|---|---|
| Enzymes | Phospolipase A2 | 10–12 |
| Hyaluronidase | 1–3 | |
| Acid phosphomonoesterase | 1 | |
| Lysophopholipase | 1 | |
| α-glucosidase | 0.6 | |
| Proteins and peptides | Melittin | 40–50 |
| Apamin | 1–3 | |
| Mast cell degranulating peptide | 1–2 | |
| Secapin | 0.5–2 | |
| Procamine | 1–2 | |
| Adolapin | 1.0 | |
| Protease inhibitor | 0.8 | |
| Tertiapin | 0.1 | |
| Other small peptides (<5 amino acids) | 13–15 | |
| Physiologically active amines | Histamine | 0.5–2.0 |
| Dopamine | 0.2–1.0 | |
| Noradrenalin | 0.1–0.7 | |
| Amino acids | Aminobutyric acid | 0.5 |
| α-amino acids | 1 | |
| Sugars | Glucose and fructose | 2 |
| Phospholipids | 5 | |
| Volatile compounds | 4–8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajski, G.; Leonova, E.; Sjakste, N. Bee Venom: Composition and Anticancer Properties. Toxins 2024, 16, 117. https://doi.org/10.3390/toxins16030117
Gajski G, Leonova E, Sjakste N. Bee Venom: Composition and Anticancer Properties. Toxins. 2024; 16(3):117. https://doi.org/10.3390/toxins16030117
Chicago/Turabian StyleGajski, Goran, Elina Leonova, and Nikolajs Sjakste. 2024. "Bee Venom: Composition and Anticancer Properties" Toxins 16, no. 3: 117. https://doi.org/10.3390/toxins16030117
APA StyleGajski, G., Leonova, E., & Sjakste, N. (2024). Bee Venom: Composition and Anticancer Properties. Toxins, 16(3), 117. https://doi.org/10.3390/toxins16030117







