Alleviative Effect of Rutin on Zearalenone-Induced Reproductive Toxicity in Male Mice by Preventing Spermatogenic Cell Apoptosis and Modulating Gene Expression in the Hypothalamic–Pituitary–Gonadal Axis
Abstract
1. Introduction
2. Results
2.1. Effects of RUT on the Testes Tissue Morphology and Sperm Quality of Mice Fed with ZEN
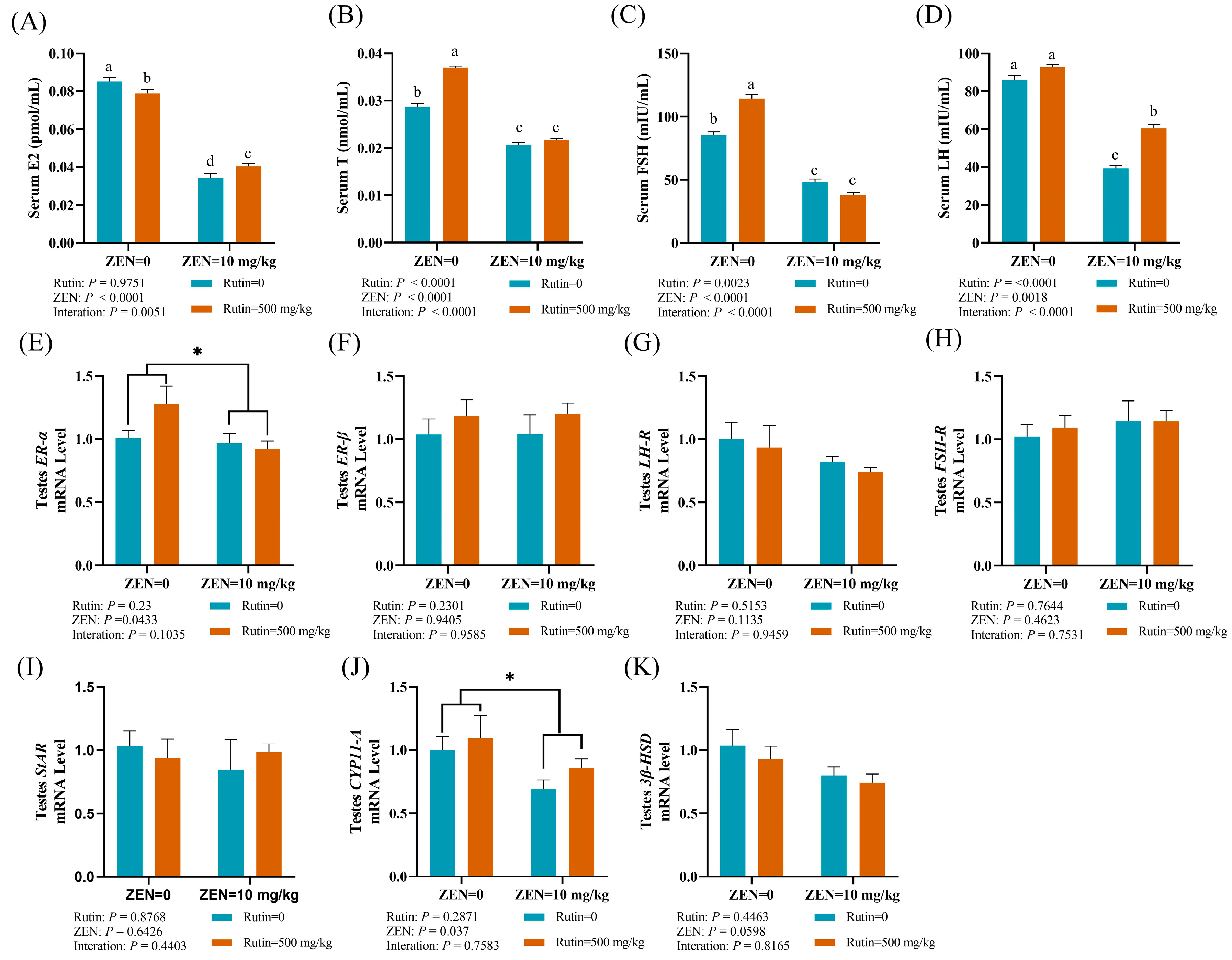
2.2. Effects of RUT on Serum Hormones, the mRNA Expression Levels of Reproductive Hormone Receptor and Steroid Synthesis-Related Genes in Testes of Mice Fed with ZEN
2.3. Effect of RUT on the Cell Apoptosis and the mRNA and Protein Expression Levels of Genes Related to Apoptosis in the Testes of Mice Fed with ZEN
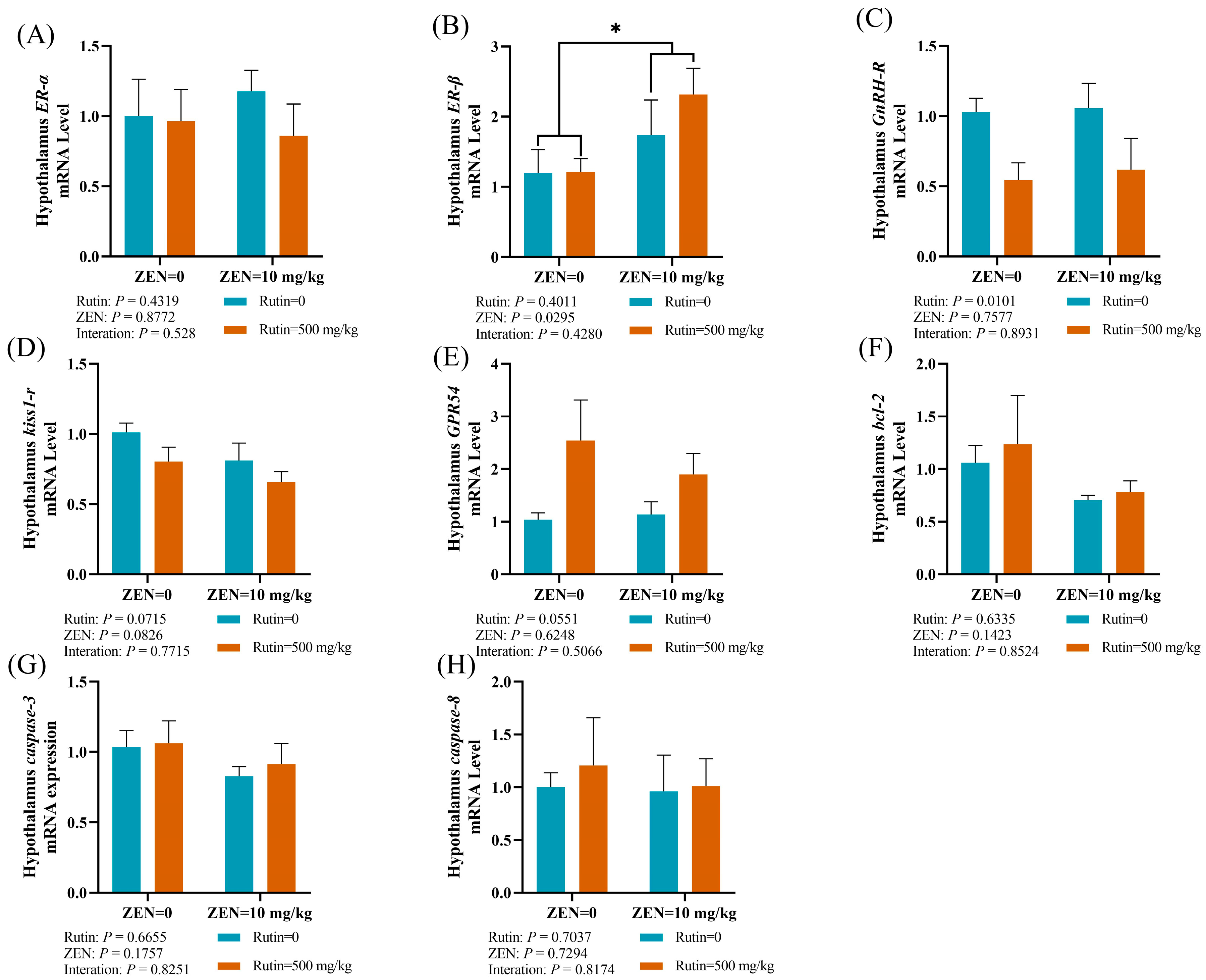
2.4. Effects of RUT on the mRNA Expression Levels of Reproductive Hormone Receptors and Apoptosis-Related Genes in the Hypothalamus of Male Mice Fed with ZEN
2.5. Effects of RUT on the mRNA Expression Levels of Reproductive-Related Genes in the Pituitary Gland of Male Mice Fed with ZEN
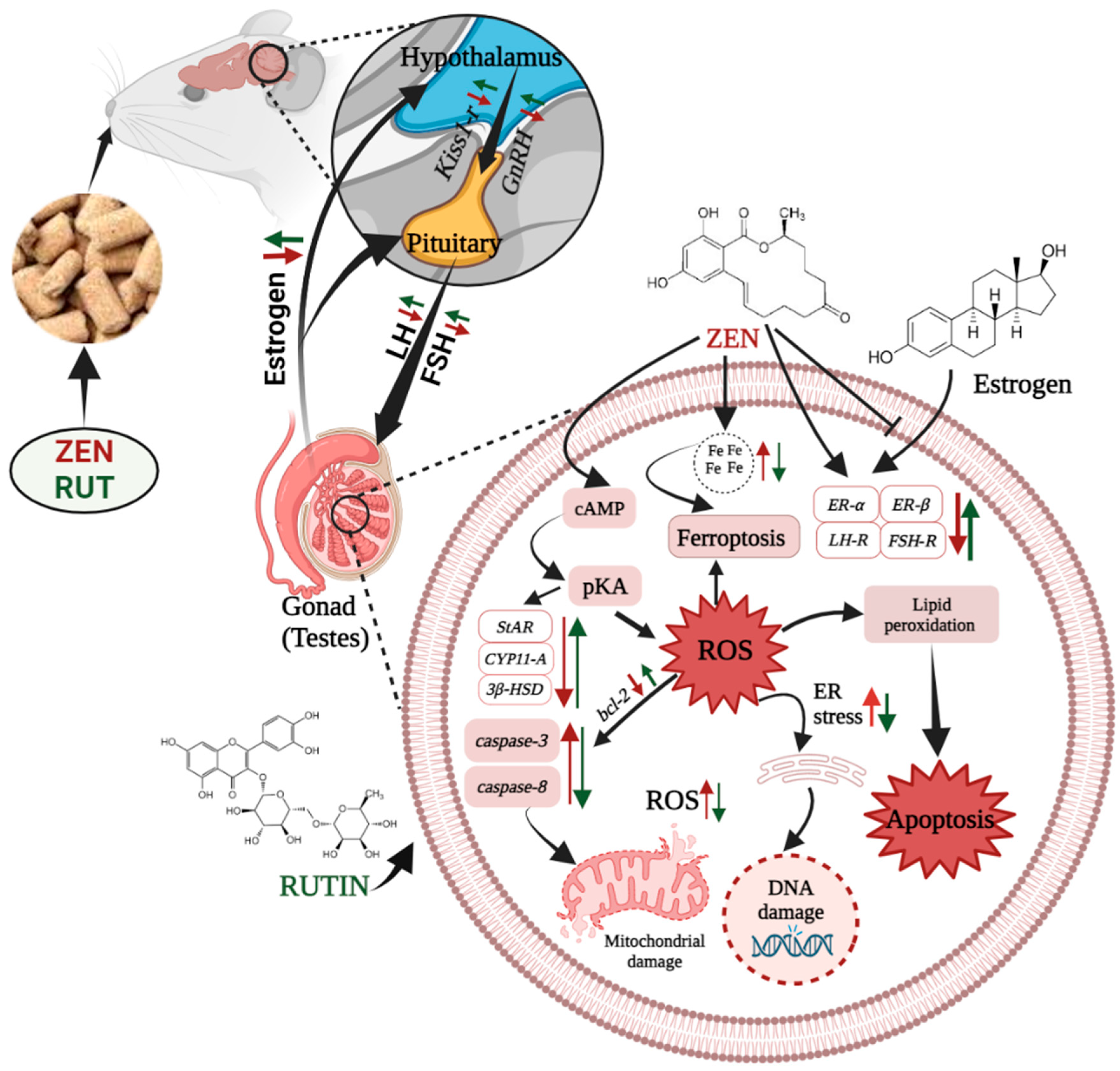
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals
5.2. Experimental Animals and Design
5.3. Collection of Sample
5.4. Determination of Mouse Sperm Quality
5.5. Reproductive Hormone Indicators of Serum
5.6. Histopathological Examinations of Testes
5.7. Observation of Testis Cell Apoptosis via the In Situ Terminal Transferase Labeling Method (TUNEL Method)
5.8. Quantitative Real-Time PCR (RT-qPCR) to Determine the mRNA Expression Levels of Genes Related to the Reproductive Hormone, Steroid Synthesis, and Cell Apoptosis
5.9. Western Blot Analysis
5.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamle, M.; Mahato, D.K.; Devi, S.; Lee, K.E.; Kang, S.G.; Kumar, P. Fumonisins: Impact on Agriculture, Food, and Human Health and Their Management Strategies. Toxins 2019, 11, 328. [Google Scholar] [CrossRef] [PubMed]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide Contamination of Food-Crops with Mycotoxins: Validity of the Widely Cited ‘FAO Estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef] [PubMed]
- Zinedine, A.; Soriano, J.M.; Moltó, J.C.; Mañes, J. Review on the Toxicity, Occurrence, Metabolism, Detoxification, Regulations and Intake of Zearalenone: An Oestrogenic Mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zatecka, E.; Ded, L.; Elzeinova, F.; Kubatova, A.; Dorosh, A.; Margaryan, H.; Dostalova, P.; Korenkova, V.; Hoskova, K.; Peknicova, J. Effect of Zearalenone on Reproductive Parameters and Expression of Selected Testicular Genes in Mice. Reprod. Toxicol. 2014, 45, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Gajęcka, M.; Zielonka, Ł.; Gajęcki, M. The Effect of Low Monotonic Doses of Zearalenone on Selected Reproductive Tissues in Pre-Pubertal Female Dogs-A Review. Molecules 2015, 20, 20669–20687. [Google Scholar] [CrossRef] [PubMed]
- Kinkade, C.W.; Rivera-Núñez, Z.; Gorcyzca, L.; Aleksunes, L.M.; Barrett, E.S. Impact of Fusarium-Derived Mycoestrogens on Female Reproduction: A Systematic Review. Toxins 2021, 13, 373. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Wang, G.X.; Liu, J.L.; Fan, J.J.; Cui, S. Toxic Effects of Zearalenone and Its Derivatives α-Zearalenol on Male Reproductive System in Mice. Reprod. Toxicol. 2007, 24, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, Y.; Gu, J.; Yuan, Y.; Liu, X.; Zheng, W.; Huang, Q.; Liu, Z.; Bian, J. Zearalenone Inhibits Testosterone Biosynthesis in Mouse Leydig Cells via the Crosstalk of Estrogen Receptor Signaling and Orphan Nuclear Receptor Nur77 Expression. Toxicol. Vitr. 2014, 28, 647–656. [Google Scholar] [CrossRef]
- Long, M.; Yang, S.; Wang, Y.; Li, P.; Zhang, Y.; Dong, S.; Chen, X.; Guo, J.; He, J.; Gao, Z.; et al. The Protective Effect of Selenium on Chronic Zearalenone-Induced Reproductive System Damage in Male Mice. Molecules 2016, 21, 1687. [Google Scholar] [CrossRef]
- Boeira, S.P.; Funck, V.R.; Borges Filho, C.; Del’Fabbro, L.; De Gomes, M.G.; Donato, F.; Royes, L.F.F.; Oliveira, M.S.; Jesse, C.R.; Furian, A.F. Lycopene Protects against Acute Zearalenone-Induced Oxidative, Endocrine, Inflammatory and Reproductive Damages in Male Mice. Chem. Biol. Interact. 2015, 230, 50–57. [Google Scholar] [CrossRef]
- Su, Y.; Sun, Y.; Ju, D.; Chang, S.; Shi, B.; Shan, A. The Detoxification Effect of Vitamin C on Zearalenone Toxicity in Piglets. Ecotoxicol. Environ. Saf. 2018, 158, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yang, S.; Wang, M.; Chen, J.; Huang, S.; Wei, Z.; Cheng, Z.; Wang, H.; Long, M.; Li, P. Curcumin Inhibits Zearalenone-Induced Apoptosis and Oxidative Stress in Leydig Cells via Modulation of the PTEN/Nrf2/Bip Signaling Pathway. Food Chem. Toxicol. 2020, 141, 111385. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Wang, H.; Zhu, J.; Wang, T.; Nepovimova, E.; Long, M.; Li, P.; Kuca, K.; Wu, W. Procyanidins Inhibit Zearalenone-Induced Apoptosis and Oxidative Stress of Porcine Testis Cells through Activation of Nrf2 Signaling Pathway. Food Chem. Toxicol. 2022, 165, 113061. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Jia, H.; Zhai, Z.; Li, J.; Song, Z.; Yang, Q.; Ren, F.; Wu, Z. Protective Effect of Glucosamine on Zearalenone-Induced Reproductive Toxicity and Placental Dysfunction in Mice. Food Chem. Toxicol. 2023, 172, 113539. [Google Scholar] [CrossRef] [PubMed]
- Farha, A.K.; Gan, R.Y.; Li, H.B.; Wu, D.T.; Atanasov, A.G.; Gul, K.; Zhang, J.R.; Yang, Q.Q.; Corke, H. The Anticancer Potential of the Dietary Polyphenol Rutin: Current Status, Challenges, and Perspectives. Crit. Rev. Food Sci. Nutr. 2020, 62, 832–859. [Google Scholar] [CrossRef] [PubMed]
- Mirani, N.; Nagma; Ashraf, J.; Siddique, J.; Rub, A. Protective Effect of Rutin against Cadmium Induced Hepatotoxicity in Swiss Albino Mice. J. Pharmacol. Toxicol. 2012, 7, 150–157. [Google Scholar] [CrossRef][Green Version]
- Akondi, R.B.; Kumar, P.; Annapurna, A.; Pujari, M. Protective Effect of Rutin and Naringin on Sperm Quality in Streptozotocin (STZ) Induced Type 1 Diabetic Rats. Iran. J. Pharm. Res. 2011, 10, 585–596. [Google Scholar]
- Abarikwu, S.O.; Otuechere, C.A.; Ekor, M.; Monwuba, K.; Osobu, D. Rutin Ameliorates Cyclophosphamide-Induced Reproductive Toxicity in Male Rats. Toxicol. Int. 2012, 19, 207–214. [Google Scholar] [CrossRef]
- Abarikwu, S.O.; Iserhienrhien, B.O.; Badejo, T.A. Rutin- and Selenium-Attenuated Cadmium-Induced Testicular Pathophysiology in Rats. Hum. Exp. Toxicol. 2013, 32, 395–406. [Google Scholar] [CrossRef]
- Aksu, E.H.; Kandemir, F.M.; Özkaraca, M.; Ömür, A.D.; Küçükler, S.; Çomaklı, S. Rutin Ameliorates Cisplatin-Induced Reproductive Damage via Suppression of Oxidative Stress and Apoptosis in Adult Male Rats. Andrologia 2017, 49, e12593. [Google Scholar] [CrossRef]
- Abarikwu, S.O.; Njoku, R.C.C.; John, I.G.; Amadi, B.A.; Mgbudom-Okah, C.J.; Onuah, C.L. Antioxidant and Anti-Inflammatory Protective Effects of Rutin and Kolaviron against Busulfan-Induced Testicular Injuries in Rats. Syst. Biol. Reprod. Med. 2022, 68, 151–161. [Google Scholar] [CrossRef]
- Arjumand, W.; Seth, A.; Sultana, S. Rutin Attenuates Cisplatin Induced Renal Inflammation and Apoptosis by Reducing NFκB, TNF-α and Caspase-3 Expression in Wistar Rats. Food Chem. Toxicol. 2011, 49, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Kandemir, F.M.; Ozkaraca, M.; Yildirim, B.A.; Hanedan, B.; Kirbas, A.; Kilic, K.; Aktas, E.; Benzer, F. Rutin Attenuates Gentamicin-Induced Renal Damage by Reducing Oxidative Stress, Inflammation, Apoptosis, and Autophagy in Rats. Ren. Fail. 2015, 37, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Elsawy, H.; Badr, G.M.; Sedky, A.; Abdallah, B.M.; Alzahrani, A.M.; Abdel-Moneim, A.M. Rutin Ameliorates Carbon Tetrachloride (CCl4)-Induced Hepatorenal Toxicity and Hypogonadism in Male Rats. PeerJ 2019, 7, e7011. [Google Scholar] [CrossRef] [PubMed]
- Ropejko, K.; Twarużek, M. Zearalenone and Its Metabolites—General Overview, Occurrence, and Toxicity. Toxins 2021, 13, 35. [Google Scholar] [CrossRef]
- Zhu, L.; Yuan, H.; Guo, C.; Lu, Y.; Deng, S.; Yang, Y.; Wei, Q.; Wen, L.; He, Z. Zearalenone Induces Apoptosis and Necrosis in Porcine Granulosa Cells via a Caspase-3- and Caspase-9-Dependent Mitochondrial Signaling Pathway. J. Cell. Physiol. 2012, 227, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Chen, F.; Sun, J.; Zhou, J.; Wang, X.; Wang, N.; Li, X.; Zhang, Z.; Wang, A.; Jin, Y.P. Mycotoxin Zearalenone Induces Apoptosis in Mouse Leydig Cells via an Endoplasmic Reticulum Stress-Dependent Signalling Pathway. Reprod. Toxicol. 2015, 52, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Muthulakshmi, S.; Hamideh, P.F.; Habibi, H.R.; Maharajan, K.; Kadirvelu, K.; Mudili, V. Mycotoxin Zearalenone Induced Gonadal Impairment and Altered Gene Expression in the Hypothalamic–Pituitary–Gonadal Axis of Adult Female Zebrafish (Danio rerio). J. Appl. Toxicol. 2018, 38, 1388–1397. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.; Jaremko, M. Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Nassiri-Asl, M. Review of the Protective Effects of Rutin on the Metabolic Function as an Important Dietary Flavonoid. J. Endocrinol. Investig. 2014, 37, 783–788. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2016, 25, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, Z.; Cui, H.; Ding, K.; Zhao, Y.; Ma, X.; Adetunji, A.O.; Min, L. Effect of Zearalenone-Induced Ferroptosis on Mice Spermatogenesis. Animals 2022, 12, 3026. [Google Scholar] [CrossRef]
- Ben Salah-Abbès, J.; Abbès, S.; Abdel-Wahhab, M.A.; Oueslati, R. Raphanus Sativus Extract Protects against Zearalenone Induced Reproductive Toxicity, Oxidative Stress and Mutagenic Alterations in Male Balb/c Mice. Toxicon 2009, 53, 525–533. [Google Scholar] [CrossRef]
- Kuiper-Goodman, T.; Scott, P.M.; Watanabe, H. Risk Assessment of the Mycotoxin Zearalenone. Regul. Toxicol. Pharmacol. 1987, 7, 253–306. [Google Scholar] [CrossRef] [PubMed]
- Wan, B.; Yuan, X.; Yang, W.; Jiao, N.; Li, Y.; Liu, F.; Liu, M.; Yang, Z.; Huang, L.; Jiang, S. The Effects of Zearalenone on the Localization and Expression of Reproductive Hormones in the Ovaries of Weaned Gilts. Toxins 2021, 13, 626. [Google Scholar] [CrossRef] [PubMed]
- de Mattos, K.; Pierre, K.J.; Tremblay, J.J. Hormones and Signaling Pathways Involved in the Stimulation of Leydig Cell Steroidogenesis. Endocrines 2023, 4, 573–594. [Google Scholar] [CrossRef]
- Martin, L.J.; Touaibia, M. Improvement of Testicular Steroidogenesis Using Flavonoids and Isoflavonoids for Prevention of Late-Onset Male Hypogonadism. Antioxidants 2020, 9, 237. [Google Scholar] [CrossRef]
- Chimento, A.; Sirianni, R.; Casaburi, I.; Pezzi, V. Role of Estrogen Receptors and G Protein-Coupled Estrogen Receptor in Regulation of Hypothalamus-Pituitary-Testis Axis and Spermatogenesis. Front. Endocrinol. 2014, 5, 1. [Google Scholar] [CrossRef]
- Osawe, S.O.; Farombi, E.O. Quercetin and Rutin Ameliorates Sulphasalazine-Induced Spermiotoxicity, Alterations in Reproductive Hormones and Steroidogenic Enzyme Imbalance in Rats. Andrologia 2018, 50, e12981. [Google Scholar] [CrossRef]
- Yuan, H.; Deng, Y.; Yuan, L.; Wu, J.; Yuan, Z.; Yi, J.; Zhang, M.; Guo, C.; Wen, L.; Li, R.; et al. Gynostemma Pentaphyllum Protects Mouse Male Germ Cells against Apoptosis Caused by Zearalenone via Bax and Bcl-2 Regulation. Toxicol. Mech. Methods 2010, 20, 153–158. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, W.; Bian, X.; Yuan, Y.; Gu, J.; Liu, X.; Liu, Z.; Bian, J. Zearalenone Induces Apoptosis and Cytoprotective Autophagy in Primary Leydig Cells. Toxicol. Lett. 2014, 226, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xu, M.; Dai, Y.; Ding, X.; Xiao, C.; Ji, H.; Xu, Y. Exploration of Bcl-2 Family and Caspases-Dependent Apoptotic Signaling Pathway in Zearalenone-Treated Mouse Endometrial Stromal Cells. Biochem. Biophys. Res. Commun. 2016, 476, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Oh, S. Zearalenone Induces Endothelial Cell Apoptosis through Activation of a Cytosolic Ca2+/ERK1/2/P53/Caspase 3 Signaling Pathway. Toxins 2021, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Graceli, J.B.; Dettogni, R.S.; Merlo, E.; Niño, O.; da Costa, C.S.; Zanol, J.F.; Ríos Morris, E.A.; Miranda-Alves, L.; Denicol, A.C. The Impact of Endocrine-Disrupting Chemical Exposure in the Mammalian Hypothalamic-Pituitary Axis. Mol. Cell. Endocrinol. 2020, 518, 110997. [Google Scholar] [CrossRef] [PubMed]
- Tiemann, U.; Tomek, W.; Schneider, F.; Vanselow, J. Effects of the Mycotoxins α- and β-Zearalenol on Regulation of Progesterone Synthesis in Cultured Granulosa Cells from Porcine Ovaries. Reprod. Toxicol. 2003, 17, 673–681. [Google Scholar] [CrossRef]
- Takemura, H.; Shim, J.Y.; Sayama, K.; Tsubura, A.; Zhu, B.T.; Shimoi, K. Characterization of the Estrogenic Activities of Zearalenone and Zeranol in Vivo and in Vitro. J. Steroid Biochem. Mol. Biol. 2007, 103, 170–177. [Google Scholar] [CrossRef]
- Sellamani, M.; Kalagatur, N.K.; Siddaiah, C.; Mudili, V.; Krishna, K.; Natarajan, G.; Rao Putcha, V.L. Antifungal and Zearalenone Inhibitory Activity of Pediococcus Pentosaceus Isolated from Dairy Products on Fusarium Graminearum. Front. Microbiol. 2016, 7, 890. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



| Gene | Forward Primer (5′-3′) | Reverse Primer (3′-5′) |
|---|---|---|
| ER-α | TCTGGAGTGTGCCTGGTTGGAG | GCGGAATCGACTTGACGTAGCC |
| ER-β | ACGTCAGGCACATCAGTAACAAGG | CATCTCCAGCAGCAGGTCATACAC |
| LH-R | TCTTGGAAATGCTACACAGCAAC | GGAGGAGGGCAAAATACACAAA |
| FSH-R | CAAGATAGCAAGGTGACCGAGA | GCAAGYYGGGYAGGYYGGAGA |
| StAR | GGGCATACTCAACAACCAGGAA | CTTGACATTTGGGTTCCACTCTC |
| CYP11-A | CATTCCTGCTGGAAACTGTGAGC | ATCTCGCTGAGCTTTCTTGTAGG |
| 3β-HSD | AGCAAAAAGATGGCCGAGAA | GGCACAAGTATGCAATGTGCC |
| Bcl-2 | TGAAGCGGTCCGGTGGATA | CAGCATTTGCAGAAGTCCTGTGA |
| Caspase-3 | AGAGACATTCATGGGCCTGAAATAC | CACCATGGCTTAGAATCACACACAC |
| Caspase-8 | TGGGACCTGCTGGTCAACTTC | AAAGATCTCAATTCCAACTCGCTCA |
| GnRH-R | CTGCCTTCAATGCTTCCTTC | CAGTGGCATGACGATCAGAG |
| Kiss1-r | TCTGGGCTCACTGCATGTCCTAC | CTGTCTGAAGTGTGAACCCAGGAAG |
| GAPDH | AGGTCGGTGTGAACGGATTTG | GGGGTCGTTGATGGCAACA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayed, H.; Zhang, Q.; Tang, Y.; Wang, Y.; Guo, Y.; Zhang, J.; Ji, C.; Ma, Q.; Zhao, L. Alleviative Effect of Rutin on Zearalenone-Induced Reproductive Toxicity in Male Mice by Preventing Spermatogenic Cell Apoptosis and Modulating Gene Expression in the Hypothalamic–Pituitary–Gonadal Axis. Toxins 2024, 16, 121. https://doi.org/10.3390/toxins16030121
Sayed H, Zhang Q, Tang Y, Wang Y, Guo Y, Zhang J, Ji C, Ma Q, Zhao L. Alleviative Effect of Rutin on Zearalenone-Induced Reproductive Toxicity in Male Mice by Preventing Spermatogenic Cell Apoptosis and Modulating Gene Expression in the Hypothalamic–Pituitary–Gonadal Axis. Toxins. 2024; 16(3):121. https://doi.org/10.3390/toxins16030121
Chicago/Turabian StyleSayed, Hira, Qiongqiong Zhang, Yu Tang, Yanan Wang, Yongpeng Guo, Jianyun Zhang, Cheng Ji, Qiugang Ma, and Lihong Zhao. 2024. "Alleviative Effect of Rutin on Zearalenone-Induced Reproductive Toxicity in Male Mice by Preventing Spermatogenic Cell Apoptosis and Modulating Gene Expression in the Hypothalamic–Pituitary–Gonadal Axis" Toxins 16, no. 3: 121. https://doi.org/10.3390/toxins16030121
APA StyleSayed, H., Zhang, Q., Tang, Y., Wang, Y., Guo, Y., Zhang, J., Ji, C., Ma, Q., & Zhao, L. (2024). Alleviative Effect of Rutin on Zearalenone-Induced Reproductive Toxicity in Male Mice by Preventing Spermatogenic Cell Apoptosis and Modulating Gene Expression in the Hypothalamic–Pituitary–Gonadal Axis. Toxins, 16(3), 121. https://doi.org/10.3390/toxins16030121







