Examination of Microcystin Adsorption by the Type of Plastic Materials Used during the Procedure of Microcystin Analysis
Abstract
:1. Introduction
2. Results
2.1. Method Validation for Microcystins
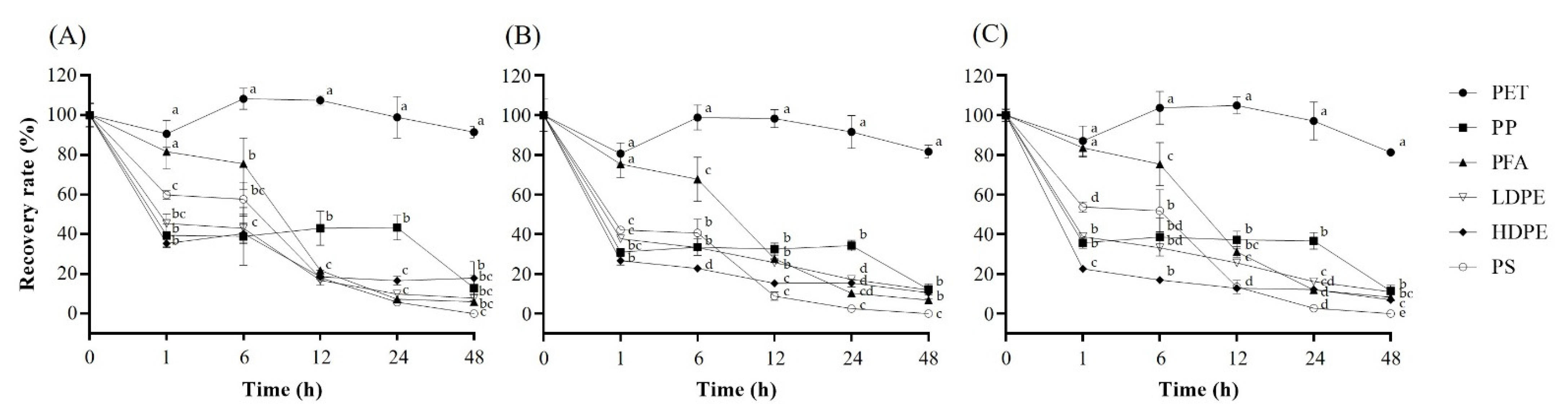
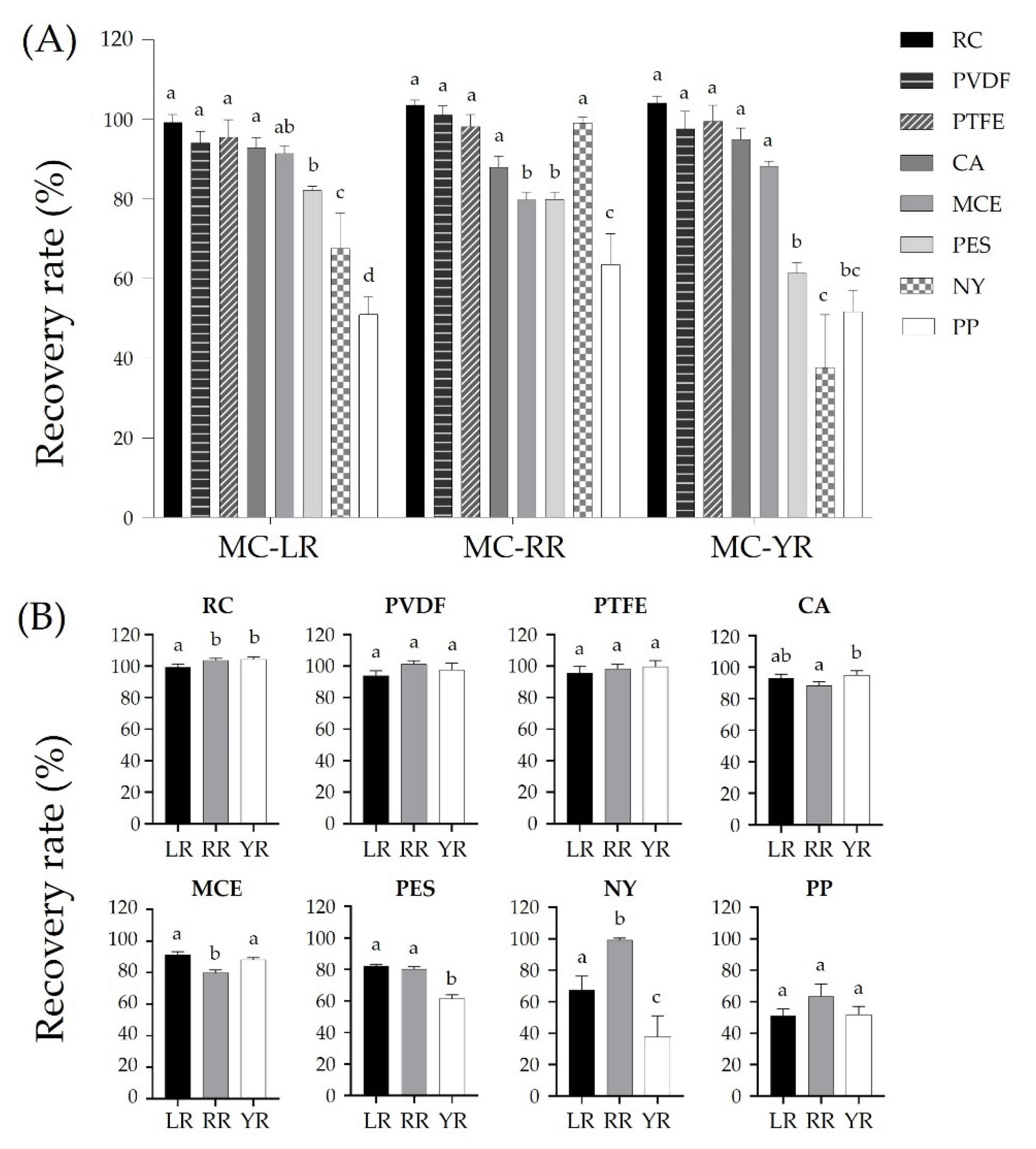
2.2. Adsorption Effects of Six Plastic Containers on Analysis of Microcystins
2.3. Effects of Plastic Materials Used for Eight Membrane Filters on Adsorption of Microcystins
3. Discussion
4. Conclusions
5. Materials and Methods
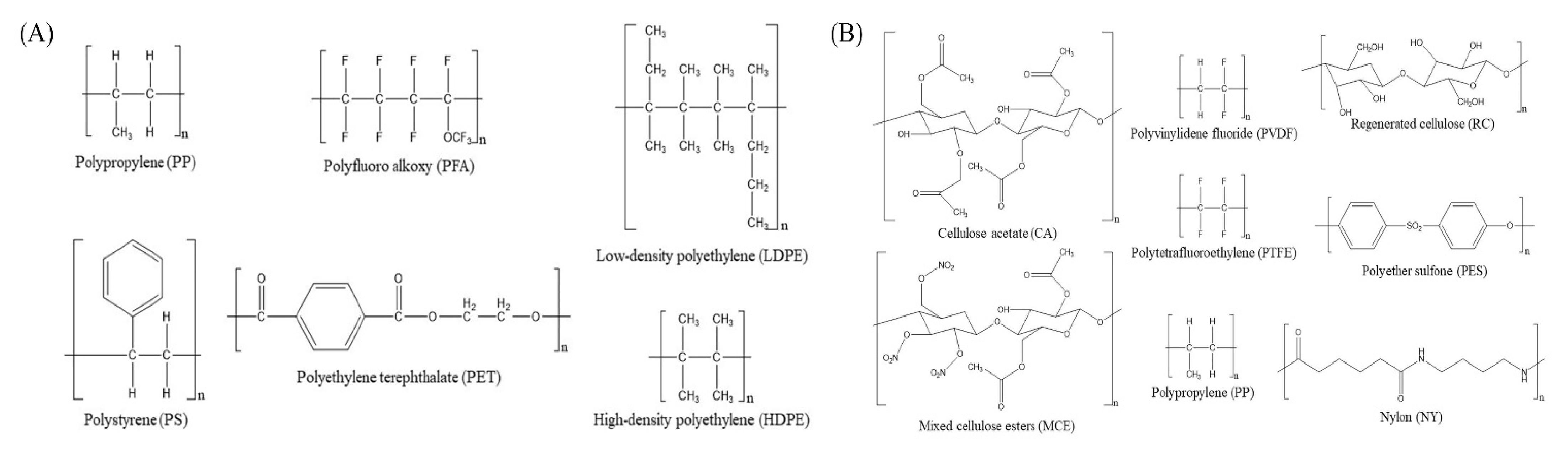
5.1. Chemicals and Materials
5.2. Preparation of Standard Solution
5.3. Adsorption of Microcystins on Different Plastic Materials of Storage Containers
5.4. Adsorption of Microcystins by Different Plastic Materials of Membrane Syringe Filters
5.5. Microcystin Analysis Using UPLC-MS/MS
5.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, H.-K. Survey method relating freshwater phytoplantkon for the management of water resources. Daehan Hwangyeong Gonghag Hoeji 2007, 29, 593–609. [Google Scholar]
- Moss, B.; Kosten, S.; Meerhoff, M.; Battarbee, R.W.; Jeppesen, E.; Mazzeo, N.; Havens, K.; Lacerot, G.; Liu, Z.; De Meester, L. Allied attack: Climate change and eutrophication. Inland Waters 2011, 1, 101–105. [Google Scholar] [CrossRef]
- Cheung, M.Y.; Liang, S.; Lee, J. Toxin-producing cyanobacteria in freshwater: A review of the problems, impact on drinking water safety, and efforts for protecting public health. J. Microbiol. 2013, 51, 1–10. [Google Scholar] [CrossRef]
- De Senerpont Domis, L.N.; Elser, J.J.; Gsell, A.S.; Huszar, V.L.; Ibelings, B.W.; Jeppesen, E.; Kosten, S.; Mooij, W.M.; Roland, F.; Sommer, U. Plankton dynamics under different climatic conditions in space and time. Freshw. Biol. 2013, 58, 463–482. [Google Scholar] [CrossRef]
- Huang, W.; Bi, Y.; Hu, Z. Effects of fertilizer-urea on growth, photosynthetic activity and microcystins production of Microcystis aeruginosa isolated from Dianchi lake. Bull. Environ. Contam. Toxicol. 2014, 92, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.H.; Kim, J.D.; Kim, B.H.; Kong, D.S.; Han, M.S. Isolation and characterization of a bio-agent antagonistic to diatom, Stephanodiscus hantzschii. J. Appl. Microbiol. 2005, 98, 1030–1038. [Google Scholar] [CrossRef]
- Negro, A.I.; Hoyos, C.D.; Vega, J.C. Phytoplankton structure and dynamics in Lake Sanabria and Valparaíso reservoir (NW Spain). In The Trophic Spectrum Revisited; Springer: Berlin/Heidelberg, Germany, 2000; pp. 25–37. [Google Scholar]
- Watson, S.B.; McCauley, E.; Downing, J.A. Patterns in phytoplankton taxonomic composition across temperate lakes of differing nutrient status. Limnol. Oceanogr. 1997, 42, 487–495. [Google Scholar] [CrossRef]
- Zhang, S.; Du, X.; Liu, H.; Losiewic, M.D.; Chen, X.; Ma, Y.; Wang, R.; Tian, Z.; Shi, L.; Guo, H. The Latest Advances in the Reproductive Toxicity of Microcystin-LR. Environ. Res. 2021, 192, 110254. [Google Scholar] [CrossRef]
- Codd, G.A.; Bell, S.G.; Kaya, K.; Ward, C.J.; Beattie, K.A.; Metcalf, J.S. Cyanobacterial toxins, exposure routes and human health. Eur. J. Phycol. 1999, 34, 405–415. [Google Scholar] [CrossRef]
- Ressom, R. Health Effects of Toxic Cyanobacteria (Blue-Green Algae); National Health and Medical Research Council: Canberra, Australia, 1994. [Google Scholar]
- Spoof, L.; Catherine, A. Appendix 3: Tables of microcystins and nodularins. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Wiley: Hoboken, NJ, USA, 2016; pp. 526–537. [Google Scholar]
- Chen, L.; Giesy, J.P.; Adamovsky, O.; Svirčev, Z.; Meriluoto, J.; Codd, G.A.; Mijovic, B.; Shi, T.; Tuo, X.; Li, S.-C. Challenges of using blooms of Microcystis spp. in animal feeds: A comprehensive review of nutritional, toxicological and microbial health evaluation. Sci. Total Environ. 2021, 764, 142319. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, S.; Jiang, X. Cyanobacterial toxins in freshwater and food: Important sources of exposure to humans. Annu. Rev. Food Sci. Technol. 2017, 8, 281–304. [Google Scholar] [CrossRef]
- Shi, L.; Du, X.; Liu, H.; Chen, X.; Ma, Y.; Wang, R.; Tian, Z.; Zhang, S.; Guo, H.; Zhang, H. Update on the adverse effects of microcystins on the liver. Environ. Res. 2021, 195, 110890. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Pant, S.; Vijayaraghavan, R.; Rao, P.L. Comparative toxicity evaluation of cyanobacterial cyclic peptide toxin microcystin variants (LR, RR, YR) in mice. Toxicology 2003, 188, 285–296. [Google Scholar] [CrossRef]
- Molica, R.J.; Oliveira, E.J.; Carvalho, P.V.; Costa, A.N.; Cunha, M.C.; Melo, G.L.; Azevedo, S.M. Occurrence of saxitoxins and an anatoxin-a (s)-like anticholinesterase in a Brazilian drinking water supply. Harmful Algae 2005, 4, 743–753. [Google Scholar] [CrossRef]
- Yang, S.; Chen, L.; Wen, C.; Zhang, X.; Feng, X.; Yang, F. MicroRNA expression profiling involved in MC-LR-induced hepatotoxicity using high-throughput sequencing analysis. J. Toxicol. Environ. Health Part A 2018, 81, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Massey, I.Y.; Feng, H.; Yang, F. A review of cardiovascular toxicity of microcystins. Toxins 2019, 11, 507. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Xu, S.; Huang, F.; Wen, C.; Zheng, S.; Feng, H.; Guo, J.; Chen, J.; Feng, X.; Yang, F. Effects of chronic exposure to microcystin-LR on kidney in mice. Int. J. Environ. Res. Public Health 2019, 16, 5030. [Google Scholar] [CrossRef]
- Gorham, T.; Root, E.D.; Jia, Y.; Shum, C.; Lee, J. Relationship between cyanobacterial bloom impacted drinking water sources and hepatocellular carcinoma incidence rates. Harmful Algae 2020, 95, 101801. [Google Scholar] [CrossRef]
- Abderrahim, L.A.; Taibi, K.; Boussaid, M.; Al-Shara, B.; Abderrahim, N.A.; Abderrahim, S.A. Allium sativum mitigates oxidative damages induced by Microcystin-LR in heart and liver tissues of mice. Toxicon 2021, 200, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Zheng, S.; Yang, Y.; Feng, X.; Chen, J.; Tang, Y.; Wang, H.; Yang, F. Chronic exposure to low concentration of MC-LR caused hepatic lipid metabolism disorder. Ecotoxicol. Environ. Saf. 2022, 239, 113649. [Google Scholar] [CrossRef]
- de la Cruz, A.A.; Antoniou, M.G.; Hiskia, A.; Pelaez, M.; Song, W.; O’Shea, K.E.; He, X.; Dionysiou, D.D. Can we effectively degrade microcystins?-Implications on human health. Anticancer Agents Med. Chem. 2011, 11, 19–37. [Google Scholar] [CrossRef]
- Poste, A.E.; Hecky, R.E.; Guildford, S.J. Evaluating microcystin exposure risk through fish consumption. Environ. Sci. Technol. 2011, 45, 5806–5811. [Google Scholar] [CrossRef]
- Schmidt, J.R.; Shaskus, M.; Estenik, J.F.; Oesch, C.; Khidekel, R.; Boyer, G.L. Variations in the microcystin content of different fish species collected from a eutrophic lake. Toxins 2013, 5, 992–1009. [Google Scholar] [CrossRef]
- Wijewickrama, M.M.; Manage, P.M. Accumulation of Microcystin-LR in grains of two rice varieties (Oryza sativa L.) and a leafy vegetable, Ipomoea aquatica. Toxins 2019, 11, 432. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Cyanobacterial Toxins: Microcystins; WHO: Geneva, Switzerland, 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/338066/WHO-HEP-ECH-WSH-2020.6-eng.pdf (accessed on 1 June 2020).
- Greer, B.; McNamee, S.E.; Boots, B.; Cimarelli, L.; Guillebault, D.; Helmi, K.; Marcheggiani, S.; Panaiotov, S.; Breitenbach, U.; Akçaalan, R. A validated UPLC–MS/MS method for the surveillance of ten aquatic biotoxins in European brackish and freshwater systems. Harmful Algae 2016, 55, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Dahlmann, J.; Budakowski, W.R.; Luckas, B. Liquid chromatography–electrospray ionisation-mass spectrometry based method for the simultaneous determination of algal and cyanobacterial toxins in phytoplankton from marine waters and lakes followed by tentative structural elucidation of microcystins. J. Chromatogr. A 2003, 994, 45–57. [Google Scholar] [CrossRef]
- Codd, G.A.; Bell, S. The Occurrence and Fate of Blue-Green Algal Toxins in Freshwaters; National Rivers Authority: Wales, UK, 1996. [Google Scholar]
- Hyenstrand, P.; Metcalf, J.; Beattie, K.; Codd, G. Effects of adsorption to plastics and solvent conditions in the analysis of the cyanobacterial toxin microcystin-LR by high performance liquid chromatography. Water Res. 2001, 35, 3508–3511. [Google Scholar] [CrossRef]
- Kamp, L.; Church, J.L.; Carpino, J.; Faltin-Mara, E.; Rubio, F. The effects of water sample treatment, preparation, and storage prior to cyanotoxin analysis for cylindrospermopsin, microcystin and saxitoxin. Chem.-Biol. Interact. 2016, 246, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, E.; Ibáñez, M.; Sancho, J.V.; Hernández, F. Determination of six microcystins and nodularin in surface and drinking waters by on-line solid phase extraction–ultra high pressure liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2012, 1266, 61–68. [Google Scholar] [CrossRef]
- Svirčev, Z.; Baltić, V.; Gantar, M.; Juković, M.; Stojanović, D.; Baltić, M. Molecular aspects of microcystin-induced hepatotoxicity and hepatocarcinogenesis. J. Environ. Sci. Health C 2010, 28, 39–59. [Google Scholar] [CrossRef]
- Pillai, S.A.; Chobisa, D.; Urimi, D.; Ravindra, N. Filters and filtration: A review of mechanisms that impact cost, product quality and patient safety. J. Pharm. Sci. Res. 2016, 8, 271. [Google Scholar]
- Moura, D.S.; Pestana, C.J.; Moffat, C.F.; Hui, J.; Irvine, J.T.; Edwards, C.; Lawton, L.A. Adsorption of cyanotoxins on polypropylene and polyethylene terephthalate: Microplastics as vector of eight microcystin analogues. Environ. Pollut. 2022, 303, 119135. [Google Scholar] [CrossRef]
- Yao, S.; Cao, H.; Arp, H.P.H.; Li, J.; Bian, Y.; Xie, Z.; Cherubini, F.; Jiang, X.; Song, Y. The role of crystallinity and particle morphology on the sorption of dibutyl phthalate on polyethylene microplastics: Implications for the behavior of phthalate plastic additives. Environ. Pollut. 2021, 283, 117393. [Google Scholar] [CrossRef] [PubMed]
- Hüffer, T.; Hofmann, T. Sorption of non-polar organic compounds by micro-sized plastic particles in aqueous solution. Environ. Pollut. 2016, 214, 194–201. [Google Scholar] [CrossRef]
- Grinsted, R.A.; Clark, L.; Koenig, J.L. Study of cyclic sorption-desorption into poly (methyl methacrylate) rods using NMR imaging. Macromolecules 1992, 25, 1235–1241. [Google Scholar] [CrossRef]
- Pestana, C.J.; Moura, D.S.; Capelo-Neto, J.; Edwards, C.; Dreisbach, D.; Spengler, B.; Lawton, L.A. Potentially poisonous plastic particles: Microplastics as a vector for cyanobacterial toxins microcystin-LR and microcystin-LF. Environ. Sci. Technol. 2021, 55, 15940–15949. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Hentschel, B.T.; Kaye, S. Long-term field measurement of sorption of organic contaminants to five types of plastic pellets: Implications for plastic marine debris. Environ. Sci. Technol. 2013, 47, 1646–1654. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Zhang, H. Adsorption of antibiotics on microplastics. Environ. Pollut. 2018, 237, 460–467. [Google Scholar] [CrossRef]
- Amborski, L.E.; Flierl, D.W. Physical properties of polyethylene terephthalate films. Ind. Eng. Chem. Res. 1953, 45, 2290–2295. [Google Scholar] [CrossRef]
- Ravindranath, K.; Mashelkar, R. Polyethylene terephthalate—I. Chemistry, thermodynamics and transport properties. Chem. Eng. Sci. 1986, 41, 2197–2214. [Google Scholar] [CrossRef]
- O’Hagan, D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 2008, 37, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, X.; Zhou, X.; Kong, X.; Tao, S.; Xing, B. Sorption of four hydrophobic organic compounds by three chemically distinct polymers: Role of chemical and physical composition. Environ. Sci. Technol. 2012, 46, 7252–7259. [Google Scholar] [CrossRef] [PubMed]
- Mei, W.; Chen, G.; Bao, J.; Song, M.; Li, Y.; Luo, C. Interactions between microplastics and organic compounds in aquatic environments: A mini review. Sci. Total Environ. 2020, 736, 139472. [Google Scholar] [CrossRef] [PubMed]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R. Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2027–2045. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Shih, K.M.; Li, X.Y. The partition behavior of perfluorooctanesulfonate (PFOS) and perfluorooctanesulfonamide (FOSA) on microplastics. Chemosphere 2015, 119, 841–847. [Google Scholar] [CrossRef]
- Velzeboer, I.; Kwadijk, C.; Koelmans, A. Strong sorption of PCBs to nanoplastics, microplastics, carbon nanotubes, and fullerenes. Environ. Sci. Technol. 2014, 48, 4869–4876. [Google Scholar] [CrossRef] [PubMed]
- Kyte, J.; Doolittle, R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef]
- Sivonen, K.; Jones, G. Toxic Cyanobacteria in Water; A Guide to Their Public Health Consequences Monitoring and Management; Wiley: London, UK, 1999. [Google Scholar]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef]
- Lu, D.R.; Lee, S.J.; Park, K. Calculation of solvation interaction energies for protein adsorption on polymer surfaces. J. Biomater. Sci. Polym. Ed. 1992, 3, 127–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hou, D.; Wang, J.; Wang, Z.; Tian, B.; Liang, P. Hydrophilic surface coating on hydrophobic PTFE membrane for robust anti-oil-fouling membrane distillation. Appl. Surf. Sci. 2018, 450, 57–65. [Google Scholar] [CrossRef]
- Tran, H.N.; Wang, Y.-F.; You, S.-J.; Chao, H.-P. Insights into the mechanism of cationic dye adsorption on activated charcoal: The importance of π–π interactions. Process Saf. Environ. Prot. 2017, 107, 168–180. [Google Scholar] [CrossRef]
- Zugenmaier, P. Conformation and packing of various crystalline cellulose fibers. Prog. Polym. Sci. 2001, 26, 1341–1417. [Google Scholar] [CrossRef]
- Lindman, B.; Karlström, G.; Stigsson, L. On the mechanism of dissolution of cellulose. J. Mol. Liq. 2010, 156, 76–81. [Google Scholar] [CrossRef]
- Carlson, M.; Thompson, R. Analyte loss due to membrane filter adsorption as determined by high-performance liquid chromatography. J. Chromatogr. Sci. 2000, 38, 77–83. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, J.; Massey, I.Y.; Peng, T.; Yang, F. Immobilization of Microbes for Biodegradation of Microcystins: A Mini Review. Toxins 2022, 14, 573. [Google Scholar] [CrossRef]
| Analyte | RT | Ionization Mode | Precursor Ion | Product Ion | Calibration Range (μg/L) | Linearity a (R2) | LOD | LOQ | Accuracy b (%) | Repeatability c (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| (m/z) | μg/L | |||||||||
| MC-RR | 3.79 | + | 520.0 | 134.9 | 1–20 | 0.999 | 0.001 | 0.004 | 0.7 | 6.2 |
| MC-LR | 4.07 | + | 995.4 | 134.9 | 1–20 | 0.999 | 0.001 | 0.002 | −3.4 | 8.5 |
| MC-YR | 4.16 | + | 1045.4 | 134.9 | 1–20 | 0.999 | 0.002 | 0.005 | −2.9 | 3.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, C.; Lee, J.W.; Jung, W.-K.; Lee, Y.-M.; Lee, S.; Lee, S.G. Examination of Microcystin Adsorption by the Type of Plastic Materials Used during the Procedure of Microcystin Analysis. Toxins 2022, 14, 625. https://doi.org/10.3390/toxins14090625
Seo C, Lee JW, Jung W-K, Lee Y-M, Lee S, Lee SG. Examination of Microcystin Adsorption by the Type of Plastic Materials Used during the Procedure of Microcystin Analysis. Toxins. 2022; 14(9):625. https://doi.org/10.3390/toxins14090625
Chicago/Turabian StyleSeo, Chan, Joo Won Lee, Won-Kyo Jung, Yoon-Mi Lee, Seungjun Lee, and Sang Gil Lee. 2022. "Examination of Microcystin Adsorption by the Type of Plastic Materials Used during the Procedure of Microcystin Analysis" Toxins 14, no. 9: 625. https://doi.org/10.3390/toxins14090625
APA StyleSeo, C., Lee, J. W., Jung, W.-K., Lee, Y.-M., Lee, S., & Lee, S. G. (2022). Examination of Microcystin Adsorption by the Type of Plastic Materials Used during the Procedure of Microcystin Analysis. Toxins, 14(9), 625. https://doi.org/10.3390/toxins14090625







