Abstract
Chronic kidney disease (CKD) is an incurable disease in which renal function gradually declines, resulting in no noticeable symptoms during the early stages and a life-threatening disorder in the latest stage. The changes that accompany renal failure are likely to influence the gut microbiota, or the ecosystem of micro-organisms resident in the intestine. Altered gut microbiota can display metabolic changes and become harmful to the host. To study the gut–kidney axis in vivo, animal models should ideally reproduce the disorders affecting both the host and the gut microbiota. Murine models of CKD, but not dog, manifest slowed gut transit, similarly to patient. Animal models of CKD also reproduce altered intestinal barrier function, as well as the resulting leaky gut syndrome and bacterial translocation. CKD animal models replicate metabolic but not compositional changes in the gut microbiota. Researchers investigating the gut–kidney axis should pay attention to the selection of the animal model (disease induction method, species) and the setting of the experimental design (control group, sterilization method, individually ventilated cages) that have been shown to influence gut microbiota.
Key Contribution:
In this review, we evaluate how animal models of CKD perform changes in the gut and gut microbiota. The article also discusses how animal models of CKD can be adapted to study the gut–kidney axis.
1. Introduction
Chronic kidney disease (CKD) is defined as abnormalities in kidney structure or function that last over 3 months and have health implications [1]. In CKD, the decrease in renal function leads to the accumulation of various compounds in the blood, known as uremic solutes. Increased blood concentrations of uremic solutes promote uremic syndrome, which comprises many organ disorders and cellular dysfunctions [2,3]. CKD patients have been reported to display reduced gut motility, increased intestinal permeability, bacterial overgrowth, bacterial translocation, and inflammation of intestinal origin [4,5,6], which are consistent with dysbiosis. Although the main cause of intestinal dysbiosis in CKD patients remains unknown, there is evidence that a low-fiber [7], low-protein diet [8], medications and treatments [9,10,11], and reduced renal function alter the biochemical environment of the gut and modify the gut microbiota (GM), or the ecosystem of micro-organisms inhabiting the intestine. Uremic syndrome affects the gastrointestinal tract with manifestations including anorexia, nausea, ulcers, malnutrition, and protein-energy wasting [12]. CKD may favor the growth of uremic toxin-producing bacteria. In parallel, the reduced abundance of carbohydrate-fermenting bacteria leads to the decreased production of beneficial short-chain fatty acids (SCFAs) [13], which are involved in the regulation of glycemic and lipid metabolism, immune system response, blood pressure, and intestinal barrier functions [14]. Alterations in the gut–kidney axis (Figure 1) may adversely affect the development of CKD and increase the risk of comorbidities, such as cardiovascular disease [15], which is the leading cause of mortality in patients with CKD.
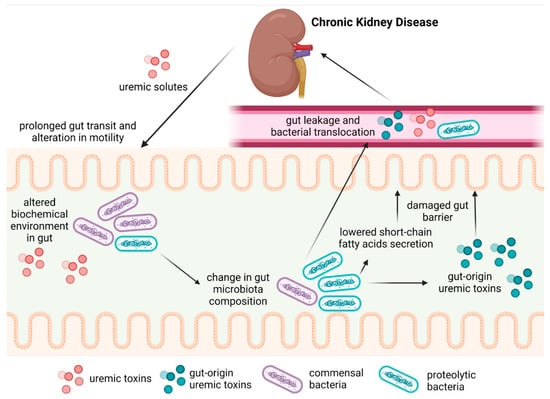
Figure 1.
The gut–kidney axis during CKD.
With the ultimate goals of prevention and treatment in mind, changes in the gut and GM deserve further characterization, for which in vivo studies are needed. To adequately study the gut–kidney axis, animal models of CKD and experimental setups should meet the requirements specific to GM research and adequately reproduce the disorders affecting the host and GM. In the present review, we will evaluate how animal models of CKD perform with regard to changes in the gut and GM and discuss the validity of the models.
2. Materials and Methods
In order to test the validity of using animal models of CKD to study alterations to the gut–kidney axis, an electronic search was performed on material published from 1 January 1990 to 1 April 2022, in PubMed. We searched combinations of the terms: “chronic kidney disease”, “chronic renal disease”, “chronic renal failure”, “gut microbiota”, “gut microbiome”, “intestinal microbiota”, “tight junction”, “gut barrier”, “gut barrier transport”, “gut motility,” “intestinal transit,” “leaky gut”, “gut hyperpermeability”, “bacterial translocation”, and “endotoxemia”. Search results were limited to English-language publications. In addition, references of selected studies were screened to identify eligible trials that were not retrieved by database searches. We included studies on animal models of CKD only. Each search result was independently reviewed for eligibility by the author (P.B.). We excluded trials that reported: (1) no details on CKD induction in animal models; (2) no results on intestinal disturbances, and (3), for articles showing gut microbiota alterations, no indication of the effect of compositional changes. We retrieved 23 studies (Table 1).

Table 1.
Main characteristics and results of CKD animal studies assessing gut and GI alterations retrieved by the search.
3. CKD Models Used to Assess the Gut–Kidney Axis
A variety of animal models were used to explore the pathophysiology of CKD and gut-specific alterations (Table 1). Although rodent models of CKD are predominant, other species are used, including dogs [16]. Furthermore, a range of CKD induction methods is available to mimic desirable aspects of the disease, such as a CKD model induced by an adenine-enriched diet. Adenine is metabolized to uric acid [39] and 2,8-dihydroxyadenine [40], which accumulate as tubular-shaped crystals in the kidney, causing damage to its parenchyma [40], leading to fibrosis and, ultimately, uremia [41]. Surgical models have been developed as an alternative to diet-based models. A subtotal five-sixths nephrectomy (SNx) generally consists of the removal of one kidney and two-thirds of the contralateral. Renal mass reduction results in increasing the single-nephron glomerular filtration rate in the remaining nephrons, which subsequently leads to their injury and a gradual decline in renal function [42]. Unilateral ureteral obstruction (UUO) is another surgical model in which the ureter is durably or transiently ligated. Obstructed urinary flow results in upstream changes, mechanical stretching, and cell apoptosis [43]. These changes are related to tubular cell damage, kidney inflammation, and fibrosis [44]. UUO may model the transition from acute renal failure to CKD.
4. Motility Dysfunction and Gut Barrier
The intestinal barrier plays an important role in the host–GM relationship, as it separates the gut lumen environment from the host organism. The intestinal barrier passively and actively regulates nutrients’ absorption and prevents the transfer of harmful substances into the bloodstream [45]. Gut motility is essential for the progression of food down the gastrointestinal tract during digestion, absorption of nutrients, regulation of bacterial growth, and removal of undigested and harmful compounds [5].
The manometric examination of the bowel in patients with CKD revealed abnormal motility patterns in the small intestine, which correlated with a higher risk of bacterial overgrowth [5]. Colon motility, aside from small intestine motility, might be impaired in CKD. The colonic transit time increased from 24 ± 12 h in healthy subjects to 33 ± 14 h in continuous ambulatory peritoneal dialysis patients and 43 ± 22 h in hemodialysis patients [46]. Elongated intestinal transit was observed in the SNx rat model [17,18,19] and the mouse model induced by an adenine-enriched diet [20]. In contrast, oro-anal transit and colonic transit times were decreased in SNx dogs [16]. The effect of the dialysis modality on gut motility in animal models was not assessed.
Leaky gut, or intestinal hyperpermeability, is a phenomenon that occurs when the structure of tight junctions and/or transcellular transport is impaired, enabling harmful substances to enter the bloodstream [47]. It can be detected by the presence in the blood of significant amounts of molecules normally present in the gut lumen but absent in blood (unable to cross the gut barrier), such as diamine-oxidase. Diamine-oxidase has been found to be present in the blood of both CKD and end-stage renal disease (ESRD) patients, whilst it is not observed in healthy subjects [48]. Using orally administered markers (sucrose, sucralose, or lactulose/mannitol ratio) and measuring urinary recovery, a higher colon permeability has been shown in CKD patients compared to healthy individuals [4]. The administration of a symbiotic formula containing prebiotics, probiotics, and antioxidants reduced small intestine permeability in CKD patients [4]. In animal studies, gut permeability is usually determined by the level of circulating endotoxins, mainly lipopolysaccharides (LPS). In uremic rats, serum LPS levels were greatly increased compared to controls [21]. However, LPS is not an ideal marker of leaky gut, as it can be produced by blood bacteria in the case of infection [49]. Using orally administered exogenous markers (e.g., FITC-dextran) is a more precise alternative that has confirmed the higher gut leakage in SNx mice compared to sham [22,23].
One of the consequences of a leaky gut is greater bacterial translocation, which is defined as the passage of viable bacteria, bacterial cell elements, and bacterial products through the gut barrier to normally sterile tissues, such as blood, mesenteric lymph nodes, and internal organs [50]. In two studies, about 20% of ESRD patients were found to have bacteria-derived DNA fragments in their blood, which is a sign of bacterial translocation [6,51]. The bacterial DNA found in the blood of ESRD patients belonged to the taxa Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Staphylococcus epidermis, Enterococcus faecalis, Proteus mirabilis, Staphylococcus haemolyticus [51], and Klebsiella [6]. Translocation of bacterial DNA was correlated with inflammatory markers, such as serum IL-6 and C-reactive protein [6,51]. In animal models, bacterial translocation can be seen in rats with CKD induced by an adenine-enriched diet [24] and by SNx [25,26]. Interestingly, in SNx rats, translocated bacterial DNA showed similarity in taxa to those found in humans. The frequency of translocations was much higher in animal models than in humans [25]. The passage of viable bacteria from the gastrointestinal tract to extra-intestinal sites, including blood, was reported in SNx rats [26]; however, this was not confirmed in another study [25], and no viable bacteria were found in hemocultures of CKD patients [51].
The intestinal barrier consists of a monolayer of tightly sealed epithelial cells covered at their apical poles by a mucus layer produced by goblet cells [45]. Tight junctions strengthen the cell-to-cell contact, limiting the paracellular route for penetration of molecules into the organism [45]. In a small cohort of CKD patients, the histological examination of tight junction proteins in the colon showed a moderate reduction in occludin, while the zo-1, claudin-1, and claudin-4 proteins were unchanged [52]. In hemodialyzed patients, a decrease in claudin-1 and zo-1 proteins was observed in the histological analysis, and the reduction in occludin was greater compared to that in CKD patients [52]. Changes in the abundance of tight junction proteins in the colon and other parts of the intestine [27] were observed in CKD rats [21,24,27,28,29,30,31,32] and mice [22,23,33], regardless of the method of induction [29]. The abundance of tight junction proteins was reduced [21,23,24,27,28,29,30,31,32,33] and structural alterations of tight junctions, such as reduced density and widened intercellular space, were observed [22]. Interestingly, mRNA expressions of tight junction components were decreased in the colon of SNx mice [22] and increased in SNx rats despite lower protein levels [29]. Although it seems that protein level and gene expression should be positively associated, they are actually hardly comparable due to the post-transcriptional mechanisms involved in the conversion of mRNA to protein, the different half-lives of mRNAs and proteins in vivo, and, finally, the different technical properties of detection methods of protein and mRNA make them difficult to compare [53]. Reduced concentrations of SCFAs (products of commensal bacteria) in serum and feces have been reported in CKD patients compared to controls [54]. In animal models of CKD, SCFAs levels were also reduced [34,35]. In SNx rats, supplementation with the SCFA butyrate restored the number of tight junction proteins [21]. Similar effects were observed after supplementation with non-digestible carbohydrates [33,36] that are fermented by the GM, resulting in SCFAs production. The effects of SCFAs or indigestible carbohydrates’ supplementation on tight junction protein abundance have not been examined in CKD patients.
Interestingly, the mucus layer has been reported to be altered in CKD animals. A reduced expression of mucin-2 (a main component of mucus) and lower mucus production were found [21]. Mucus plays the dual role of reducing contact between the GM and gut epithelial cells and providing a source of nutrients for bacteria [55]. To our knowledge, potential changes in mucus secretion and composition in CKD patients have not been reported.
Considerably less is known about changes in the transcellular pathway in CKD. The transcellular route depends on a complex network of channels and transporters regulated by sensing and signaling machinery [56]. Chronic changes in the biochemical milieu associated with renal disease may result in dysfunction of the transcellular route [56]. ABCG2, a urate transporter present in both kidneys and intestines, appears to play an important role in CKD. In ESRD patients, a group with dysfunctional variants of the ABCG2 gene had reduced ABCG2-mediated intestinal urate excretion resulting in increased hyperuricemia [57]. Increased expression of the ABCG2 transporter in the ileum was observed in SNx rats [36], suggesting that intestinal urate excretion may increase with renal insufficiency.
Overall, CKD appears to be associated with an impairment of paracellular and transcellular transport, which contributes to the phenomenon of leaky gut, bacterial translocation, and associated inflammation of gut origin.
5. Gut Microbiota Alterations in CKD
CKD may also modify the composition of the GM. In ESRD patients, the expansion of proteolytic bacteria involved in uremic toxins’ metabolism was observed [13]. Among 19 overgrowing bacterial families, 12 possessed the urease gene (Alteromonadaceae, Cellulomonadaceae, Clostridiaceae, Dermabacteraceae, Enterobacteriaceae, Halomonadaceae, Methylococcaceae, Micrococcaceae, Moraxellaceae, Polyangiaceae, Pseudomonadaceae, and Xanthomonadaceae), and 5 harbored the uricase gene (Cellulomonadaceae, Dermabacteraceaea, Micrococcaceae, Polyangiaceae, and Xanthomonadaceae) producing ammonia that is harmful to the intestinal epithelium. In addition, families expressing the tryptophanase gene (Clostridiaceae, Enterobacteriaceae, and Verrucomicrobiaceaea), necessary for the conversion of tryptophan into indole, which is then metabolized by the liver into uremic toxins, were also overrepresented. Clostridiaceae and Enterobacteriaceae also possess enzymes that produce p-cresyl from tyrosine. In contrast, the relative abundance of carbohydrate-fermenting bacteria (Lactobacillacease and Prevotellaceae families) decreased in ESRD patients [13]. In CKD and ESRD patients, the reduced concentrations of SCFAs in blood and stool samples were associated with a lower abundance of Enterobacter, Enterococcus, Bifidobacterium, Bacteroides, Clostridium, Faecalibacterium, and Roseburia, most of which are SCFAs-producing taxa [54]. Interestingly, the SCFAs’ concentration in the blood was inversely correlated with the degree of renal insufficiency in patients [54].
Due to the different genetic backgrounds of the host and environmental conditions, it might be difficult to reproduce compositional changes in animal models. The compositional diversity of the GM varied according to the method of induction: it increased in the SNx model [37], did not change in the UUO model [34], and decreased in the adenine-enriched diet [35], while it mostly decreased in CKD and ESRD patients [58]. In the UUO rat model, bacteria belonging to the taxa Acetatifactor, Blautia, Intestinimonas, and Oscillibacter were more abundant, while the taxa Lactobacillales, Clostridium, Streptococcus, and Pseudomonas were reduced [34]. A decrease in the plasma tryptophan level and an increase in tryptophan metabolites were associated with a lower abundance of Clostridium, Pseudomonas, and Lactobacillales, which hold the tryptophan synthase gene, and a higher abundance of Oscilibacter, Blautia, and Intestinimonas, which are able to catabolize tryptophan into indoles [34]. In adenine rats, changes in the GM were associated with an increase in the trimethylamine N-oxide (TMAO) blood level [35]. GM changes included a reduced abundance of the carbohydrate-fermenting bacteria Bacteroidetes and an increase in Lachnospiraceae, Erysipelatoclostridium, Tannerellaceae, Akkermansiaceae, Verrucomicrobiae, Oscillibacter, Flavonifractor, and Parabacteroides, which were positively associated with TMAO levels [35]. In SNx rats, the GM composition was modified with an increased abundance of Methanosphaera, Akkermansia, Christensenella, Adlercreutzia, Corynebacterium, and Coprobacillus, as well as genera from the class Mollicutes and family Clostridiacea, which was correlated with amino acids’ metabolism [37].
Interestingly, the animal models display changes in the composition of GM that result in metabolic changes comparable to clinical observations. Altogether, animal models of CKD manifest changes in the GM composition and function that are consistent with the increased production of uremic toxins and decreased production of SCFAs, as in humans.
Overall, the alterations in the gut structure and functions, as well as in the GM, that have been reported in CKD patients appear to be reproduced, at least partly, in animal models designed to reproduce CKD. For observations from animal models that have not been shown in humans, it is highly relevant to evaluate the validity of the model and experiment to assess how representative of the human disease they are, and, therefore, how the results can be extrapolated from preclinical models to humans.
6. Adaptation of CKD Animal Models to GM–Kidney Research
The value of an animal experiment is defined by the accuracy of the translation of the results into clinical research [59]. Successful translation is based on proper experiment design and the selection of an adequate animal model [59]. Internal validity addresses the consistency of the experimental design and its reproducibility [60]. To minimize the researcher’s influence on the obtained results, randomization techniques and blind testing may be applied [59]. A meta-analysis of 290 animal studies showed that experiments with randomization and/or blind-testing were more likely to report reproducible results [61]. These methods can be implemented in the research on the gut–kidney axis to avoid biases, although disease induction is often associated with important phenotypic changes, thereby limiting the blinding of model induction.
External validity, or how the results obtained in the preclinical models can be translated into a better understanding and predictability of human conditions, is also important [59]. When designing animal models, the first thing that needs to be taken into account is the animal species. To mimic human CKD, various species can be used that differ in kidney structure, body size, blood volume, and genetics, which impact the choice of CKD induction methods, the similarity of CKD disease progression, and the choice of analytical techniques [62]. The specificity of GM investigation also requires considering the anatomical and physiological characteristics of the animal used for the study. The similarity of the intestinal tract as an environment of GM between animals and humans is a prerequisite. Mammals manifest great functional and anatomical similarities between orders, though species variation needs to be acknowledged. Among them, differences in the length and volume of parts of the gastrointestinal tract, species-specific intestinal wall structures [63], variable pH, and luminal enzyme secretions are observed [64]. As the digestive tract strongly reflects the adaptation of the species to the diet, omnivorous animals, such as rats, mice, pigs, or monkeys, are preferable. Furthermore, the choice of omnivorous animals allows the implementation of a wider spectrum of dietary interventions. Other limitations include the physical capacities of the gastrointestinal tract that exist in humans but not in other species, such as the ability to vomit (absent from rodents) [65]. Another major obstacle is behaviors and eating habits manifested by animals and not by humans, such as coprophagy, which has a strong impact on GM composition but cannot be prevented without serious health implications [66].
Laboratory animal breeders provide specific pathogen-free (SPF) animals that are free of commonly occurring pathogens and parasites and free of the corresponding diseases. Due to their genetic homogeneity and similar living environment, results obtained on colony animals are relatively easy to replicate. However, the low genetic diversity does not reflect the broader and more varied population of CKD patients. For these reasons, the idea of “dirty” animal models may better represent human populations, where genetic differences, contact with pathogens, and living environment shape GM composition at the expense of a lower control [67]. The use of pet animals with greater genetic diversity, different ages, and health conditions would better reflect an unselected population. Still, it remains controversial, as uncontrolled and unknown factors shaping the GM composition may bias the results [67]. The method of induction of CKD also needs to be thought upon. In GM research, methods of CKD induction are based on surgery or diet modifications (Table 2). The changes in GM composition reported by an adenine-enriched diet are variable, ranging from clearly observed [68] to minimal [38] or absent [69]. However, animals fed an adenine-rich diet have significantly lower body masses [38,68,69]. A lower food intake is also observed, probably due to both CKD and diet aversion [70], which result in vitamin deficiency and malnutrition [38] and can directly cause shifts in the GM composition. A major limitation of the adenine-enriched diet-induced model is the impossibility of setting up a control group to distinguish the effect of diet on GM from the CKD–GM interactions. In surgical models, the use of drugs, such as antibiotics [71], anesthetics [72], and analgesics [73], can affect the GM composition up to several weeks after surgery. Moreover, in some studies, it has been observed that the body weight of operated animals is lower compared to control animals [27]. Still, sham surgery can help distinguish the effects of surgery and drugs on GM from those of CKD.

Table 2.
Summary of gut alterations observed in animal models of CKD induced by adenine-enriched diet or by surgery.
Special attention should also be paid to the animal environment. Diet shapes the GM composition [75], and GM mediates nutrient absorption and host metabolism [76]. To avoid fluctuations in GM composition, commercially available diets with a fixed composition should be considered. The quality of drinking water must also be maintained constant during the experiment. In particular, the presence of residual micro-organisms (usually from Proteobacteria, Bacterioidetes, or Actinobacteria, such as Acinetobacter, Pseudomonas, and Mycobacterium) [77], the pH of the water [78], and the concentration and ion ratio [78,79] may also influence the GM composition. For rodents, the effect of bedding and supplementation with fiber-rich wooden bricks or nests should also be evaluated as their consumption leads to reduced nutrient absorption [80], growth of fiber-fermenting bacteria, and increased production of metabolites, such as SCFAs [81]. Bedding consumption can be reduced by ad libitum feeding [81]. Diet, water, and bedding can be sterilized to decrease the inoculation of animals with environmental bacteria. Water may be decontaminated by reverse osmosis or UV radiation [82], although the former method alters the ion input provided by the water. UV radiation can also be used to decontaminate bedding and diet, as this method does not lead to nutrient degradation or moisture absorption [83].
To keep SPF conditions during an experiment, it is possible to rely on individually ventilated cages (IVC). Microenvironmental parameters, such as temperature, humidity [84], and light–dark cycle [85], which may lead to GM alteration, can be maintained by the IVC controller. However, noise and airflow conditioned by air filters may be potential stressors for animals [86]. The use of IVC is also limited to species with small body sizes. IVC are helpful to avoid the cage effect, which leads to GM homogenization and the loss of individual traits in a group of animals by mutual inoculation during contact with skin or feces (especially during coprophagy) [87]. The adopted strategy to deal with this is housing animals in individual cages, which discriminates against using herd animals with strong social needs. Nevertheless, social isolation and SPF conditions negatively affect the immune system’s maturation [88], potentially leading to GM dysbiosis.
Considering all of this, we reviewed the designs of animal experiments on the gut–kidney axis that were retrieved by the search (Table 1). Most of the studies were randomized (15 out of 23), performed on omnivorous colony animals (21 out of 23), or in a controlled environment (16 out of 23) with surgically induced CKD (17 out of 23). The weaknesses of the studies were a lack of information about diet composition, water source, sterilization methods, animal co-housing, and sterility of cages. Improving the reporting of GM-relevant study design parameters would improve the reproducibility and verifiability of results.
7. Conclusions
The co-evolution of GM and the host has led to highly complex and multifactorial relationships [89]. The holobiont, an organism composed of a host and a microbiome, combines a highly conserved human genome and a dynamic genome of the microbiome that can change rapidly in response to external factors, by a variety of means (increasing or reducing the number of particular microbes, acquisition of novel microbes, horizontal gene transfer, mutations) [90]. In health, the GM composition changes dynamically within alternative stable states with positive impacts on the host [91]. However, unfavorable factors may induce a critical transition of GM composition to dysbiosis, where GM metabolism is adverse to the host [91].
CKD models in rodents, but not in dogs, reproduce the elongated intestinal transit time observed in humans. Alterations of the gut barrier and endotoxemia have also been reproduced in these models. Interestingly, predominantly translocated taxa are similar in clinical and preclinical studies. During CKD, a shift towards a uremic toxin-producing GM and reduced production of beneficial SCFAs is observed. Despite GM compositional differences between human and animal models, the changes in GM metabolism in CKD have been well reproduced regardless of the approach used to induce CKD (diet or surgery). Different methods of inducing CKD in animal models are associated with different problems and limitations. The adenine-enriched diet causes crystal formation and tubulointerstitial damage. However, the altered content of diet itself may strongly influence the composition and metabolism of the GM. Moreover, the adenine-enriched diet adversely affects body weight and causes malnutrition. The biggest obstacle to studying the gut–kidney axis with this model appears to be the difficulty in having a control group able to distinguish the effects of the diet from those of CKD. Surgically induced CKD animal models (such as SNx and UUO) require the use of medication, which influenced the GM composition. In contrast to the diet-induced models, it is possible to perform sham surgery to control the effect of surgery and medications.
GM investigations have specific requirements that need to be taken into consideration when designing animal experiments. Preferably, omnivorous species with small body sizes that allow for housing in controlled conditions, ideally in IVC, should be used. Methods of disinfection of diet and bedding should be implemented. It is crucial to set up a control group that allows for distinguishing the GM effects of CKD from those linked to the CKD induction method. Thus, surgically induced CKD would be preferable to adenine-enriched diet-induced CKD for GM studies.
Author Contributions
Conceptualization and methodology, P.B., N.G., S.B., A.A., M.C.-S. and F.D.; investigation, P.B.; writing—original draft preparation, P.B.; writing—review and editing, P.B., N.G., S.B., C.D., A.A., M.C.-S. and F.D.; visualization, P.B.; supervision, N.G., S.B., C.D., A.A., M.C.-S. and F.D.; project administration, N.G., S.B., C.D., A.A., M.C.-S. and F.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant EU RESEARCH FRAMEWORK PROGRAMME: Innovative Training Network “Strategy-CKD” (MSCA-ITN-2019-860329).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- De Boer, I.H.; Caramori, M.L.; Chan, J.C.N.; Heerspink, H.J.L.; Hurst, C.; Khunti, K.; Liew, A.; Michos, E.D.; Navaneethan, S.D.; Olowu, W.A.; et al. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020, 98, S1–S115. [Google Scholar] [CrossRef] [PubMed]
- Alméras, C.; Argilés, A. The General Picture of Uremia. Semin Dial. 2009, 22, 329–333. [Google Scholar] [CrossRef]
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A.; European Uremic Toxin Work Group. Normal and Pathologic Concentrations of Uremic Toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Cosola, C.; Rocchetti, M.T.; di Bari, I.; Acquaviva, P.M.; Maranzano, V.; Corciulo, S.; Di Ciaula, A.; Di Palo, D.M.; La Forgia, F.M.; Fontana, S.; et al. An Innovative Synbiotic Formulation Decreases Free Serum Indoxyl Sulfate, Small Intestine Permeability and Ameliorates Gastrointestinal Symptoms in a Randomized Pilot Trial in Stage IIIb-IV CKD Patients. Toxins 2021, 13, 334. [Google Scholar] [CrossRef]
- Strid, H.; Simrén, M.; Stotzer, P.-O.; Ringström, G.; Abrahamsson, H.; Björnsson, E.S. Patients with Chronic Renal Failure Have Abnormal Small Intestinal Motility and a High Prevalence of Small Intestinal Bacterial Overgrowth. Digestion 2003, 67, 129–137. [Google Scholar] [CrossRef]
- Wang, F.; Jiang, H.; Shi, K.; Ren, Y.; Zhang, P.; Cheng, S. Gut Bacterial Translocation Is Associated with Microinflammation in End-Stage Renal Disease Patients: Bacterial Translocation in ESRD. Nephrology 2012, 17, 733–738. [Google Scholar] [CrossRef]
- Chiavaroli, L.; Mirrahimi, A.; Sievenpiper, J.L.; Jenkins, D.J.A.; Darling, P.B. Dietary Fiber Effects in Chronic Kidney Disease: A Systematic Review and Meta-Analysis of Controlled Feeding Trials. Eur. J. Clin. Nutr. 2015, 69, 761–768. [Google Scholar] [CrossRef]
- Hsu, C.-K.; Su, S.-C.; Chang, L.-C.; Shao, S.-C.; Yang, K.-J.; Chen, C.-Y.; Chen, Y.-T.; Wu, I.-W. Effects of Low Protein Diet on Modulating Gut Microbiota in Patients with Chronic Kidney Disease: A Systematic Review and Meta-Analysis of International Studies. Int. J. Med. Sci. 2021, 18, 3839–3850. [Google Scholar] [CrossRef]
- Kortman, G.A.M.; Reijnders, D.; Swinkels, D.W. Oral Iron Supplementation: Potential Implications for the Gut Microbiome and Metabolome in Patients with CKD. Hemodial. Int. 2017, 21, S28–S36. [Google Scholar] [CrossRef]
- Yoshifuji, A.; Wakino, S.; Irie, J.; Matsui, A.; Hasegawa, K.; Tokuyama, H.; Hayashi, K.; Itoh, H. Oral Adsorbent AST-120 Ameliorates Gut Environment and Protects against the Progression of Renal Impairment in CKD Rats. Clin. Exp. Nephrol. 2018, 22, 1069–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, D.; Zhao, W.; Lin, Z.; Wu, J.; Lin, H.; Li, Y.; Song, J.; Zhang, J.; Peng, H. The Effects of Hemodialysis and Peritoneal Dialysis on the Gut Microbiota of End-Stage Renal Disease Patients, and the Relationship Between Gut Microbiota and Patient Prognoses. Front. Cell. Infect. Microbiol. 2021, 11, 579386. [Google Scholar] [CrossRef]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A Proposed Nomenclature and Diagnostic Criteria for Protein—Energy Wasting in Acute and Chronic Kidney Disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Piceno, Y.M.; DeSantis, T.Z.; Pahl, M.; Andersen, G.L.; Vaziri, N.D. Expansion of Urease- and Uricase-Containing, Indole- and p-Cresol-Forming and Contraction of Short Chain Fatty Acid-Producing Intestinal Microbiota in ESRD. Am. J. Nephrol. 2014, 39, 230–237. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Huang, Y.; Xin, W.; Xiong, J.; Yao, M.; Zhang, B.; Zhao, J. The Intestinal Microbiota and Metabolites in the Gut-Kidney-Heart Axis of Chronic Kidney Disease. Front. Pharmacol. 2022, 13, 837500. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, H.P.; Ferré, J.P.; Watson, A.D.; Brown, C.A.; Serthelon, J.P.; Laroute, V.; Concordet, D.; Toutain, P.L. Small Bowel Motility and Colonic Transit Are Altered in Dogs with Moderate Renal Failure. Am. J. Physiol Regul Integr Comp. Physiol 2001, 281, R230–R238. [Google Scholar] [CrossRef]
- Da Graça, J.R.V.; Parente, C.C.; Fiúza, R.F.; Da Silva, P.A.F.; Mota, B.T.; Salles, L.D.; Silva, C.M.D.S.; da Silva, M.T.B.; Oliveira, R.; Dos Santos, A.A. Subtotal Nephrectomy Inhibits the Gastric Emptying of Liquid in Awake Rats. Physiol. Rep. 2015, 3, e12291. [Google Scholar] [CrossRef]
- Wang, S.C.; Lu, K.Y.; Chen, S.M.; Young, T.K. Gastric Emptying and Intestinal Transit of Liquid and Solid Markers in Rats with Chronic Uremia. Chin. J. Physiol. 2001, 44, 81–87. [Google Scholar] [PubMed]
- Yu, C.; Tan, S.; Wang, Z.; Deng, B.; Li, J.; Wang, Q.; Zhou, C.; Kang, X.; Yu, Z.; Zhuang, S. Chronic Kidney Disease Elicits an Intestinal Inflammation Resulting in Intestinal Dysmotility Associated with the Activation of Inducible Nitric Oxide Synthesis in Rat. Digestion 2018, 97, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Hoibian, E.; Florens, N.; Koppe, L.; Vidal, H.; Soulage, C.O. Distal Colon Motor Dysfunction in Mice with Chronic Kidney Disease: Putative Role of Uremic Toxins. Toxins 2018, 10, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, A.; Krieg, R.; Massey, H.D.; Carl, D.; Ghosh, S.; Gehr, T.W.B.; Ghosh, S.S. Sodium Butyrate Ameliorates Insulin Resistance and Renal Failure in CKD Rats by Modulating Intestinal Permeability and Mucin Expression. Nephrol. Dial. Transplant. 2019, 34, 783–794. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, J.; Wang, S.; Xiong, J.; Chen, Y.; Liu, Y.; Xiao, T.; Li, Y.; He, T.; Li, Y.; et al. Indoxyl Sulfate Induces Intestinal Barrier Injury through IRF1-DRP1 Axis-Mediated Mitophagy Impairment. Theranostics 2020, 10, 7384–7400. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lim, S.Y.; Ko, Y.S.; Lee, H.Y.; Oh, S.W.; Kim, M.G.; Cho, W.Y.; Jo, S.K. Intestinal Barrier Disruption and Dysregulated Mucosal Immunity Contribute to Kidney Fibrosis in Chronic Kidney Disease. Nephrol. Dial. Transplant. 2019, 34, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Yuan, J.; Khazaeli, M.; Masuda, Y.; Ichii, H.; Liu, S. Oral Activated Charcoal Adsorbent (AST-120) Ameliorates Chronic Kidney Disease-Induced Intestinal Epithelial Barrier Disruption. Am. J. Nephrol. 2013, 37, 518–525. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, P.; Jiang, H.; Cheng, S. Gut Bacterial Translocation Contributes to Microinflammation in Experimental Uremia. Dig. Dis. Sci. 2012, 57, 2856–2862. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Duarte, J.B.; de Aguilar-Nascimento, J.E.; Nascimento, M.; Nochi, R.J. Bacterial Translocation in Experimental Uremia. Urol. Res. 2004, 32, 266–270. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Yuan, J.; Nazertehrani, S.; Ni, Z.; Liu, S. Chronic Kidney Disease Causes Disruption of Gastric and Small Intestinal Epithelial Tight Junction. Am. J. Nephrol. 2013, 38, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Yoshifuji, A.; Wakino, S.; Irie, J.; Tajima, T.; Hasegawa, K.; Kanda, T.; Tokuyama, H.; Hayashi, K.; Itoh, H. Gut Lactobacillus Protects against the Progression of Renal Damage by Modulating the Gut Environment in Rats. Nephrol. Dial. Transplant. 2016, 31, 401–412. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Yuan, J.; Rahimi, A.; Ni, Z.; Said, H.; Subramanian, V.S. Disintegration of Colonic Epithelial Tight Junction in Uremia: A Likely Cause of CKD-Associated Inflammation. Nephrol. Dial. Transplant. 2012, 27, 2686–2693. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Nunes, A.C.F.; Said, H.; Khazaeli, M.; Liu, H.; Zhao, Y.; Jing, W.; Cogburn, K.; Alikhani, L.; Lau, W.L. Route of Intestinal Absorption and Tissue Distribution of Iron Contained in the Novel Phosphate Binder Ferric Citrate. Nephrol. Dial. Transplant. 2020, 35, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.L.; Liu, S.-M.; Pahlevan, S.; Yuan, J.; Khazaeli, M.; Ni, Z.; Chan, J.Y.; Vaziri, N.D. Role of Nrf2 Dysfunction in Uremia-Associated Intestinal Inflammation and Epithelial Barrier Disruption. Dig. Dis. Sci. 2015, 60, 1215–1222. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Liu, S.-M.; Lau, W.L.; Khazaeli, M.; Nazertehrani, S.; Farzaneh, S.H.; Kieffer, D.A.; Adams, S.H.; Martin, R.J. High Amylose Resistant Starch Diet Ameliorates Oxidative Stress, Inflammation, and Progression of Chronic Kidney Disease. PLoS ONE 2014, 9, e114881. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.V.; Suzuki, T. Dietary Fermentable Fibers Attenuate Chronic Kidney Disease in Mice by Protecting the Intestinal Barrier. J. Nutr. 2018, 148, 552–561. [Google Scholar] [CrossRef]
- Chen, L.; Chen, D.-Q.; Liu, J.-R.; Zhang, J.; Vaziri, N.D.; Zhuang, S.; Chen, H.; Feng, Y.-L.; Guo, Y.; Zhao, Y.-Y. Unilateral Ureteral Obstruction Causes Gut Microbial Dysbiosis and Metabolome Disorders Contributing to Tubulointerstitial Fibrosis. Exp. Mol. Med. 2019, 51, 1–18. [Google Scholar] [CrossRef]
- Hsu, C.-N.; Yang, H.-W.; Hou, C.-Y.; Chang-Chien, G.-P.; Lin, S.; Tain, Y.-L. Maternal Adenine-Induced Chronic Kidney Disease Programs Hypertension in Adult Male Rat Offspring: Implications of Nitric Oxide and Gut Microbiome Derived Metabolites. Int. J. Mol. Sci. 2020, 21, 7237. [Google Scholar] [CrossRef]
- Yano, H.; Tamura, Y.; Kobayashi, K.; Tanemoto, M.; Uchida, S. Uric Acid Transporter ABCG2 Is Increased in the Intestine of the 5/6 Nephrectomy Rat Model of Chronic Kidney Disease. Clin. Exp. Nephrol. 2014, 18, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Deng, Y.; Yang, A.; Lu, Z.; Chen, Y.; Liu, X.; Han, L.; Zou, C. Rhubarb Enema Improved Colon Mucosal Barrier Injury in 5/6 Nephrectomy Rats May Associate With Gut Microbiota Modification. Front. Pharmacol. 2020, 11, 1092. [Google Scholar] [CrossRef] [PubMed]
- Mishima, E.; Fukuda, S.; Shima, H.; Hirayama, A.; Akiyama, Y.; Takeuchi, Y.; Fukuda, N.N.; Suzuki, T.; Suzuki, C.; Yuri, A.; et al. Alteration of the Intestinal Environment by Lubiprostone Is Associated with Amelioration of Adenine-Induced CKD. J. Am. Soc. Nephrol. 2015, 26, 1787–1794. [Google Scholar] [CrossRef]
- Coe, F.L. Uric Acid and Calcium Oxalate Nephrolithiasis. Kidney Int. 1983, 24, 392–403. [Google Scholar] [CrossRef]
- Koeda, T.; Wakaki, K.; Koizumi, F.; Yokozawa, T.; Oura, H. Early Changes of Proximal Tubules in the Kidney of Adenine-Ingesting Rats, with Special Reference to Biochemical and Electron Microscopic Studies. Nihon Jinzo Gakkai Shi. 1988, 30, 239–246. [Google Scholar] [PubMed]
- Yokozawa, T.; Zheng, P.D.; Oura, H.; Koizumi, F. Animal Model of Adenine-Induced Chronic Renal Failure in Rats. Nephron 1986, 44, 230–234. [Google Scholar] [CrossRef]
- Brenner, B.M.; Lawler, E.V.; Mackenzie, H.S. The Hyperfiltration Theory: A Paradigm Shift in Nephrology. Kidney Int. 1996, 49, 1774–1777. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Klimova, E.; Aparicio-Trejo, O.E.; Tapia, E.; Pedraza-Chaverri, J. Unilateral Ureteral Obstruction as a Model to Investigate Fibrosis-Attenuating Treatments. Biomolecules 2019, 9, 141. [Google Scholar] [CrossRef]
- Ucero, A.C.; Benito-Martin, A.; Izquierdo, M.C.; Sanchez-Niño, M.D.; Sanz, A.B.; Ramos, A.M.; Berzal, S.; Ruiz-Ortega, M.; Egido, J.; Ortiz, A. Unilateral Ureteral Obstruction: Beyond Obstruction. Int. Urol. Nephrol. 2014, 46, 765–776. [Google Scholar] [CrossRef]
- Meijers, B.; Farré, R.; Dejongh, S.; Vicario, M.; Evenepoel, P. Intestinal Barrier Function in Chronic Kidney Disease. Toxins 2018, 10, 298. [Google Scholar] [CrossRef]
- Wu, M.-J.; Chang, C.-S.; Cheng, C.-H.; Chen, C.-H.; Lee, W.-C.; Hsu, Y.-H.; Shu, K.-H.; Tang, M.-J. Colonic Transit Time in Long-Term Dialysis Patients. Am. J. Kidney Dis. 2004, 44, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Vanuytsel, T.; Tack, J.; Farre, R. The Role of Intestinal Permeability in Gastrointestinal Disorders and Current Methods of Evaluation. Front. Nutr. 2021, 8, 717925. [Google Scholar] [CrossRef]
- Lemesch, S.; Ribitsch, W.; Schilcher, G.; Spindelböck, W.; Hafner-Gießauf, H.; Marsche, G.; Pasterk, L.; Payerl, D.; Schmerböck, B.; Tawdrous, M.; et al. Mode of Renal Replacement Therapy Determines Endotoxemia and Neutrophil Dysfunction in Chronic Kidney Disease. Sci. Rep. 2016, 6, 34534. [Google Scholar] [CrossRef]
- Munford, R.S. Endotoxemia—Menace, Marker, or Mistake? J. Leukoc. Biol. 2016, 100, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Berg, R.D.; Garlington, A.W. Translocation of Certain Indigenous Bacteria from the Gastrointestinal Tract to the Mesenteric Lymph Nodes and Other Organs in a Gnotobiotic Mouse Model. Infect. Immun. 1979, 23, 403–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bossola, M.; Sanguinetti, M.; Scribano, D.; Zuppi, C.; Giungi, S.; Luciani, G.; Torelli, R.; Posteraro, B.; Fadda, G.; Tazza, L. Circulating Bacterial-Derived DNA Fragments and Markers of Inflammation in Chronic Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-K.; Lim, P.-S.; Jin, J.-S.; Wu, M.-Y.; Chen, C.-H. Impaired Gut Epithelial Tight Junction Expression in Hemodialysis Patients Complicated with Intradialytic Hypotension. Biomed. Res. Int. 2018, 2018, 2670312. [Google Scholar] [CrossRef]
- Greenbaum, D.; Colangelo, C.; Williams, K.; Gerstein, M. Comparing Protein Abundance and MRNA Expression Levels on a Genomic Scale. Genome Biol. 2003, 4, 117. [Google Scholar] [CrossRef]
- Wang, S.; Lv, D.; Jiang, S.; Jiang, J.; Liang, M.; Hou, F.; Chen, Y. Quantitative Reduction in Short-Chain Fatty Acids, Especially Butyrate, Contributes to the Progression of Chronic Kidney Disease. Clin. Sci. 2019, 133, 1857–1870. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal Epithelial Cells: Regulators of Barrier Function and Immune Homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Nigam, S.K.; Bush, K.T. Uraemic Syndrome of Chronic Kidney Disease: Altered Remote Sensing and Signalling. Nat. Rev. Nephrol. 2019, 15, 301–316. [Google Scholar] [CrossRef]
- Matsuo, H.; Tsunoda, T.; Ooyama, K.; Sakiyama, M.; Sogo, T.; Takada, T.; Nakashima, A.; Nakayama, A.; Kawaguchi, M.; Higashino, T.; et al. Hyperuricemia in Acute Gastroenteritis Is Caused by Decreased Urate Excretion via ABCG2. Sci. Rep. 2016, 6, 31003. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ning, X.; Liu, B.; Dong, R.; Bai, M.; Sun, S. Specific Alterations in Gut Microbiota in Patients with Chronic Kidney Disease: An Updated Systematic Review. Ren. Fail. 2021, 43, 102–112. [Google Scholar] [CrossRef]
- Van der Worp, H.B.; Howells, D.W.; Sena, E.S.; Porritt, M.J.; Rewell, S.; O’Collins, V.; Macleod, M.R. Can Animal Models of Disease Reliably Inform Human Studies? PLoS Med. 2010, 7, e1000245. [Google Scholar] [CrossRef] [PubMed]
- Belzung, C.; Lemoine, M. Criteria of Validity for Animal Models of Psychiatric Disorders: Focus on Anxiety Disorders and Depression. Biol. Mood Anxiety Disord. 2011, 1, 9. [Google Scholar] [CrossRef] [Green Version]
- Bebarta, V.; Luyten, D.; Heard, K. Emergency Medicine Animal Research: Does Use of Randomization and Blinding Affect the Results? Acad. Emerg. Med. 2003, 10, 684–687. [Google Scholar] [CrossRef]
- Becker, G.J.; Hewitson, T.D. Animal Models of Chronic Kidney Disease: Useful but Not Perfect. Nephrol. Dial. Transplant. 2013, 28, 2432–2438. [Google Scholar] [CrossRef] [PubMed]
- Treuting, P.M.; Valasek, M.A.; Dintzis, S.M. 11—Upper gastrointestinal tract. In Comparative Anatomy and Histology; Treuting, P.M., Dintzis, S.M., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2017; pp. 155–175. ISBN 978-0-12-381361-9. [Google Scholar]
- Kararli, T.T. Comparison of the Gastrointestinal Anatomy, Physiology, and Biochemistry of Humans and Commonly Used Laboratory Animals. Biopharm. Drug Dispos. 1995, 16, 351–380. [Google Scholar] [CrossRef]
- Horn, C.C.; Kimball, B.A.; Wang, H.; Kaus, J.; Dienel, S.; Nagy, A.; Gathright, G.R.; Yates, B.J.; Andrews, P.L.R. Why Can’t Rodents Vomit? A Comparative Behavioral, Anatomical, and Physiological Study. PLoS ONE 2013, 8, e60537. [Google Scholar] [CrossRef]
- Bo, T.-B.; Zhang, X.-Y.; Kohl, K.D.; Wen, J.; Tian, S.-J.; Wang, D.-H. Coprophagy Prevention Alters Microbiome, Metabolism, Neurochemistry, and Cognitive Behavior in a Small Mammal. ISME J. 2020, 14, 2625–2645. [Google Scholar] [CrossRef]
- Jarett, J.K.; Kingsbury, D.D.; Dahlhausen, K.E.; Ganz, H.H. Best Practices for Microbiome Study Design in Companion Animal Research. Front. Vet. Sci. 2021, 8, 644836. [Google Scholar] [CrossRef] [PubMed]
- Al-Asmakh, M.; Sohail, M.U.; Al-Jamal, O.; Shoair, B.M.; Al-Baniali, A.Y.; Bouabidi, S.; Nasr, S.; Bawadi, H. The Effects of Gum Acacia on the Composition of the Gut Microbiome and Plasma Levels of Short-Chain Fatty Acids in a Rat Model of Chronic Kidney Disease. Front. Pharmacol. 2020, 11, 569402. [Google Scholar] [CrossRef]
- Sueyoshi, M.; Fukunaga, M.; Mei, M.; Nakajima, A.; Tanaka, G.; Murase, T.; Narita, Y.; Hirata, S.; Kadowaki, D. Effects of Lactulose on Renal Function and Gut Microbiota in Adenine-Induced Chronic Kidney Disease Rats. Clin. Exp. Nephrol. 2019, 23, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Olauson, H.; Lindberg, K.; Amin, R.; Edvardsson, K.; Lindholm, B.; Andersson, G.; Wernerson, A.; Sabbagh, Y.; Schiavi, S.; et al. A Novel Model of Adenine-Induced Tubulointerstitial Nephropathy in Mice. BMC Nephrol. 2013, 14, 116. [Google Scholar] [CrossRef]
- Tirelle, P.; Breton, J.; Riou, G.; Déchelotte, P.; Coëffier, M.; Ribet, D. Comparison of Different Modes of Antibiotic Delivery on Gut Microbiota Depletion Efficiency and Body Composition in Mouse. BMC Microbiol. 2020, 20, 340. [Google Scholar] [CrossRef]
- Serbanescu, M.A.; Mathena, R.P.; Xu, J.; Santiago-Rodriguez, T.; Hartsell, T.L.; Cano, R.J.; Mintz, C.D. General Anesthesia Alters the Diversity and Composition of the Intestinal Microbiota in Mice. Anesth. Analg. 2019, 129, e126–e129. [Google Scholar] [CrossRef]
- Wang, F.; Meng, J.; Zhang, L.; Johnson, T.; Chen, C.; Roy, S. Morphine Induces Changes in the Gut Microbiome and Metabolome in a Morphine Dependence Model. Sci. Rep. 2018, 8, 3596. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Yuan, J.; Norris, K. Role of Urea in Intestinal Barrier Dysfunction and Disruption of Epithelial Tight Junction in CKD. Am. J. Nephrol. 2013, 37, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Fan, R.; Zhao, L.; Tong, C.; Qian, X.; Zhang, M.; Xiao, R.; Ma, W. Trans-Fatty Acids Alter the Gut Microbiota in High-Fat-Diet-Induced Obese Rats. Br. J. Nutr. 2020, 124, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 2018, 173, 1728–1741.e13. [Google Scholar] [CrossRef]
- Dias, M.F.; Reis, M.P.; Acurcio, L.B.; Carmo, A.O.; Diamantino, C.F.; Motta, A.M.; Kalapothakis, E.; Nicoli, J.R.; Nascimento, A.M.A. Changes in Mouse Gut Bacterial Community in Response to Different Types of Drinking Water. Water Res. 2018, 132, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Whipple, B.; Agar, J.; Zhao, J.; Pearce, D.A.; Kovács, A.D. The Acidified Drinking Water-Induced Changes in the Behavior and Gut Microbiota of Wild-Type Mice Depend on the Acidification Mode. Sci. Rep. 2021, 11, 2877. [Google Scholar] [CrossRef]
- Zhou, K.; Liu, W.; Chen, Z.; Yang, D.; Qiu, Z.; Feng, H.; Li, C.; Jin, M.; Li, J.; Xu, Q.; et al. The Effect of Different Drinking Water in Culture Medium on Feces Microbiota Diversity. J. Water Health 2020, 19, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Ambery, A.G.; Tackett, L.; Penque, B.A.; Hickman, D.L.; Elmendorf, J.S. Effect of Corncob Bedding on Feed Conversion Efficiency in a High-Fat Diet-Induced Prediabetic Model in C57Bl/6J Mice. J. Am. Assoc. Lab. Anim. Sci. 2014, 53, 449–451. [Google Scholar] [PubMed]
- Gregor, A.; Fragner, L.; Trajanoski, S.; Li, W.; Sun, X.; Weckwerth, W.; König, J.; Duszka, K. Cage Bedding Modifies Metabolic and Gut Microbiota Profiles in Mouse Studies Applying Dietary Restriction. Sci. Rep. 2020, 10, 20835. [Google Scholar] [CrossRef] [PubMed]
- Bidot, W.A.; Ericsson, A.C.; Franklin, C.L. Effects of Water Decontamination Methods and Bedding Material on the Gut Microbiota. PLoS ONE 2018, 13, e0198305. [Google Scholar] [CrossRef]
- Carter, R.L.; Lipman, N.S. Feed and bedding. In Management of Animal Care and Use Programs in Research, Education, and Testing; Weichbrod, R.H., Thompson, G.A. (Heidbrink), Norton, J.N., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Abingdon, UK, 2018; ISBN 978-1-4987-4844-5. [Google Scholar]
- Chen, S.; Zheng, Y.; Zhou, Y.; Guo, W.; Tang, Q.; Rong, G.; Hu, W.; Tang, J.; Luo, H. Gut Dysbiosis with Minimal Enteritis Induced by High Temperature and Humidity. Sci. Rep. 2019, 9, 18686. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Snijders, A.M.; Brislawn, C.J.; Stratton, K.G.; Zink, E.M.; Fansler, S.J.; Metz, T.O.; Mao, J.-H.; Jansson, J.K. Light-Stress Influences the Composition of the Murine Gut Microbiome, Memory Function, and Plasma Metabolome. Front. Mol. Biosci. 2019, 6, 108. [Google Scholar] [CrossRef] [PubMed]
- David, J.M.; Knowles, S.; Lamkin, D.M.; Stout, D.B. Individually Ventilated Cages Impose Cold Stress on Laboratory Mice: A Source of Systemic Experimental Variability. J. Am. Assoc. Lab. Anim. Sci. 2013, 52, 738–744. [Google Scholar] [PubMed]
- Deloris Alexander, A.; Orcutt, R.P.; Henry, J.C.; Baker, J.; Bissahoyo, A.C.; Threadgill, D.W. Quantitative PCR Assays for Mouse Enteric Flora Reveal Strain-Dependent Differences in Composition That Are Influenced by the Microenvironment. Mamm. Genome 2006, 17, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Beura, L.K.; Hamilton, S.E.; Bi, K.; Schenkel, J.M.; Odumade, O.A.; Casey, K.A.; Thompson, E.A.; Fraser, K.A.; Rosato, P.C.; Filali-Mouhim, A.; et al. Normalizing the Environment Recapitulates Adult Human Immune Traits in Laboratory Mice. Nature 2016, 532, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.R.; Schluter, J.; Coyte, K.Z.; Rakoff-Nahoum, S. The Evolution of the Host Microbiome as an Ecosystem on a Leash. Nature 2017, 548, 43–51. [Google Scholar] [CrossRef]
- Rosenberg, E.; Zilber-Rosenberg, I. The Hologenome Concept of Evolution after 10 Years. Microbiome 2018, 6, 78. [Google Scholar] [CrossRef]
- Van de Guchte, M.; Blottière, H.M.; Doré, J. Humans as Holobionts: Implications for Prevention and Therapy. Microbiome 2018, 6, 81. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).