Microcystin-LR in Primary Liver Cancers: An Overview
Abstract
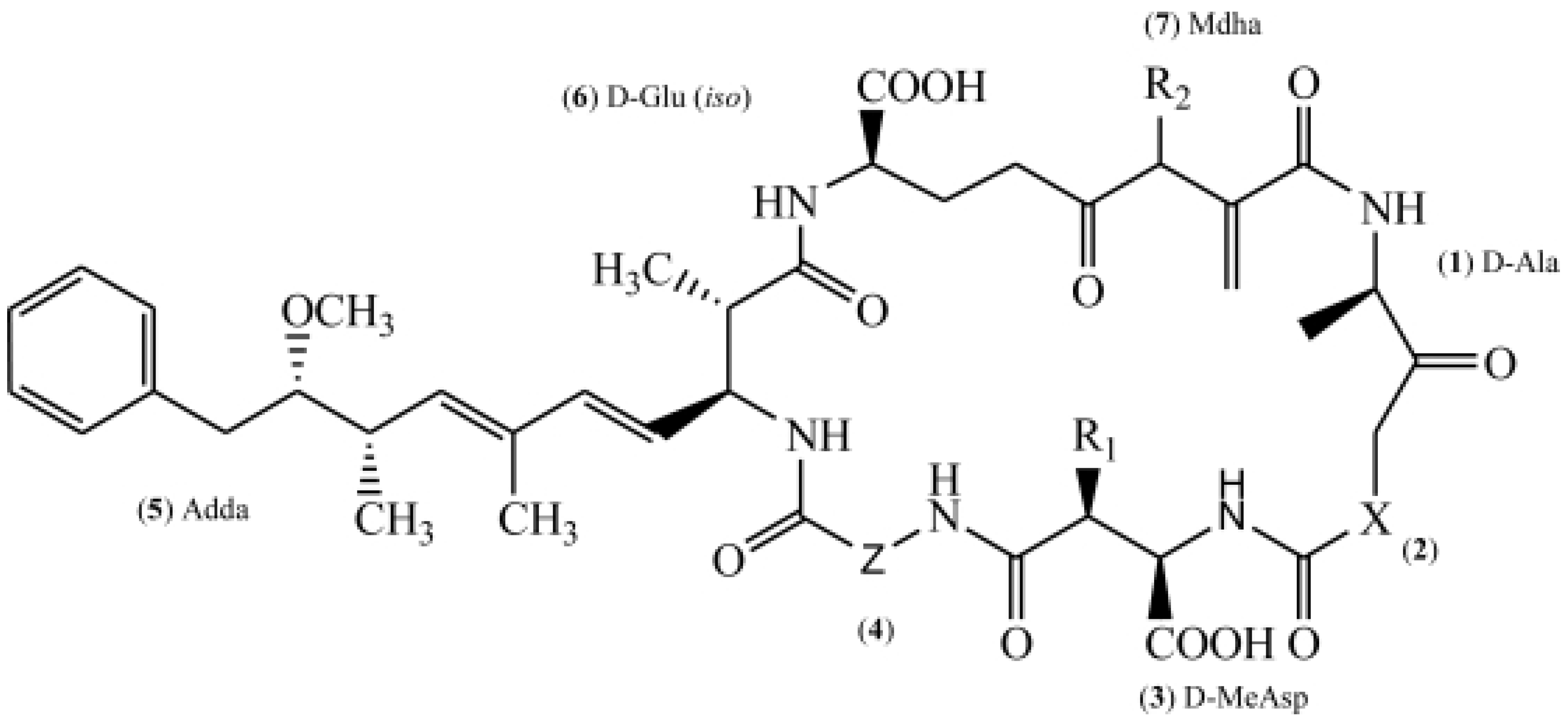
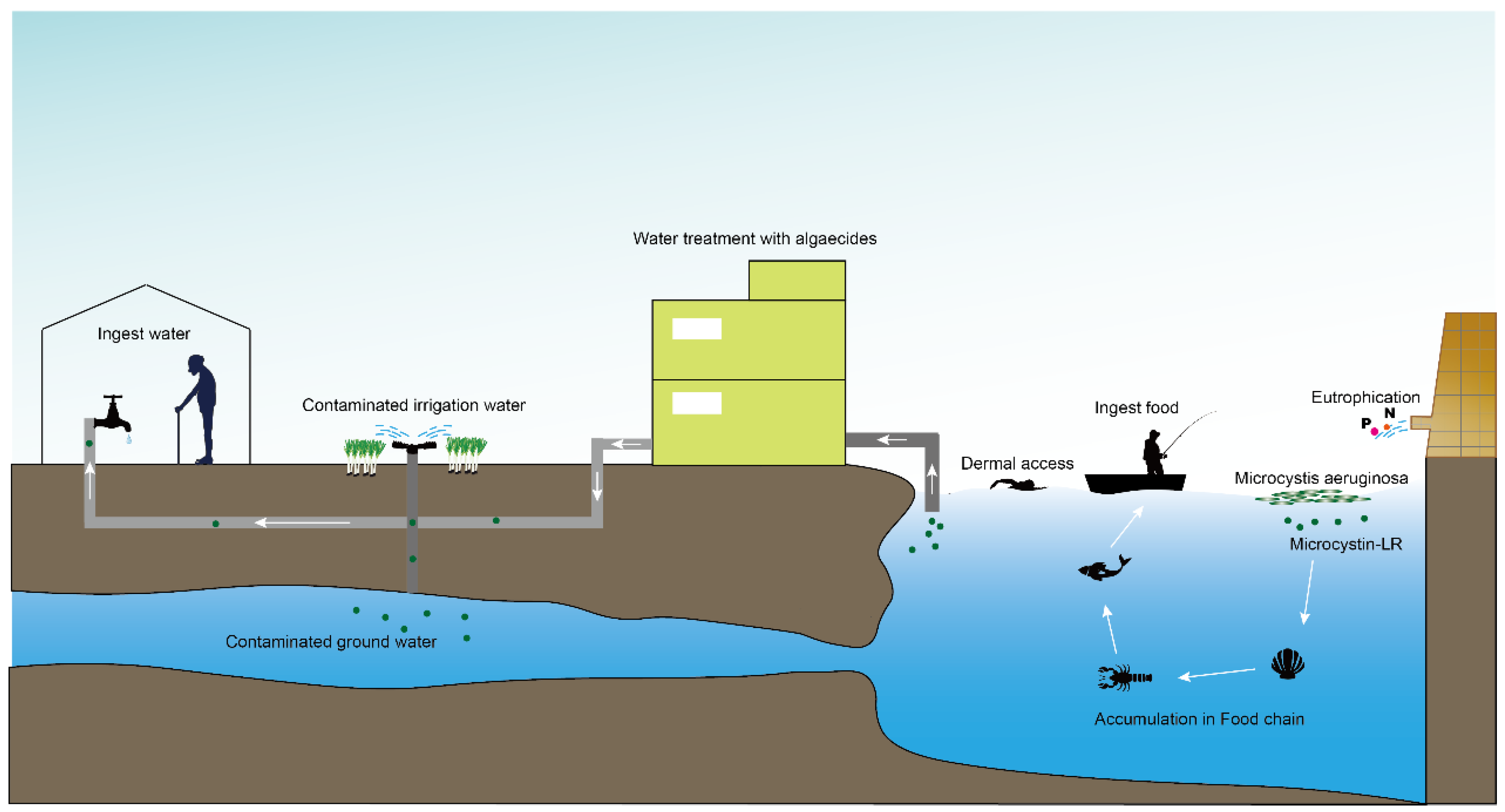
1. Introduction
2. Liver Fibrosis and Cirrhosis induced by Microcystin-LR
3. Microcystin-LR in Hepatocellular Carcinoma
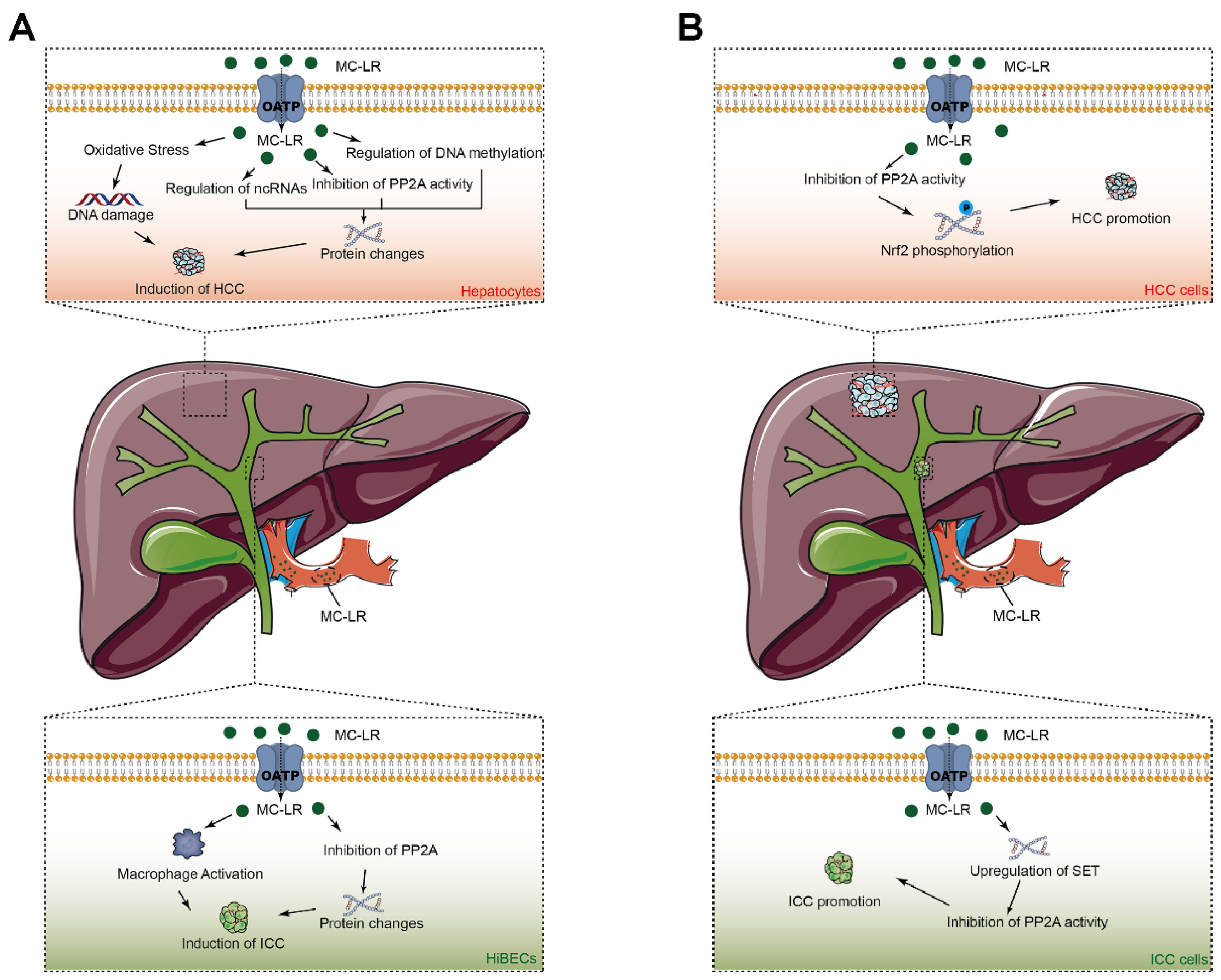
3.1. MC-LR in the Origin of HCC
3.1.1. Experimental Research
MC-LR Acts as an Inhibitor of Phosphatases 2A in Hepatocytes
MC-LR Induces Oxidative Stress in Hepatocytes
MC-LR Influences Tumor-Associated Non-Coding RNAs in Hepatocytes
MC-LR Induces DNA Methylation in Hepatocytes
3.1.2. Clinical and Epidemiological Research
3.2. MC-LR in the Prognosis of HCC
4. Microcystin-LR in Intrahepatic Cholangiocarcinoma
4.1. MC-LR in the Origin of ICC
4.2. MC-LR in the Prognosis of ICC
5. Summary
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melaram, R.; Newton, A.R.; Chafin, J. Microcystin Contamination and Toxicity: Implications for Agriculture and Public Health. Toxins 2022, 14, 350. [Google Scholar] [CrossRef] [PubMed]
- Codd, G.A.; Bell, S.G.; Brooks, W.P. Cyanobacterial Toxins in Water. Water Science and Technology 1989, 21, 1–13. [Google Scholar] [CrossRef]
- Kenefick, S.L.; Hrudey, S.E.; Peterson, H.G.; Prepas, E.E. Toxin Release from Microcystis Aeruginosa after Chemical Treatment. Water Science and Technology 1993, 27, 433–440. [Google Scholar] [CrossRef]
- del Campo, F.F.; Ouahid, Y. Identification of microcystins from three collection strains of Microcystis aeruginosa. Environ. Pollut. 2010, 158, 2906–2914. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.A.; Banack, S.A.; Murch, S.J.; Rasmussen, U.; Tien, G.; Bidigare, R.R.; Metcalf, J.S.; Morrison, L.F.; Codd, G.A.; Bergman, B. Diverse taxa of cyanobacteria produce beta-N-methylamino-L-alanine, a neurotoxic amino acid. Proc. Natl. Acad. Sci. U S A 2005, 102, 5074–5078. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Giesy, J.P.; Adamovsky, O.; Svircev, Z.; Meriluoto, J.; Codd, G.A.; Mijovic, B.; Shi, T.; Tuo, X.; Li, S.C.; et al. Challenges of using blooms of Microcystis spp. in animal feeds: A comprehensive review of nutritional, toxicological and microbial health evaluation. Sci. Total Environ. 2021, 764, 142319. [Google Scholar] [CrossRef]
- Bouaicha, N.; Miles, C.O.; Beach, D.G.; Labidi, Z.; Djabri, A.; Benayache, N.Y.; Nguyen-Quang, T. Structural Diversity, Characterization and Toxicology of Microcystins. Toxins 2019, 11, 714. [Google Scholar] [CrossRef]
- Campos, A.; Vasconcelos, V. Molecular mechanisms of microcystin toxicity in animal cells. Int. J. Mol. Sci. 2010, 11, 268–287. [Google Scholar] [CrossRef]
- Puerto, M.; Pichardo, S.; Jos, A.; Camean, A.M. Comparison of the toxicity induced by microcystin-RR and microcystin-YR in differentiated and undifferentiated Caco-2 cells. Toxicon 2009, 54, 161–169. [Google Scholar] [CrossRef]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef]
- de la Cruz, A.A.; Antoniou, M.G.; Hiskia, A.; Pelaez, M.; Song, W.; O′Shea, K.E.; He, X.; Dionysiou, D.D. Can we effectively degrade microcystins?--Implications on human health. Anticancer Agents Med. Chem. 2011, 11, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Niu, X.; Zhang, D.; Song, X.; Li, Y.; Ma, J.; Lai, S.; Yang, Z.; Zhou, S. The behaviors of Microcystis aeruginosa and microcystins during the Fe(2+)/persulfate (PS) preoxidation-coagulation and flocs storage period. Environ. Res. 2020, 186, 109549. [Google Scholar] [CrossRef] [PubMed]
- AlKahtane, A.A.; Abushouk, A.I.; Mohammed, E.T.; M, A.L.; Alarifi, S.; Ali, D.; Alessia, M.S.; Almeer, R.S.; AlBasher, G.; Alkahtani, S.; et al. Fucoidan alleviates microcystin-LR-induced hepatic, renal, and cardiac oxidative stress and inflammatory injuries in mice. Environ. Sci. Pollut. Res. Int. 2020, 27, 2935–2944. [Google Scholar] [CrossRef] [PubMed]
- Nishiwaki-Matsushima, R.; Ohta, T.; Nishiwaki, S.; Suganuma, M.; Kohyama, K.; Ishikawa, T.; Carmichael, W.W.; Fujiki, H. Liver tumor promotion by the cyanobacterial cyclic peptide toxin microcystin-LR. J. Cancer Res. Clin. Oncol. 1992, 118, 420–424. [Google Scholar] [CrossRef]
- Huynh-Delerme, C.; Edery, M.; Huet, H.; Puiseux-Dao, S.; Bernard, C.; Fontaine, J.J.; Crespeau, F.; de Luze, A. Microcystin-LR and embryo-larval development of medaka fish, Oryzias latipes. I. Effects on the digestive tract and associated systems. Toxicon 2005, 46, 16–23. [Google Scholar] [CrossRef]
- Cao, L.; Huang, F.; Massey, I.Y.; Wen, C.; Zheng, S.; Xu, S.; Yang, F. Effects of Microcystin-LR on the Microstructure and Inflammation-Related Factors of Jejunum in Mice. Toxins 2019, 11, 482. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Sayed, A.A.; Abdeen, A.; Aleya, L.; Ali, D.; Alkahtane, A.A.; Alarifi, S.; Alkahtani, S. Piperine Enhances the Antioxidant and Anti-Inflammatory Activities of Thymoquinone against Microcystin-LR-Induced Hepatotoxicity and Neurotoxicity in Mice. Oxid. Med. Cell Longev. 2019, 2019, 1309175. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Zhang, C.; Xiang, Z.; Ding, J.; Han, X. Learning and memory deficits and alzheimer′s disease-like changes in mice after chronic exposure to microcystin-LR. J. Hazard. Mater. 2019, 373, 504–518. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, L.; Hou, J.; Ma, T.; Pan, C.; Zhou, Y.; Han, R.; Ding, Y.; Peng, H.; Xiang, Z.; et al. The mechanisms in the altered ontogenetic development and lung-related pathology in microcystin-leucine arginine (MC-LR)-paternal-exposed offspring mice. Sci. Total Environ. 2020, 736, 139678. [Google Scholar] [CrossRef]
- Li, X.; Xu, L.; Zhou, W.; Zhao, Q.; Wang, Y. Chronic exposure to microcystin-LR affected mitochondrial DNA maintenance and caused pathological changes of lung tissue in mice. Environ. Pollut. 2016, 210, 48–56. [Google Scholar] [CrossRef]
- Wang, C.; Gu, S.; Yin, X.; Yuan, M.; Xiang, Z.; Li, Z.; Cao, H.; Meng, X.; Hu, K.; Han, X. The toxic effects of microcystin-LR on mouse lungs and alveolar type II epithelial cells. Toxicon 2016, 115, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Ait Abderrahim, L.; Taibi, K.; Boussaid, M.; Al-Shara, B.; Ait Abderrahim, N.; Ait Abderrahim, S. Allium sativum mitigates oxidative damages induced by Microcystin-LR in heart and liver tissues of mice. Toxicon 2021, 200, 30–37. [Google Scholar] [CrossRef]
- Shu, Y.; Jiang, H.; Yuen, C.N.T.; Wang, W.; He, J.; Zhang, H.; Liu, G.; Wei, L.; Chen, L.; Wu, H. Microcystin-leucine arginine induces skin barrier damage and reduces resistance to pathogenic bacteria in Lithobates catesbeianus tadpoles. Ecotoxicol. Environ. Saf. 2022, 238, 113584. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Zhang, Q.; Xiang, Z.; Li, D.; Han, X. Microcystin-leucine arginine exhibits immunomodulatory roles in testicular cells resulting in orchitis. Environ. Pollut. 2017, 229, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Zhang, L.; Gan, W.; Fu, K.; Jiang, K.; Ding, J.; Wu, J.; Han, X.; Li, D. piRNA-DQ722010 contributes to prostate hyperplasia of the male offspring mice after the maternal exposed to microcystin-leucine arginine. Prostate 2019, 79, 798–812. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhao, X.; Guo, M.; Zhu, J.; Li, D.; Ding, J.; Han, X.; Wu, J. Microcystin-leucine arginine (MC-LR) induces mouse ovarian inflammation by promoting granulosa cells to produce inflammatory cytokine via activation of cGAS-STING signaling. Toxicol. Lett. 2022, 358, 6–16. [Google Scholar] [CrossRef]
- Pan, C.; Zhang, L.; Meng, X.; Qin, H.; Xiang, Z.; Gong, W.; Luo, W.; Li, D.; Han, X. Chronic exposure to microcystin-LR increases the risk of prostate cancer and induces malignant transformation of human prostate epithelial cells. Chemosphere 2021, 263, 128295. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, M.; Chen, M.; Zhu, Q.; Zhou, L.; Qin, W.; Wang, T. Microcystin-LR promotes epithelial-mesenchymal transition in colorectal cancer cells through PI3-K/AKT and SMAD2. Toxicol. Lett. 2017, 265, 53–60. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.X.; Qin, W.; Xu, L.; Wang, T.; Cheng, S.; Yang, L. Effects of microcystin-LR exposure on matrix metalloproteinase-2/-9 expression and cancer cell migration. Ecotoxicol. Environ. Saf. 2012, 77, 88–93. [Google Scholar] [CrossRef]
- Ding, W.; Shangguan, Y.; Zhu, Y.; Sultan, Y.; Feng, Y.; Zhang, B.; Liu, Y.; Ma, J.; Li, X. Negative impacts of microcystin-LR and glyphosate on zebrafish intestine: Linked with gut microbiota and microRNAs? Environ. Pollut. 2021, 286, 117685. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.X.; Yang, T.W.; Yin, J.M.; Yang, P.X.; Kou, B.X.; Chai, M.Y.; Liu, X.N.; Chen, D.X. Progress and prospects of biomarkers in primary liver cancer (Review). Int. J. Oncol. 2020, 57, 54–66. [Google Scholar] [CrossRef]
- Gravitz, L. Liver cancer. Nature 2014, 516, S1. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; He, W.; Yan, M.; He, J.; Zhou, Q.; Yan, X.; Fu, X.; Chen, J.; Han, X.; Qiu, Y. Higher content of microcystin-leucine-arginine promotes the survival of intrahepatic cholangiocarcinoma cells via regulating SET resulting in the poorer prognosis of patients. Cell Prolif. 2021, 54, e12961. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Yan, M.; Wang, C.; Meng, X.; Xiang, Z.; Qiu, Y.; Han, X. Microcystin-leucine-arginine induces liver fibrosis by activating the Hedgehog pathway in hepatic stellate cells. Biochem. Biophys. Res. Commun. 2020, 533, 770–778. [Google Scholar] [CrossRef]
- Clements, O.; Eliahoo, J.; Kim, J.U.; Taylor-Robinson, S.D.; Khan, S.A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J. Hepatol. 2020, 72, 95–103. [Google Scholar] [CrossRef]
- Labine, M.; Gong, Y.; Minuk, G.Y. Long-Term, Low-Dose Exposure to Microcystin-LR Does not Cause or Increase the Severity of Liver Disease in Rodents. Ann. Hepatol. 2017, 16, 959–965. [Google Scholar] [CrossRef]
- Sedan, D.; Laguens, M.; Copparoni, G.; Aranda, J.O.; Giannuzzi, L.; Marra, C.A.; Andrinolo, D. Hepatic and intestine alterations in mice after prolonged exposure to low oral doses of Microcystin-LR. Toxicon 2015, 104, 26–33. [Google Scholar] [CrossRef]
- Lad, A.; Su, R.C.; Breidenbach, J.D.; Stemmer, P.M.; Carruthers, N.J.; Sanchez, N.K.; Khalaf, F.K.; Zhang, S.; Kleinhenz, A.L.; Dube, P.; et al. Chronic Low Dose Oral Exposure to Microcystin-LR Exacerbates Hepatic Injury in a Murine Model of Non-Alcoholic Fatty Liver Disease. Toxins 2019, 11, 486. [Google Scholar] [CrossRef]
- Lad, A.; Hunyadi, J.; Connolly, J.; Breidenbach, J.D.; Khalaf, F.K.; Dube, P.; Zhang, S.; Kleinhenz, A.L.; Baliu-Rodriguez, D.; Isailovic, D.; et al. Antioxidant Therapy Significantly Attenuates Hepatotoxicity following Low Dose Exposure to Microcystin-LR in a Murine Model of Diet-Induced Non-Alcoholic Fatty Liver Disease. Antioxidants 2022, 11, 1625. [Google Scholar] [CrossRef]
- He, J.; Li, G.; Chen, J.; Lin, J.; Zeng, C.; Chen, J.; Deng, J.; Xie, P. Prolonged exposure to low-dose microcystin induces nonalcoholic steatohepatitis in mice: A systems toxicology study. Arch. Toxicol. 2017, 91, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Arman, T.; Baron, J.A.; Lynch, K.D.; White, L.A.; Aldan, J.; Clarke, J.D. MCLR-elicited hepatic fibrosis and carcinogenic gene expression changes persist in rats with diet-induced nonalcoholic steatohepatitis through a 4-week recovery period. Toxicology 2021, 464, 153021. [Google Scholar] [CrossRef] [PubMed]
- Banreti, A.; Lukacsovich, T.; Csikos, G.; Erdelyi, M.; Sass, M. PP2A regulates autophagy in two alternative ways in Drosophila. Autophagy 2012, 8, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Mumby, M. PP2A: Unveiling a reluctant tumor suppressor. Cell 2007, 130, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Possemato, R.; Campbell, K.T.; Plattner, C.A.; Pallas, D.C.; Hahn, W.C. Identification of specific PP2A complexes involved in human cell transformation. Cancer Cell 2004, 5, 127–136. [Google Scholar] [CrossRef]
- Baribault, H.; Blouin, R.; Bourgon, L.; Marceau, N. Epidermal growth factor-induced selective phosphorylation of cultured rat hepatocyte 55-kD cytokeratin before filament reorganization and DNA synthesis. J. Cell Biol. 1989, 109, 1665–1676. [Google Scholar] [CrossRef]
- Ohta, T.; Nishiwaki, R.; Yatsunami, J.; Komori, A.; Suganuma, M.; Fujiki, H. Hyperphosphorylation of cytokeratins 8 and 18 by microcystin-LR, a new liver tumor promoter, in primary cultured rat hepatocytes. Carcinogenesis 1992, 13, 2443–2447. [Google Scholar] [CrossRef]
- Liu, J.; Wang, B.; Huang, P.; Wang, H.; Xu, K.; Wang, X.; Xu, L.; Guo, Z. Microcystin-LR promotes cell proliferation in the mice liver by activating Akt and p38/ERK/JNK cascades. Chemosphere 2016, 163, 14–21. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Wang, B.; Chen, T.; Wang, X.; Huang, P.; Xu, L.; Guo, Z. Microcystin-LR promotes proliferation by activating Akt/S6K1 pathway and disordering apoptosis and cell cycle associated proteins phosphorylation in HL7702 cells. Toxicol. Lett. 2016, 240, 214–225. [Google Scholar] [CrossRef]
- Uchida, D.; Takaki, A.; Oyama, A.; Adachi, T.; Wada, N.; Onishi, H.; Okada, H. Oxidative Stress Management in Chronic Liver Diseases and Hepatocellular Carcinoma. Nutrients 2020, 12, 1576. [Google Scholar] [CrossRef]
- Bouaicha, N.; Maatouk, I.; Plessis, M.J.; Perin, F. Genotoxic potential of Microcystin-LR and nodularin in vitro in primary cultured rat hepatocytes and in vivo in rat liver. Environ. Toxicol. 2005, 20, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Doostdar, H.; Duthie, S.J.; Burke, M.D.; Melvin, W.T.; Grant, M.H. The influence of culture medium composition on drug metabolising enzyme activities of the human liver derived Hep G2 cell line. FEBS Lett. 1988, 241, 15–18. [Google Scholar] [CrossRef]
- Ma, J.; Li, Y.; Duan, H.; Sivakumar, R.; Li, X. Chronic exposure of nanomolar MC-LR caused oxidative stress and inflammatory responses in HepG2 cells. Chemosphere 2018, 192, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Nong, Q.; Komatsu, M.; Izumo, K.; Indo, H.P.; Xu, B.; Aoyama, K.; Majima, H.J.; Horiuchi, M.; Morimoto, K.; Takeuchi, T. Involvement of reactive oxygen species in Microcystin-LR-induced cytogenotoxicity. Free Radic. Res. 2007, 41, 1326–1337. [Google Scholar] [CrossRef]
- Sun, X.; Mi, L.; Liu, J.; Song, L.; Chung, F.L.; Gan, N. Sulforaphane prevents microcystin-LR-induced oxidative damage and apoptosis in BALB/c mice. Toxicol. Appl. Pharmacol. 2011, 255, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Duthie, S.J.; Collins, A.R. The influence of cell growth, detoxifying enzymes and DNA repair on hydrogen peroxide-mediated DNA damage (measured using the comet assay) in human cells. Free Radic. Biol. Med. 1997, 22, 717–724. [Google Scholar] [CrossRef]
- Floyd, R.A. The role of 8-hydroxyguanine in carcinogenesis. Carcinogenesis 1990, 11, 1447–1450. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.L.; Loeb, L.A. The contribution of endogenous sources of DNA damage to the multiple mutations in cancer. Mutat. Res. 2001, 477, 7–21. [Google Scholar] [CrossRef]
- Zegura, B.; Sedmak, B.; Filipic, M. Microcystin-LR induces oxidative DNA damage in human hepatoma cell line HepG2. Toxicon 2003, 41, 41–48. [Google Scholar] [CrossRef]
- Cheng, K.C.; Cahill, D.S.; Kasai, H.; Nishimura, S.; Loeb, L.A. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G----T and A----C substitutions. J. Biol. Chem. 1992, 267, 166–172. [Google Scholar] [CrossRef]
- Yan, H.; Bu, P. Non-coding RNA in cancer. Essays Biochem 2021, 65, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Christodoulou, F.; Raible, F.; Tomer, R.; Simakov, O.; Trachana, K.; Klaus, S.; Snyman, H.; Hannon, G.J.; Bork, P.; Arendt, D. Ancient animal microRNAs and the evolution of tissue identity. Nature 2010, 463, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, Y.; Yao, L.; Li, X. Analysis of MicroRNA Expression Profiling Involved in MC-LR-Induced Cytotoxicity by High-Throughput Sequencing. Toxins 2017, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Chen, X.; Ma, J.; Zhang, B.; Li, X. Aberrant Expressional Profiling of Known MicroRNAs in the Liver of Silver Carp (Hypophthalmichthys molitrix) Following Microcystin-LR Exposure Based on samllRNA Sequencing. Toxins 2020, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chen, L.; Wen, C.; Zhang, X.; Feng, X.; Yang, F. MicroRNA expression profiling involved in MC-LR-induced hepatotoxicity using high-throughput sequencing analysis. J. Toxicol Environ. Health A 2018, 81, 89–97. [Google Scholar] [CrossRef]
- El-Abd, N.E.; Fawzy, N.A.; El-Sheikh, S.M.; Soliman, M.E. Circulating miRNA-122, miRNA-199a, and miRNA-16 as Biomarkers for Early Detection of Hepatocellular Carcinoma in Egyptian Patients with Chronic Hepatitis C Virus Infection. Mol. Diagn. Ther. 2015, 19, 213–220. [Google Scholar] [CrossRef]
- Shen, S.; Lin, Y.; Yuan, X.; Shen, L.; Chen, J.; Chen, L.; Qin, L.; Shen, B. Biomarker MicroRNAs for Diagnosis, Prognosis and Treatment of Hepatocellular Carcinoma: A Functional Survey and Comparison. Sci. Rep. 2016, 6, 38311. [Google Scholar] [CrossRef]
- Xu, L.; Li, T.; Ding, W.; Cao, Y.; Ge, X.; Wang, Y. Combined seven miRNAs for early hepatocellular carcinoma detection with chronic low-dose exposure to microcystin-LR in mice. Sci. Total Environ. 2018, 628-629, 271–281. [Google Scholar] [CrossRef]
- Guo, J.U.; Agarwal, V.; Guo, H.; Bartel, D.P. Expanded identification and characterization of mammalian circular RNAs. Genome Biol. 2014, 15, 409. [Google Scholar] [CrossRef]
- Jeck, W.R.; Sharpless, N.E. Detecting and characterizing circular RNAs. Nat. Biotechnol. 2014, 32, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Wen, C.; Yang, S.; Yang, Y.; Yang, F. Circular RNA expression profiles following MC-LR treatment in human normal liver cell line (HL7702) cells using high-throughput sequencing analysis. J. Toxicol Environ. Health A 2019, 82, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Yang, S.; Zheng, S.; Feng, X.; Chen, J.; Yang, F. Analysis of long non-coding RNA profiled following MC-LR-induced hepatotoxicity using high-throughput sequencing. J. Toxicol Environ. Health A 2018, 81, 1165–1172. [Google Scholar] [CrossRef]
- Kondoh, N.; Wakatsuki, T.; Hada, A.; Shuda, M.; Tanaka, K.; Arai, M.; Yamamoto, M. Genetic and epigenetic events in human hepatocarcinogenesis. Int. J. Oncol. 2001, 18, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Baylin, S.B.; Herman, J.G. DNA hypermethylation in tumorigenesis: Epigenetics joins genetics. Trends Genet. 2000, 16, 168–174. [Google Scholar] [CrossRef]
- Chen, H.Q.; Zhao, J.; Li, Y.; He, L.X.; Huang, Y.J.; Shu, W.Q.; Cao, J.; Liu, W.B.; Liu, J.Y. Gene expression network regulated by DNA methylation and microRNA during microcystin-leucine arginine induced malignant transformation in human hepatocyte L02 cells. Toxicol. Lett. 2018, 289, 42–53. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, H.Q.; Yang, H.F.; Li, Y.; Chen, D.J.; Huang, Y.J.; He, L.X.; Zheng, C.F.; Wang, L.Q.; Wang, J.; et al. Epigenetic silencing of ALX4 regulates microcystin-LR induced hepatocellular carcinoma through the P53 pathway. Sci. Total Environ. 2019, 683, 317–330. [Google Scholar] [CrossRef]
- Yu, S.Z. Primary prevention of hepatocellular carcinoma. J. Gastroenterol. Hepatol. 1995, 10, 674–682. [Google Scholar] [CrossRef]
- Shunzhang, Y.; Gang, C. BLUE-GREEN ALGAE TOXINS AND LIVER CANCER. Chinese Journal of Cancer Research 1994, 01, 9–15. [Google Scholar] [CrossRef]
- Svircev, Z.; Krstic, S.; Miladinov-Mikov, M.; Baltic, V.; Vidovic, M. Freshwater cyanobacterial blooms and primary liver cancer epidemiological studies in Serbia. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 36–55. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zeng, H.; Lin, H.; Wang, J.; Feng, X.; Qiu, Z.; Chen, J.A.; Luo, J.; Luo, Y.; Huang, Y.; et al. Serum microcystin levels positively linked with risk of hepatocellular carcinoma: A case-control study in southwest China. Hepatology 2017, 66, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Gan, N.; Sun, X.; Song, L. Activation of Nrf2 by microcystin-LR provides advantages for liver cancer cell growth. Chem. Res. Toxicol. 2010, 23, 1477–1484. [Google Scholar] [CrossRef]
- Lei, F.; Lei, X.; Li, R.; Tan, H. Microcystin-LR in peripheral circulation worsens the prognosis partly through oxidative stress in patients with hepatocellular carcinoma. Clin. Exp. Med. 2019, 19, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Jesper, D.; Heyn, S.G.; Schellhaas, B.; Pfeifer, L.; Goertz, R.S.; Zopf, S.; Neurath, M.F.; Strobel, D. Effects of liver cirrhosis and patient condition on clinical outcomes in intrahepatic cholangiocarcinoma: A retrospective analysis of 156 cases in a single center. Eur. J. Gastroenterol. Hepatol. 2018, 30, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Sirica, A.E.; Gores, G.J. Desmoplastic stroma and cholangiocarcinoma: Clinical implications and therapeutic targeting. Hepatology 2014, 59, 2397–2402. [Google Scholar] [CrossRef]
- Yan, M.; Shen, G.; Zhou, Y.; Meng, X.; Han, X. The role of ERK-RSK signaling in the proliferation of intrahepatic biliary epithelial cells exposed to microcystin-leucine arginine. Biochem. Biophys. Res. Commun. 2020, 521, 492–498. [Google Scholar] [CrossRef]
- Yan, M.; Gu, S.; Pan, C.; Chen, Y.; Han, X. MC-LR-induced interaction between M2 macrophage and biliary epithelial cell promotes biliary epithelial cell proliferation and migration through regulating STAT3. Cell Biol. Toxicol. 2021, 37, 935–949. [Google Scholar] [CrossRef]
- Mavros, M.N.; Economopoulos, K.P.; Alexiou, V.G.; Pawlik, T.M. Treatment and Prognosis for Patients With Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg. 2014, 149, 565–574. [Google Scholar] [CrossRef]
- Sirica, A.E. The role of cancer-associated myofibroblasts in intrahepatic cholangiocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 9, 44–54. [Google Scholar] [CrossRef]
- Affo, S.; Yu, L.X.; Schwabe, R.F. The Role of Cancer-Associated Fibroblasts and Fibrosis in Liver Cancer. Annu Rev. Pathol. 2017, 12, 153–186. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, S.; Jiang, M.; Zhang, B. Microcystin-LR in Primary Liver Cancers: An Overview. Toxins 2022, 14, 715. https://doi.org/10.3390/toxins14100715
Gu S, Jiang M, Zhang B. Microcystin-LR in Primary Liver Cancers: An Overview. Toxins. 2022; 14(10):715. https://doi.org/10.3390/toxins14100715
Chicago/Turabian StyleGu, Shen, Mingxuemei Jiang, and Bo Zhang. 2022. "Microcystin-LR in Primary Liver Cancers: An Overview" Toxins 14, no. 10: 715. https://doi.org/10.3390/toxins14100715
APA StyleGu, S., Jiang, M., & Zhang, B. (2022). Microcystin-LR in Primary Liver Cancers: An Overview. Toxins, 14(10), 715. https://doi.org/10.3390/toxins14100715




