1. Introduction
Aquaculture production represents a significant source of animal protein to millions of people worldwide. According to the Food and Agriculture Organization of the United Nations [
1], the total world aquaculture production has risen to 51.4 million tons by volume and
$60.0 billion by value. The increase in the production of cultured fish has also led to a significant decrease in the landings of several capture fisheries, a direct consequence of the use of fish as the source of animal proteins in aquafeeds [
2,
3,
4]. To mitigate this negative trend, plant-based proteins are increasingly being used as sustainable alternatives to fish-meal-based proteins in aquafeeds [
5].
In Africa, the quality of the plant products used in fish feed formulations is said to be a limiting factor in the progressive increase in aquaculture productivity; in addition, these feed ingredients are, very often, ideal substrates for the growth of fungi, such as
Aspergillus spp. and
Fusarium spp. [
6,
7]. These fungi, under favorable conditions (that is, those generally present in the tropical regions of the world), may result in the synthesis of mycotoxins, such as aflatoxin B1 and fumonisin B1 [
4].
The aflatoxins are a group of mycotoxins produced by the blue-green molds,
Aspergillus flavus and
Aspergillus parasiticus [
8,
9]. These molds are common contaminants in the feed ingredients of agricultural origin (such as cotton seed, ground nut, maize, wheat, soya bean and the respective by-products from the agricultural processing of these commodities). The aflatoxins have also been reported in fish meal. Four major aflatoxins (AFB
1, AFB
2, AFG
1 and AFG
2) have been reported to be direct contaminants of feed ingredients and formulated agricultural and aquacultural feeds [
10,
11]. Of the aflatoxins, AFB
1 is reported to be the most prevalent, most potent and the most carcinogenic [
12,
13,
14], and has been classified as a group 1 carcinogen by the International Agency for Research on Cancer [
15].
Aflatoxicosis, a disease state caused by the effects of aflatoxins, has been noted to be common in aquaculture [
16]. According to [
17],
Oncorhynchus mykiss fed cotton seed meals contaminated with aflatoxins developed liver tumors and exhibited a mortality pattern of up to 85%. Other information available on the effects of AFB
1 in the cultivable species of fin and non-fin fishes, i.e.,
Oncorhynchus mykiss [
18];
Ictalurus punctatus [
19,
20];
Oreochromis niloticus [
21];
Labeo rohita [
22] and
Penaeus monodons [
23], suggests that fish exhibit a wide plasticity in the susceptibility to AFB
1, and that cold water species are more sensitive when compared to warm water fishes [
18,
20]. These species-specific differences in sensitivity to AFB
1 have been attributed to the differences in the metabolism of aflatoxin B1 in the liver and the affinity of AFB
1-derived metabolites to hepatic macromolecules [
14,
24].
Fusariumverticillioides, the mold primarily associated with the production of the fumonisins, is prevalent in hot–humid regions of the world, and its occurrence has been related to the presence of invading insects [
24,
25]. Though maize is most frequently contaminated by fumonisins, these mycotoxins have been found at high concentrations in wheat, asparagus, tea and cowpea [
26]. According to [
27], it is very difficult to obtain uncontaminated maize, even if the contamination level is not significant. In most investigations, FB
1 is the most prevalent toxin, with a co-occurrence of FB
2 and FB
3 [
25]. Several countries in Africa, North and South America, Asia and Europe have reported FB
1 in cereals at levels from 0.02 to 25.9 ng/kg, and FB
2 at levels from 0.05 to 11.3 ng/kg [
26,
28]. FB
1 is stable in acetonitrile-water (1:1), at food-processing temperatures and light, but unstable in methanol [
26].
Feed additives are used world-wide for many different reasons. Some help to cover the need for essential nutrients, and others help to increase animal performance, feed intake and thereby optimize feed utilization. The rare earth elements (REE), composed of about 15 elements with atomic numbers ranging from 57 (lanthanum) to 71 (lutetium), are a promising set of feed additives in animal production [
29,
30]. The REE are reported as being used as performance enhancers in animal production, without affecting the quality of the final produce [
31,
32].
The growth-promoting effects of REE are reported to be based on the type and concentration of the REE applied [
33,
34]. It is reported that the application of REE additives, with concentrations ranging from 100 to 200 mg/kg, in the diets of 40–50 days old piglets, significantly improved the daily body weight gain [
35]. It is reported that diets supplemented with mixtures of lanthanum chloride at 100, 200 and 300 mg/kg per diet improved the activities of proteinase, lipase and amylase in the liver and pancreas of the adults and fry of carp (
Cyprinus carpio) [
36]. Furthermore, dietary lanthanum at 75 mg/kg has been reported to result in a 2–5% increase in body weight and a 7% increase in the feed conversion ratio of piglets [
31].
Clarias gariepinus, also called the African sharp tooth fish, is widely farmed in the West African sub-region; this fish is cultured based on the aquafeeds produced from maize and soybean cake [
37,
38], resulting in the risk of inadvertent dietary AFB
1 and/or FB
1 exposures. In a previous study, we observed a marked decrease in weight gain following dietary exposures to mixtures of AFB
1 and FB
1, and reported the tolerable limits for dietary exposures to mixtures of AFB
1 and FB
1 in juvenile
Clarias gariepinus to be 17.6 μg AFB
1/kg and 24.5 mg FB
1/kg [
39].
The addition of rare earth metals to animal diets as growth promoters is considered to be a promising alternative to the use of antibiotics and other chemicals [
29,
40,
41]. There are reports on the potential for the use of lanthanum chloride (LC), either as immunostimulants and/or growth-promoters in agriculture, as well as in aquaculture [
31,
42]. The present study was set up to determine the effects of lanthanum chloride on the growth performance, hematology and serum chemistry of the juvenile
Clarias gariepinus, when fed diets contaminated with mixtures of aflatoxin B1 and fumonisin B1.
3. Discussion
The water quality parameters of the culture tanks in the present study were determined to be within the range recommended for the culture of clariid catfishes [
39]; thus, may not have contributed to the pathophysiological observations recorded in this study. The proximate and mycotoxin (AFB
1 + FB
1) content of the feed was not altered by the addition of the lanthanum chloride; furthermore, the AFB
1 and FB
1 content of the final feed was higher than the respective concentrations of the purified mycotoxins introduced into the diets at formulation. This is an expected result, as it has been previously noted that agricultural products are often contaminated with various mycotoxins, and that these mycotoxins occur at varying concentrations; hence, the difference in the AFB
1 and FB
1 contents of the produced diets reflects the concentrations of these mycotoxins in the agricultural materials used in the production of the feed [
9,
43].
The fish fed diet A (the control diet) consumed the most quantity of feed, while the fish fed diet B (19.7 µg AFB
1 + 28.5 mg FB
1/kg) consumed the lowest quantities of fed. This is an expected result, as mycotoxins, especially aflatoxins, are reported to cause a reduction in feeding, or an outright feed refusal, with a consequent decrease in the performance in the animals [
41,
44]. This agrees with the findings of [
32], who observed that dietary rare earth elements improve the body weight gain and feed conversion ratio, without increasing feed intake. The results of the present study also show that the fish fed diets containing 400 mg/kg lanthanum chloride consumed more feed compared with the fish fed 200 mg/kg lanthanum chloride; thus, indicating that the feed consumption increased with the dietary concentration of lanthanum chloride.
The fish fed the diets containing AFB
1 and FB
1 exhibited the lowest weight gain. This is similar to the findings of our earlier study [
39], where poor growth performance was recorded in the juvenile
Clarias gariepinus catfish when fed diets amended with doses of mixtures AFB
1 and FB
1. The aflatoxins are reported to cause gastrointestinal dysfunctions marked by significant changes in the gut morphology, reduced digestive ability and a disruption of the digestive enzymes and intestinal innate immunity [
23]. The fumonisins are reported to negatively influence growth performance by their abilities to interfere with cellular growth and cell–cell interactions [
20,
45]. The poor growth recorded for fish when fed diets containing mixtures of AFB
1 and FB
1 may be as a consequence of the combined activities of the two mycotoxins.
The fish fed diet D (400 mg/kg lanthanum chloride) exhibited a superior weight gain compared with the fish fed diet C (200 mg/kg lanthanum chloride), suggesting that the weight gain in the juvenile
Clarias gariepinus, fed diets contaminated with mixtures of aflatoxin B1 and fumonisin B1, was influenced by the concentration of the dietary inclusion of lanthanum chloride. The exact mechanisms of the growth promotion by lanthanum chloride are yet to be described. It is, however, reported that lanthanum chloride may promote weight gain in animals by improving the utilization of dietary nutrients, such as total energy, crude protein and fat [
46]. It is also reported that dietary lanthanum chloride increases the secretion of gastric juices in the exposed animals [
47]; thus, the increased weight gain observed in the present study may be a function of the increased activities of gastric enzymes in the exposed fish [
47] and, since the fish fed the diets containing lanthanum chloride at 400 mg/kg diet consumed more feed compared with those fed the diets containing lanthanum chloride at 200 mg/kg diet, it is therefore reasonable for them to gain better weight.
The fish fed diet B (19.7 µg AFB
1 + 28.5 mg FB
1/kg diet) exhibited the highest (3.268), and the lowest feed conversion ratio (30.60 ± 1.60) compared with the fish fed the other diets. This is a result of the deleterious effects of the mixed mycotoxins in the diets [
13,
40,
48,
49]. The feed conversion ratio and the feed conversion efficiency were significantly improved by the addition of the lanthanum chloride into the diets. The results of the present study further show that fish fed diet C (lanthanum chloride 200 mg/kg diet), exhibited the lowest (1.981 ± 0.07) feed conversion ratio. Hence, the lanthanum chloride at 200 mg/kg inclusion rates produced the best nutrient utilization in the juvenile
Clarias gariepinus fed diets contaminated with mixtures of aflatoxin B1 and fumonisin B1.
The evaluations of the hematological parameters of the fish are required in the physiological assessment of the effects of exposure to sub-chronic concentrations of contaminants [
50] and/or the physiological response to the dietary intake of essential nutrients [
51]. This is because the determination of the erythrocytes count, the hematocrit values, and the hemoglobin concentration in the fish aids in the assessment and prognostication of anemias [
52].
The results of the present study show significant decreases in the erythrocytes counts, the hematocrit values and the hemoglobin concentrations of the fish fed the diets contaminated with mixtures of AFB
1 and FB
1 compared with the corresponding values for the fish fed the control diets at days 7, 28 and 56 of the trial. This is similar to the findings of [
8], who reported a disruption in the protein digestion and absorption in Nile tilapia following dietary exposures to AFB
1.
There were no significant variations in the erythrocytes counts, the hematocrit values and the hemoglobin concentrations of the fish fed the low (200 mg/kg) or high (400 mg/kg) concentrations of lanthanum chloride; however, the fish fed the mycotoxin-contaminated diets amended with lanthanum chloride exhibited significantly higher erythrocytes counts, hematocrit values and hemoglobin concentrations compared with their corresponding values in the fish fed diets contaminated with the mixtures of AFB
1 + FB
1 only. This indicated that dietary lanthanum chloride may ameliorate the depression of erythropoiesis induced by dietary exposures to mixtures of AFB
1 and FB
1. This finding may be a result of the increases in feed consumption and improved utilization of dietary nutrients [
53,
54], or due to the anti-oxidative effects of lanthanum chloride, wherein lanthanum chloride is able to protect the oxidation of dietary fatty acids, such as omega-3 fatty acids, thereby making it more available and/or enhancing their absorption [
55].
There were significant and sustained leukocytopenia in the fish fed the diets contaminated with AFB
1 and FB
1 throughout the duration of the study. This agrees with the reports [
21,
45,
48,
49], where it was reported that the dietary mycotoxins elicit a suppression of the immune response of exposed animals. Furthermore, the fish fed the diets contaminated with AFB
1 and FB
1 and containing lanthanum chloride exhibited significantly higher leucocytes counts compared with the fish fed diets contaminated AFB
1 and FB
1 alone, indicating lanthanum chloride may have some ameliorative effect on the leucocytes counts of juvenile
Clarias gariepinus fed diets contaminated with mixtures of AFB
1 and FB
1. The mechanism for these immunoprotective effects may not be unconnected with the antioxidant activities of lanthanum chloride [
53,
55] and/or the increased nutrient absorption and utilization effects of lanthanum chloride [
56,
57].
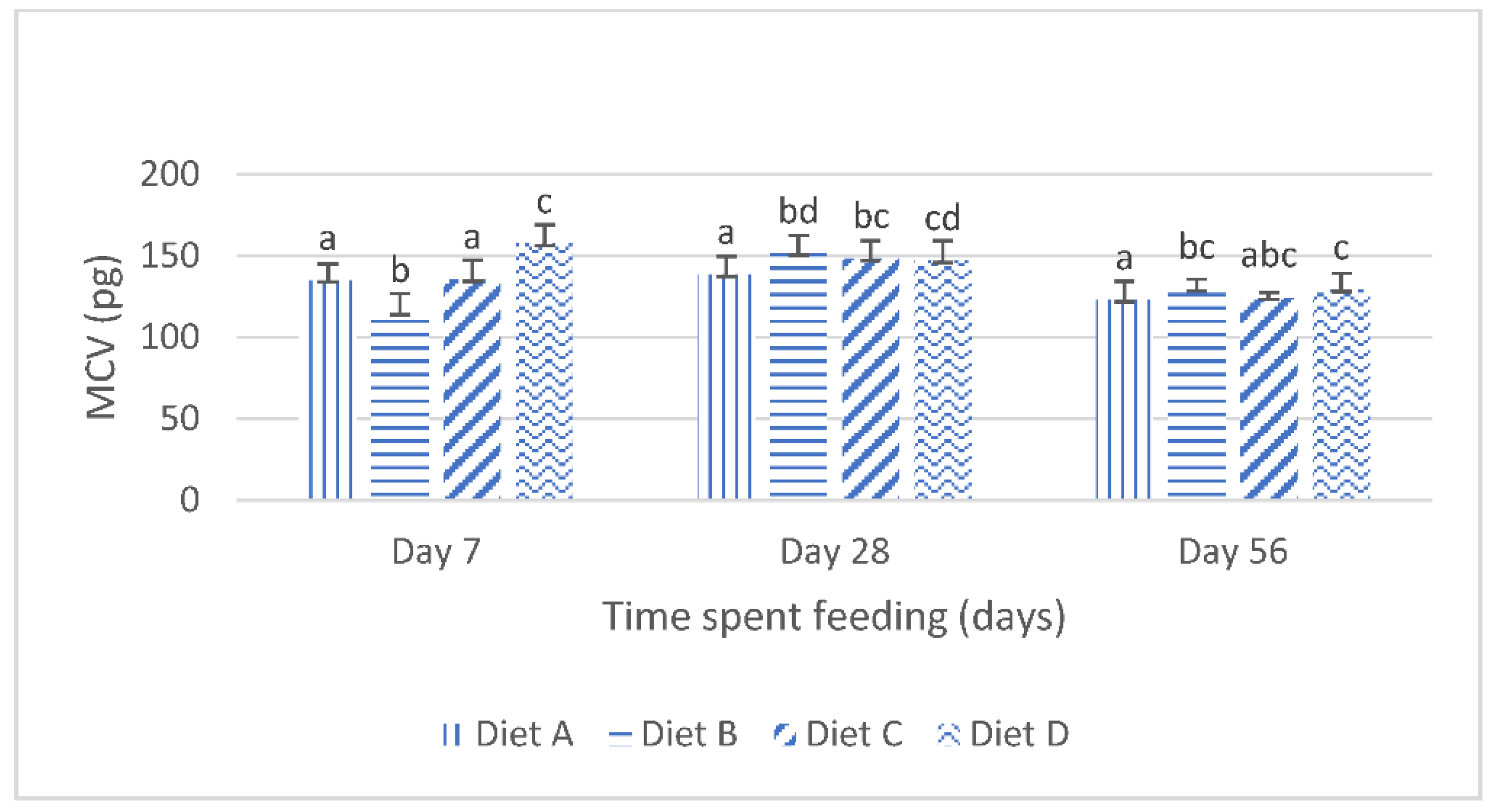
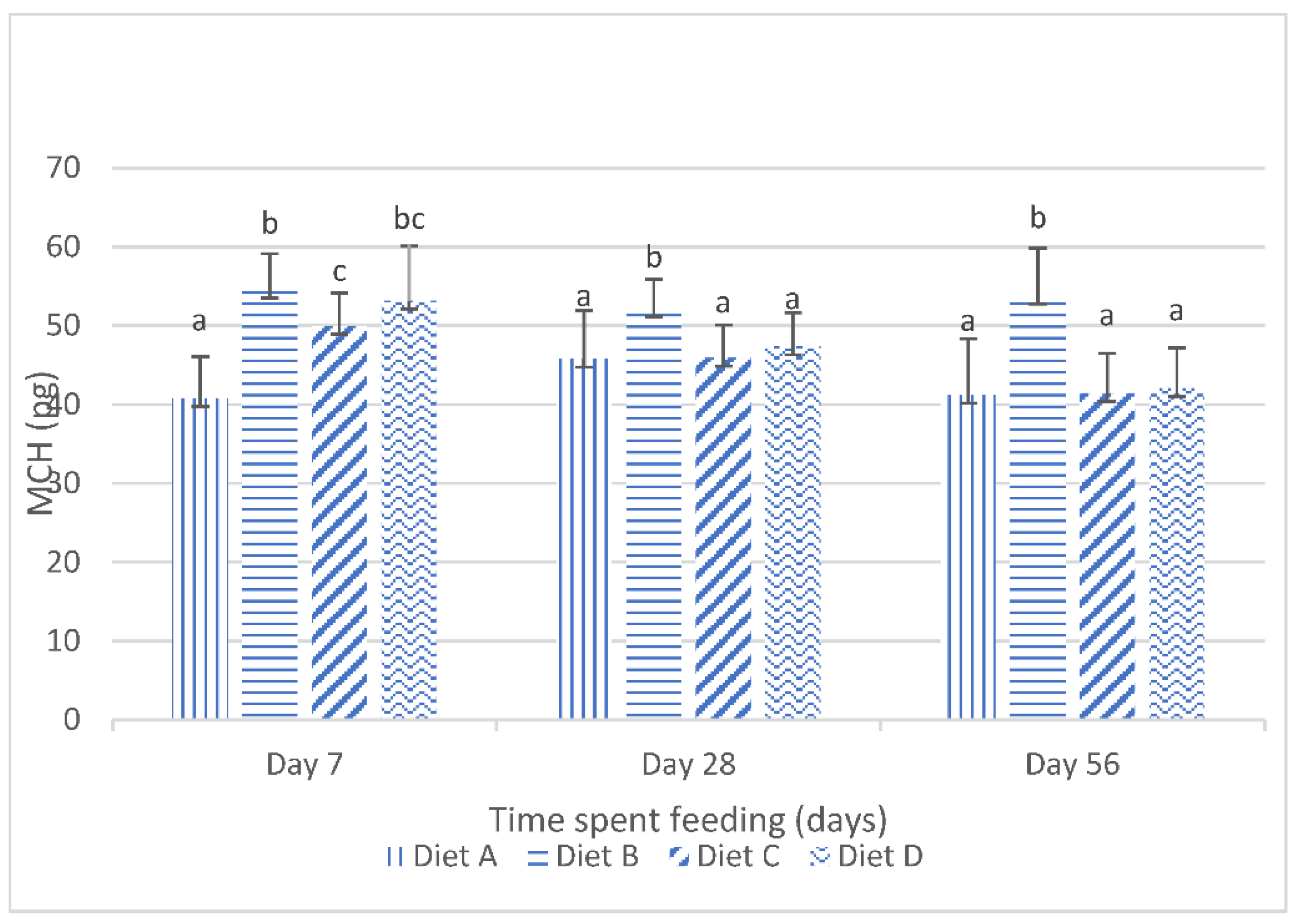
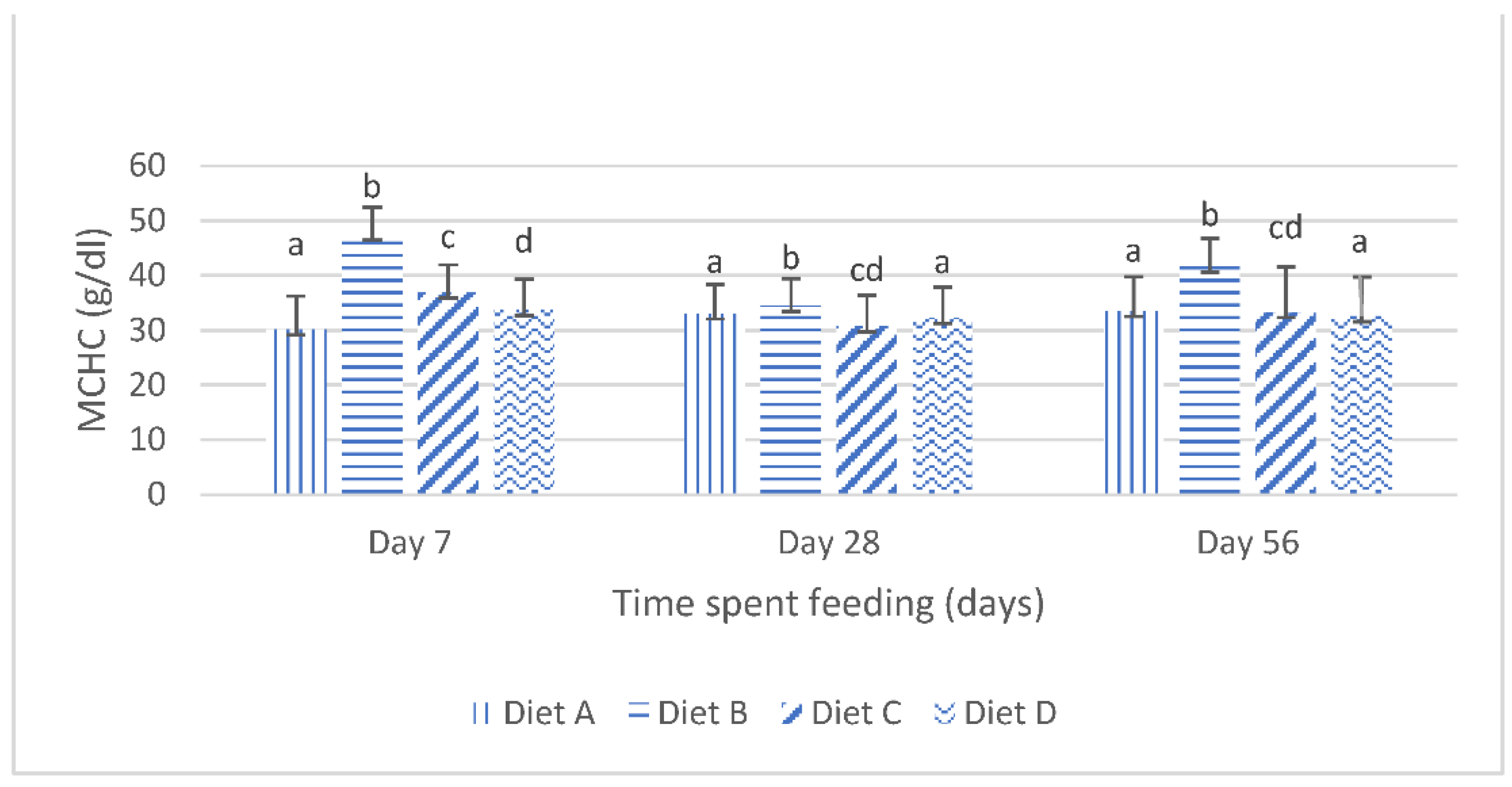
Although the results of the present study show that the dietary lanthanum chloride elicited significant changes in the erythrocytic indices (the mean corpuscular volume, the mean corpuscular hemoglobin values and the mean corpuscular hemoglobin concentration) of juvenile
Clarias gariepinus fed with the contaminated diets, the observed changes were well within the scope of the hematological reference intervals for juvenile
Clarias gariepinus [
39]. It is probable that these changes may have been more pronounced if the duration of the study had extended beyond the 56 days duration, as it is generally reported that the effects of dietary exposures to mycotoxins are dependent on the duration of the exposure and the concentration of the mycotoxins [
5,
13,
20].
The serum total proteins, consisting of the albumin and globulin concentrations, provide critical information reflecting the functional statuses of various organs and/or systems; since they are involved in the specific immune responses of the fish and participate in the maintenance of the acid-base balance [
58,
59], the serum proteins are also involved in the protection of the cellular integrity of cells, such as the erythrocytes, hepatocytes and the nephrocytes [
50]. The serum total proteins also provide an easy and readily available source of energy in emergencies, such as that obtained in situations of feed deprivation [
60,
61,
62].
The serum total proteins increase in cases of generalized chronic inflammation and in inflammatory disorders affecting the liver and the kidneys [
60]. The present study was marked by hyperproteinemia (observed 56 days post dietary exposure) in the fish fed the diets contaminated with mixtures of AFB
1 and FB
1; this is an expected result as both of the mycotoxins have been reported to elicit hepatic and nephrotic syndromes in exposed fish [
45,
63]. There were no significant differences in the serum total protein of the fish fed the diets amended with lanthanum chloride or bentonite clay. This may be the consequence of ingested free mycotoxins, especially of the fumonisins [
64].
The serum albumins are produced in the liver. Therefore, the synthetic capacity of the liver (which is an estimate of the protein losing nephropathy) may be estimated by the determination of the serum albumin concentration [
65]. According to [
66], malnutrition, increased protein catabolism, enteropathy and/or chronic nephropathy are marked by a reduced serum albumin concentration (termed hypoalbuminemia). The fish fed the diets contaminated with the mixtures of AFB
1 and FB
1 exhibited significantly elevated serum albumin concentrations. These may be a result of the combined effects of AFB
1 and FB
1 [
63]. As observed for the serum total proteins, the inclusion of the lanthanum chloride in the diets elicited a marginal but significant reduction in the serum albumin concentration compared with those of the fish fed the diets contaminated with only the mixtures of AFB
1 and FB
1. This may indicate that lanthanum chloride may have some hepatoprotective and nephroprotective properties in juvenile
Clarias gariepinus fed diets contaminated with mixtures of AFB
1 and FB
1.The hepatic enzymes (transaminase and alkaline phosphatase) are liberated into the serum in situations of hepatocellular or cholestatic liver injuries [
67]. In hepatopathies, such as those seen in hepatocellular degenerations, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are liberated into the serum, while alkaline phosphatase (ALP) is liberated into the serum in hepatic cholestasis [
66]. The results of the present study shows significant elevations in the serum activities of these enzymes in the fish fed the diets containing the mixtures of AFB
1 and FB
1. The highest values for the AST, ALT and ALP were obtained in the fish fed diet B (contaminated with AFB
1 and FB
1). The AST, ALT and the ALP activities were significantly lower in the fish fed the diets containing lanthanum chloride and bentonite clay, compared with the corresponding values in the fish fed AFB
1- and FB
1-contaminated diets only. The fumonisins are reported to cause cellular rupture and necrosis by the inhibition of mitochondrial respiration and the complete deregulation of calcium homeostasis [
68]; meanwhile, by its ability to preferentially bind to calcium, the lanthanum chloride may prevent tissue necrosis via this mechanism and, hence, reduce the elaboration of these enzymes into the serum, indicating some erythrocyte, hepatic and kidney protective effects of the dietary lanthanum chloride [
24,
69].
The serum creatinine concentration is increased significantly in the skeletal muscle necrosis and/or atrophy, as well as in chronic nephropathies [
58,
70]. In the present study, there were significant elevations of the serum creatinine concentration in the fish fed the diets containing the mixtures of AFB
1 and FB
1. The highest values for the serum creatinine concentration were obtained in the fish fed diet B (contaminated with AFB
1 and FB
1), indicating significant skeletal muscle necrosis and/or atrophy, as well as probable chronic nephropathy [
66]. The serum creatinine concentrations of the fish fed AFB
1 and FB
1-contaminated diets, containing lanthanum chloride or bentonite clay, were significantly lower compared with those of the fish only fed the AFB
1- and FB
1-contaminated diets. This may be indicative of some liver and kidney protective effects of the lanthanum chloride in the
Clarias gariepinus fed the diets contaminated with the mixtures of AFB
1 and FB
1 [
24,
71].
The serum urea nitrogen and the uric acid concentrations are critical analytes required in the assessments of the functional status of the kidney [
67]. It is reported that decreases in the blood urea nitrogen concentrations are usually observed in hepatic insufficiencies and in cases of malnutrition, while an increased blood urea nitrogen concentration is commonly reported in renal disease, shock and in cardiac insufficiencies [
71,
72]. The results obtained from the present study show the serum urea and uric acid concentrations of the fish fed the diets contaminated with the mixtures of AFB
1 and FB
1 were significantly higher than those of the fish fed the control diet, indicating a significant impact on the kidneys [
8,
41,
69]. The fish fed the diets contaminated with the mixtures of AFB
1 and FB
1 containing lanthanum chloride exhibited significantly lowered serum urea and uric acid concentrations compared to the fish fed the diets contaminated with only mixtures of AFB
1 and FB
1, indicating that the dietary lanthanum chloride in juvenile
Clarias gariepinus may have some ameliorating effects on the AFB
1- and FB
1-induced kidney toxicities [
70,
73].
Lactate dehydrogenase (LDH) is an enzyme found in several tissues/organs (such as the muscles, liver, heart, kidneys and the blood vessels. It catalyzes the reversible transformation of pyruvate into lactate [
74]. The increased serum activity of LDH is indicative of degenerative changes in any of the aforementioned tissues/organs [
66,
67]. The results of our study show that the serum LDH activity of the fish fed the diets contaminated with only mixtures of AFB
1 and FB
1 were significantly higher compared with those of the fish fed the control diet, indicating a significant impact on the kidneys and/or the other aforementioned organs [
67,
70]. The fish fed the diets contaminated with the mixtures of AFB
1 and FB
1 containing lanthanum chloride exhibited significantly lowered serum urea and uric acid concentrations compared to the fish fed the diets contaminated with only mixtures of AFB
1 and FB
1, indicating that the dietary lanthanum chloride in juvenile
Clarias gariepinus may have some ameliorating effects on the toxicities of the mixtures of AFB
1 and FB
1 [
40,
75].