The Effect of Foliar Fungicide and Insecticide Application on the Contamination of Fumonisins, Moniliformin and Deoxynivalenol in Maize Used for Food Purposes
Abstract
:1. Introduction
2. Results
2.1. Metereological Trends
2.2. Grain Yield
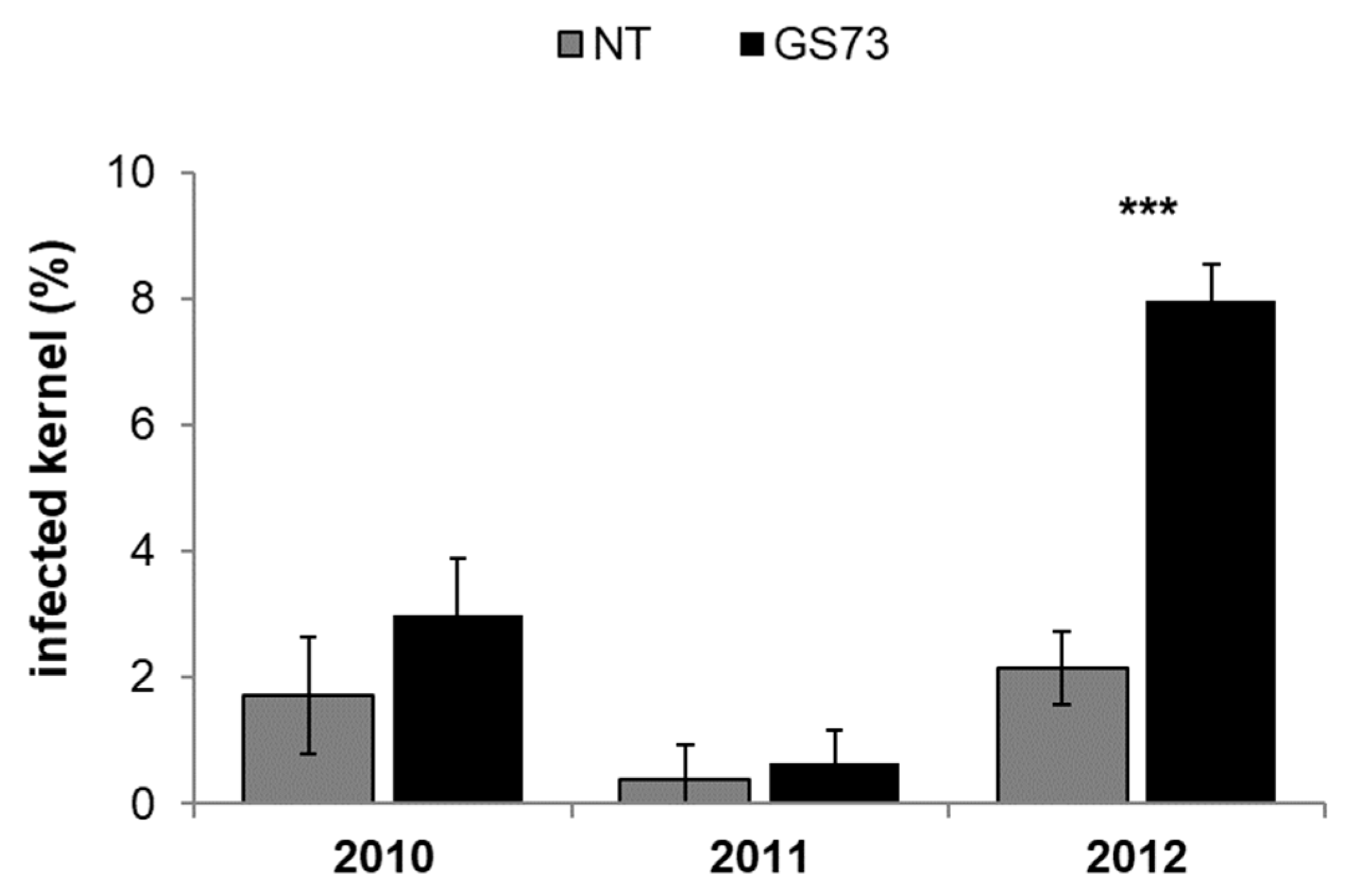
2.3. ECB and Fungal Ear Rot Symptoms and Fungal Infection
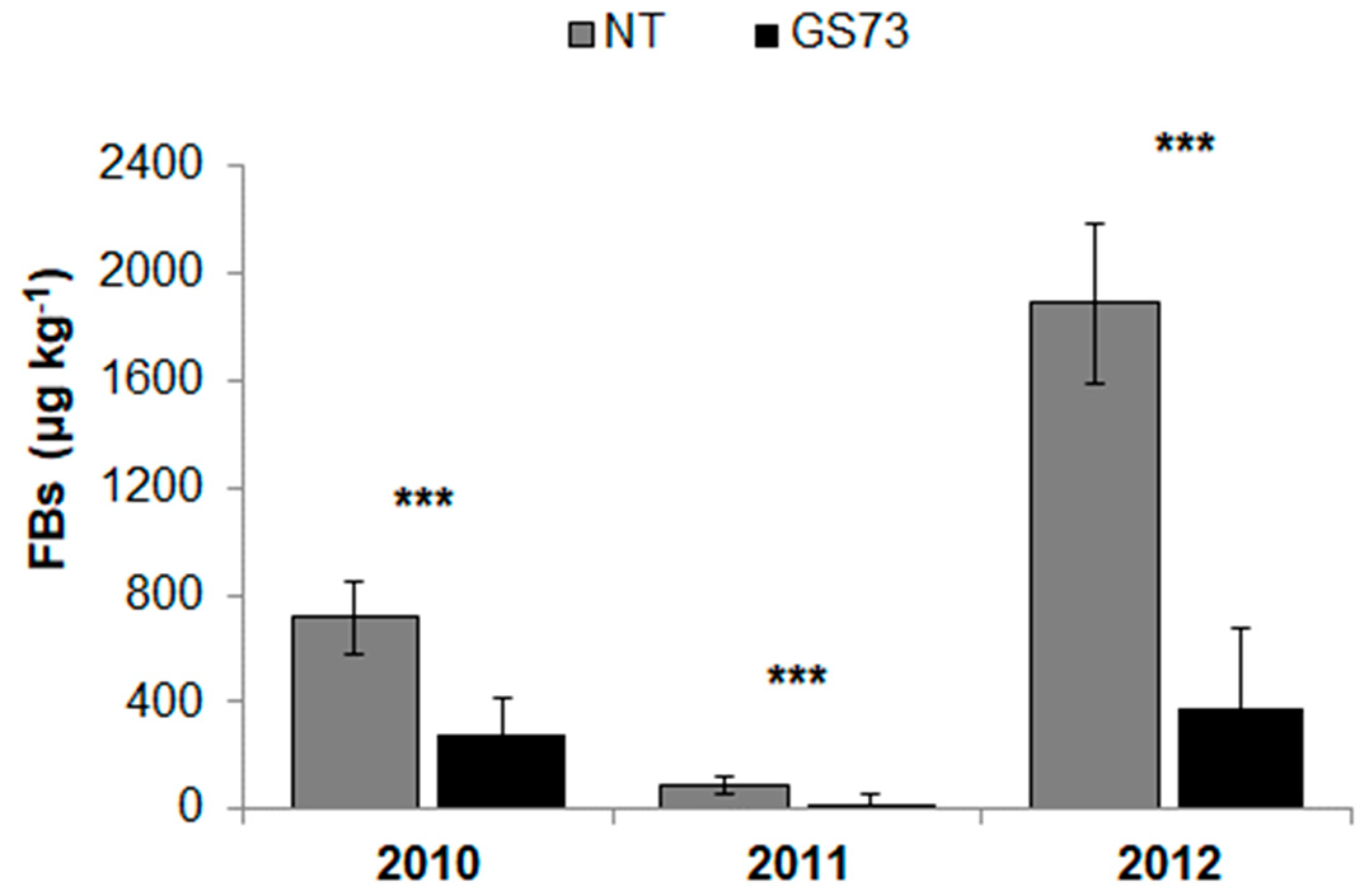
2.4. Mycotoxin Contamination
3. Discussion
4. Materials and Methods
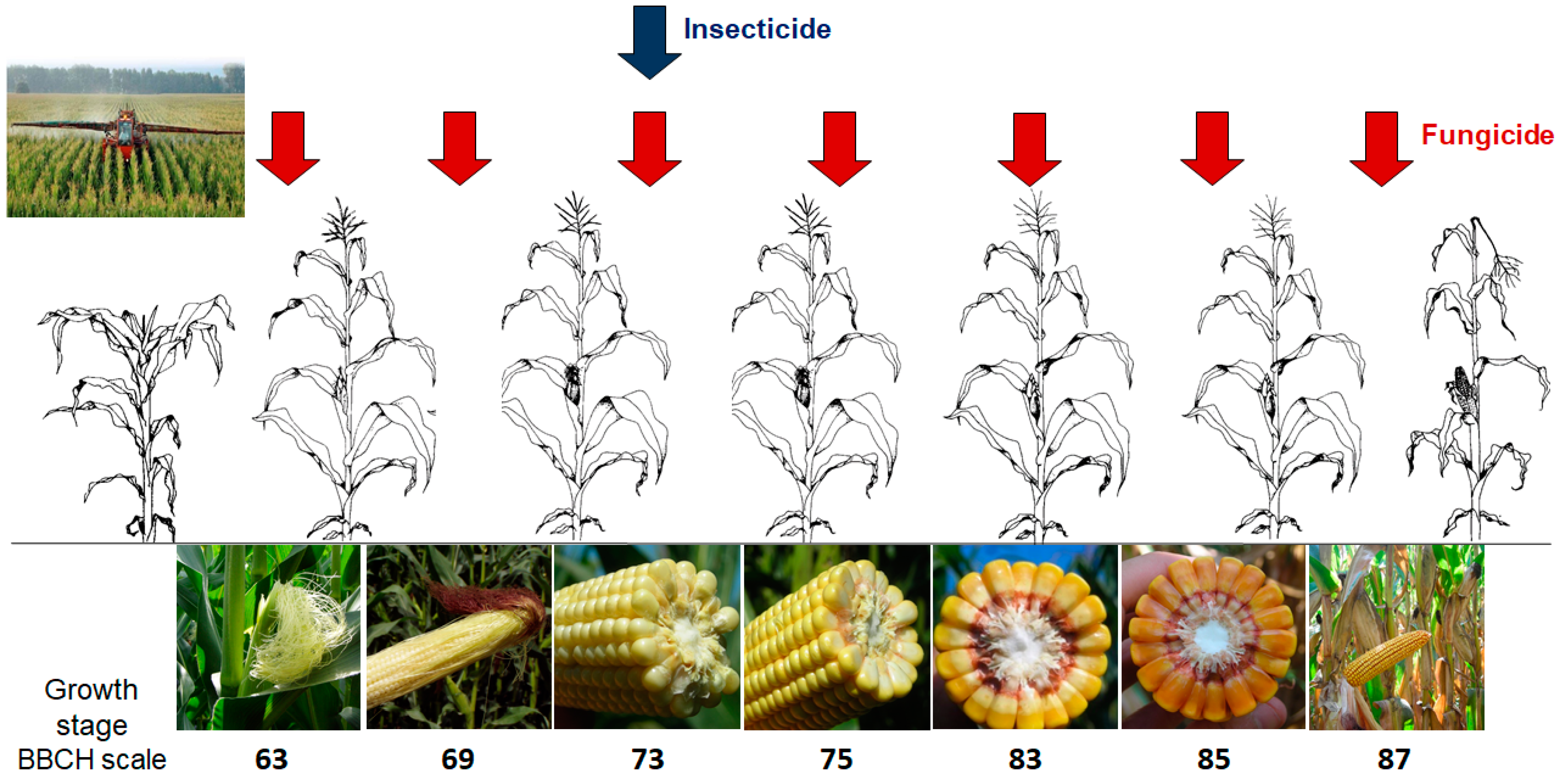
4.1. Experimental Design
- Seven fungicide application timings, which were compared with an untreated control (NT). The fungicide treatments were applied once at approximately 10-day intervals, starting from maize flowering (GS63), until physiological maturity (GS87), at the GS reported in Figure 4;
- An insecticide treatment, which was applied at the early milk stage (GS73) to minimize the ear injuries caused by ECB activity, and compared with an untreated control (NT).
4.2. Grain Yield
4.3. ECB and Fungal Ear Rot Symptoms and Fungal Infection
4.4. Mycotoxin Analysis
4.5. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. FAOSTAT Statistical Database. 2020. Available online: https://www.fao.org/faostat/en/#home (accessed on 11 May 2022).
- Pellegrini, N.; Agostoni, C. Nutritional aspects of gluten-free products. J. Sci. Food Agric. 2015, 95, 2380–2385. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.K.; Supriya, P. Value Addition in Maize. In Maize: Nutrition Dynamics and Novel Uses; Chaudhary, D., Kumar, S., Langyan, S., Eds.; Springer: New Delhi, India, 2014; pp. 141–152. [Google Scholar] [CrossRef]
- Munkvold, G. Crop management practices to minimize the risk of mycotoxins contamination in temperate-zone maize. In Mycotoxin Reduction in Grain Chains; Leslie, J.F., Logrieco, A.F., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2014; pp. 59–77. [Google Scholar]
- Battilani, P.; Toscano, P.; Van der Fels-Klerx, H.; Camardo Leggieri, M.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarpino, V.; Reyneri, A.; Sulyok, M.; Krska, R.; Blandino, M. Impact of the insecticide application to maize cultivated in different environmental conditions on emerging mycotoxins. Field Crops Res. 2018, 217, 188–198. [Google Scholar] [CrossRef]
- Commission regulation No. 1881/2006, of 10 December 2006 setting maximum levels for certain contaminants in food stuff. OJEU 2006, L364, 5–24. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:255:0014:0017:EN:PDF (accessed on 11 May 2022).
- Commission Regulation No 1126/2007 of 28 September 2007 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards Fusarium toxins in maize and maize products. OJEU 2006, L255, 14–17. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32006R1881 (accessed on 11 May 2022).
- U.S. Food and Drug Administration. Guidance for Industry: Fumonisin Levels in Human Foods and Animal Feeds; Final Guidance (6 June 2000; Revised 9 November 2001). 2001. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-fumonisin-levels-human-foods-and-animal-feeds (accessed on 11 May 2022).
- Scarpino, V.; Reyneri, A.; Vanara, F.; Scopel, C.; Causin, R.; Blandino, M. Relationship between European Corn Borer injury, Fusarium proliferatum and F. subglutinans infection and moniliformin contamination in maize. Field Crops Res. 2015, 183, 69–78. [Google Scholar] [CrossRef]
- Pfordt, A.; Ramos Romero, L.; Schiwek, S.; Karlovsky, P.; von Tiedemann, A. Impact of Environmental Conditions and Agronomic Practices on the Prevalence of Fusarium Species Associated with Ear- and Stalk Rot in Maize. Pathogens 2020, 9, 236. [Google Scholar] [CrossRef] [Green Version]
- Ma, N.; Abdul Haseeb, H.; Xing, F.; Su, Z.; Shan, L.; Guo, W. Fusarium avenaceum: A Toxigenic Pathogen Causing Ear Rot on Maize in Yunnan Province, China. Plant Dis. 2019, 103, 1424. [Google Scholar] [CrossRef]
- Jonsson, M.; Atosuo, J.; Jestoi, M.; Nathanail, A.V.; Kokkonen, U.-M.; Anttila, M.; Koivisto, P.; Lilius, E.-M.; Peltonen, K. Repeated Dose 28-Day Oral Toxicity Study of Moniliformin in Rats. Toxicol. Lett. 2015, 233, 38–44. [Google Scholar] [CrossRef]
- Blandino, M.; Reyneri, A.; Vanara, F.; Tamietti, G.; Pietri, A. Influence of agricultural practices on Fusarium infection, fumonisin and deoxynivalenol contamination of maize kernels. World Mycotoxin J. 2009, 2, 409–418. [Google Scholar] [CrossRef]
- Logrieco, A.; Battilani, P.; Camardo Leggieri, M.; Jiang, Y.; Haesaert, G.; Lanubile, A.; Mahuku, G.; Mesterházy, A.; Ortega-Beltran, A.; Pasti, M.; et al. Perspectives on Global Mycotoxin Issues and Management from the MycoKey Maize Working Group. Plant Dis. 2021, 105, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Blandino, M.; Reyneri, A.; Vanara, F.; Pascale, M.; Haidukowski, M.; Campagna, C. Management of fumonisin contamination in maize kernels through the timing of insecticide application against the European corn borer Ostrinia nubilalis Hübner. Food Addit. Contam. Part A 2009, 26, 1501–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folcher, L.; Jarry, M.; Weissenberger, A.; Gérault, F.; Eychenne, N.; Delos, M.; Regnault-Roger, C. Comparative activity of agrochemical treatments on mycotoxin levels with regard to corn borers and Fusarium mycoflora in maize (Zea mays L.) fields. Crop Prot. 2009, 28, 302–308. [Google Scholar] [CrossRef]
- Mazzoni, E.; Scandolara, A.; Giorni, P.; Pietri, A.; Battilani, P. Field control of Fusarium ear rot, Ostrinia nubilalis (Hübner), and fumonisins in maize kernels. Pest Manag. Sci. 2011, 67, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Blandino, M.; Scarpino, V.; Sulyok, M.; Krska, R.; Reyneri, A. Effect of agronomic programmes with different susceptibility to deoxynivalenol risk on emerging contamination in winter wheat. Eur. J. Agron. 2017, 85, 12–24. [Google Scholar] [CrossRef]
- Masiello, M.; Somma, S.; Ghionna, V.; Logrieco, A.F.; Moretti, A. In Vitro and in Field Response of Different Fungicides against Aspergillus flavus and Fusarium Species Causing Ear Rot Disease of Maize. Toxins 2019, 11, 11. [Google Scholar] [CrossRef] [Green Version]
- Limay-Rios, V.; Schaafsma, A.W. Effect of Prothioconazole Application Timing on Fusarium Mycotoxin Content in Maize Grain. J. Agric. Food Chem. 2018, 66, 4809–4819. [Google Scholar] [CrossRef]
- Scaglioni, P.; Blandino, M.; Scarpino, V.; Giordano, D.; Testa, G.; Badiale-Furlong, E. Application of fungicides and microalgal phenolic extracts for the direct control of fumonisin contamination in maize. J. Agric. Food Chem. 2018, 66, 4835–4841. [Google Scholar] [CrossRef]
- Eli, K.; Schaafsma, A.W.; Limay-Rios, V.; Hooker, D.C. Effect of pydiflumetofen on Gibberella ear rot and Fusarium mycotoxin accumulation in maize grain. World Mycotoxin J. 2021, 14, 495–512. [Google Scholar] [CrossRef]
- Blandino, M.; Galeazzi, M.; Savoia, W.; Reyneri, A. Timing of azoxystrobin + propiconazole application on maize to control Northern Corn Leaf Blight and maximize grain yield. Field Crops Res. 2012, 139, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Saladini, M.; Blandino, M.; Reyneri, A.; Alma, A. The impact of insecticide treatments on Ostrinia nubilalis (Hübner) (Lepidoptera: Crambidae) and their influence on the mycotoxin contamination of maize kernels. Pest Manag. Sci. 2008, 64, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Munkvold, G.P. Epidemiology of Fusarium diseases and their mycotoxins in maize ears. Eur. J. Plant Path. 2003, 109, 705–713. [Google Scholar] [CrossRef]
- Masoero, F.; Meschini, M.; Rossi, F.; Grandini, A.; Pietri, A. Nutritive value, mycotoxin contamination and in vitro rumen fermentation of normal and genetically modified corn (Cry1A(B)) grown in northern Italy. Maydica 1999, 44, 205–209. [Google Scholar]
- Folcher, L.; Delos, M.; Marengue, E.; Jarry, M.; Weissenberger, A.; Eychenne, N.; Regnault-Roger, C. Lower mycotoxin levels in Bt maize grain. Agron. Sustain. Dev. 2010, 30, 711–719. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Zhao, J.; Zhang, R.; Deng, L.; Li, J.; Gao, Y.; Liu, C. Effect of Tebuconazole Enantiomers and Environmental Factors on Fumonisin Accumulation and FUM Gene Expression in Fusarium verticillioides. J. Agric. Food Chem. 2018, 66, 13107–13115. [Google Scholar] [CrossRef] [PubMed]
- Miguel, T.A.; Bordini, J.G.; Saito, G.H.; Andrade, C.G.T.J.; Ono, M.A.; Hirooka, E.Y.; Vizoni, E.; Ono, E.Y.S. Effect of fungicide on Fusarium verticillioides mycelia morphology and fumonisin B1 production. Braz. J. Microbiol. 2015, 46, 293–299. [Google Scholar] [CrossRef] [Green Version]
- Doohan, F.M.; Weston, G.; Rezanoor, H.N.; Parry, D.W. Development and use of a reverse transcription—PCR assay to study the expression of tri5 by Fusarium species “in vitro” and “in planta”. Appl. Environ. Microbiol. 1999, 65, 3850–3854. [Google Scholar] [CrossRef] [Green Version]
- Paul, P.A.; Lipps, P.E.; Hershman, D.E.; McMullen, M.P.; Draper, M.A.; Madden, L.V. Efficacy of triazole-based fungicides for Fusarium Head Blight and deoxynivalenol control in wheat: A multivariate meta-analysis. Phytopathology 2008, 98, 999–1011. [Google Scholar] [CrossRef] [Green Version]
- Scarpino, V.; Reyneri, A.; Sulyok, M.; Krska, R.; Blandino, M. Effect of fungicide application to control Fusarium head blight and Fusarium and Alternaria mycotoxins in winter wheat (Triticum aestivum L.). World Mycotoxin J. 2015, 8, 499–510. [Google Scholar] [CrossRef]
- Andriolli, C.F.; Casa, R.T.; Kuhnem, P.R.; Kuhmen, P.R.; Bogo, A.; Luis Zancan, R.; Melo Reis, E. Timing of fungicide application for the control of Gibberella ear rot of maize. Trop. Plant Pathol. 2016, 41, 264–269. [Google Scholar] [CrossRef]
- Anderson, N.R.; Romero Luna, M.P.; Ravellette, J.D.; Wise, K.A. Impact of Foliar Fungicides on Gibberella Ear Rot and Deoxynivalenol Levels in Indiana Corn. Plant Health Prog. 2017, 18, 186–191. [Google Scholar] [CrossRef] [Green Version]
- Parker, N.S.; Anderson, N.R.; Richmond, D.S.; Long, E.Y.; Wise, K.A.; Krupke, C.H. Larval western bean cutworm feeding damage encourages the development of Gibberella ear rot on field corn. Pest Manag. Sci. 2017, 73, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Abdala, L.J.; Gerde, J.A.; Gambin, B.L.; Borrás, L. Fungicide Applications and Grain Dry Milling Quality in Late-Sown Maize. Crop Sci. 2018, 58, 892–899. [Google Scholar] [CrossRef]
- Small, I.M.; Flett, B.C.; Marasas, W.F.O.; McLeod, A.; Viljoen, A. Use of resistance elicitors to reduce Fusarium ear rot and fumonisin accumulation in maize. Crop Prot. 2012, 41, 10–16. [Google Scholar] [CrossRef]
- Janse van Rensburg, B.; Mc Laren, N.W.; Schoeman, A.; Flett, B.C. The effects of cultivar and prophylactic fungicide spray for leaf diseases on colonisation of maize ears by fumonisin producing Fusarium spp. and fumonisin synthesis in South Africa. Crop prot. 2016, 79, 56–63. [Google Scholar] [CrossRef]
- De Curtis, F.; De Cicco, V.; Haidukowski, M.; Pascale, M.; Somma, S.; Moretti, A. Effects of agrochemical treatments on the occurrence of Fusarium ear rot and fumonisin contamination of maize in Southern Italy. Field Crops Res. 2011, 123, 161–169. [Google Scholar] [CrossRef]
- Ferrigo, D.; Mondin, M.; Scopel, C.; Dal Maso, E.; Stefenatti, M.; Raiola, A.; Causin, R. Effects of a prothioconazole- and tebuconazole-based fungicide on Aspergillus flavus development under laboratory and field conditions. Eur. J. Plant Pathol. 2019, 155, 151–161. [Google Scholar] [CrossRef]
- Lagogianni, S.; Tsitsigiannis, D.I. Effective chemical management for prevention of aflatoxins in maize. Phytopathol. Mediterr. 2018, 57, 186–197. Available online: https://www.jstor.org/stable/26458747 (accessed on 11 May 2022).
- Lancashire, P.D.; Bleiholder, H.; Longelüddcke, P.; Stauss, R.; Van Den Boom, T.; Weber, E.; Witzenberger, A. An uniform decimal code for growth stages of crops and weeds. Ann. Appl. Biol. 1991, 119, 561–601. [Google Scholar] [CrossRef]
- Nelson, P.E.; Toussoun, T.A.; Marasas, W.F.O. Fusarium Species: An Illustrated Manual for Identification; Pennsylvania State University Press: University Park, PA, USA, 1983; p. 193. [Google Scholar]
- Scarpino, V.; Blandino, M.; Negre, M.; Reyneri, A.; Vanara, F. Moniliformin analysis in maize samples from North-West Italy using multifunctional clean-up columns and the LC-MS/MS detection method. Food. Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2013, 30, 876–884. [Google Scholar] [CrossRef] [Green Version]
- Sovrani, V.; Blandino, M.; Scarpino, V.; Reyneri, A.; Coisson, J.D.; Travaglia, F.; Locatelli, M.; Bordiga, M.; Montella, R.; Arlorio, M. Bioactive compound content, antioxidant activity, deoxynivalenol and heavy metal contamination of pearled wheat fractions. Food Chem. 2012, 135, 39–46. [Google Scholar] [CrossRef] [Green Version]

| Year | Month | Rainfall | Rainy Days | GDDs 1 |
|---|---|---|---|---|
| mm | n° | Σ °C−day | ||
| 2010 | March | 66 | 8 | 54 |
| April | 59 | 8 | 143 | |
| May | 202 | 10 | 205 | |
| June | 146 | 8 | 313 | |
| July | 10 | 1 | 439 | |
| August | 59 | 5 | 345 | |
| September | 55 | 4 | 235 | |
| October | 119 | 5 | 114 | |
| April–September | 530 | 36 | 1680 | |
| Flowering−Harvest | 158 | 11 | 867 | |
| 2011 | March | 211 | 11 | 68 |
| April | 53 | 5 | 198 | |
| May | 42 | 5 | 269 | |
| June | 157 | 11 | 303 | |
| July | 71 | 7 | 344 | |
| August | 17 | 2 | 411 | |
| September | 64 | 5 | 336 | |
| October | 22 | 2 | 169 | |
| April–September | 405 | 35 | 1861 | |
| Flowering−Harvest | 152 | 14 | 1025 | |
| 2012 | March | 26 | 2 | 148 |
| April | 155 | 11 | 120 | |
| May | 96 | 7 | 236 | |
| June | 48 | 6 | 365 | |
| July | 47 | 7 | 404 | |
| August | 20 | 3 | 423 | |
| September | 77 | 8 | 249 | |
| October | 112 | 5 | 148 | |
| April–September | 444 | 42 | 1797 | |
| Flowering−Harvest | 128 | 17 | 1065 |
| Factor | Source of | Grain | ECB | ECB | Ear rot | Ear rot | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variation | Yield | Incidence 1 | Severity 2 | Incidence 3 | Severity 4 | ||||||
| (t ha−1) | (%) | (%) | (%) | (%) | |||||||
| Insecticide (I) | NT | 15.0 | b | 65.2 | a | 15.1 | a | 65.6 | a | 8.4 | a |
| GS73 | 15.6 | a | 25.0 | b | 3.9 | b | 30.3 | b | 2.7 | b | |
| p-value | 0.006 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
| SEM | 1.6 | 13.2 | 5.2 | 13.8 | 3.5 | ||||||
| Fungicide (F) | NT | 15.2 | a | 48.8 | a | 10.5 | a | 50.1 | a | 5.9 | a |
| GS63 | 15.6 | a | 47.7 | a | 10.2 | a | 50.9 | a | 6.0 | a | |
| GS69 | 15.4 | a | 44.5 | a | 9.1 | a | 45.8 | a | 5.0 | a | |
| GS73 | 15.3 | a | 43.1 | a | 8.7 | a | 45.2 | a | 5.5 | a | |
| GS75 | 15.3 | a | 44.4 | a | 9.7 | a | 47.5 | a | 6.1 | a | |
| GS83 | 15.0 | a | 43.1 | a | 8.6 | a | 47.0 | a | 5.2 | a | |
| GS85 | 15.2 | a | 44.0 | a | 9.0 | a | 48.8 | a | 5.1 | a | |
| GS87 | 15.7 | a | 45.2 | a | 10.3 | a | 48.1 | a | 5.9 | a | |
| p-value | 0.730 | 0.690 | 0.779 | 0.784 | 0.893 | ||||||
| SEM | 3.0 | 24.7 | 9.7 | 25.8 | 6.6 | ||||||
| Year (Y) | 2010 | 14.3 | c | 44.1 | b | 9.3 | b | 50.9 | b | 4.3 | b |
| 2011 | 16.4 | a | 20.3 | c | 4.8 | c | 21.6 | c | 3.1 | c | |
| 2012 | 15.4 | b | 70.9 | a | 14.4 | a | 71.3 | a | 9.3 | a | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
| SEM | 2.0 | 16.2 | 6.4 | 16.9 | 4.3 | ||||||
| I × F | p-value | 0.915 | 0.964 | 0.951 | 0.971 | 0.425 | |||||
| I × Y | p-value | 0.534 | <0.001 | <0.001 | 0.001 | <0.001 | |||||
| F × Y | p-value | 0.065 | 0.979 | 0.769 | 0.960 | 0.904 | |||||
| I × F × Y | p-value | 0.929 | 0.895 | 0.535 | 0.852 | 0.195 | |||||
| Factor | Source of | Fusarium spp. Section (%) | |||
|---|---|---|---|---|---|
| Variation | Liseola | Discolor | |||
| Insecticide (I) | NT | 63.7 | a | 1.4 | b |
| GS73 | 34.2 | b | 3.9 | a | |
| p-value | <0.001 | 0.004 | |||
| SEM | 10.8 | 4.0 | |||
| Fungicide (F) | NT | 61.7 | a | 2.2 | a |
| GS63 | 44.4 | a | 3.4 | a | |
| GS69 | 36.2 | a | 3.3 | a | |
| GS73 | 47.8 | a | 2.2 | a | |
| GS75 | 49.6 | a | 3.0 | a | |
| GS83 | 45.1 | a | 1.0 | a | |
| GS85 | 48.1 | a | 2.2 | a | |
| GS87 | 58.9 | a | 3.8 | a | |
| p-value | 0.168 | 0.749 | |||
| SEM | 20.2 | 7.5 | |||
| Year (Y) | 2010 | 41.1 | b | 2.3 | b |
| 2011 | 34.9 | b | 0.5 | b | |
| 2012 | 71.0 | a | 5.1 | a | |
| p-value | <0.001 | <0.001 | |||
| SEM | 13.2 | 4.9 | |||
| I × F | p-value | 0.674 | 0.546 | ||
| I × Y | p-value | 0.082 | 0.018 | ||
| F × Y | p-value | 0.696 | 0.485 | ||
| I × F × Y | p-value | 0.442 | 0.295 | ||
| Factor | Source of | FBs | MON | DON | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variation | T | NT (µg kg−1) | T | NT (µg kg−1) | T | NT (µg kg−1) | ||||
| Insecticide (I) | NT | 6.0 | a | 898 | 5.2 | a | 300 | 4.3 | b | 128 |
| GS73 | 4.4 | b | 226 | 3.2 | b | 63 | 4.6 | a | 205 | |
| p-value | <0.001 | <0.001 | 0.041 | |||||||
| SEM | 1.1 | 1.3 | 1.0 | |||||||
| Fungicide (F) | NT | 5.6 | a | 603 | 4.7 | a | 261 | 4.2 | a | 133 |
| GS63 | 5.5 | a | 715 | 4.3 | a | 151 | 4.2 | a | 113 | |
| GS69 | 4.9 | a | 499 | 3.8 | a | 222 | 4.4 | a | 145 | |
| GS73 | 5.2 | a | 432 | 3.9 | a | 128 | 4.4 | a | 169 | |
| GS75 | 4.9 | a | 447 | 4.2 | a | 190 | 4.9 | a | 268 | |
| GS83 | 5.2 | a | 653 | 3.8 | a | 163 | 4.7 | a | 212 | |
| GS85 | 5.2 | a | 680 | 4.6 | a | 195 | 4.4 | a | 129 | |
| GS87 | 5.3 | a | 464 | 4.2 | a | 142 | 4.6 | a | 162 | |
| p-value | 0.212 | 0.080 | 0.204 | |||||||
| SEM | 2.0 | 2.5 | 1.9 | |||||||
| Year (Y) | 2010 | 6.0 | b | 497 | 4.2 | b | 143 | 4.5 | b | 115 |
| 2011 | 3.2 | c | 55 | 3.1 | c | 55 | 3.4 | c | 54 | |
| 2012 | 6.5 | a | 1133 | 5.2 | a | 347 | 5.5 | a | 331 | |
| p-value | <0.001 | <0.001 | <0.001 | |||||||
| SEM | 1.3 | 1.7 | 1.3 | |||||||
| I × F | p-value | 0.293 | 0.742 | 0.702 | ||||||
| I × Y | p-value | 0.049 | 0.206 | 0.289 | ||||||
| F × Y | p-value | 0.249 | 0.877 | 0.546 | ||||||
| I × F × Y | p-value | 0.198 | 0.999 | 0.508 | ||||||
| Fungicide Treatment | Maize Growth Stage (GS) 1 | 2010 | 2011 | 2012 |
|---|---|---|---|---|
| NT | Untreated control | - | - | - |
| GS63 | Flowering: tips of stigmata visible | 12th July | 4th July | 4th July |
| GS69 | End of flowering: stigmata completely dry | 22nd July | 15th July | 15th July |
| GS73 | Early milk | 29th July | 25th July | 26th July |
| GS75 | Milk ripening, about 40% dry matter | 5th August | 3rd August | 5th August |
| GS83 | Early dough, about 45% dry matter | 19th August | 16th August | 16th August |
| GS85 | Dough stage, about 55% dry matter | 27th August | 26th August | 28th August |
| GS87 | Physiological maturity | 7th September | 5th September | 9th September |
| Other agronomic information | ||||
| Insecticide application | Early milk | 29th July | 25th July | 26th July |
| Sowing date | 2nd April | 9th April | 28th March | |
| Harvesting date | 6th October | 27th September | 4th October | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blandino, M.; Scarpino, V.; Testa, G.; Vanara, F.; Reyneri, A. The Effect of Foliar Fungicide and Insecticide Application on the Contamination of Fumonisins, Moniliformin and Deoxynivalenol in Maize Used for Food Purposes. Toxins 2022, 14, 422. https://doi.org/10.3390/toxins14070422
Blandino M, Scarpino V, Testa G, Vanara F, Reyneri A. The Effect of Foliar Fungicide and Insecticide Application on the Contamination of Fumonisins, Moniliformin and Deoxynivalenol in Maize Used for Food Purposes. Toxins. 2022; 14(7):422. https://doi.org/10.3390/toxins14070422
Chicago/Turabian StyleBlandino, Massimo, Valentina Scarpino, Giulio Testa, Francesca Vanara, and Amedeo Reyneri. 2022. "The Effect of Foliar Fungicide and Insecticide Application on the Contamination of Fumonisins, Moniliformin and Deoxynivalenol in Maize Used for Food Purposes" Toxins 14, no. 7: 422. https://doi.org/10.3390/toxins14070422
APA StyleBlandino, M., Scarpino, V., Testa, G., Vanara, F., & Reyneri, A. (2022). The Effect of Foliar Fungicide and Insecticide Application on the Contamination of Fumonisins, Moniliformin and Deoxynivalenol in Maize Used for Food Purposes. Toxins, 14(7), 422. https://doi.org/10.3390/toxins14070422






