Effects of Turmeric Powder on Aflatoxin M1 and Aflatoxicol Excretion in Milk from Dairy Cows Exposed to Aflatoxin B1 at the EU Maximum Tolerable Levels
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects on Milk Yield, Milk Composition and Somatic Cell Count
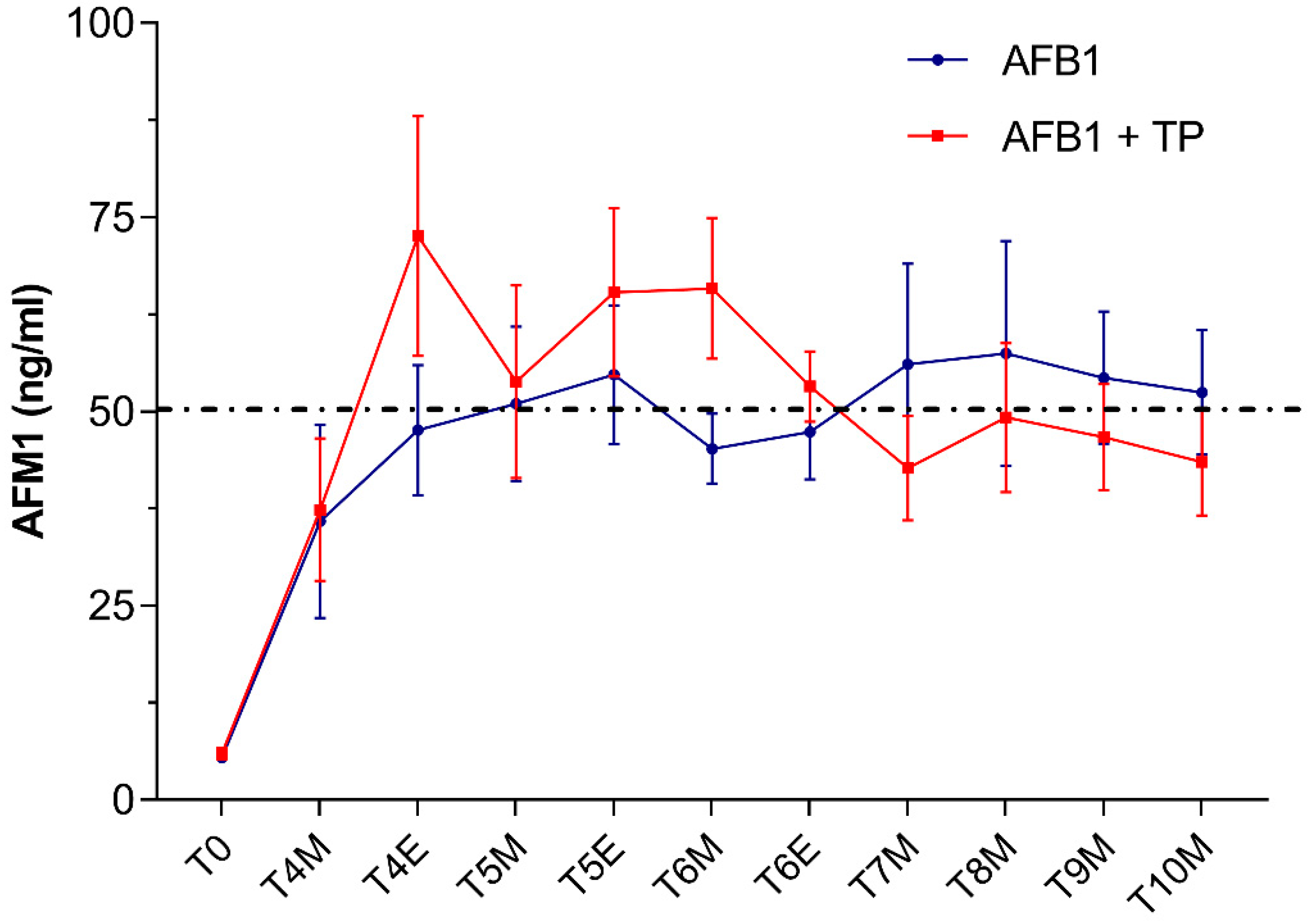
2.2. Effects on Milk Concentration of AFM1
2.3. Effects on Milk Concentration of AFL
3. Conclusions
4. Materials and Methods
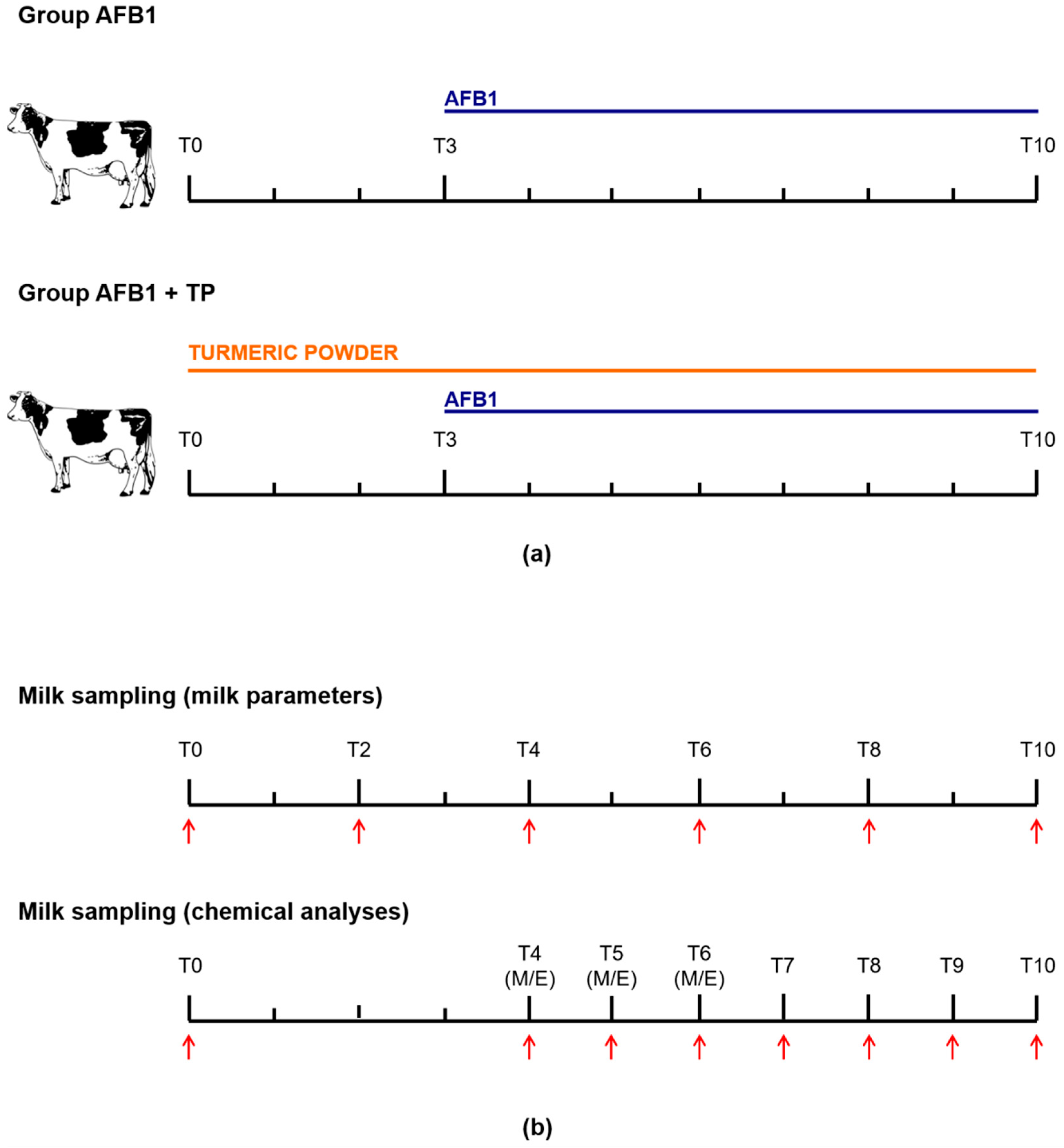
4.1. Experimental Design and Sample Collection
4.2. Chemicals
4.3. Analytical Determinations
4.4. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nazhand, A.; Durazzo, A.; Lucarini, M.; Souto, E.B.; Santini, A. Characteristics, occurrence, detection and detoxification of aflatoxins in foods and feeds. Foods 2020, 9, 644. [Google Scholar] [CrossRef] [PubMed]
- Battilani, P.; Toscano, P.; van der Fels-Klerx, H.J.; Moretti, A.; Camardo Leggieri, M.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328–24334. [Google Scholar] [CrossRef] [Green Version]
- Valencia-Quintana, R.; Milić, M.; Jakšić, D.; Klarić, M.Š.; Tenorio-Arvide, M.G.; Pérez-Flores, G.A.; Bonassi, S.; Sánchez-Alarcón, J. Environment changes, aflatoxins, and health issues, a review. Int. J. Environ. Res. Public Health 2020, 17, 7850. [Google Scholar] [CrossRef] [PubMed]
- Dohnal, V.; Wu, Q.; Kuča, K. Metabolism of aflatoxins: Key enzymes and interindividual as well as interspecies differences. Arch. Toxicol. 2014, 88, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Benkerroum, N. Chronic and acute toxicities of aflatoxins: Mechanisms of action. Int. J. Environ. Res. Public Health 2020, 17, 423. [Google Scholar] [CrossRef] [Green Version]
- WHO; IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Aflatoxins. In Chemical Agents and Related Occupations, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2012; Volume 100F, pp. 225–248. [Google Scholar]
- EFSA. Risk assessment of aflatoxins in food. EFSA J. 2020, 18, e06040. [Google Scholar] [CrossRef]
- Theumer, M.G.; Henneb, Y.; Khoury, L.; Snini, S.P.; Tadrist, S.; Canlet, C.; Puel, O.; Oswald, I.P.; Audebert, M. Genotoxicity of aflatoxins and their precursors in human cells. Toxicol. Lett. 2018, 287, 100–107. [Google Scholar] [CrossRef]
- Carvajal-Moreno, M.; Vargas-Ortiz, M.; Hernández-Camarillo, E.; Ruiz-Velasco, S.; Rojo-Callejas, F. Presence of unreported carcinogens, aflatoxins and their hydroxylated metabolites, in industrialized Oaxaca cheese from Mexico City. Food Chem. Toxicol. 2019, 124, 128–138. [Google Scholar] [CrossRef]
- Van Herwaarden, A.E.; Wagenaar, E.; Karnekamp, B.; Merino, G.; Jonker, J.W.; Schinkel, A.H. Breast cancer resistance protein (Bcrp1/Abcg2) reduces systemic exposure of the dietary carcinogens aflatoxin B1, IQ and Trp-P-1 but also mediates their secretion into breast milk. Carcinogenesis 2006, 27, 123–130. [Google Scholar] [CrossRef]
- Manzini, L.; Halwachs, S.; Girolami, F.; Badino, P.; Honscha, W.; Nebbia, C. Interaction of mammary bovine ABCG2 with AFB1 and its metabolites and regulation by PCB 126 in a MDCKII in vitro model. J. Vet. Pharmacol. Ther. 2017, 40, 591–598. [Google Scholar] [CrossRef]
- Čolović, R.; Puvača, N.; Cheli, F.; Avantaggiato, G.; Greco, D.; Đuragić, O.; Kos, J.; Pinotti, L. Decontamination of mycotoxin-contaminated feedstuffs and compound feed. Toxins 2019, 11, 617. [Google Scholar] [CrossRef] [Green Version]
- Kihal, A.; Rodriguez-Prado, M.; Godoy, C.; Cristofol, C.; Calsamiglia, S. In vitro assessment of the capacity of certain mycotoxin binders to adsorb some amino acids and water-soluble vitamins. J. Dairy Sci. 2020, 103, 3125–3132. [Google Scholar] [CrossRef]
- Solcan, C.; Gogu, M.; Floristean, V.; Oprisan, B.; Solcan, G. The hepatoprotective effect of sea buckthorn (Hippophae rhamnoides) berries on induced aflatoxin B1 poisoning in chickens. Poultry Sci. 2013, 92, 966–974. [Google Scholar] [CrossRef]
- Carter, A.C.; King, J.B.; Mattes, A.O.; Cai, S.; Singh, N.; Cichewicz, R.H. Natural-product-inspired compounds as countermeasures against the liver carcinogen Aflatoxin B1. J. Nat. Prod. 2019, 82, 1694–1703. [Google Scholar] [CrossRef]
- Umaya, S.R.; Vijayalakshmi, Y.C.; Sejian, V. Exploration of plant products and phytochemicals against aflatoxin toxicity in broiler chicken production: Present status. Toxicon 2021, 200, 55–68. [Google Scholar] [CrossRef]
- Holanda, D.M.; Kim, Y.I.; Parnsen, W.; Kim, S.W. Phytobiotics with adsorbent to mitigate toxicity of multiple mycotoxins on health and growth of pigs. Toxins 2021, 13, 442. [Google Scholar] [CrossRef]
- Lam, P.; Cheung, F.; Tan, H.Y.; Wang, N.; Yuen, M.F.; Feng, Y. Hepatoprotective Effects of Chinese medicinal herbs: A focus on anti-inflammatory and anti-oxidative activities. Int. J. Mol. Sci. 2016, 17, 465. [Google Scholar] [CrossRef] [Green Version]
- Dai, C.; Ciccotosto, G.D.; Cappai, R.; Tang, S.; Li, D.; Xie, S.; Xiao, X.; Velkov, T. Curcumin attenuates colistin-induced neurotoxicity in N2a cells via anti-inflammatory activity, suppression of oxidative stress, and apoptosis. Mol. Neurobiol. 2018, 55, 421–434. [Google Scholar] [CrossRef]
- Lee, S.E.; Campbell, B.C.; Molyneux, R.J.; Hasegawa, S.; Lee, H.S. Inhibitory effects of naturally occurring compounds on aflatoxin B1 biotransformation. J. Agric. Food Chem. 2001, 49, 5171–5177. [Google Scholar] [CrossRef]
- Limaye, A.; Yu, R.C.; Chou, C.C.; Liu, J.R.; Cheng, K.C. Protective and detoxifying effects conferred by dietary selenium and curcumin against AFB1-mediated toxicity in livestock: A review. Toxins 2018, 10, 25. [Google Scholar] [CrossRef] [Green Version]
- Murakami, M.; Ohnuma, S.; Fukuda, M.; Chufan, E.E.; Kudoh, K.; Kanehara, K.; Sugisawa, N.; Ishida, M.; Naitoh, T.; Shibata, H.; et al. Synthetic analogs of curcumin modulate the function of multidrug resistance–linked ATP-binding cassette transporter ABCG2. Drug Metab. Dispos. 2017, 45, 1166–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, P.; Ishfaq, M.; Yu, H.; Yang, Y.; Li, S.; Li, X.; Fazlani, S.A.; Guo, W.; Zhang, X. Curcumin ameliorates duodenal toxicity of AFB1 in chicken through inducing P-glycoprotein and downregulating cytochrome P450 enzymes. Poult. Sci. 2020, 99, 7035–7045. [Google Scholar] [CrossRef] [PubMed]
- Pauletto, M.; Giantin, M.; Tolosi, R.; Bassan, I.; Barbarossa, A.; Zaghini, A.; Dacasto, M. Curcumin mitigates afb1-induced hepatic toxicity by triggering cattle antioxidant and anti-inflammatory pathways: A whole transcriptomic in vitro study. Antioxidants 2020, 9, 1059. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.L.; Wang, Y.M.; Nennich, T.D.; Li, Y.; Liu, J.X. Transfer of dietary aflatoxin B1 to milk aflatoxin M1 and effect of inclusion of adsorbent in the diet of dairy cows. J. Dairy Sci. 2015, 98, 2545–2554. [Google Scholar] [CrossRef]
- Xiong, J.L.; Wang, Y.M.; Zhou, H.L.; Liu, J.X. Effects of dietary adsorbent on milk aflatoxin M1 content and the health of lactating dairy cows exposed to long-term aflatoxin B1 challenge. J. Dairy Sci. 2018, 101, 8944–8953. [Google Scholar] [CrossRef] [Green Version]
- Maki, C.R.; Monteiro, A.P.A.; Elmore, S.E.; Tao, S.; Bernard, J.K.; Harvey, R.B.; Romoser, A.A.; Phillips, T.D. Calcium montmorillonite clay in dairy feed reduces aflatoxin concentrations in milk without interfering with milk quality, composition or yield. Anim. Feed Sci. Technol. 2016, 214, 130–135. [Google Scholar] [CrossRef]
- Masoero, F.; Gallo, A.; Moschini, M.; Piva, G.; Diaz, D. Carryover of aflatoxin from feed to milk in dairy cows with low or high somatic cell counts. Animal 2007, 1, 1344–1350. [Google Scholar] [CrossRef] [Green Version]
- Applebaum, R.S.; Brackett, R.E.; Wiseman, D.W.; Marth, E.H. Aflatoxin: Toxicity to dairy cattle and occurrence in milk and milk products—A review. J. Food Prot. 1982, 45, 752–777. [Google Scholar] [CrossRef]
- Oh, J.; Wall, E.H.; Bravo, D.M.; Hristov, A.N. Host-mediated effects of phytonutrients in ruminants: A review. J. Dairy Sci. 2017, 100, 5974–5983. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.; Hristov, A.N.; Lee, C.; Cassidy, T.; Heyler, K.; Varga, G.A.; Pate, J.; Walusimbi, S.; Brzezicka, E.; Toyokawa, K.; et al. Immune and production responses of dairy cows to postruminal supplementation with phytonutrients. J. Dairy Sci. 2013, 96, 7830–7843. [Google Scholar] [CrossRef] [Green Version]
- Diaz, D.E.; Hagler, W.M.; Blackwelder, J.T.; Eve, J.A.; Hopkins, B.A.; Anderson, K.L.; Jones, F.T.; Whitlow, L.W. Aflatoxin binders II: Reduction of aflatoxin M1 in milk by sequestering agents of cows consuming aflatoxin in feed. Mycopathologia 2004, 157, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Flores-Flores, M.E.; Lizarraga, E.; López de Cerain, A.; González-Peñas, E. Presence of mycotoxins in animal milk: A review. Food Control 2015, 53, 163–176. [Google Scholar] [CrossRef]
- EFSA. Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission related to Aflatoxin B1 as undesirable substance in animal feed. EFSA J. 2004, 39, 1–27. [Google Scholar] [CrossRef]
- Frazzoli, C.; Gherardi, P.; Saxena, N.; Belluzzi, G.; Mantovani, A. The hotspot for (global) One Health in primary food production: Aflatoxin M1 in dairy products. Front. Public Health 2017, 4, 294. [Google Scholar] [CrossRef] [Green Version]
- Pauletto, M.; Tolosi, R.; Giantin, M.; Guerra, G.; Barbarossa, A.; Zaghini, A.; Dacasto, M. Insights into aflatoxin B1 toxicity in cattle An in vitro whole-transcriptomic approach. Toxins 2020, 12, 429. [Google Scholar] [CrossRef]
- Ghadiri, S.; Spalenza, V.; Dellafiora, L.; Badino, P.; Barbarossa, A.; Dall’Asta, C.; Nebbia, C.; Girolami, F. Modulation of aflatoxin B1 cytotoxicity and aflatoxin M1 synthesis by natural antioxidants in a bovine mammary epithelial cell line. Toxicol. In Vitro 2019, 57, 174–183. [Google Scholar] [CrossRef]
- Olagaray, K.E.; Bradford, B.J. Plant flavonoids to improve productivity of ruminants—A review. Anim. Feed Sci. Technol. 2019, 251, 21–36. [Google Scholar] [CrossRef]
- Gessner, D.K.; Ringseis, R.; Eder, K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J. Anim. Physiol. Anim. Nutr. 2017, 101, 605–628. [Google Scholar] [CrossRef]
- Winkler, A.; Gessner, D.K.; Koch, C.; Romberg, F.J.; Dusel, G.; Herzog, E.; Most, E.; Eder, K. Effects of a plant product consisting of green tea and curcuma extract on milk production and the expression of hepatic genes involved in endoplasmic stress response and inflammation in dairy cows. Arch. Anim. Nutr. 2015, 69, 425–441. [Google Scholar] [CrossRef]
- Schneider, C.; Gordon, O.N.; Edwards, R.L.; Luis, P.B. Degradation of curcumin: From mechanism to biological implications. J. Agric. Food Chem. 2015, 63, 7606–7614. [Google Scholar] [CrossRef] [Green Version]
- Mirzaei, H.; Shakeri, A.; Rashidi, B.; Jalili, A.; Banikazemi, Z.; Sahebkar, A. Phytosomal curcumin: A review of pharmacokinetic, experimental and clinical studies. Biomed. Pharmacother. 2017, 85, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wan, Y.; Li, P.; Xue, Y.; Cui, W.; Chen, Q.; Chen, J.; Wang, F.; Mao, D. Effect of curcumin supplement in summer diet on blood metabolites, antioxidant status, immune response, and testicular gene expression in hu sheep. Animals 2019, 9, 720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.Y.; Qi, M.; Zhao, L.; Zhu, M.K.; Guo, J.; Liu, J.; Gu, C.Q.; Rajput, S.A.; Krumm, C.S.; Qi, D.S.; et al. Curcumin prevents aflatoxin B1 hepatoxicity by inhibition of cytochrome P450 isozymes in chick liver. Toxins 2016, 8, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuilman, M.E.M.; Maas, R.F.M.; Judah, D.J.; Fink-Gremmels, J. Bovine hepatic metabolism of aflatoxin B1. J. Agric. Food Chem. 1998, 46, 2707–2713. [Google Scholar] [CrossRef]
- Helferich, W.G.; Baldwin, R.L.; Hsieh, D.P.H. [14C]-Aflatoxin B1 metabolism in lactating goats and rats. J. Anim. Sci. 1986, 62, 697–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trucksess, M.W.; Richard, J.L.; Stoloff, L.; McDonald, J.S.; Brumley, W.C. Absorption and distribution patterns of aflatoxicol and aflatoxins B1 and M1 in blood and milk of cows given aflatoxin B1. Am. J. Vet. Res. 1983, 44, 1753–1756. [Google Scholar] [PubMed]
- Carvajal, M.; Rojo, F.; Méndez, I.; Bolaños, A. Aflatoxin B1 and its interconverting metabolite aflatoxicol in milk: The situation in Mexico. Food Addit. Contam. 2003, 20, 1077–1086. [Google Scholar] [CrossRef]
- Bailey, G.S.; Dashwood, R.; Loveland, P.M.; Pereira, C.; Hendricks, J.D. Molecular dosimetry in fish: Quantitative target organ DNA adduction and hepatocarcinogenicity for four aflatoxins by two exposure routes in rainbow trout. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1998, 399, 233–244. [Google Scholar] [CrossRef]
- Cavallini, D.; Mammi, L.M.E.; Fustini, M.; Palmonari, A.; Heinrichs, A.J.; Formigoni, A. Effects of ad libitum or restricted access to total mixed ration with supplemental long hay on production, intake, and rumination. J. Dairy Sci. 2018, 101, 10922–10928. [Google Scholar] [CrossRef] [Green Version]
- Cavallini, D.; Mammi, L.M.E.; Biagi, G.; Fusaro, I.; Giammarco, M.; Formigoni, A.; Palmonari, A. Effects of 00-rapeseed meal inclusion in Parmigiano Reggiano hay-based ration on dairy cows’ production, reticular pH and fibre digestibility. It. J. Anim. Sci. 2021, 20, 295–303. [Google Scholar] [CrossRef]
- Amirahmadi, M.; Shoeibi, S.; Rastegar, H.; Elmi, M.; Mousavi Khaneghah, A. Simultaneous analysis of mycotoxins in corn flour using LC/MS-MS combined with a modified QuEChERS procedure. Toxin Rev. 2018, 37, 187–195. [Google Scholar] [CrossRef]
| Parameter | T0 | T4 | T10 | |||
|---|---|---|---|---|---|---|
| AFB1 | AFB1 + TP | AFB1 | AFB1 + TP | AFB1 | AFB1 + TP | |
| Milk yield (L) | 19.1 ± 4.5 | 19.7 ± 4.8 | 17.7 ± 4.6 | 20.16 ± 4.7 | 16.9 ± 3.9 | 17.8 ± 4.1 |
| Lipids (g/100 g) | 3.37 ± 0.76 | 3.64 ± 0.52 | 3.46 ± 0.61 | 4.15 ± 1.52 | 4.08 ± 0.76 | 3.52 ± 0.75 |
| Proteins (g/100 g) | 3.44 ± 0.32 | 3.55 ± 0.37 | 3.52 ± 0.39 | 3.48 ± 0.34 | 3.58 ± 0.39 | 3.53 ± 0.38 |
| Lactose (g/100 g) | 4.54 ± 0.60 | 4.70 ± 0.23 | 4.55 ± 0.56 | 4.57 ± 0.16 | 4.57 ± 0.29 | 4.78 ± 0.42 |
| Somatic cells (cell/mL × 1000) | 258 ± 160 | 203 ± 97 | 187 ± 106 | 316 ± 246 | 323 ± 211 | 193 ± 117 |
| Urea (mg/dL) | 23.4 ± 6.8 | 24.4 ± 5.6 | 25.0 ± 6.2 | 27.1 ± 7.9 | 25.9 ± 4.0 | 23.6 ± 4.8 |
| Time Points | Cow 1 | Cow 2 | Cow 3 | Cow 4 | Cow 5 | Cow 6 | Cow 7 | Cow 8 | |
|---|---|---|---|---|---|---|---|---|---|
| AFB1 + TP | T4M | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| T4E | n.d. | n.d. | <LOQ | n.d. | n.d. | n.d. | n.d. | n.d. | |
| T5M | n.d. | n.d. | <LOQ | n.d. | n.d. | n.d. | <LOQ | n.d. | |
| T5E | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| T6M | <LOQ | n.d. | n.d. | n.d. | n.d. | n.d. | <LOQ | n.d. | |
| T6E | <LOQ | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| T7M | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| T8M | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | <LOQ | n.d. | |
| T9M | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| T10M | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| AFB1 | T4M | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | <LOQ |
| T4E | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | <LOQ | |
| T5M | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| T5E | n.d. | <LOQ | n.d. | n.d. | n.d. | n.d. | 19 | n.d. | |
| T6M | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| T6E | n.d. | n.d. | <LOQ | n.d. | n.d. | n.d. | n.d. | n.d. | |
| T7M | <LOQ | 14 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| T8M | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 26 | n.d. | |
| T9M | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 21 | n.d. | |
| T10M | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girolami, F.; Barbarossa, A.; Badino, P.; Ghadiri, S.; Cavallini, D.; Zaghini, A.; Nebbia, C. Effects of Turmeric Powder on Aflatoxin M1 and Aflatoxicol Excretion in Milk from Dairy Cows Exposed to Aflatoxin B1 at the EU Maximum Tolerable Levels. Toxins 2022, 14, 430. https://doi.org/10.3390/toxins14070430
Girolami F, Barbarossa A, Badino P, Ghadiri S, Cavallini D, Zaghini A, Nebbia C. Effects of Turmeric Powder on Aflatoxin M1 and Aflatoxicol Excretion in Milk from Dairy Cows Exposed to Aflatoxin B1 at the EU Maximum Tolerable Levels. Toxins. 2022; 14(7):430. https://doi.org/10.3390/toxins14070430
Chicago/Turabian StyleGirolami, Flavia, Andrea Barbarossa, Paola Badino, Shiva Ghadiri, Damiano Cavallini, Anna Zaghini, and Carlo Nebbia. 2022. "Effects of Turmeric Powder on Aflatoxin M1 and Aflatoxicol Excretion in Milk from Dairy Cows Exposed to Aflatoxin B1 at the EU Maximum Tolerable Levels" Toxins 14, no. 7: 430. https://doi.org/10.3390/toxins14070430
APA StyleGirolami, F., Barbarossa, A., Badino, P., Ghadiri, S., Cavallini, D., Zaghini, A., & Nebbia, C. (2022). Effects of Turmeric Powder on Aflatoxin M1 and Aflatoxicol Excretion in Milk from Dairy Cows Exposed to Aflatoxin B1 at the EU Maximum Tolerable Levels. Toxins, 14(7), 430. https://doi.org/10.3390/toxins14070430








