Simultaneous Removal of Mycotoxins by a New Feed Additive Containing a Tri-Octahedral Smectite Mixed with Lignocellulose
Abstract
:1. Introduction
2. Results and Discussion
2.1. Screening of Adsorbent Materials as Multi-Mycotoxin Detoxifying Agents
2.2. Evaluation of Cytotoxicity and Antioxidant Properties of Selected Multi-Mycotoxin Detoxifying Agents
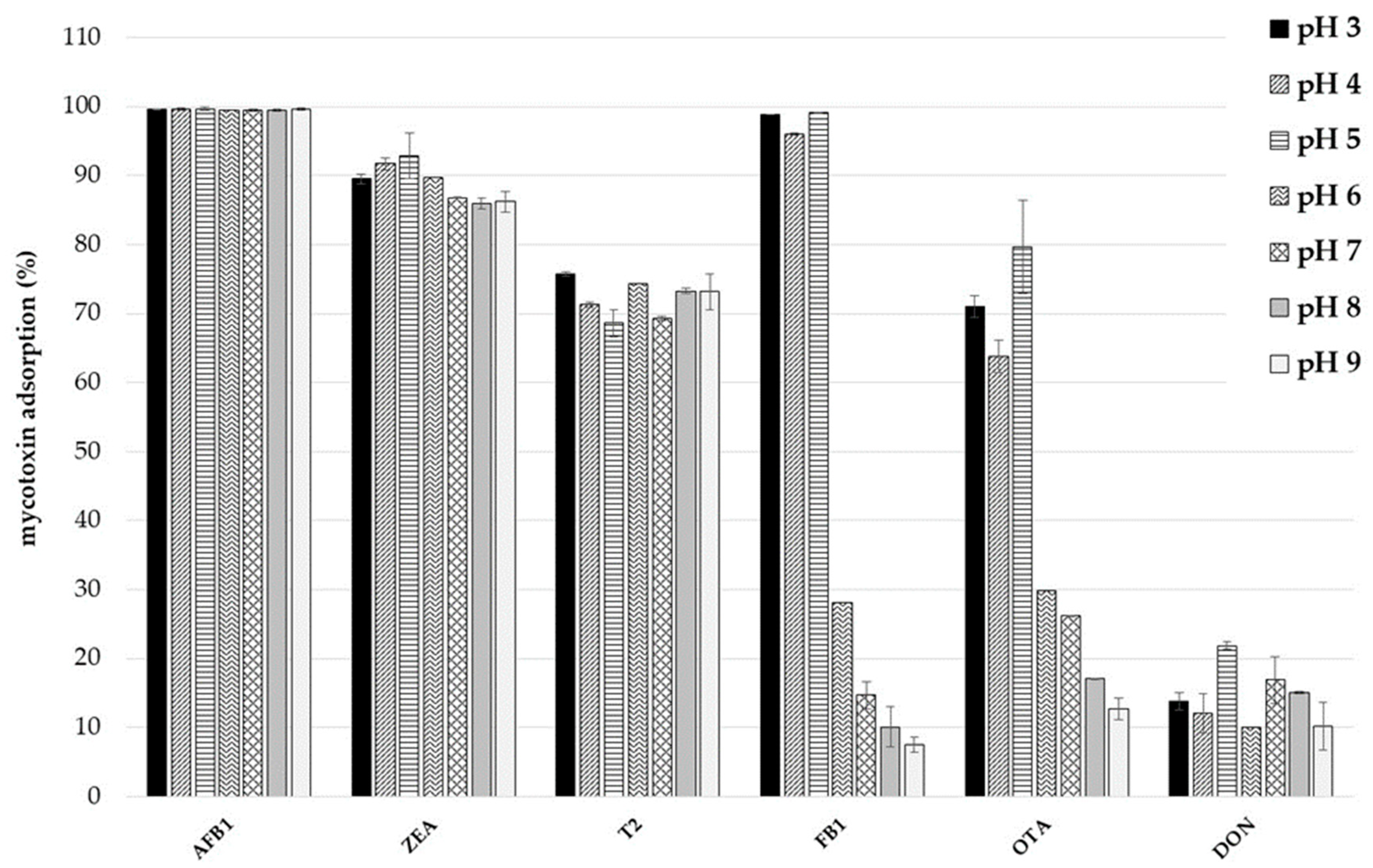
2.3. Effect of pH on Mycotoxin Adsorption and Desorption Study
2.4. Effect of Adsorbent Dosage and Toxin Concentration on Adsorption of AFB1, ZEA, OTA, FB1 and T-2
3. Conclusions
4. Materials and Methods
4.1. Reagents and Samples
4.2. Mycotoxin Adsorption Experiments
4.3. LC Analysis of Mycotoxins
4.4. Evaluation of Cytotoxicity and Antioxidant Properties of Selected MMDAs
4.5. Effect of pH and Desorption Study
4.6. Equilibrium Adsorption Isotherms
4.7. Data Calculations and Curve Fitting
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vila-Donat, P.; Marín, S.; Sanchis, V.; Ramos, A.J. A review of the mycotoxin adsorbing agents, with an emphasis on their multi-binding capacity, for animal feed decontamination. Food Chem. Toxicol. 2018, 114, 246–259. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Commission Regulation (EC) No. 386/2009 of 12 May 2009 amending Regulation (EC) No. 1831/2003 of the European Parliament and of the Council as regards the establishment of a new functional group of feed additives. Off. J. Eur. Union 2009, 118, 66. Available online: http://data.europa.eu/eli/reg/2009/386/oj (accessed on 13 May 2009).
- Boudergue, C.; Burel, C.; Dragacci, S.; Favrot, M.C.; Fremy, J.M.; Massimi, C.; Prigent, P.; Debongnie, P.; Pussemier, L.; Boudra, H.; et al. Review of Mycotoxin-detoxifying Agents used as Feed Additives: Mode of Action, Efficacy and Feed/Food Safety. EFSA Support. Publ. 2019, 6, 22E. [Google Scholar] [CrossRef]
- Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; Bastos, M.L.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G.; Gropp, J.; et al. Scientific Opinion on the safety and efficacy of bentonite as a feed additive for all animal species. EFSA J. 2017, 15, 5096–5109. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Commission Implementing Regulation (EU) No 1060/2013 of 29 October 2013 concerning the authorisation of bentonite as a feed additive for all animal species. Off. J. Eur. Union 2013, 289, 31–37. [Google Scholar]
- D’Ascanio, V.; Greco, D.; Menicagli, E.; Santovito, E.; Catucci, L.; Logrieco, A.F.; Avantaggiato, G. The role of geological origin of smectites and of their physico-chemical properties on aflatoxin adsorption. Appl. Clay Sci. 2019, 181, 105209. [Google Scholar] [CrossRef]
- Phillips, T.D.; Afriyie-Gyawu, E.; Williams, J.; Huebner, H.; Ankrah, N.A.; Ofori-Adjei, D.; Jolly, P.; Johnson, N.; Taylor, J.; Marroquin-Cardona, A. Reducing human exposure to aflatoxin through the use of clay: A review. Food Addit. Contam. Part A 2008, 25, 134–145. [Google Scholar] [CrossRef]
- Phillips, T.D.; Wang, M.; Elmore, S.E.; Hearon, S.; Wang, J.S. NovaSil clay for the protection of humans and animals from aflatoxins and other contaminants. Clays Clay Miner. 2019, 67, 99–110. [Google Scholar] [CrossRef]
- Kolosova, A.; Stroka, J. Substances for reduction of the contamination of feed by mycotoxins: A review. World Mycotoxin J. 2011, 4, 225–256. [Google Scholar] [CrossRef]
- Li, Y.; Tian, G.; Dong, G.; Bai, S.; Han, X.; Liang, J.; Meng, J.; Zhang, H. Research progress on the raw and modified montmorillonites as adsorbents for mycotoxins: A review. Appl. Clay Sci. 2018, 163, 299–311. [Google Scholar] [CrossRef]
- Wang, G.; Miao, Y.; Sun, Z.; Zheng, S. Simultaneous adsorption of aflatoxin B1 and zearalenone by mono- and di-alkyl cationic surfactants modified montmorillonites. J. Colloid Interface Sci. 2018, 511, 67–76. [Google Scholar] [CrossRef]
- Vila-Donat, P.; Marín, S.; Sanchis, V.; Ramos, A.J. New mycotoxin adsorbents based on trioctahedral bentonites for animal feed. Anim. Feed Sci. Technol. 2019, 255, 114228. [Google Scholar] [CrossRef]
- Vila-Donat, P.; Marín, S.; Sanchis, V.; Ramos, A.J. Tri-octahedral bentonites as potential technological feed additive for Fusarium mycotoxin reduction. Food Addit. Contam. Part A 2020, 37, 1374–1387. [Google Scholar] [CrossRef] [PubMed]
- Aoudia, N.; Tangni, E.K.; Larondelle, Y. Distribution of ochratoxin A in plasma and tissues of rats fed a naturally contaminated diet amended with micronized wheat fibres: Effectiveness of mycotoxin sequestering activity. Food Chem. Toxicol. 2008, 46, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Aoudia, N.; Callu, P.; Grosjean, F.; Larondelle, Y. Effectiveness of mycotoxin sequestration activity of micronized wheat fibres on distribution of ochratoxin A in plasma, liver and kidney of piglets fed a naturally contaminated diet. Food Chem. Toxicol. 2009, 47, 1485–1489. [Google Scholar] [CrossRef]
- Smith, T.K. Influence of Dietary Fiber, Protein and Zeolite on Zearalenone Toxicosis in Rats and Swine. J. Anim. Sci. 1980, 50, 278–285. [Google Scholar] [CrossRef] [Green Version]
- Tranquil, E.; Kanarskaya, Z.A.; Tikhomirov, D.F.; Kanarsky, A.V. Compositions and Methods for Decontamination of Animal Feed Containing Mycotoxins Typical for Both Northern and Southern Climates. U.S. Patent 8,496,984 B2, 30 July 2013. [Google Scholar]
- Greco, D.; D’Ascanio, V.; Santovito, E.; Logrieco, A.F.; Avantaggiato, G. Comparative efficacy of agricultural by-products in sequestering mycotoxins: Multi-mycotoxin adsorption efficacy of agricultural by-products. J. Sci. Food Agric. 2019, 99, 1623–1634. [Google Scholar] [CrossRef]
- Avantaggiato, G.; Greco, D.; Damascelli, A.; Solfrizzo, M.; Visconti, A. Assessment of Multi-mycotoxin Adsorption Efficacy of Grape Pomace. J. Agric. Food Chem. 2014, 62, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Gambacorta, L.; Pinton, P.; Avantaggiato, G.; Oswald, I.P.; Solfrizzo, M. Grape Pomace, an Agricultural Byproduct Reducing Mycotoxin Absorption: In Vivo assessment in pig using urinary biomarkers. J. Agric. Food Chem. 2016, 64, 6762–6771. [Google Scholar] [CrossRef]
- Adunphatcharaphon, S.; Petchkongkaew, A.; Greco, D.; D’Ascanio, V.; Visessanguan, W.; Avantaggiato, G. The Effectiveness of Durian Peel as a Multi-Mycotoxin Adsorbent. Toxins 2020, 12, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Scientific Opinion on the Safety and Efficacy of Lignosulphonate as a Feed Additive for All Animal Species. EFSA J. 2015, 13, 4160. [Google Scholar] [CrossRef]
- Baurhoo, B.; Ruiz-Feria, C.A.; Zhao, X. Purified lignin: Nutritional and health impacts on farm animals—A review. Anim. Feed Sci. Technol. 2008, 144, 175–184. [Google Scholar] [CrossRef]
- Ugartondo, V.; Mitjans, M.; Vinardell, M.P. Comparative antioxidant and cytotoxic effects of lignins from different sources. Bioresour. Technol. 2008, 99, 6683–6687. [Google Scholar] [CrossRef]
- Dong, X.; Dong, M.; Lu, Y.; Turley, A.; Jin, T.; Wu, C. Antimicrobial and antioxidant activities of lignin from residue of corn stover to ethanol production. Ind. Crop. Prod. 2011, 34, 1629–1634. [Google Scholar] [CrossRef]
- Ayyachamy, M.; Cliffe, F.E.; Coyne, J.M.; Collier, J.; Tuohy, M.G. Lignin: Untapped biopolymers in biomass conversion technologies. Biomass Convers. Biorefin. 2013, 3, 255–269. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G.; Dobado, J.A.; Isac-Garcia, J.; Marin-Martinez, F. Applications of Modified and Unmodified Lignins. Lignin and Lignans as Renewable Raw Materials: Chemistry, Technology and Applications; John Wiley & Sons Ltd.: New York, NY, USA, 2015; pp. 247–288. [Google Scholar] [CrossRef]
- Ravi Kanth Reddy, P.; Elghandour, M.M.M.Y.; Salem, A.Z.M.; Yasaswini, D.; Pandu Ranga Reddy, P.; Nagarjuna Reddy, A.; Hyder, I. Plant secondary metabolites as feed additives in calves for antimicrobial stewardship. Anim. Feed Sci. Technol. 2020, 264, 114469. [Google Scholar] [CrossRef]
- Di Gregorio, M.C.; de Neeff, D.V.; Jager, A.V.; Corassin, C.H.; de Pinho Carão, A.C.; de Albuquerque, R.; de Azevedo, A.C.; Fernandes Oliveira, C.A. Mineral adsorbent for prevention mycotoxins in animal feed. Toxin Rev. 2014, 33, 125–135. [Google Scholar] [CrossRef]
- Bueno, D.J.; Di Marco, L.; Oliver, G.; Bardón, A. In vitro binding of zearalenone to different adsorbents. J. Food Prot. 2005, 68, 613–615. [Google Scholar] [CrossRef]
- Dakovic, A.; Tomasevic-Canovic, M.; Dondur, V.; Stojsic, D.; Rottinghaus, G. 32-O-04-In Vitro adsorption of zearalenone by octadecyldimethylbenzyl ammonium-exchanged clinoptilolite-heulandite tuff and bentonite. Stud. Surf. Sci. Catal. 2001, 135, 171. [Google Scholar] [CrossRef]
- Thimm, N.; Schwaighofer, B.; Ottner, F.; Fröschl, H.; Greifenender, S.; Binder, E.M. Adsorption of mycotoxins. Mycotox Res. 2001, 17, 219–223. [Google Scholar] [CrossRef]
- Stojšić, D.; Stojković, M.; Daković, A.S.; Adamović, M.; Tomašević-Čanović, M.R. Efficacy of organozeolite to ameliorate the toxic effects of zearalenone in lambs. Acta Vet. 2004, 54, 53–62. [Google Scholar] [CrossRef]
- Daković, A.; Tomašević-Čanović, M.; Rottinghaus, G.E.; Matijašević, S.; Sekulić, Ž. Fumonisin B1 adsorption to octadecyldimethylbenzyl ammonium-modified clinoptilolite-rich zeolitic tuff. Microporous Mesoporous Mater. 2007, 105, 285–290. [Google Scholar] [CrossRef]
- Daković, A.; Tomašević-Čanović, M.; Rottinghaus, G.; Dondur, V.; Mašić, Z. Adsorption of ochratoxin A on octadecyldimethyl benzyl ammonium exchanged-clinoptilolite-heulandite tuff. Colloids Surf. B Biointerfaces 2003, 30, 157–165. [Google Scholar] [CrossRef]
- Santos, R.R.; Vermeulen, S.; Haritova, A.; Fink-Gremmels, J. Isotherm modeling of organic activated bentonite and humic acid polymer used as mycotoxin adsorbents. Food Addit. Contam. 2011, 28, 1578–1589. [Google Scholar] [CrossRef]
- Döll, S.; Dänicke, S.; Valenta, H.; Flachowsky, G. In vitro studies on the evaluation of mycotoxin detoxifying agents for their efficacy on deoxynivalenol and zearalenone. Arch. Anim. Nutr. 2004, 58, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Sabater-Vilar, M.; Malekinejad, H.; Selman, M.H.J.; van der Doelen, M.A.M.; Fink-Gremmels, J. In Vitro assessment of adsorbents aiming to prevent deoxynivalenol and zearalenone mycotoxicoses. Mycopathologia 2007, 163, 81–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avantaggiato, G.; Greco, D.; D’Ascanio, V.; Logrieco, A.F. Advances and Criticisms on the Use of Mycotoxin Detoxifying Agents. In Mycotoxins in Food and Beverage: Innovations and Advances, Part II, 1st ed.; Montet, D., Brabet, C., Schorr-Galindo, S., Ray, R.C., Eds.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2021; Volume 1, pp. 122–153. [Google Scholar] [CrossRef]
- Dos Santos, P.S.B.; da Silva, S.H.F.; Erdocia, X.; Gatto, D.A.; Labidi, J. Characterization of Kraft Lignin Precipitated with Different Alcohols. Chem. Eng. Trans. 2015, 43, 469–474. [Google Scholar] [CrossRef]
- García, A.; Amendola, D.; González, M.; Spigno, G.; Labidi, J. Lignin as natural radical scavenger. Study of the antioxidant capacity of apple tree pruning lignin obtained by different methods. Chem. Eng. Trans. 2011, 24, 925–930. [Google Scholar] [CrossRef]
- USDA, United State Department of Agriculture. Database for the Proanthocyanidin Content of Selected Foods. 2004. Available online: http://www.nal.usda.gov/fnic/foodcomp (accessed on 13 March 2018).
- Dakovic, A.; Matijasevic, S.; Rottinghaus, G.E.; Ledoux, D.R.; Butkeraitis, P.; Sekulic, Z. Aflatoxin B-1 adsorption by natural and copper modified montmorillonite. Colloids Surf. B Biointerfaces 2008, 66, 20–25. [Google Scholar] [CrossRef]
- Akinrinmade, F.J.; Akinrinde, A.S.; Amid, A. Changes in serum cytokine levels, hepatic and intestinal morphology in aflatoxin B1-induced injury: Modulatory roles of melatonin and flavonoid-rich fractions from Chromolena odorata. Mycotoxin Res. 2016, 32, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Taranu, I.; Marin, D.E.; Palade, M.; Pistol, G.C.; Chedea, V.S.; Gras, M.A.; Rotar, C. Assessment of the efficacy of a grape seed waste in counteracting the changes induced by aflatoxin B1 contaminated diet on performance, plasma, liver and intestinal tissues of pigs after weaning. Toxicon 2019, 162, 24–31. [Google Scholar] [CrossRef]
- Romero, A.; Ares, I.; Ramos, E.; Castellano, V.; Martinez, M.; Martinez-Larrañga, M.R.; Anadón, A.; Martínez, M.A. Mycotoxins modify the barrier function of Caco-2 cells through differential gene expression of specific claudin isoforms: Protective effect of illite mineral clay. Toxicology 2016, 353, 21–23. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Longobardi, C.; Andretta, E.; Romano, V.; Lauritano, C.; Avantaggiato, G.; Schiavone, A.; Jarriyawattanachaikul, W.; Florio, S.; Ciarcia, R.; Damiano, S. Effects of Some New Antioxidants on Apoptosis and ROS Production in AFB1 Treated Chickens. Med. Sci. Forum 2021, 2, 12. [Google Scholar] [CrossRef]
- EFSA. Statement on the establishment of guidelines for the assessment of additives from the functional group ‘substances for reduction of the contamination of feed by mycotoxins’. EFSA J. 2010, 8, 1693–1701. [Google Scholar] [CrossRef]
- Pascale, M.; Panzarini, G.; Visconti, A. Determination of HT-2 and T-2 toxins in oats and wheat by ultra-performance liquid chromatography with photodiode array detection. Talanta 2012, 89, 231–236. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Balance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static In Vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Barberis, A.; Garbetta, A.; Cardinali, A.; Bazzu, G.; D’Antuono, I.; Rocchitta, G.; Fadda, A.; Linsalata, V.; D’Hallewin, G.; Serra, P.A. Real-time monitoring of glucose and phenols intestinal absorption through an integrated Caco-2TC7 cells/biosensors telemetric device: Hypoglycemic effect of fruit phytochemicals. Biosens. Bioelectron. 2017, 88, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Minervini, F.; Garbetta, A.; D’Antuono, I.; Cardinali, A.; Martino, N.A.; Debellis, L.; Visconti, A. Toxic mechanisms induced by Fumonisin B1 mycotoxin on human intestinal cell line. Arch. Environ. Contam. Toxicol. 2014, 67, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef]
- Garbetta, A.; Nicassio, L.; D’Antuono, I.; Cardinali, A.; Linsalata, V.; Attolico, G.; Minervini, F. Influence of In Vitro digestion process on polyphenolic profile of skin grape (cv. Italia) and on antioxidant activity in basal or stressed conditions of human intestinal cell line (HT-29). Food. Res. Int. 2018, 106, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdicphosphotungstic acid reagents. Am. J. Enol. Vitic. 1956, 16, 144–158. [Google Scholar]


| Materials | Mycotoxin Adsorption (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| AFB1 | ZEA | OTA | FB1 | |||||
| pH 3 | pH 7 | pH 3 | pH 7 | pH 3 | pH 7 | pH 3 | pH 7 | |
| Lignocellulose 1 | 76 | 82 | 67 | 70 | 73 | 1 | 23 | 3 |
| Lignocellulose 2 | 70 | 82 | 49 | 61 | 56 | 2 | 19 | 8 |
| Lignocellulose 3 | 79 | 70 | 76 | 67 | 66 | 0 | 23 | 3 |
| Lignocellulose 4 | 59 | 60 | 61 | 63 | 62 | 3 | 0 | 7 |
| Lignocellulose 5 | 47 | 87 | 37 | 68 | 35 | 0 | 25 | 8 |
| Lignocellulose 6 | 81 | 71 | 77 | 71 | 72 | 0 | 39 | 6 |
| Na-smectite 1 (di-octahedral) | 99 | 97 | 4 | 7 | 29 | 2 | 92 | 9 |
| Na-smectite 2 (di-octahedral) | 87 | 84 | 15 | 6 | 1 | 1 | 27 | 1 |
| Na-smectite 3 (di-octahedral) | 100 | 100 | 18 | 4 | 55 | 3 | 100 | 8 |
| Na-smectite 4 (di-octahedral) | 100 | 98 | 28 | 8 | 74 | 3 | 96 | 8 |
| Na-smectite 5 (di-octahedral) | 100 | 100 | 34 | 14 | 77 | 4 | 100 | 4 |
| Na-smectite 6 (di-octahedral) | 100 | 99 | 38 | 24 | 75 | 1 | 99 | 8 |
| Ca-smectite 7 (di-octahedral) | 100 | 100 | 36 | 20 | 71 | 2 | 100 | 20 |
| Na-smectite 8 (tri-octahedral) | 99 | 83 | 2 | 3 | 10 | 0 | 88 | 6 |
| Na-smectite 9 (tri-octahedral) | 98 | 98 | 77 | 81 | 90 | 14 | 100 | 26 |
| Na-smectite 10 (tri-octahedral) | 99 | 99 | 79 | 77 | 88 | 6 | 100 | 27 |
| Na-smectite 11 (tri-octahedral) | 100 | 99 | 84 | 76 | 77 | 4 | 100 | 13 |
| Na-smectite 12 (tri-octahedral) | 100 | 99 | 89 | 82 | 80 | 25 | 99 | 25 |
| pH | Mycotoxin Adsorption (%) | ||||||
|---|---|---|---|---|---|---|---|
| AFB1 | FB1 | ZEA | OTA | T-2 | DON | HT-2 | |
| 7 | 100.0 ± 0.0 | 1.5 ± 1.3 | 51.5 ± 0.0 | 2.8 ± 0.3 | 33.4 ± 2.9 | 8.1 ± 0.8 | 0.0 ± 0.0 |
| 5 | 96.7 ± 1.4 | 91.0 ± 0.9 | 64.5 ± 0.2 | 31.9 ± 0.6 | 23.4 ± 1.6 | 0.5 ± 0.4 | 0.0 ± 0.0 |
| 3 | 100.0 ± 0.0 | 94.2 ± 0.9 | 65.8 ± 0.7 | 84.0 ± 0.5 | 44.9 ± 0.8 | 13.6 ± 1.3 | 0.0 ± 0.0 |
| Toxin | Adsorption pH 3 (%) | Desorption pH 7 (%) |
|---|---|---|
| AFB1 | 96.6 ± 0.6 | 1.7 ± 0.3 |
| ZEA | 91.6 ± 0.0 | 4.1 ± 0.7 |
| FB1 | 99.6 ± 0.1 | 30.6 ± 2.7 |
| OTA | 91.7 ± 0.4 | 38.1 ± 4.6 |
| T-2 | 81.2± 1.6 | 10.6 ± 0.2 |
| Dosage (mg mL−1) | Mycotoxin Adsorption (%) | ||||||
|---|---|---|---|---|---|---|---|
| AFB1 | FB1 | ZEA | OTA | DON | T-2 | HT-2 | |
| 0.005 | 8.5 ± 2.3 | 56.2 ± 2.5 | 9.2 ± 0.8 | 5.6 ± 0.1 | 0.7 ± 1.0 | 5.7 ± 1.6 | 2.5 ± 1.6 |
| 0.01 | 20.2 ± 0.6 | 54.8 ± 4.9 | 14.7 ± 2.2 | 8.6 ± 0.7 | 4.4 ± 3.0 | 7.5± 3.4 | 6.7± 3.4 |
| 0.025 | 40.9 ± 2.0 | 59.8 ± 4.1 | 14.5 ± 1.2 | 4.7 ± 2.0 | 2.8 ± 1.4 | 5.9 ± 0.8 | 2.9 ± 0.8 |
| 0.05 | 64.3 ± 1.5 | 69.5 ± 1.8 | 16.1 ± 3.1 | 8.7 ± 1.5 | 4.9 ± 0.8 | 15.7 ± 3.0 | 2.1 ± 3.0 |
| 0.1 | 92.3 ± 0.3 | 83.6 ± 1.8 | 28.8 ± 0.9 | 17.5 ± 0.4 | 11.0 ± 0.1 | 21.7 ± 1.0 | 11.1 ± 1.0 |
| 0.5 | 99.5 ± 0.0 | 93.8 ± 0.2 | 49.7 ± 3.2 | 21.9 ± 1.0 | 3.9 ± 0.5 | 11.7 ± 1.3 | 0.0 ± 0.0 |
| 1 | 99.1 ± 0.0 | 96.8 ± 0.2 | 68.3 ± 0.6 | 37.6 ± 0.1 | 8.6 ± 0.4 | 26.0 ± 3.2 | 0.0 ± 0.0 |
| 5 | 95.1 ± 1.2 | 99.1 ± 0.1 | 92.9 ± 3.3 | 79.7 ± 6.8 | 21.9 ± 0.6 | 62.6 ± 2.0 | 0.0 ± 0.0 |
| 10 | 94.1 ± 0.3 | 99.5 ± 0.1 | 95.1 ± 0.1 | 84.6 ± 0.2 | 22.0 ± 0.6 | 69.7 ± 0.9 | 0.0 ± 0.0 |
| Toxin | Adsmax | C50 |
|---|---|---|
| AFB1 | 103 ± 3 | 0.03 |
| FB1 | 96 ± 3 | 0.01 |
| ZEA | 96 ± 6 | 0.3 |
| OTA | 99 ± 8 | 1.5 |
| T-2 | 78 ± 9 | 2.3 |
| Adsorption Parameters | AFB1 | AFB1 | ZEA | OTA | T-2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| pH 7 | pH 3 | pH 7 | pH 3 | pH 7 | pH 3 | pH 7 | pH 3 | pH 7 | pH 3 | |
| Adsmax 1 | 67.9 | 24.6 | 0.6 | 91.0 | 7.2 | 4.6 | 0.3 | 11.1 | 1.0 | 1.0 |
| KL 2 | 0.6 | 1.3 | 1.8 | 1.6 | 0.5 | 0.9 | 0.4 | 1.6 | - | - |
| Dosage 3 | 0.05 | 0.05 | 1 | 0.05 | 0.5 | 0.5 | 1 | 0.05 | 5 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, D.; D’Ascanio, V.; Abbasciano, M.; Santovito, E.; Garbetta, A.; Logrieco, A.F.; Avantaggiato, G. Simultaneous Removal of Mycotoxins by a New Feed Additive Containing a Tri-Octahedral Smectite Mixed with Lignocellulose. Toxins 2022, 14, 393. https://doi.org/10.3390/toxins14060393
Greco D, D’Ascanio V, Abbasciano M, Santovito E, Garbetta A, Logrieco AF, Avantaggiato G. Simultaneous Removal of Mycotoxins by a New Feed Additive Containing a Tri-Octahedral Smectite Mixed with Lignocellulose. Toxins. 2022; 14(6):393. https://doi.org/10.3390/toxins14060393
Chicago/Turabian StyleGreco, Donato, Vito D’Ascanio, Mariagrazia Abbasciano, Elisa Santovito, Antonella Garbetta, Antonio F. Logrieco, and Giuseppina Avantaggiato. 2022. "Simultaneous Removal of Mycotoxins by a New Feed Additive Containing a Tri-Octahedral Smectite Mixed with Lignocellulose" Toxins 14, no. 6: 393. https://doi.org/10.3390/toxins14060393
APA StyleGreco, D., D’Ascanio, V., Abbasciano, M., Santovito, E., Garbetta, A., Logrieco, A. F., & Avantaggiato, G. (2022). Simultaneous Removal of Mycotoxins by a New Feed Additive Containing a Tri-Octahedral Smectite Mixed with Lignocellulose. Toxins, 14(6), 393. https://doi.org/10.3390/toxins14060393






