Genomics of Maize Resistance to Fusarium Ear Rot and Fumonisin Contamination
Abstract
1. Introduction
2. Mapping of QTL for FER and/or Fumonisin Content in Bi-Parental Populations
3. Genome-Wide Association Studies for FER and Fumonisin Content
4. Gene Expression Studies
5. Reverse Genetics to Uncover Metabolic Pathways Involved in Resistance to F. verticillioides and Fumonisin Contamination
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bhat, R.V.; Miller, J. Mycotoxins and food supply. Foodnutr. Agric. 1991, 1, 27–31. [Google Scholar]
- Bhatnagar, D.; Payne, G.; Klich, M.; Leslie, J.F. Identification of toxigenic Aspergillus and Fusarium species in the maize grain chain. In Mycotoxin Reduction in Grain Chains; Leslie, J.F., Logrieco, A.F., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 9–25. [Google Scholar]
- CAST. Mycotoxins: Risks in Plant, Animal, and Human Systems; Council for Agricultural Science and Technology: Ames, IA, USA, 2003. [Google Scholar]
- Braun, M.S.; Wink, M. Exposure, occurrence, and chemistry of fumonisins and their cryptic derivatives. Compr. Rev. Food Sci. Food Saf. 2018, 17, 769–791. [Google Scholar] [CrossRef]
- Munkvold, G.P.; Arias, S.; Taschl, I.; Gruber-Dorninger, C.; Serna-Saldivar, S.O. Chapter 9—Mycotoxins in corn: Occurrence, impacts, and management. In Corn, 3rd ed.; AACC International Press: Oxford, UK, 2019; pp. 235–287. [Google Scholar]
- Lanubile, A.; Maschietto, V.; Marocco, A. Breeding maize for resistance to mycotoxins. In Mycotoxin Reduction in Grain Chains; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 37–58. [Google Scholar]
- Blacutt, A.A.; Gold, S.E.; Voss, K.A.; Gao, M.; Glenn, A.E. Fusarium verticillioides: Advancements in understanding the toxicity, virulence, and niche adaptations of a model mycotoxigenic pathogen of maize. Phytopathology 2018, 108, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Munkvold, G.P.; Desjardins, A.E. Fumonisins in maizecan—We reduce their occurrence? Plant Dis. 1997, 81, 556–565. [Google Scholar] [CrossRef]
- Marasas, W.F. Discovery and occurrence of the fumonisins: A historical perspective. Environ. Health Perspect. 2001, 109. [Google Scholar]
- Voss, K.A.; Smith, G.W.; Haschek, W.M. Fumonisins: Toxicokinetics, mechanism of action and toxicity. Anim. Feed Sci. Technol. 2007, 137, 299–325. [Google Scholar] [CrossRef]
- Rheeder, J.P.; Marasas, W.F.O.; Vismer, H.F. Production of fumonisin analogs by Fusarium species. Appl. Environ. Microbiol. 2002, 68, 2101–2105. [Google Scholar] [CrossRef]
- Sun, G.; Wang, S.; Hu, X.; Su, J.; Huang, T.; Yu, J.; Tang, L.; Gao, W.; Wang, J.-S. Fumonisin B1 contamination of home-grown corn in high-risk areas for esophageal and liver cancer in China. Food Addit. Contam. 2007, 24, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.M.; Rohandel, G.; Roudbarmohammadi, S.; Roudbary, M.; Sohanaki, H.; Ghiasian, S.A.; Taherkhani, A.; Semnani, S.; Aghasi, M. Fumonisin B1 contamination of cereals and risk of esophageal cancer in a high risk area in northeastern Iran. Asian Pac. J. Cancer Prev. 2012, 13, 2625–2628. [Google Scholar] [CrossRef] [PubMed]
- Gelineau-van Waes, J.; Starr, L.; Maddox, J.; Aleman, F.; Voss, K.A.; Wilberding, J.; Riley, R.T. Maternal fumonisin exposure and risk for neural tube defects: Mechanisms in an in vivo mouse model. Birth Defects Res. Part A Clin. Mol. Teratol. 2005, 73, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Missmer, S.A.; Suarez, L.; Felkner, M.; Wang, E.; Merrill, A.H.; Rothman, K.J.; Hendricks, K.A. Exposure to fumonisins and the occurrence of neural tube defects along the Texas-Mexico border. Environ. Health Perspect. 2006, 114, 237–241. [Google Scholar] [CrossRef] [PubMed]
- IARC. Fumonisin b1. Sometraditional herbalmedicines, somemycotoxins, naphthalene and styrene. In 82 Monograph of the International Agency for Research of Cancer on the Evaluation of Carcinogenic Risks to Humans; World Health Organization: Lyon, France, 2002; pp. 301–306. [Google Scholar]
- FDA. Guidance for Industry: Fumonisin Levels in Human Foods and Animal Feeds. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-fumonisin-levels-human-foods-and-animal-feeds (accessed on 28 June 2020).
- Anukul, N.; Vangnai, K.; Mahakarnchanakul, W. Significance of regulation limits in mycotoxin contamination in Asia and risk management programs at the national level. J. Food Drug Anal. 2013, 21, 227–241. [Google Scholar] [CrossRef]
- 1126/2007/EC. Regulation (EC) no 1126/2007 of 28 September 2007 amending regulation (EC) no 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards fusarium toxins in maize and maize products. Off. J. Eur. Union 2007, L255, 14–17. [Google Scholar]
- Pascale, M.; Visconti, A.; Chelkowski, J. Ear rot susceptibility and mycotoxin contamination of maize hybrids inoculated with Fusarium species under field conditions. Eur. J. Plant Pathol. 2002, 108, 645–651. [Google Scholar] [CrossRef]
- Cao, A.; Santiago, R.; Ramos, A.J.; Marin, S.; Reid, L.M.; Butron, A. Environmental factors related to fungal infection and fumonisin accumulation during the development and drying of white maize kernels. Int. J. Food Microbiol. 2013, 164, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Santiago, R.; Cao, A.; Butron, A. Genetic factors involved in fumonisin accumulation in maize kernels and their implications in maize agronomic management and breeding. Toxins 2015, 7, 3267–3296. [Google Scholar] [CrossRef] [PubMed]
- Afolabi, C.G.; Ojiambo, P.S.; Ekpo, E.J.A.; Menkir, A.; Bandyopadhyay, R. Evaluation of maize inbred lines for resistance to fusarium ear rot and fumonisin accumulation in grain in tropical Africa. Plant Dis. 2007, 91, 279–286. [Google Scholar] [CrossRef]
- Kleinschmidt, C.E.; Clements, M.J.; Maragos, C.M.; Pataky, J.K.; White, D.G. Evaluation of food-grade dent corn hybrids for severity of fusarium ear rot and fumonisin accumulation in grain. Plant Dis. 2005, 89, 291–297. [Google Scholar] [CrossRef]
- Presello, D.A.; Iglesias, J.; Botta, G.; Eyherabide, G.H. Severity of fusarium ear rot and concentration of fumonisin in grain of Argentinian maize hybrids. Crop Prot. 2007, 26, 852–855. [Google Scholar] [CrossRef]
- Presello, D.A.; Reid, L.M.; Mather, D.E. Resistance of Argentine maize germpiasm to gibberella and fusarium ear rots. Maydica 2004, 49, 73–81. [Google Scholar]
- Henry, W.B.; Williams, W.P.; Windham, G.L.; Hawkins, L.K. Evaluation of maize inbred lines for resistance to Aspergillus and Fusarium ear rot and mycotoxin accumulation. Agron. J. 2009, 101, 1219–1226. [Google Scholar] [CrossRef]
- Clements, M.J.; Maragos, C.A.; Pataky, J.K.; White, D.G. Sources of resistance to fumonisin accumulation in grain and fusarium ear and kernel rot of corn. Phytopathology 2004, 94, 251–260. [Google Scholar] [CrossRef]
- Clements, M.J.; White, D.G. Identifying sources of resistance to aflatoxin and fumonisin contamination in corn grain. J. Toxicol. Toxin Rev. 2004, 23, 381–396. [Google Scholar] [CrossRef]
- Löffler, M.; Kessel, B.; Ouzunova, M.; Miedaner, T. Covariation between line and testcross performance for reduced mycotoxin concentrations in European maize after silk channel inoculation of two Fusarium species. Theor. Appl. Genet. 2011, 122, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Löffler, M.; Kessel, B.; Ouzunova, M.; Miedaner, T. Population parameters for resistance to Fusarium graminearum and Fusarium verticillioides ear rot among large sets of early, mid-late and late maturing European maize (Zea mays l.) inbred lines. Theor. Appl. Genet. 2010, 120, 1053–1062. [Google Scholar] [CrossRef]
- Robertson, L.A.; Kleinschmidt, C.E.; White, D.G.; Payne, G.A.; Maragos, C.M.; Holland, J.B. Heritabilities and correlations of fusarium ear rot resistance and fumonisin contamination resistance in two maize populations. Crop Sci. 2006, 46, 353–361. [Google Scholar] [CrossRef]
- Bolduan, C.; Miedaner, T.; Schipprack, W.; Dhillon, B.S.; Melchinger, A.E. Genetic variation for resistance to ear rots and mycotoxins contamination in early European maize inbred lines. Crop Sci. 2009, 49, 2019–2028. [Google Scholar] [CrossRef]
- Santiago, R.; Cao, A.; Malvar, R.A.; Reid, L.M.; Butron, A. Assessment of corn resistance to fumonisin accumulation in a broad collection of inbred lines. Field Crop. Res. 2013, 149, 193–202. [Google Scholar] [CrossRef]
- Rose, L.J.; Mouton, M.; Beukes, I.; Flett, B.C.; van der Vyver, C.; Viljoen, A. Multi-environment evaluation of maize inbred lines for resistance to fusarium ear rot and fumonisins. Plant Dis. 2016, 100, 2134–2144. [Google Scholar] [CrossRef]
- Balconi, C.; Berardo, N.; Locatelli, S.; Lanzanova, C.; Torri, A.; Redaelli, R. Evaluation of ear rot (Fusarium verticillioides) resistance and fumonisin accumulation in Italian maize inbred lines. Phytopathol. Mediterr. 2014, 53, 14–26. [Google Scholar]
- Cao, A.; Butron, A.; Ramos, A.J.; Marin, S.; Souto, C.; Santiago, R. Assessing white maize resistance to fumonisin contamination. Eur. J. Plant Pathol. 2014, 138, 283–292. [Google Scholar] [CrossRef]
- Jouany, J.P. Methods for preventing, decontaminating and minimizing the toxicity of mycotoxins in feeds. Anim. Feed Sci. Technol. 2007, 137, 342–362. [Google Scholar] [CrossRef]
- Eller, M.S.; Holland, J.B.; Payne, G.A. Breeding for improved resistance to fumonisin contamination in maize. Toxin Rev. 2008, 27, 371–389. [Google Scholar] [CrossRef]
- Shephard, G.S. Determination of mycotoxins in maize. In Mycotoxin Reduction in Grain Chains; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 26–36. [Google Scholar]
- Löffler, M.; Miedaner, T.; Kessel, B.; Ouzunova, M. Mycotoxin accumulation and corresponding ear rot rating in three maturity groups of European maize inoculated by two fusarium species. Euphytica 2010, 174, 153–164. [Google Scholar] [CrossRef]
- Williams, W.P.; Windham, G.L. Diallel analysis of fumonisin accumulation in maize. Field Crop. Res. 2009, 114, 324–326. [Google Scholar] [CrossRef]
- Pérez-Brito, D.; Jeffers, D.; González-de-León, D.; Khairallah, M.; Cortés-Cruz, M.; Velázquez-Cardelas, G.; Azpíroz-Rivero, S.; Srinivasan, G. QTL mapping of Fusarium moniliforme ear rot resistance in highland maize, Mexico. Agrociencia 2001, 35, 181–196. [Google Scholar]
- Hung, H.-Y.; Holland, J.B. Diallel analysis of resistance to fusarium ear rot and fumonisin contamination in maize. Crop Sci. 2012, 52, 2173–2181. [Google Scholar] [CrossRef]
- Butron, A.; Reid, L.M.; Santiago, R.; Cao, A.; Malvar, R.A. Inheritance of maize resistance to gibberella and fusarium ear rots and kernel contamination with deoxynivalenol and fumonisins. Plant Pathol. 2015, 64, 1053–1060. [Google Scholar] [CrossRef]
- Netshifhefhe, N.E.I.; Flett, B.C.; Viljoen, A.; Rose, L.J. Inheritance and genotype by environment analyses of resistance to Fusarium verticillioides and fumonisin contamination in maize F1 hybrids. Euphytica 2018, 214, 20. [Google Scholar] [CrossRef]
- Eller, M.S.; Payne, G.A.; Holland, J.B. Selection for reduced fusarium ear rot and fumonisin content in advanced backcross maize lines and their topcross hybrids. Crop Sci. 2010, 50, 2249–2260. [Google Scholar] [CrossRef]
- Presello, D.A.; Pereyra, A.O.; Iglesias, J.; Fauguel, C.M.; Sampietro, D.A.; Eyherabide, G.H. Responses to selection of S5 inbreds for broad-based resistance to ear rots and grain mycotoxin contamination caused by Fusarium spp. in maize. Euphytica 2011, 178, 23–29. [Google Scholar] [CrossRef]
- Horne, D.W.; Eller, M.S.; Holland, J.B. Responses to recurrent index selection for reduced fusarium ear rot and lodging and for increased yield in maize. Crop Sci. 2016, 56, 85–94. [Google Scholar] [CrossRef]
- Chiuraise, N.; Derera, J.; Yobo, K.S.; Magorokosho, C.; Nunkumar, A.; Qwabe, N.F.P. Progress in stacking aflatoxin and fumonisin contamination resistance genes in maize hybrids. Euphytica 2016, 207, 49–67. [Google Scholar] [CrossRef]
- Gaikpa, D.S.; Miedaner, T. Genomics-assisted breeding for ear rot resistances and reduced mycotoxin contamination in maize: Methods, advances and prospects. Theor. Appl. Genet. 2019, 132, 2721–2739. [Google Scholar] [CrossRef]
- VanOpdorp, N.J.; Koehler, K.L. Genetic loci associated with Fusarium ear rot (FKR) resistance in maize and generation of improved FKR resistant maize inbred lines c Agrigenetics. Inc. Patent 2010. US20100269212A1, 21 October 2010. [Google Scholar]
- Robertson-Hoyt, L.A.; Jines, M.P.; Balint-Kurti, P.J.; Kleinschmidt, C.E.; White, D.G.; Payne, G.A.; Maragos, C.M.; Molnar, T.L.; Holland, J.B. QTL mapping for fusarium ear rot and fumonisin contamination resistance in two maize populations. Crop Sci. 2006, 46, 1734–1743. [Google Scholar] [CrossRef]
- Ding, J.-Q.; Wang, X.-M.; Chander, S.; Yan, J.-B.; Li, J.-S. QTL mapping of resistance to fusarium ear rot using a RIL population in maize. Mol. Breed. 2008, 22, 395–403. [Google Scholar] [CrossRef]
- Chen, J.F.; Ding, J.Q.; Li, H.M.; Li, Z.M.; Sun, X.D.; Li, J.J.; Wang, R.X.; Dai, X.D.; Dong, H.F.; Song, W.B.; et al. Detection and verification of quantitative trait loci for resistance to fusarium ear rot in maize. Mol. Breed. 2012, 30, 1649–1656. [Google Scholar] [CrossRef]
- Li, Z.M.; Ding, J.Q.; Wang, R.X.; Chen, J.F.; Sun, X.D.; Chen, W.; Song, W.B.; Dong, H.F.; Dai, X.D.; Xia, Z.L.; et al. A new QTL for resistance to fusarium ear rot in maize. J. Appl. Genet. 2011, 52, 403–406. [Google Scholar] [CrossRef]
- Chen, J.; Shrestha, R.; Ding, J.Q.; Zheng, H.J.; Mu, C.H.; Wu, J.Y.; Mahuku, G. Genome-wide association study and QTL mapping reveal genomic loci associated with fusarium ear rot resistance in tropical maize germplasm. G3-Genes Genomes Genet. 2016, 6, 3803–3815. [Google Scholar] [CrossRef]
- Morales, L.; Zila, C.T.; Mejia, D.E.M.; Arbelaez, M.M.; Balint-Kurti, P.J.; Holland, J.B.; Nelson, R.J. Diverse components of resistance to Fusarium verticillioides infection and fumonisin contamination in four maize recombinant inbred families. Toxins 2019, 11, 17. [Google Scholar] [CrossRef]
- Giomi, G.M.; Kreff, E.D.; Iglesias, J.; Fauguel, C.M.; Fernandez, M.; Oviedo, M.S.; Presello, D.A. Quantitative trait loci for fusarium and gibberella ear rot resistance in Argentinian maize germplasm. Euphytica 2016, 211, 287–294. [Google Scholar] [CrossRef]
- Maschietto, V.; Colombi, C.; Pirona, R.; Pea, G.; Strozzi, F.; Marocco, A.; Rossini, L.; Lanubile, A. QTL mapping and candidate genes for resistance to fusarium ear rot and fumonisin contamination in maize. BMC Plant Biol. 2017, 17. [Google Scholar] [CrossRef]
- Xiang, K.; Zhang, Z.M.; Reid, L.M.; Zhu, X.Y.; Yuan, G.S.; Pan, G.T. A meta-analysis of QTL associated with ear rot resistance in maize. Maydica 2010, 55, 281–290. [Google Scholar]
- Morales, L.; Marino, T.P.; Wenndt, A.J.; Fouts, J.Q.; Holland, J.B.; Nelson, R.J. Dissecting symptomatology and fumonisin contamination produced by Fusarium verticillioides in maize ears. Phytopathology 2018, 108, 1475–1485. [Google Scholar] [CrossRef]
- Wu, Y.B.; Zhou, Z.J.; Dong, C.P.; Chen, J.F.; Ding, J.Q.; Zhang, X.C.; Mu, C.; Chen, Y.N.; Li, X.P.; Li, H.M.; et al. Linkage mapping and genome-wide association study reveals conservative QTL and candidate genes for fusarium rot resistance in maize. BMC Genom. 2020, 21, 11. [Google Scholar] [CrossRef]
- Zila, C.T.; Fernando Samayoa, L.; Santiago, R.; Butron, A.; Holland, J.B. A genome-wide association study reveals genes associated with fusarium ear rot resistance in a maize core diversity panel. G3-Genes Genomes Genet. 2013, 3, 2095–2104. [Google Scholar] [CrossRef]
- Zila, C.T.; Ogut, F.; Romay, M.C.; Gardner, C.A.; Buckler, E.S.; Holland, J.B. Genome-wide association study of fusarium ear rot disease in the U.S.A. maize inbred line collection. BMC Plant Biol. 2014. [Google Scholar] [CrossRef]
- Coan, M.M.D.; Senhorinho, H.J.C.; Pinto, R.J.B.; Scapim, C.A.; Tessmann, D.J.; Williams, W.P.; Warburton, M.L. Genome-wide association study of resistance to ear rot by Fusarium verticillioides in a tropical field maize and popcorn core collection. Crop Sci. 2018, 58, 564–578. [Google Scholar] [CrossRef]
- de Jong, G.; Pamplona, A.K.A.; Von Pinho, R.G.; Balestre, M. Genome-wide association analysis of ear rot resistance caused by Fusarium verticillioides in maize. Genomics 2018, 110, 291–303. [Google Scholar] [CrossRef]
- Butron, A.; Santiago, R.; Cao, A.; Samayoa, L.F.; Malvar, R.A. QTLs for resistance to fusarium ear rot in a multiparent advanced generation intercross (MAGIC) maize population. Plant Dis. 2019, 103, 897–904. [Google Scholar] [CrossRef]
- Samayoa, L.F.; Cao, A.; Santiago, R.; Malvar, R.A.; Butron, A. Genome-wide association analysis for fumonisin content in maize kernels. BMC Plant Biol. 2019, 19. [Google Scholar] [CrossRef]
- Munkvold, G.P.; McGee, D.C.; Carlton, W.M. Importance of different pathways for maize kernel infection by Fusarium moniliforme. Phytopathology 1997, 87, 209–217. [Google Scholar] [CrossRef]
- Munkvold, G.P.; Carlton, W.M. Influence of inoculation method on systemic Fusarium moniliforme infection of maize plants grown from infected seeds. Plant Dis. 1997, 81, 211–216. [Google Scholar] [CrossRef]
- Venturini, G.; Assante, G.; Vercesi, A. Fusarium verticillioides contamination patterns in northern Italian maize during the growing season. Phytopathol. Mediterr. 2011, 50, 110–120. [Google Scholar]
- Oren, L.; Ezrati, S.; Cohen, D.; Sharon, A. Early events in the Fusarium verticillioides-maize interaction characterized by using a green fluorescent protein-expressing transgenic isolate. Appl. Environ. Microbiol. 2003, 69, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Ju, M.; Zhou, Z.J.; Mu, C.; Zhang, X.C.; Gao, J.Y.; Liang, Y.K.; Chen, J.F.; Wu, Y.B.; Li, X.P.; Wang, S.W.; et al. Dissecting the genetic architecture of Fusarium verticillioides seed rot resistance in maize by combining QTL mapping and genome-wide association analysis. Sci. Rep. 2017, 7, 46446. [Google Scholar] [CrossRef]
- Septiani, P.; Lanubile, A.; Stagnati, L.; Busconi, M.; Nelissen, H.; Pe, M.E.; Dell’Acqua, M.; Marocco, A. Unravelling the genetic basis of fusarium seedling rot resistance in the magic maize population: Novel targets for breeding. Sci. Rep. 2019, 9, 5665. [Google Scholar] [CrossRef]
- Stagnati, L.; Lanubile, A.; Samayoa, L.F.; Bragalanti, M.; Giorni, P.; Busconi, M.; Holland, J.B.; Marocco, A. A genome wide association study reveals markers and genes associated with resistance to Fusarium verticillioides infection of seedlings in a maize diversity panel. G3 (BethesdaMd.) 2019, 9, 571–579. [Google Scholar] [CrossRef]
- Mu, C.; Gao, J.Y.; Zhou, Z.J.; Wang, Z.; Sun, X.D.; Zhang, X.C.; Dong, H.F.; Han, Y.A.; Li, X.P.; Wu, Y.B.; et al. Genetic analysis of cob resistance to F. verticillioides: Another step towards the protection of maize from ear rot. Theor. Appl. Genet. 2019, 132, 1049–1059. [Google Scholar] [CrossRef]
- Ye, J.R.; Zhong, T.; Zhang, D.F.; Ma, C.Y.; Wang, L.N.; Yao, L.S.; Zhang, Q.Q.; Zhu, M.; Xu, M.L. The auxin-regulated protein ZmAuxRP1 coordinates the balance between root growth and stalk rot disease resistance in maize. Mol. Plant. 2019, 12, 360–373. [Google Scholar] [CrossRef]
- Glenn, A.E.; Hinton, D.M.; Yates, I.E.; Bacon, C.W. Detoxification of corn antimicrobial compounds as the basis for isolating Fusarium verticillioides and some other Fusarium species from corn. Appl. Environ. Microbiol. 2001, 67, 2973–2981. [Google Scholar] [CrossRef] [PubMed]
- Lanubile, A.; Maschietto, V.; Borrelli, V.M.; Stagnati, L.; Logrieco, A.F.; Marocco, A. Molecular basis of resistance to fusarium ear rot in maize. Front. Plant Sci. 2017, 8, 1774. [Google Scholar] [CrossRef]
- Lanubile, A.; Pasini, L.; Marocco, A. Differential gene expression in kernels and silks of maize lines with contrasting levels of ear rot resistance after Fusarium verticillioides infection. J. Plant Physiol. 2010, 167, 1398–1406. [Google Scholar] [PubMed]
- Lanubile, A.; Bernardi, J.; Marocco, A.; Logrieco, A.; Paciolla, C. Differential activation of defense genes and enzymes in maize genotypes with contrasting levels of resistance to Fusarium verticillioides. Environ. Exp. Bot. 2012, 78, 39–46. [Google Scholar] [CrossRef]
- Lanubile, A.; Bernardi, J.; Battilani, P.; Logrieco, A.; Marocco, A. Resistant and susceptible maize genotypes activate different transcriptional responses against Fusarium verticillioides. Physiol. Mol. Plant Pathol. 2012, 77, 52–59. [Google Scholar] [CrossRef]
- Yuan, G.S.; Zhang, Z.M.; Xiang, K.; Zhao, M.J.; Shen, Y.O.; Pan, G.T. Large-scale identification of differentially expressed genes in maize inbreds susceptible and resistant to fusarium ear rot. Plant Omics 2012, 5, 471–475. [Google Scholar]
- Yuan, G.S.; Zhang, Z.M.; Xiang, K.; Shen, Y.O.; Du, J.; Lin, H.J.; Liu, L.; Zhao, M.J.; Pan, G.T. Different gene expressions of resistant and susceptible maize inbreds in response to Fusarium verticillioides infection. Plant Mol. Biol. Rep. 2013, 31, 925–935. [Google Scholar] [CrossRef]
- Campos-Bermudez, V.A.; Fauguel, C.M.; Tronconi, M.A.; Casati, P.; Presello, D.A.; Andreo, C.S. Transcriptional and metabolic changes associated to the infection by Fusarium verticillioides in maize inbreds with contrasting ear rot resistance. PLoS ONE 2013, 8, e61580. [Google Scholar] [CrossRef]
- Lanubile, A.; Ferrarini, A.; Maschietto, V.; Delledonne, M.; Marocco, A.; Bellin, D. Functional genomic analysis of constitutive and inducible defense responses to Fusarium verticillioides infection in maize genotypes with contrasting ear rot resistance. BMC Genom. 2014, 15, 710. [Google Scholar] [CrossRef]
- Wang, Y.P.; Zhou, Z.J.; Gao, J.Y.; Wu, Y.B.; Xia, Z.L.; Zhang, H.Y.; Wu, J.Y. The mechanisms of maize resistance to Fusarium verticillioides by comprehensive analysis of RNA-seq data. Front. Plant Sci. 2016, 7, 1654. [Google Scholar] [CrossRef]
- Yuan, G.S.; Xiang, K.; Zhang, Z.M.; Shen, Y.O.; Du, J.; Lin, H.J.; Liu, L.; Pan, G.T. Analysis on the relationship between Fusarium verticillioides infection-induced genes and ear rot resistance in maize. J. Food Agric. Environ. 2013, 11, 363–366. [Google Scholar]
- Bravo, J.M.; Campo, S.; Murillo, I.; Coca, M.; Segundo, B.S. Fungus- and wound-induced accumulation of mRNA containing a class ii chitinase of the pathogenesis-related protein 4 (PR-4) family of maize. Plant Mol. Biol. 2003, 52, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Murillo, I.; Cavallarin, L.; San Segundo, B. Cytology of infection of maize seedlings by Fusarium moniliforme and immunolocalization of the pathogenesis-related PRMs protein. Phytopathology 1999, 89, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Maschietto, V.; Lanubile, A.; De Leonardis, S.; Marocco, A.; Paciolla, C. Constitutive expression of pathogenesis-related proteins and antioxydant enzyme activities triggers maize resistance towards Fusarium verticillioides. J. Plant Physiol. 2016, 200, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Lanubile, A.; Maschietto, V.; De Leonardis, S.; Battilani, P.; Paciolla, C.; Marocco, A. Defense responses to mycotoxin-producing fungi Fusarium proliferatum, F. subglutinans, and Aspergillus flavus in kernels of susceptible and resistant maize genotypes. Mol. Plant-Microbe Interact. 2015, 28, 546–557. [Google Scholar] [CrossRef]
- Battilani, P.; Lanubile, A.; Scala, V.; Reverberi, M.; Gregori, R.; Falavigna, C.; Dall’asta, C.; Park, Y.S.; Bennett, J.; Borrego, E.J.; et al. Oxylipins from both pathogen and host antagonize jasmonic acid-mediated defence via the 9-lipoxygenase pathway in Fusarium verticillioides infection of maize. Mol. Plant Pathol. 2018, 19, 2162–2176. [Google Scholar] [CrossRef]
- Maschietto, V.; Marocco, A.; Malachova, A.; Lanubile, A. Resistance to Fusarium verticillioides and fumonisin accumulation in maize inbred lines involves an earlier and enhanced expression of lipoxygenase (lox) genes. J. Plant Physiol. 2015, 188, 9–18. [Google Scholar] [CrossRef]
- Lanubile, A.; Logrieco, A.; Battilani, P.; Proctor, R.H.; Marocco, A. Transcriptional changes in developing maize kernels in response to fumonisin-producing and nonproducing strains of Fusarium verticillioides. Plant Sci. 2013, 210, 183–192. [Google Scholar] [CrossRef]
- Fauguel, C.M.; Bermudez, V.A.C.; Iglesias, J.; Fernandez, M.; Farroni, A.; Andreo, C.S.; Presello, D.A. Volatile compounds released by maize grains and silks in response to infection by Fusarium verticillioides and its association with pathogen resistance. Plant Pathol. 2017, 66, 1128–1138. [Google Scholar] [CrossRef]
- Gao, X.Q.; Shim, W.B.; Gobel, C.; Kunze, S.; Feussner, I.; Meeley, R.; Balint-Kurti, P.; Kolomiets, M. Disruption of a maize 9-lipoxygenase results in increased resistance to fungal pathogens and reduced levels of contamination with mycotoxin fumonisin. Mol. Plant-Microbe Interact. 2007, 20, 922–933. [Google Scholar] [CrossRef]
- Christensen, S.A.; Nemchenko, A.; Park, Y.S.; Borrego, E.; Huang, P.C.; Schmelz, E.A.; Kunze, S.; Feussner, I.; Yalpani, N.; Meeley, R.; et al. The novel monocot-specific 9-lipoxygenase zmlox12 is required to mount an effective jasmonate-mediated defense against Fusarium verticillioides in maize. Mol. Plant-Microbe Interact. 2014, 27, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Scala, V.; Beccaccioli, M.; Dall’Asta, C.; Giorni, P.; Fanelli, C. Analysis of the expression of genes related to oxylipin biosynthesis in Fusarium verticillioides and maize kernels during their interaction. J. Plant Pathol. 2015, 97, 193–197. [Google Scholar]
- Constantino, N.N.; Mastouri, F.; Damarwinasis, R.; Borrego, E.J.; Moran-Diez, M.E.; Kenerley, C.M.; Gao, X.Q.; Kolomiets, M.V. Root-expressed maize lipoxygenase 3 negatively regulates induced systemic resistance to Colletotrichum graminicola in shoots. Front. Plant Sci. 2013, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Agostini, R.B.; Postigo, A.; Rius, S.P.; Rech, G.E.; Campos-Bermudez, V.A.; Vargas, W.A. Long-lasting primed state in maize plants: Salicylic acid and steroid signaling pathways as key players in the early activation of immune responses in silks. Mol. Plant-Microbe Interact. 2019, 32, 95–106. [Google Scholar] [CrossRef]
- Atanasova-Penichon, V.; Barreau, C.; Richard-Forget, F. Antioxidant secondary metabolites in cereals: Potential involvement in resistance to Fusarium and mycotoxin accumulation. Front. Microbiol. 2016, 7, 16. [Google Scholar] [CrossRef]
- Venturini, G.; Babazadeh, L.; Casati, P.; Pilu, R.; Salomoni, D.; Toffolatti, S.L. Assessing pigmented pericarp of maize kernels as possible source of resistance to fusarium ear rot, Fusarium spp. infection and fumonisin accumulation. Int. J. Food Microbiol. 2016, 227, 56–62. [Google Scholar] [CrossRef]
- Christensen, S.A.; Sims, J.; Vaughan, M.M.; Hunter, C.; Block, A.; Willett, D.; Alborn, H.T.; Huffaker, A.; Schmelz, E.A. Commercial hybrids and mutant genotypes reveal complex protective roles for inducible terpenoid defenses in maize. J. Exp. Bot. 2018, 69, 1693–1705. [Google Scholar] [CrossRef]
- Diaz-Gomez, J.; Marin, S.; Nogareda, C.; Sanchis, V.; Ramos, A.J. The effect of enhanced carotenoid content of transgenic maize grain on fungal colonization and mycotoxin content. Mycotoxin Res. 2016, 32, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Blandino, M.; Reyneri, A. Comparison between normal and waxy maize hybrids for Fusarium-toxin contamination in NW Italy. Maydica 2007, 52, 127–134. [Google Scholar]
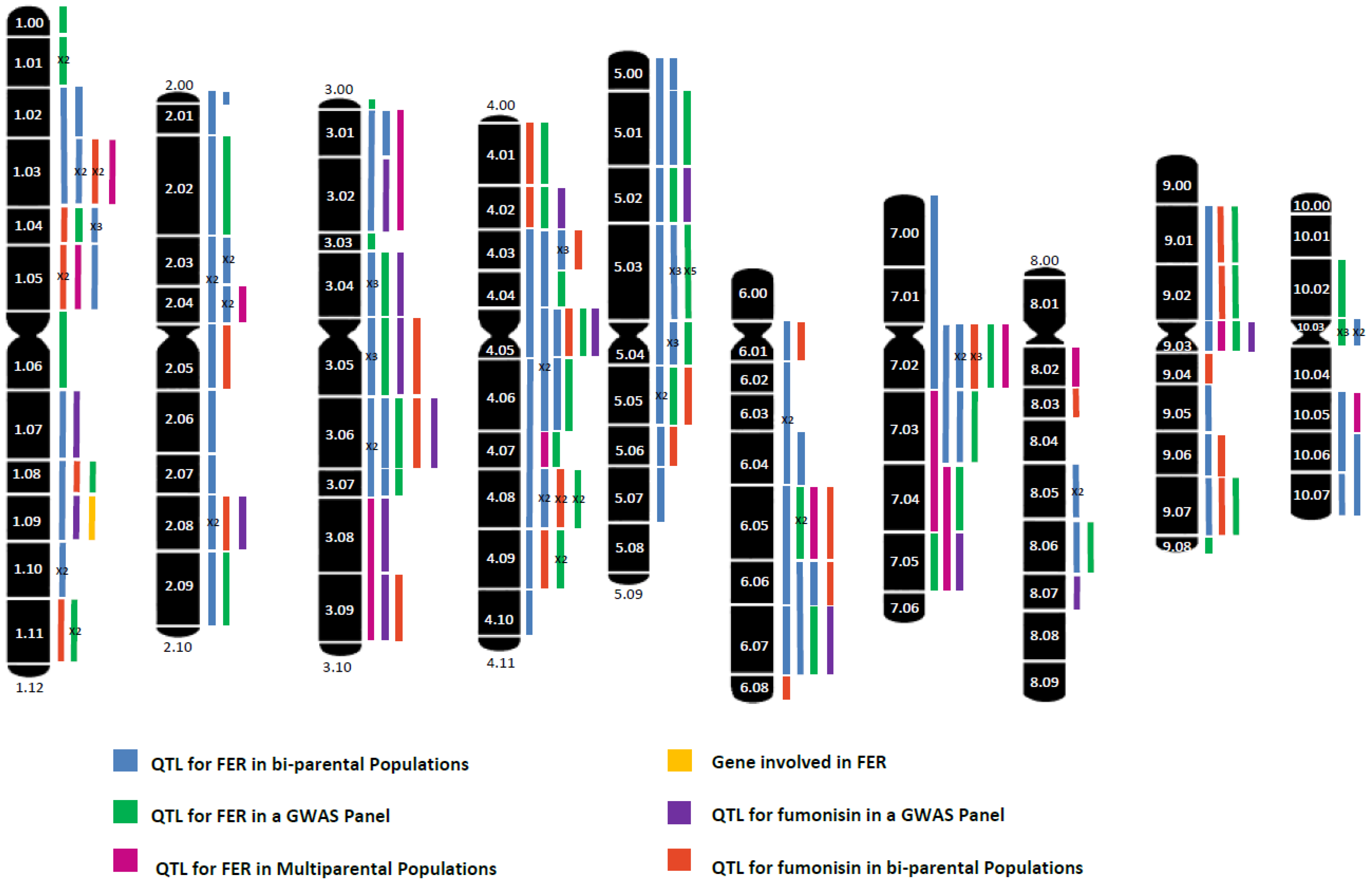
| Traits | Type of QTL Mapping | Mapping Population | Number and Type of Markers | Reference |
|---|---|---|---|---|
| FER | Linkage mapping | 238-individuals F2 | 149 RFLP 5 | Pérez-Brito et al. 2001, [43] |
| FER | Linkage mapping | 206-individuals F2 | 106 RFLP | Pérez-Brito et al. 2001, [43] |
| FER and FUM | Linkage mapping | 213 BC1F1:2 families from (GE440 × FR1064) × FR1064 | 105 SSR 6 | Robertson-Hoyt et al. 2006, [53] |
| FER and FUM | Linkage mapping | 143 RIL 2 from NC300 × B104 | 113 SSR | Robertson-Hoyt et al. 2006, [53] |
| FER | Linkage mapping | 187 RIL from 87-1 × Zong 3 | 246 SSR | Ding et al. 2008, [54] |
| FER | Linkage mapping | 210 F2:3 from BT-1 × Xi502 | 178 SSR | Chen et al. 2012, [55] |
| FER | Linkage mapping | 250 RIL from BT-1 × N6 | 207 SSR | Li et al. 2011, [56] |
| FER | Linkage mapping | 201 DH from CML495 × susceptible parent | 166 SNP 7 | Chen et al. 2016, [57] |
| FER | Linkage mapping | 277 F2:3 families from CML492 × LPSMT | 154 SNP | Chen et al. 2016, [57] |
| FER | Linkage mapping | 268 F2:3 families from CML495 × LPSMT t | 118 SNP | Chen et al. 2016, [57] |
| FER | Linkage mapping | 272 F2:3 families from CML449 × LPSMT | 93 SNP | Chen et al. 2016, [57] |
| FER | GWAS 1 | 854 tropical inbreds | 43,424 SNP | Chen et al. 2016, [57] |
| FER and FUM | Stepwise regression | Four RIL populations from a NAM 3 | 7386 GBS 8 markers | Morales et al. 2019, [58] |
| FER | Linkage mapping | 298 RIL from LP4637 × L4674 | 250 SNP | Giomi et al. 2016, [59] |
| FER and FUM | Linkage mapping | 188 F2:3 families from CO441 × CO354 | 41 SSR and 342 SNP | Maschietto et al. 2017, [60] |
| FER | Linkage mapping | 250 RIL from BT-1 × N6 | 222 SSR | Wu et al. 2020, [63] |
| FER | GWAS | 265 inbreds | 224,152 SSR | Wu et al. 2020, [63] |
| FER | GWAS | 267 inbreds from the “Goodman” association panel | 47,445 SNP | Zila et al. 2013, [64] |
| FER | GWAS | 1687 inbreds from the USDA maize gene bank | 200,978 SNP | Zila et al. 2014, [65] |
| FER | GWAS | 183 tropical inbreds (85 popcorn inbreds) | 267,525 SNP | Coan et al. 2018, [66] |
| FER | GWAS | 242 inbreds | 23,153 DArT-seq 9 markers | de Jong et al. 2018, [67] |
| FER | GWAS | 339 RIL from a MAGIC 4 | 58,556 SNP | Butrón et al. 2019, [68] |
| FUM | GWAS | 256 inbreds from the “Goodman” association panel | 226,446 SNP | Samayoa et al. 2019, [69] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago, R.; Cao, A.; Malvar, R.A.; Butrón, A. Genomics of Maize Resistance to Fusarium Ear Rot and Fumonisin Contamination. Toxins 2020, 12, 431. https://doi.org/10.3390/toxins12070431
Santiago R, Cao A, Malvar RA, Butrón A. Genomics of Maize Resistance to Fusarium Ear Rot and Fumonisin Contamination. Toxins. 2020; 12(7):431. https://doi.org/10.3390/toxins12070431
Chicago/Turabian StyleSantiago, Rogelio, Ana Cao, Rosa Ana Malvar, and Ana Butrón. 2020. "Genomics of Maize Resistance to Fusarium Ear Rot and Fumonisin Contamination" Toxins 12, no. 7: 431. https://doi.org/10.3390/toxins12070431
APA StyleSantiago, R., Cao, A., Malvar, R. A., & Butrón, A. (2020). Genomics of Maize Resistance to Fusarium Ear Rot and Fumonisin Contamination. Toxins, 12(7), 431. https://doi.org/10.3390/toxins12070431






