Fluorescence Polarization Immunoassay for the Determination of T-2 and HT-2 Toxins and Their Glucosides in Wheat
Abstract
1. Introduction
2. Results and Discussion
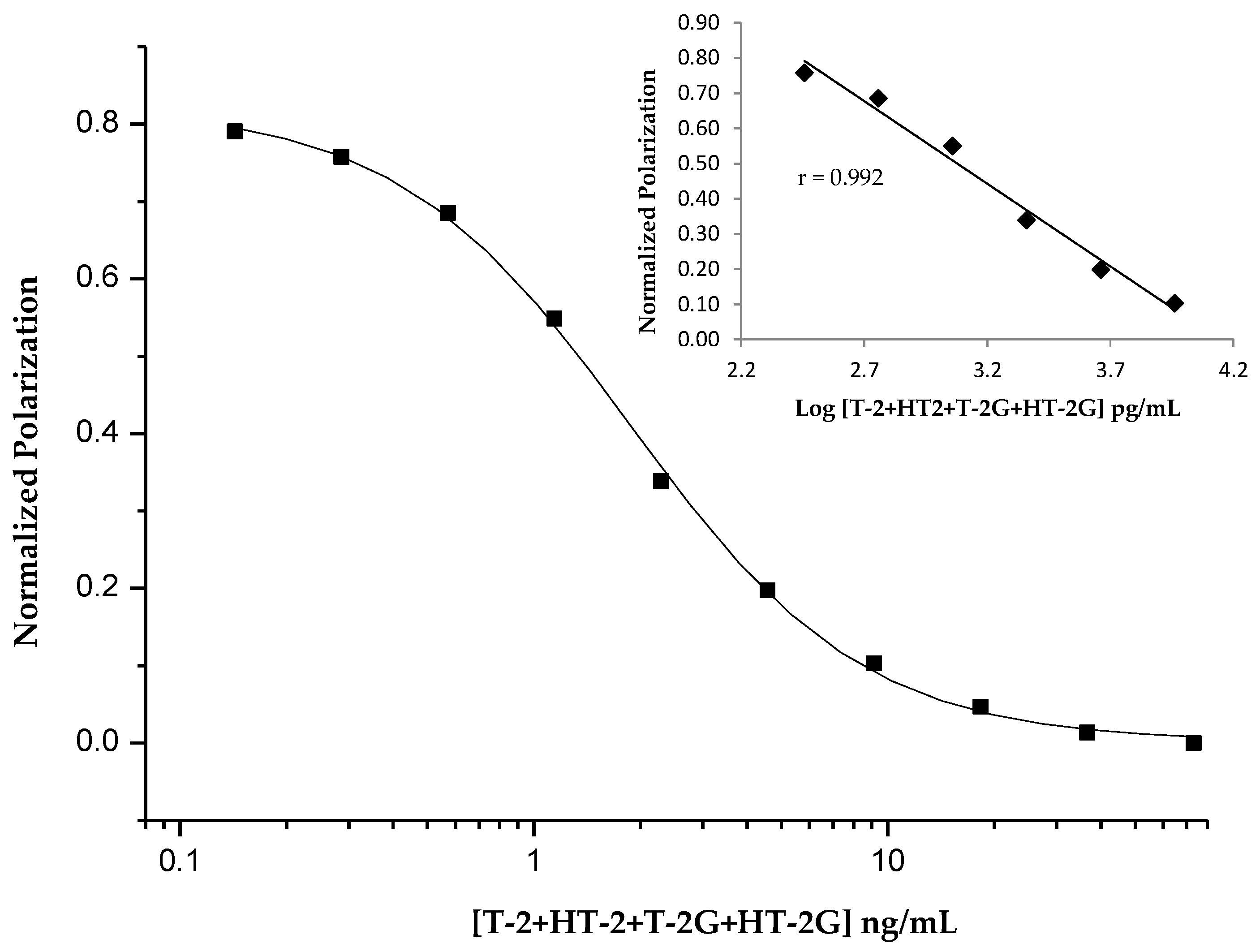
2.1. Development of the FPIA
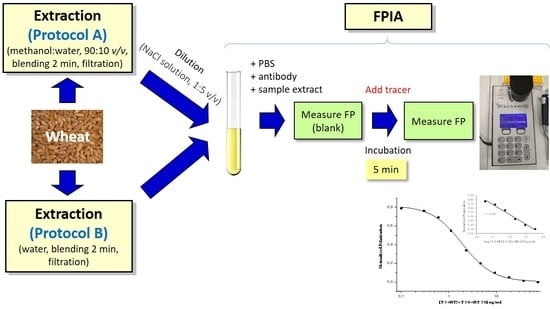
2.2. Testing of Extraction Protocols and Evaluation of Matrix Effects
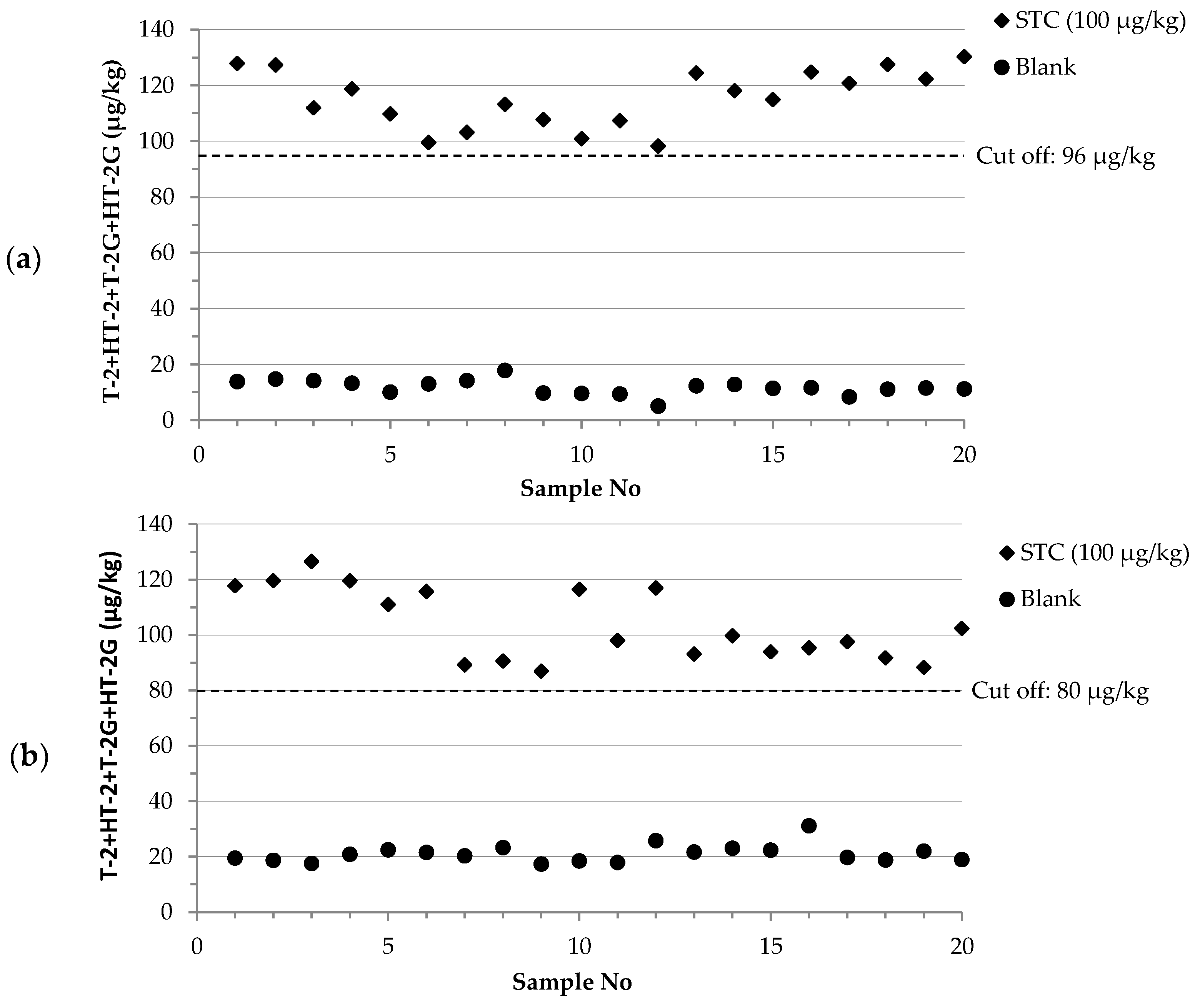
2.3. Validation of the Methods
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Immunoassay Reagent Solutions
4.3. Sample Preparations
4.4. FPIA Analysis
4.5. Evaluation of Matrix Effects
4.6. Validation as Quantitative Methods
4.7. Validation as Screening Methods
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Disclaimer
References
- Canady, R.A.; Coker, R.D.; Egan, S.K.; Krska, R.; Olsen, M.; Resnik, S.; Schlatter, J. T-2 and HT-2 toxins. In Safety Evaluation of Certain Mycotoxins in Food; WHO/FAO, Ed.; WHO Food Additives Series 47, FAO Food and Nutrition Paper 74; WHO: Geneva, Switzerland, 2001; pp. 557–638. Available online: http://www.fao.org/3/a-bc528e.pdf (accessed on 24 April 2019).
- Schothorst, R.C.; van Egmond, H.P. Report from SCOOP task 3.2.10 “Collection of occurrence data of Fusarium toxins in food assessment of dietary intake by the population of EU member states” subtask: Trichothecenes. Toxicol. Lett. 2004, 153, 133–153. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Scientific opinion on the risks for animal and public health related to the presence of T-2 and HT-2 toxin in food and feed. EFSA J. 2011, 9, 2481. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Human and animal dietary exposure to T-2 and HT-2 toxin. EFSA J. 2017, 15, 4972. [Google Scholar]
- Pettersson, H. Toxicity and risks with T-2 and HT-2 toxins in cereals. Plant Breed. Seed Sci. 2011, 64, 65–74. [Google Scholar] [CrossRef]
- European Commission (EC). Commission recommendation of 27 March 2013 on the presence of T-2 and HT-2 toxin in cereals and cereal products (2013/165/EU). Off. J. Eur. Union 2013, 91, 12–15. [Google Scholar]
- European Food Safety Authority (EFSA). Appropriateness to set a group health based guidance value for T2 and HT2 toxin and its modified forms. EFSA J. 2017, 15, 4655. [Google Scholar]
- Lattanzio, V.M.T.; Visconti, A.; Haidukowski, M.; Pascale, M. Identification and characterization of new Fusarium masked mycotoxins, T2 and HT2 glycosyl derivatives, in naturally contaminated wheat and oats by liquid chromatography-high-resolution mass spectrometry. J. Mass Spectrom. 2012, 47, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Veprikova, Z.; Vaclavikova, M.; Lacina, O.; Dzuman, Z.; Zachariasova, M.; Hajslova, J. Occurrence of mono- and di-glycosylated conjugates of T-2 and HT-2 toxins in naturally contaminated cereals. World Mycotoxin J. 2012, 5, 231–240. [Google Scholar] [CrossRef]
- Nakagawa, H.; Sakamoto, S.; Sago, Y.; Kushiro, M.; Nagashima, H. The use of LC-Orbitrap MS for the detection of Fusarium masked mycotoxins: The case of type A trichothecenes. World Mycotoxin J. 2012, 5, 271–280. [Google Scholar] [CrossRef]
- Lattanzio, V.M.T.; Ciasca, B.; Terzi, V.; Ghizzoni, R.; McCormick, S.P.; Pascale, M. Study of the natural occurrence of T-2 and HT-2 toxins and their glucosyl derivatives from field barley to malt by high resolution Orbitrap mass spectrometry. Food Addit. Contam. Part A 2015, 32, 1647–1655. [Google Scholar] [CrossRef]
- Bryla, M.; Waskiewicz, A.; Ksieniewicz-Wozniak, E.; Szymczyk, K.; Jedrzejczak, R. Modified Fusarium mycotoxins in cereals and their products-metabolism, occurrence, and toxicity: An updated review. Molecules 2018, 23, 963. [Google Scholar] [CrossRef] [PubMed]
- Meneely, J.P.; Ricci, F.; van Egmond, H.P.; Elliott, C.T. Current methods of analysis for the determination of trichothecene mycotoxins in food. TrAC Trends Anal. Chem. 2011, 30, 192–203. [Google Scholar] [CrossRef]
- Pascale, M.; Panzarini, G.; Visconti, A. Determination of HT-2 and T-2 toxins in oats and wheat by ultra-performance liquid chromatography with photodiode array detection. Talanta 2012, 89, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Krska, R.; Malachova, A.; Berthiller, F.; Van Egmond, H.P. Determination of T-2 and HT-2 toxins in food and feed: An update. World Mycotoxin J. 2014, 7, 131–142. [Google Scholar] [CrossRef]
- Nathanail, A.V.; Syvähuoko, J.; Malachová, A.; Jestoi, M.; Varga, E.; Michlmayr, H.; Adam, G.; Sieviläinen, E.; Berthiller, F.; Peltonen, K. Simultaneous determination of major type A and B trichothecenes, zearalenone and certain modified metabolites in Finnish cereal grains with a novel liquid chromatography-tandem mass spectrometric method. Anal. Bioanal. Chem. 2015, 407, 4745–4755. [Google Scholar] [CrossRef]
- Leslie, J.F.; Lattanzio, V.; Audenaert, K.; Battilani, P.; Cary, J.; Chulze, S.N.; De Saeger, S.; Gerardino, A.; Karlovsky, P.; Liao, Y.-C.; et al. Mycokey round table discussions on future directions in research on chemical detection methods, genetics and biodiversity of mycotoxins. Toxins 2018, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Chang, F.; Wang, Y.; Chen, D.; Liu, Z.; Zhou, X.; Feng, L.; Yuan, Z. Development of a sensitive monoclonal-based enzyme-linkedimmunosorbentassay for monitoring T-2 toxin in food and feed. Food Addit. Contam. A 2016, 33, 683–692. [Google Scholar] [CrossRef]
- Li, Y.; Luo, X.; Yang, S.; Cao, X.; Wang, Z.; Shi, W.; Zhang, S. High specific monoclonal antibody production and development of an ELISA method for monitoring T-2 toxin in rice. J. Agric. Food Chem. 2014, 62, 1492–1497. [Google Scholar] [CrossRef]
- Wang, J.; Duan, S.; Zhang, Y.; Wang, S. Enzyme-linked immunosorbent assay for the determination of T-2 toxin in cereals and feedstuff. Microchim. Acta 2010, 169, 137–144. [Google Scholar] [CrossRef]
- Yoshizawa, T.; Kohno, H.; Ikeda, K.; Shinoda, T.; Yokohama, H.; Morita, K.; Kusada, O.; Kobayashi, Y. A practical method for measuring deoxynivalenol, nivalenol, and T-2 + HT-2 toxin in foods by an enzyme-linked immunosorbent assay using monoclonal antibodies. Biosci. Biotechnol. Biochem. 2004, 68, 2076–2085. [Google Scholar] [CrossRef]
- Molinelli, A.; Grossalber, K.; Führer, M.; Baumgartner, S.; Sulyok, M.; Krska, R. Development of qualitative and semiquantitative immunoassay-based rapid strip tests for the detection of T-2 toxin in wheat and oat. J. Agric. Food Chem. 2008, 56, 2589–2594. [Google Scholar] [CrossRef] [PubMed]
- Lippolis, V.; Pascale, M.; Valenzano, S.; Pluchinotta, V.; Baumgartner, S.; Krska, R.; Visconti, A. A rapid fluorescence polarization immunoassay for the determination of T-2 and HT-2 toxins in wheat. Anal. Bioanal. Chem. 2011, 401, 2561–2571. [Google Scholar] [CrossRef] [PubMed]
- Porricelli, A.C.R.; Lippolis, L.; Valenzano, S.; Cortese, M.; Suman, M.; Zanardi, S.; Pascale, M. Optimization and validation of a fluorescence polarization immunoassay for rapid detection of T-2 and HT-2 toxins in cereals and cereal-based products. Food Anal. Methods 2016, 9, 3310–3318. [Google Scholar] [CrossRef]
- Meneely, J.P.; Sulyok, M.; Baumgartner, S.; Krska, R.; Elliott, C.T. A rapid optical immunoassay for the screening of T-2 and HT-2 toxin in cereals and maize-based baby food. Talanta 2010, 81, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, N.; Ning, B.; Liu, M.; Lv, Z.; Sun, Z.; Peng, Y.; Chen, C.; Li, J.; Gao, Z. Simultaneous and rapid detection of six different mycotoxins using an immunochip. Biosens. Bioelectron. 2012, 34, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.M.; Zhao, S.; Niazi, S.; Mohsin, A.; Shoaib, M.; Duan, N.; Wu, S.; Wang, Z. Silver nanoclusters based FRET aptasensor for sensitive and selective fluorescent detection of T-2 toxin. Sensor. Actuators B Chem. 2018, 277, 328–335. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.; Yuan, S.; Sun, X.; Huo, B.; Bai, J.; Peng, Y.; Ning, B.; Liu, B.; Gao, Z. Competitive fluorometric assay for the food toxin T-2 by using DNA-modified silver nanoclusters, aptamer-modified magnetic beads, and exponential isothermal amplification. Microchim. Acta 2019, 186, 219. [Google Scholar] [CrossRef]
- Maragos, C.M.; Kurtzman, C.; Busman, M.; Price, N.; McCormick, S. Development and evaluation of monoclonal antibodies for the Glucoside of T-2 Toxin (T2-Glc). Toxins 2013, 5, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- Lippolis, V.; Maragos, C. Fluorescence polarization immunoassays for rapid, accurate and sensitive determination of mycotoxins. World Mycotoxin J. 2014, 7, 479–489. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, S.; De Ruyck, K.; Beloglazova, N.; Eremin, S.A.; De Saeger, S.; Zhang, S.; Shen, J.; Wang, Z. Fluorescence polarization assays for chemical contaminants in food and environmental analyses. TrAC Trends Anal. Chem. 2019, 114, 293–313. [Google Scholar] [CrossRef]
- Lippolis, V.; Porricelli, A.C.R.; Cortese, M.; Suman, M.; Zanardi, S.; Pascale, M. Determination of ochratoxin A in Rye and Rye-Based products by Fluorescence polarization immunoassay. Toxins 2017, 9, 305. [Google Scholar] [CrossRef] [PubMed]
- European Commission (EC). Commission Regulation (EU) 519/2014 of 16 May 2014 amending Regulation (EC) No 401/2006 as regards methods of sampling of large lots, spices and food supplements, performance criteria for T-2, HT-2 toxin and citrinin and screening methods of analysis. Off. J. Eur. Union 2014, 147, 29–43. [Google Scholar]
- Lattanzio, V.M.T.; Solfrizzo, M.; Visconti, A. Enzymatic hydrolysis of T-2 toxin for the quantitative determination of total T-2 and HT-2 toxins in cereals. Anal. Bioanal. Chem. 2009, 395, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, D.; Li, J.; Zhang, Q.; Li, P. Monoclonal antibody–europium conjugate-based lateral flow time-resolved fluoroimmunoassay for quantitative determination of T-2 toxin in cereals and feed. Anal. Methods 2015, 7, 2822–2829. [Google Scholar] [CrossRef]
- McCormick, S.P.; Price, N.P.J.; Kurtzman, C.P. Glucosylation and other biotransformations of T-2 toxin by yeasts of the Trichomonascus Clade. Appl. Environ. Microbiol. 2012, 78, 8694–8702. [Google Scholar] [CrossRef] [PubMed]
- Soliani, L. Statistica applicata per la ricerca e professioni scientifiche. In Manuale di Statistica Univariata e Bivariata Parametrica e Non-Parametrica; Uninova-Gruppo Pegaso: Parma, Italy, 2007. [Google Scholar]
| MAb | Clone | [MAb] (µg/mL) | ∆Pmax (mP) 1 | |||
|---|---|---|---|---|---|---|
| T2-FL (Dilution 1:3600) 2 | HT2-FL1a (Dilution 1:3000) 2 | HT2-FL1b (Dilution 1:400) 2 | HT2-FL2 (Dilution 1:3600) 2 | |||
| Anti-T2G | 1–2 | 190 | 138 3 | - | 59 | 57 |
| 1–3 | 40 | 280 3 | 39 | 262 3 | 159 3 | |
| 1–4 | 104 | 225 3 | 28 | 253 3 | 176 3 | |
| 2–5 | 89 | 116 | 53 | 246 2 | 27 | |
| 2–11 | 118 | 144 3 | 18 | 50 | 18 | |
| 2–13 | 134 | 191 3 | 23 | 28 | 19 | |
| 2–16 | 90 | 200 3 | 37 | 82 | 21 | |
| 2–17 | 120 | 129 | 27 | 156 3 | 18 | |
| 2–21 | 132 | 159 3 | 22 | 14 | 16 | |
| 2–44 | 155 | 176 3 | 133 3 | 246 3 | 49 | |
| Anti-T2 | 1 | 6 | 282 3 | 217 3 | 93 | 111 |
| Anti-HT2 | H10-A10 | 8 | 205 3 | 230 3 | 122 | 20 |
| Spiking Levels (µg/kg) | FPIA | |||
|---|---|---|---|---|
| Protocol A | Protocol B | |||
| Recovery | RSD 1 (%) | Recovery | RSD 1 (%) | |
| 50 | 102 | 13 | 89 | 7 |
| 100 | 92 | 5 | 98 | 6 |
| 200 | 96 | 4 | 89 | 6 |
| Overall average | 97 | 9 | 92 | 7 |
| Performances | Protocol A | Protocol B | ||
|---|---|---|---|---|
| Blank | STC 1 (100 µg/kg) | Blank | STC 1 (100 µg/kg) | |
| Mean value 2 (µg/kg) | 12 | 115 | 21 | 104 |
| RSDr 3 (%) | 16 | 5 | 14 | 9 |
| RSDRI 4 (%) | 25 | 10 | 16 | 13 |
| Cut-off level | 96 | 80 | ||
| Rate of false suspect results (%) | <0.1 | <0.1 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lippolis, V.; Porricelli, A.C.R.; Mancini, E.; Ciasca, B.; Lattanzio, V.M.T.; De Girolamo, A.; Maragos, C.M.; McCormick, S.; Li, P.; Logrieco, A.F.; et al. Fluorescence Polarization Immunoassay for the Determination of T-2 and HT-2 Toxins and Their Glucosides in Wheat. Toxins 2019, 11, 380. https://doi.org/10.3390/toxins11070380
Lippolis V, Porricelli ACR, Mancini E, Ciasca B, Lattanzio VMT, De Girolamo A, Maragos CM, McCormick S, Li P, Logrieco AF, et al. Fluorescence Polarization Immunoassay for the Determination of T-2 and HT-2 Toxins and Their Glucosides in Wheat. Toxins. 2019; 11(7):380. https://doi.org/10.3390/toxins11070380
Chicago/Turabian StyleLippolis, Vincenzo, Anna C. R. Porricelli, Erminia Mancini, Biancamaria Ciasca, Veronica M. T. Lattanzio, Annalisa De Girolamo, Chris M. Maragos, Susan McCormick, Peiwu Li, Antonio F. Logrieco, and et al. 2019. "Fluorescence Polarization Immunoassay for the Determination of T-2 and HT-2 Toxins and Their Glucosides in Wheat" Toxins 11, no. 7: 380. https://doi.org/10.3390/toxins11070380
APA StyleLippolis, V., Porricelli, A. C. R., Mancini, E., Ciasca, B., Lattanzio, V. M. T., De Girolamo, A., Maragos, C. M., McCormick, S., Li, P., Logrieco, A. F., & Pascale, M. (2019). Fluorescence Polarization Immunoassay for the Determination of T-2 and HT-2 Toxins and Their Glucosides in Wheat. Toxins, 11(7), 380. https://doi.org/10.3390/toxins11070380