In Vitro and In Vivo Antimalarial Activity of LZ1, a Peptide Derived from Snake Cathelicidin
Abstract
1. Introduction
2. Results
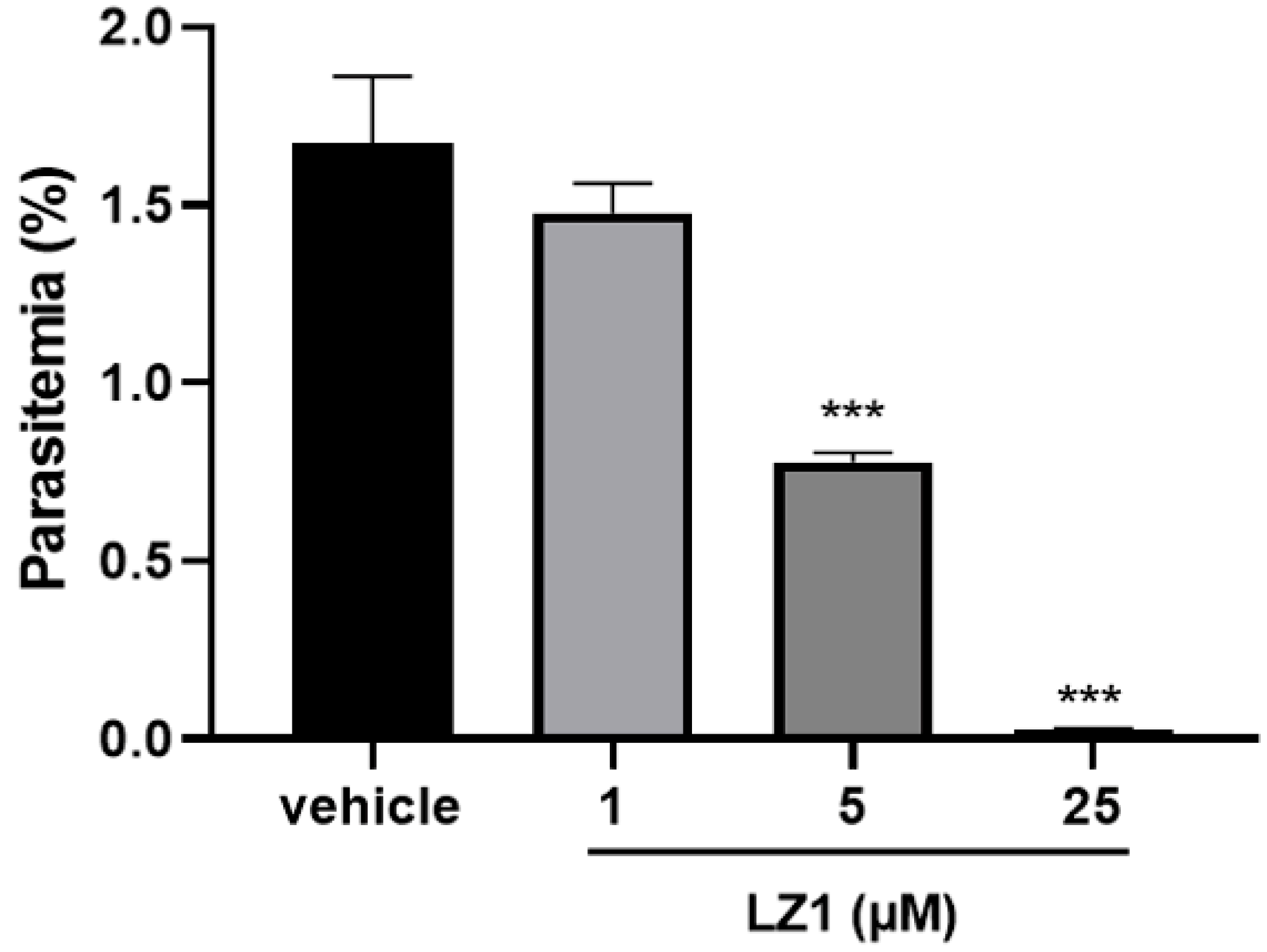
2.1. In Vitro Anti-Plasmodial Activity of LZ1
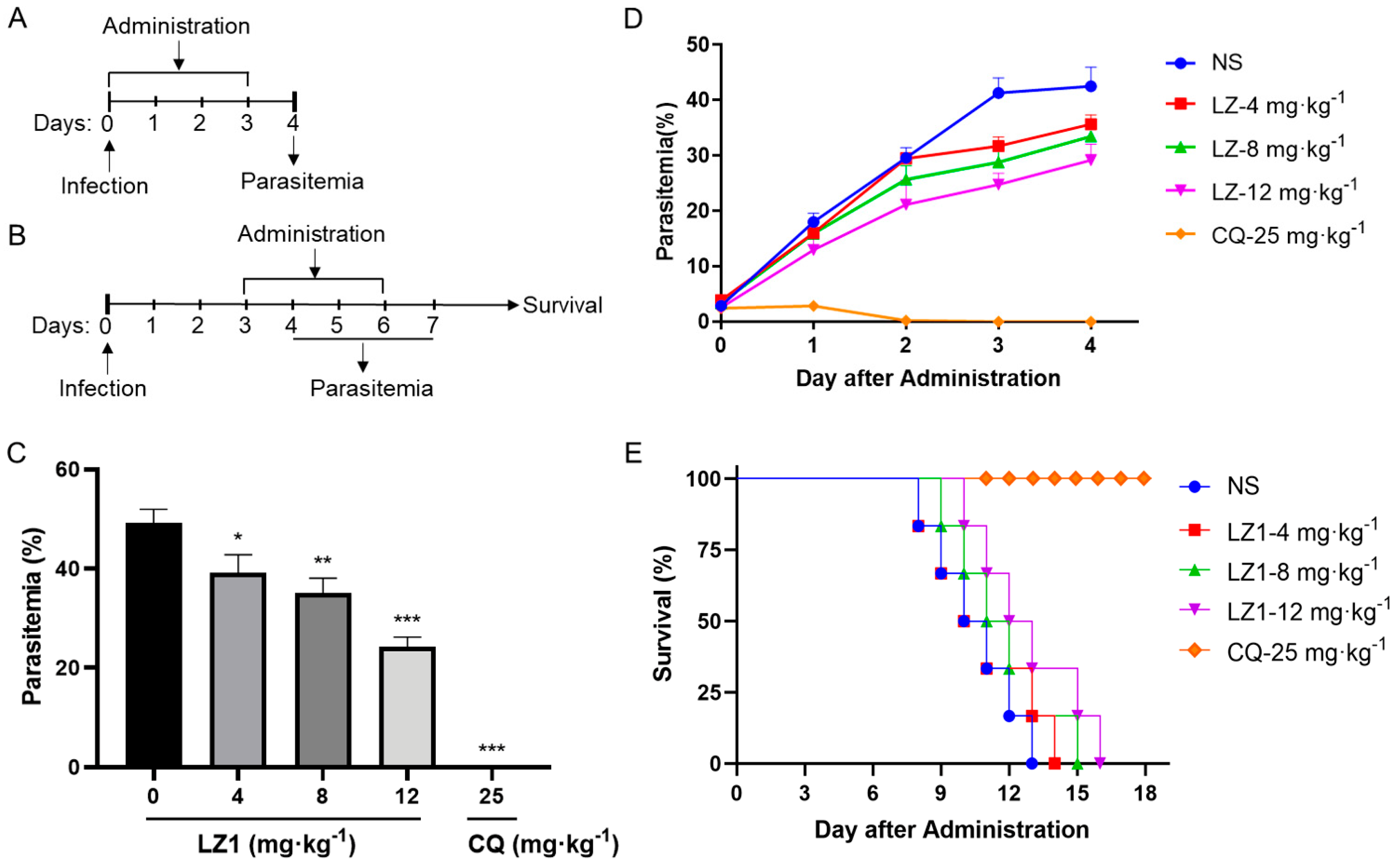
2.2. In Vivo Anti-Plasmodial Activity of LZ1
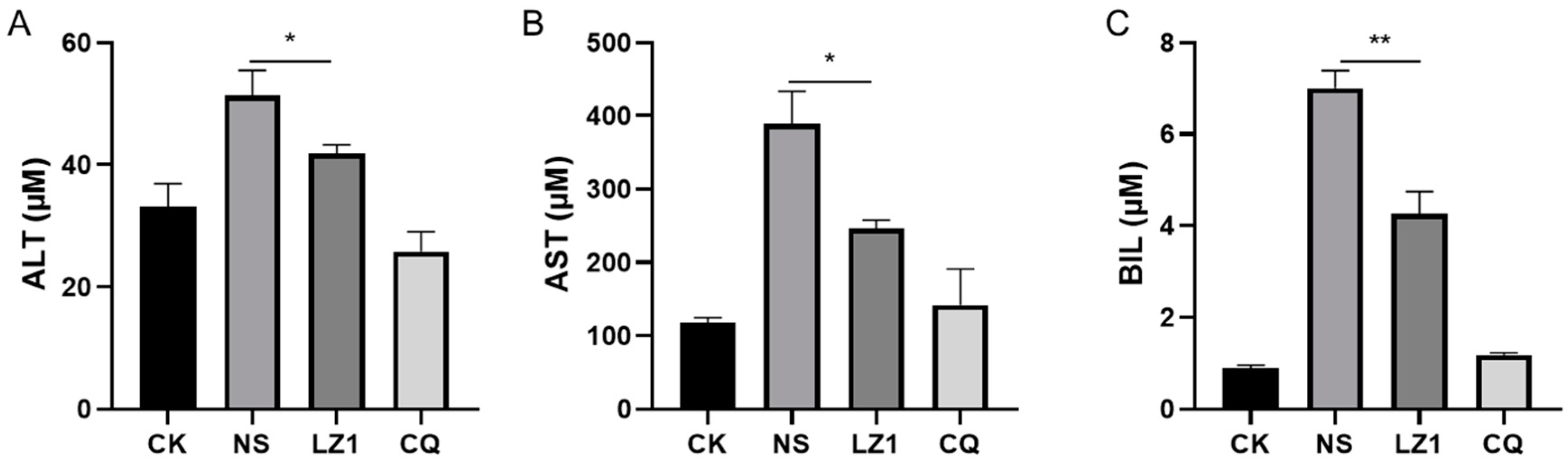
2.3. LZ1 Regulates Cytokines Expression and Liver Function
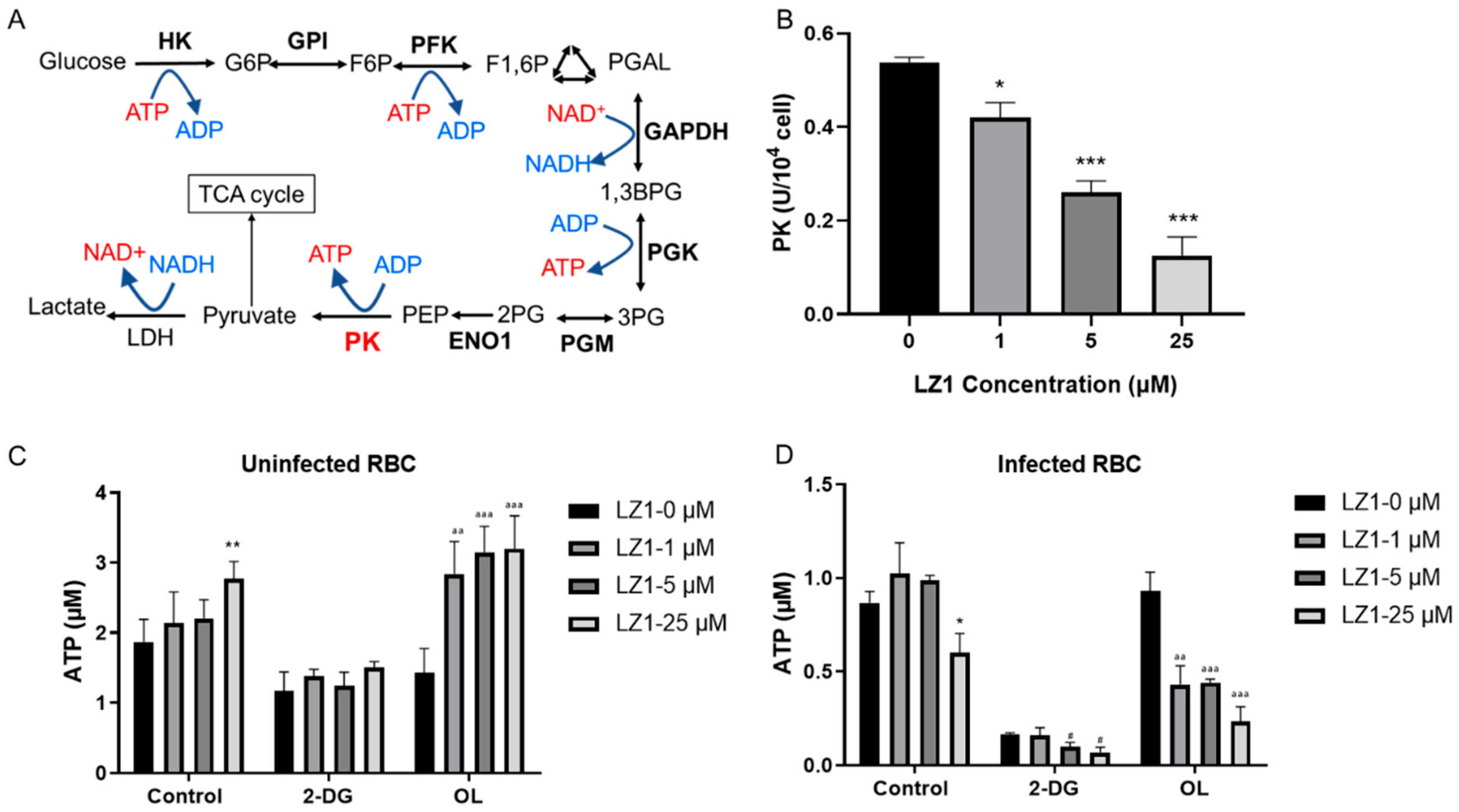
2.4. LZ1 Inhibits the Pyruvate Kinase Activity and ATP Synthesis During Plasmodium Infection
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animals and Parasite
5.2. Peptides Synthesis
5.3. In Vitro Antimalarial Assay
5.4. In Vivo Antimalarial Assay
5.5. Measurement of Serum Cytokines and Liver-Function
5.6. ATP Assays
5.7. Pyruvate Kinase Activity Assay
5.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Global Malaria Programme. World Malaria Report. World Health Organization, 2018. Available online: https://www.who.int/malaria/publications/world-malaria-report-2018/en/ (accessed on 30 June 2019).
- Shretta, R.; Yadav, P. Stabilizing supply of artemisinin and artemisinin-based combination therapy in an era of wide-spread scale-up. Malar. J. 2012, 11, 399. [Google Scholar] [CrossRef] [PubMed]
- Dondorp, A.M.; Nosten, F.; Yi, P.; Das, D.; Phyo, A.P.; Tarning, J.; Lwin, K.M.; Ariey, F.; Hanpithakpong, W.; Lee, S.J.; et al. Artemisinin resistance in Plasmodium falciparum malaria. New Engl. J. Med. 2009, 361, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, P.; Mor, A. Peptides as weapons against microorganisms in the chemical defense system of vertebrates. Annu. Rev. Microbiol. 1995, 49, 277–304. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E. Peptide antibiotics. Lancet 1997, 349, 418–422. [Google Scholar] [CrossRef]
- Kuroda, K.; Caputo, G.A. Antimicrobial polymers as synthetic mimics of host-defense peptides. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 49–66. [Google Scholar] [CrossRef]
- Kraus, D.; Peschel, A. Molecular mechanisms of bacterial resistance to antimicrobial peptides. Curr. Top. Microbiol. Immunol. 2006, 306, 231–250. [Google Scholar] [PubMed]
- Rotem, S.; Mor, A. Antimicrobial peptide mimics for improved therapeutic properties. Biochim. Biophys. Acta. 2009, 1788, 1582–1592. [Google Scholar] [CrossRef]
- Gwadz, R.W.; Kaslow, D.; Lee, J.Y.; Maloy, W.L.; Zasloff, M.; Miller, L.H. Effects of magainins and cecropins on the sporogonic development of malaria parasites in mosquitoes. Infect. Immun. 1989, 57, 2628–2633. [Google Scholar]
- Vizioli, J.; Bulet, P.; Hoffmann, J.A.; Kafatos, F.C.; Muller, H.M.; Dimopoulos, G. Gambicin: A novel immune responsive antimicrobial peptide from the malaria vector Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2001, 98, 12630–12635. [Google Scholar] [CrossRef]
- Conde, R.; Zamudio, F.Z.; Rodriguez, M.H.; Possani, L.D. Scorpine, an anti-malaria and anti-bacterial agent purified from scorpion venom. FEBS Lett. 2000, 471, 165–168. [Google Scholar] [CrossRef]
- Parra, M.; Liu, X.; Derrick, S.C.; Yang, A.; Tian, J.; Kolibab, K.; Kumar, S.; Morris, S.L. Molecular analysis of non-specific protection against murine malaria induced by BCG vaccination. PLoS ONE 2013, 8, e66115. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hong, J.; Liu, X.; Yang, H.; Liu, R.; Wu, J.; Wang, A.; Lin, D.; Lai, R. Snake cathelicidin from Bungarus fasciatus is a potent peptide antibiotics. PLoS ONE 2008, 3, e3217. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.; Chen, L.; Guang, H.; Li, Z.; Yang, H.; Li, J.; You, D.; Yu, H.; Lai, R. Cathelicidin-BF, a snake cathelicidin-derived antimicrobial peptide, could be an excellent therapeutic agent for acne vulgaris. PLoS ONE 2011, 6, e22120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mu, L.; Tang, J.; Duan, Z.; Wang, F.; Wei, L.; Rong, M.; Lai, R. A small peptide with therapeutic potential for inflammatory acne vulgaris. PLoS ONE 2013, 8, e72923. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Bai, X.; Luan, N.; Yao, H.; Zhang, Z.; Liu, W.; Chen, Y.; Yan, X.; Rong, M.; Lai, R. A Designed Tryptophan- and Lysine/Arginine-Rich Antimicrobial Peptide with Therapeutic Potential for Clinical Antibiotic-Resistant Candida albicans Vaginitis. J. Med. Chem. 2016, 59, 1791–1799. [Google Scholar] [CrossRef]
- Day, N.P.; Hien, T.T.; Schollaardt, T.; Loc, P.P.; Chuong, L.V.; Chau, T.T.; Mai, N.T.; Phu, N.H.; Sinh, D.X.; White, N.J.; et al. The prognostic and pathophysiologic role of pro- and antiinflammatory cytokines in severe malaria. J. Infect. Dis. 1999, 180, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, A.; Suri, V.; Singh, V. Malarial hepatopathy. J. Postgrad. Med. 2006, 52, 315–320. [Google Scholar]
- Jain, A.; Kaushik, R.; Kaushik, R.M. Malarial hepatopathy: Clinical profile and association with other malarial complications. Acta Trop. 2016, 159, 95–105. [Google Scholar] [CrossRef]
- Stepien, M.; Fedirko, V.; Duarte-Salles, T.; Ferrari, P.; Freisling, H.; Trepo, E.; Trichopoulou, A.; Bamia, C.; Weiderpass, E.; Olsen, A.; et al. Prospective association of liver function biomarkers with development of hepatobiliary cancers. Cancer Epidemiol. 2016, 40, 179–187. [Google Scholar] [CrossRef]
- Zanella, A.; Fermo, E.; Bianchi, P.; Valentini, G. Red cell pyruvate kinase deficiency: Molecular and clinical aspects. Br. J. Haematol 2005, 130, 11–25. [Google Scholar] [CrossRef]
- Van Niekerk, D.D.; Penkler, G.P.; du Toit, F.; Snoep, J.L. Targeting glycolysis in the malaria parasite Plasmodium falciparum. Febs J. 2016, 283, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Min-Oo, G.; Fortin, A.; Tam, M.F.; Nantel, A.; Stevenson, M.M.; Gros, P. Pyruvate kinase deficiency in mice protects against malaria. Nat. Genet. 2003, 35, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Ayi, K.; Min-Oo, G.; Serghides, L.; Crockett, M.; Kirby-Allen, M.; Quirt, I.; Gros, P.; Kain, K.C. Pyruvate kinase deficiency and malaria. New Engl. J. Med. 2008, 358, 1805–1810. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; Kurtoglu, M.; Liu, H.; Wangpaichitr, M.; You, M.; Liu, X.; Savaraj, N.; Lampidis, T.J. 2-Deoxy-D-glucose activates autophagy via endoplasmic reticulum stress rather than ATP depletion. Cancer Chemother. Pharmacol. 2011, 67, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Samy, R.P.; Foo, S.L.; Franco, O.L.; Stiles, B.G.; Kumar, A.P.; Sethi, G.; Lim, L.H. Identification of natural peptides as a new class of antimalarial drugs by in silico approaches. Front. Biosci. (Scholar edition) 2017, 9, 88–110. [Google Scholar] [CrossRef]
- Gao, B.; Xu, J.; Rodriguez Mdel, C.; Lanz-Mendoza, H.; Hernandez-Rivas, R.; Du, W.; Zhu, S. Characterization of two linear cationic antimalarial peptides in the scorpion Mesobuthus eupeus. Biochimie 2010, 92, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Parent, R.; Guillaume, C.; Deregnaucourt, C.; Delarbre, C.; Ojcius, D.M.; Montagne, J.J.; Celerier, M.L.; Phelipot, A.; Amiche, M.; et al. Isolation and characterization of Psalmopeotoxin I and II: Two novel antimalarial peptides from the venom of the tarantula Psalmopoeus cambridgei. FEBS Lett. 2004, 572, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.Y.; Chowdhury, M.; Huang, Y.D.; Yu, X.Q. Insect antimicrobial peptides and their applications. Appl. Microbiol. Biotechnol. 2014, 98, 5807–5822. [Google Scholar] [CrossRef]
- Anjum, K.; Abbas, S.Q.; Akhter, N.; Shagufta, B.I.; Shah, S.A.A.; Hassan, S.S.U. Emerging biopharmaceuticals from bioactive peptides derived from marine organisms. Chem. Biol. Drug Des. 2017, 90, 12–30. [Google Scholar] [CrossRef]
- Perez-Picaso, L.; Velasco-Bejarano, B.; Aguilar-Guadarrama, A.B.; Argotte-Ramos, R.; Rios, M.Y. Antimalarial activity of ultra-short peptides. Molecules 2009, 14, 5103–5114. [Google Scholar] [CrossRef]
- Hunt, N.H.; Grau, G.E. Cytokines: Accelerators and brakes in the pathogenesis of cerebral malaria. Trends Immunol. 2003, 24, 491–499. [Google Scholar] [CrossRef]
- Reuling, I.J.; de Jong, G.M.; Yap, X.Z.; Asghar, M.; Walk, J.; van de Schans, L.A.; Koelewijn, R.; Farnert, A.; de Mast, Q.; van der Ven, A.J.; et al. Liver Injury in Uncomplicated Malaria is an Overlooked Phenomenon: An Observational Study. EBioMedicine 2018, 36, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Maciel, C.; de Oliveira Junior, V.X.; Fazio, M.A.; Nacif-Pimenta, R.; Miranda, A.; Pimenta, P.F.; Capurro, M.L. Anti-plasmodium activity of angiotensin II and related synthetic peptides. PLoS ONE 2008, 3, e3296. [Google Scholar] [CrossRef] [PubMed]
- Kirk, K. Membrane transport in the malaria-infected erythrocyte. Physiol. Rev. 2001, 81, 495–537. [Google Scholar] [CrossRef] [PubMed]
- Oelshlegel, F.J., Jr.; Sander, B.J.; Brewer, G.J. Pyruvate kinase in malaria host-parasite interaction. Nature 1975, 255, 345–347. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grace, R.F.; Zanella, A.; Neufeld, E.J.; Morton, D.H.; Eber, S.; Yaish, H.; Glader, B. Erythrocyte pyruvate kinase deficiency: 2015 status report. Am. J. Hematol. 2015, 90, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; DeLoughery, T.G. Recurrent thromboembolic disease following splenectomy for pyruvate kinase deficiency. Am. J. Hematol. 2001, 67, 197–199. [Google Scholar] [CrossRef]
- Vivas, L.; Easton, A.; Kendrick, H.; Cameron, A.; Lavandera, J.L.; Barros, D.; de las Heras, F.G.; Brady, R.L.; Croft, S.L. Plasmodium falciparum: Stage specific effects of a selective inhibitor of lactate dehydrogenase. Exp. Parasitol. 2005, 111, 105–114. [Google Scholar] [CrossRef]
- Trager, W.; Jensen, J.B. Human malaria parasites in continuous culture. Science 1976, 193, 673–675. [Google Scholar] [CrossRef]
- Bayih, A.G.; Folefoc, A.; Mohon, A.N.; Eagon, S.; Anderson, M.; Pillai, D.R. In vitro and in vivo anti-malarial activity of novel harmine-analog heat shock protein 90 inhibitors: A possible partner for artemisinin. Malar J. 2016, 15, 579. [Google Scholar] [CrossRef]
- Ramazani, A.; Zakeri, S.; Sardari, S.; Khodakarim, N.; Djadidt, N.D. In vitro and in vivo anti-malarial activity of Boerhavia elegans and Solanum surattense. Malar J. 2010, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Shen, C.; Luan, N.; Yao, H.; Long, C.; Lai, R.; Yan, X.-W. In vivo antimalarial activity of synthetic hepcidin against Plasmodium berghei in mice. Chin. J. Nat. Med. 2017, 15, 161–167. [Google Scholar] [CrossRef]
- Moore, B.R.; Page-Sharp, M.; Stoney, J.R.; Ilett, K.F.; Jago, J.D.; Batty, K.T. Pharmacokinetics, pharmacodynamics, and allometric scaling of chloroquine in a murine malaria model. Antimicrob. Agents Chemother. 2011, 55, 3899–3907. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Y.; He, X.; Zhang, P.; Shen, C.; Mwangi, J.; Xu, C.; Mo, G.; Lai, R.; Zhang, Z. In Vitro and In Vivo Antimalarial Activity of LZ1, a Peptide Derived from Snake Cathelicidin. Toxins 2019, 11, 379. https://doi.org/10.3390/toxins11070379
Fang Y, He X, Zhang P, Shen C, Mwangi J, Xu C, Mo G, Lai R, Zhang Z. In Vitro and In Vivo Antimalarial Activity of LZ1, a Peptide Derived from Snake Cathelicidin. Toxins. 2019; 11(7):379. https://doi.org/10.3390/toxins11070379
Chicago/Turabian StyleFang, Yaqun, Xiaoqin He, Pengcheng Zhang, Chuanbin Shen, James Mwangi, Cheng Xu, Guoxiang Mo, Ren Lai, and Zhiye Zhang. 2019. "In Vitro and In Vivo Antimalarial Activity of LZ1, a Peptide Derived from Snake Cathelicidin" Toxins 11, no. 7: 379. https://doi.org/10.3390/toxins11070379
APA StyleFang, Y., He, X., Zhang, P., Shen, C., Mwangi, J., Xu, C., Mo, G., Lai, R., & Zhang, Z. (2019). In Vitro and In Vivo Antimalarial Activity of LZ1, a Peptide Derived from Snake Cathelicidin. Toxins, 11(7), 379. https://doi.org/10.3390/toxins11070379





