Cannabis: From a Plant That Modulates Feeding Behaviors toward Developing Selective Inhibitors of the Peripheral Endocannabinoid System for the Treatment of Obesity and Metabolic Syndrome
Abstract
1. Overview of Plant Cannabinoids and Endocannabinoids
2. Is Marijuana a Toxic Drug?
3. “To Eat or Not to Eat”: The Role of Cannabinoids in Feeding Behaviors
4. Targeting CB1R for Treatment of Obesity: Block Centrally or Inhibit Peripherally
5. Current View Regarding Novel Peripherally Restricted CB1R Blockers
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Pollio, A. The Name of Cannabis: A Short Guide for Nonbotanists. Cannabis Cannabinoid Res. 2016, 1, 234–238. [Google Scholar] [CrossRef]
- McPartland, J.M. Cannabis Systematics at the Levels of Family, Genus, and Species. Cannabis Cannabinoid Res. 2018, 3, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Batkai, S.; Kunos, G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. [Google Scholar] [CrossRef] [PubMed]
- Hanus, L.O.; Meyer, S.M.; Munoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef] [PubMed]
- Berman, P.; Futoran, K.; Lewitus, G.M.; Mukha, D.; Benami, M.; Shlomi, T.; Meiri, D. A new ESI-LC/MS approach for comprehensive metabolic profiling of phytocannabinoids in Cannabis. Sci. Rep. 2018, 8, 14280. [Google Scholar] [CrossRef]
- Wood, T.B.; Spivey, W.T.N.; Easterfield, T.H. Cannabinol. Part I. J. Chem. Soc. 1899, 75, 20–36. [Google Scholar] [CrossRef]
- Hanus, L.O. Pharmacological and therapeutic secrets of plant and brain (endo)cannabinoids. Med. Res. Rev. 2009, 29, 213–271. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Shvo, Y. Hashish, I. The structure of cannabidiol. Tetrahedron 1963, 19, 2073–2078. [Google Scholar] [CrossRef]
- Gaoni, Y.; Mechoulam, R. Isolation, structure, partial, synthesis of an active constituent of hashish. J. Am. Chem. Soc. 1964, 86, 1646–1647. [Google Scholar] [CrossRef]
- Mechoulam, R.; Gaoni, Y. The absolute configuration of delta-1-tetrahydrocannabinol, the major active constituent of hashish. Tetrahedron Lett. 1967, 12, 1109–1111. [Google Scholar] [CrossRef]
- Hively, R.L.; Mosher, W.A.; Hoffmann, F.W. Isolation of trans-delta-tetrahydrocannabinol from marijuana. J. Am. Chem. Soc. 1966, 88, 1832–1833. [Google Scholar] [CrossRef] [PubMed]
- Gaoni, Y.; Mechoulam, R. The structure and synthesis of cannabigerol, a new hashish constituent. Proc. Chem. Soc. 1964, 82. [Google Scholar]
- Gaoni, Y.; Mechoulam, R. Cannabichromene, a new active principle in hashish. Chem. Commun. 1966, 1, 20–21. [Google Scholar] [CrossRef]
- Crombie, L.; Ponsford, R.; Shani, A.; Yagnitinsky, B.; Mechoulam, R. Hashish components. Photochemical production of cannabicyclol from cannabichromene. Tetrahedron Lett. 1968, 5771–5772. [Google Scholar] [CrossRef]
- Devane, W.A.; Dysarz, F.A., 3rd.; Johnson, M.R.; Melvin, L.S.; Howlett, A.C. Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol. 1988, 34, 605–613. [Google Scholar]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.; Vemuri, K.; Pu, M.; Qu, L.; Han, G.W.; Wu, Y.; Zhao, S.; Shui, W.; Li, S.; Korde, A.; et al. Crystal Structure of the Human Cannabinoid Receptor CB1. Cell 2016, 167, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Yin, J.; Chapman, K.; Grzemska, M.; Clark, L.; Wang, J.; Rosenbaum, D.M. High-resolution crystal structure of the human CB1 cannabinoid receptor. Nature 2016, 540, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.; Vemuri, K.; Nikas, S.P.; Laprairie, R.B.; Wu, Y.; Qu, L.; Pu, M.; Korde, A.; Jiang, S.; Ho, J.H.; et al. Crystal structures of agonist-bound human cannabinoid receptor CB1. Nature 2017, 547, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hua, T.; Vemuri, K.; Ho, J.H.; Wu, Y.; Wu, L.; Popov, P.; Benchama, O.; Zvonok, N.; Locke, K.; et al. Crystal Structure of the Human Cannabinoid Receptor CB2. Cell 2019, 176, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.C. Cannabinoid receptor signaling. Handb. Exp. Pharmacol. 2005, 168, 53–79. [Google Scholar]
- Mackie, K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb. Exp. Pharmacol. 2005, 168, 299–325. [Google Scholar]
- Tam, J.; Hinden, L.; Drori, A.; Udi, S.; Azar, S.; Baraghithy, S. The therapeutic potential of targeting the peripheral endocannabinoid/CB1 receptor system. Eur. J. Intern. Med. 2018, 49, 23–29. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef]
- Di Marzo, V.; Piscitelli, F. The Endocannabinoid System and its Modulation by Phytocannabinoids. Neurotherapeutics 2015, 12, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Nakane, S.; Shinoda, A.; Itoh, K.; Yamashita, A.; Waku, K. 2-Arachidonoylglycerol: A possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995, 215, 89–97. [Google Scholar] [CrossRef]
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R.; et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995, 50, 83–90. [Google Scholar] [CrossRef]
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.; Di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB(1) and CB(2). Pharmacol. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef]
- Di Marzo, V.; De Petrocellis, L. Why do cannabinoid receptors have more than one endogenous ligand? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 3216–3228. [Google Scholar] [CrossRef] [PubMed]
- Murataeva, N.; Straiker, A.; Mackie, K. Parsing the players: 2-arachidonoylglycerol synthesis and degradation in the CNS. Br. J. Pharmacol. 2014, 171, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- McKinney, M.K.; Cravatt, B.F. Structure and function of fatty acid amide hydrolase. Ann. Rev. Biochem. 2005, 74, 411–432. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.P.; Carpenter, D.; Leslie, F.M.; Freund, T.F.; Katona, I.; Sensi, S.L.; Kathuria, S.; Piomelli, D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc. Natl. Acad. Sci. USA 2002, 99, 10819–10824. [Google Scholar] [CrossRef]
- Di Marzo, V. ‘Endocannabinoids’ and other fatty acid derivatives with cannabimimetic properties: Biochemistry and possible physiopathological relevance. Biochim. Biophys. Acta 1998, 1392, 153–175. [Google Scholar] [CrossRef]
- McPartland, J.M.; Guy, G.W.; Di Marzo, V. Care and feeding of the endocannabinoid system: A systematic review of potential clinical interventions that upregulate the endocannabinoid system. PLoS ONE 2014, 9, e89566. [Google Scholar] [CrossRef]
- Aran, A.; Eylon, M.; Harel, M.; Polianski, L.; Nemirovski, A.; Tepper, S.; Schnapp, A.; Cassuto, H.; Wattad, N.; Tam, J. Lower circulating endocannabinoid levels in children with autism spectrum disorder. Mol. Autism 2019, 10, 2. [Google Scholar] [CrossRef]
- Russo, E.B. Clinical endocannabinoid deficiency (CECD): Can this concept explain therapeutic benefits of cannabis in migraine, fibromyalgia, irritable bowel syndrome and other treatment-resistant conditions? Neuro Endocrinol. Lett. 2004, 25, 31–39. [Google Scholar]
- Niesters, M.; Overdyk, F.; Smith, T.; Aarts, L.; Dahan, A. Opioid-induced respiratory depression in paediatrics: A review of case reports. Br. J. Anaesth. 2013, 110, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.C.; Saukkonen, J.J. Acute respiratory failure from abused substances. J. Intensive Care Med. 2004, 19, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Kozor, R.; Grieve, S.M.; Buchholz, S.; Kaye, S.; Darke, S.; Bhindi, R.; Figtree, G.A. Regular cocaine use is associated with increased systolic blood pressure, aortic stiffness and left ventricular mass in young otherwise healthy individuals. PLoS ONE 2014, 9, e89710. [Google Scholar] [CrossRef] [PubMed]
- May, C.N.; Ham, I.W.; Heslop, K.E.; Stone, F.A.; Mathias, C.J. Intravenous morphine causes hypertension, hyperglycaemia and increases sympatho-adrenal outflow in conscious rabbits. Clin. Sci. (Lond.) 1988, 75, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Sachs, J.; McGlade, E.; Yurgelun-Todd, D. Safety and Toxicology of Cannabinoids. Neurotherapeutics 2015, 12, 735–746. [Google Scholar] [CrossRef]
- Adams, I.B.; Martin, B.R. Cannabis: Pharmacology and toxicology in animals and humans. Addiction 1996, 91, 1585–1614. [Google Scholar] [CrossRef]
- Grotenhermen, F. The toxicology of cannabis and cannabis prohibition. Chem. Biodivers. 2007, 4, 1744–1769. [Google Scholar] [CrossRef] [PubMed]
- Reece, A.S. Chronic toxicology of cannabis. Clin. Toxicol. 2009, 47, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Herkenham, M.; Lynn, A.B.; Johnson, M.R.; Melvin, L.S.; de Costa, B.R.; Rice, K.C. Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. J. Neurosci. 1991, 11, 563–583. [Google Scholar] [CrossRef]
- Simon, V.; Cota, D. Mechanisms in Endocrinology: Endocannabinoids and metabolism: Past, present and future. Eur. J. Endocrinol. 2017, 176, R309–R324. [Google Scholar] [CrossRef]
- Foltin, R.W.; Brady, J.V.; Fischman, M.W. Behavioral analysis of marijuana effects on food intake in humans. Pharmacol. Biochem. Behav. 1986, 25, 577–582. [Google Scholar] [CrossRef]
- Hollister, L.E. Hunger and appetite after single doses of marihuana, alcohol, and dextroamphetamine. Clin. Pharmacol. Ther. 1971, 12, 44–49. [Google Scholar] [CrossRef]
- Abel, E.L. Effects of marihuana on the solution of anagrams, memory and appetite. Nature 1971, 231, 260–261. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Ligresti, A.; Cristino, L. The endocannabinoid system as a link between homoeostatic and hedonic pathways involved in energy balance regulation. Int. J. Obes. (Lond.) 2009, 33 (Suppl. 2), S18–S24. [Google Scholar] [CrossRef]
- Machado Rocha, F.C.; Stefano, S.C.; De Cassia Haiek, R.; Rosa Oliveira, L.M.; Da Silveira, D.X. Therapeutic use of Cannabis sativa on chemotherapy-induced nausea and vomiting among cancer patients: Systematic review and meta-analysis. Eur. J. Cancer Care (Engl.) 2008, 17, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Badowski, M.E.; Yanful, P.K. Dronabinol oral solution in the management of anorexia and weight loss in AIDS and cancer. Ther. Clin. Risk Manag. 2018, 14, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Plasse, T.F.; Gorter, R.W.; Krasnow, S.H.; Lane, M.; Shepard, K.V.; Wadleigh, R.G. Recent clinical experience with dronabinol. Pharmacol. Biochem. Behav. 1991, 40, 695–700. [Google Scholar] [CrossRef]
- Struwe, M.; Kaempfer, S.H.; Geiger, C.J.; Pavia, A.T.; Plasse, T.F.; Shepard, K.V.; Ries, K.; Evans, T.G. Effect of dronabinol on nutritional status in HIV infection. Ann. Pharmacother. 1993, 27, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Beal, J.E.; Olson, R.; Laubenstein, L.; Morales, J.O.; Bellman, P.; Yangco, B.; Lefkowitz, L.; Plasse, T.F.; Shepard, K.V. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J. Pain Symptom Manag. 1995, 10, 89–97. [Google Scholar] [CrossRef]
- Andries, A.; Frystyk, J.; Flyvbjerg, A.; Stoving, R.K. Dronabinol in severe, enduring anorexia nervosa: A randomized controlled trial. Int. J. Eat. Disord. 2014, 47, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Volicer, L.; Stelly, M.; Morris, J.; McLaughlin, J.; Volicer, B.J. Effects of dronabinol on anorexia and disturbed behavior in patients with Alzheimer’s disease. Int. J. Geriatr. Psychiatry 1997, 12, 913–919. [Google Scholar] [CrossRef]
- Wilson, M.M.; Philpot, C.; Morley, J.E. Anorexia of aging in long term care: Is dronabinol an effective appetite stimulant?—A pilot study. J. Nutr. Health Aging 2007, 11, 195–198. [Google Scholar]
- Wiley, J.L.; Burston, J.J.; Leggett, D.C.; Alekseeva, O.O.; Razdan, R.K.; Mahadevan, A.; Martin, B.R. CB1 cannabinoid receptor-mediated modulation of food intake in mice. Br. J. Pharmacol. 2005, 145, 293–300. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, C.L.; Baile, C.A.; Bender, P.E. Cannabinols and feeding in sheep. Psychopharmacology 1979, 64, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, D.B.; Morton, E.C. Anorexia and hyperphagia produced by five pharmacologic classes of hallucinogens. Pharmacol. Biochem. Behav. 1982, 17, 539–545. [Google Scholar] [CrossRef]
- Brown, J.E.; Kassouny, M.; Cross, J.K. Kinetic studies of food intake and sucrose solution preference by rats treated with low doses of delta9-tetrahydrocannabinol. Behav. Biol. 1977, 20, 104–110. [Google Scholar] [CrossRef]
- Trojniar, W.; Wise, R.A. Facilitory effect of delta 9-tetrahydrocannabinol on hypothalamically induced feeding. Psychopharmacology 1991, 103, 172–176. [Google Scholar] [CrossRef]
- Hao, S.; Avraham, Y.; Mechoulam, R.; Berry, E.M. Low dose anandamide affects food intake, cognitive function, neurotransmitter and corticosterone levels in diet-restricted mice. Eur. J. Pharmacol. 2000, 392, 147–156. [Google Scholar] [CrossRef]
- Williams, C.M.; Kirkham, T.C. Observational analysis of feeding induced by Delta9-THC and anandamide. Physiol. Behav. 2002, 76, 241–250. [Google Scholar] [CrossRef]
- Williams, C.M.; Kirkham, T.C. Anandamide induces overeating: Mediation by central cannabinoid (CB1) receptors. Psychopharmacology 1999, 143, 315–317. [Google Scholar] [CrossRef]
- Jamshidi, N.; Taylor, D.A. Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. Br. J. Pharmacol. 2001, 134, 1151–1154. [Google Scholar] [CrossRef]
- Gallate, J.E.; Saharov, T.; Mallet, P.E.; McGregor, I.S. Increased motivation for beer in rats following administration of a cannabinoid CB1 receptor agonist. Eur. J. Pharmacol. 1999, 370, 233–240. [Google Scholar] [CrossRef]
- Kirkham, T.C.; Williams, C.M.; Fezza, F.; Di Marzo, V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: Stimulation of eating by 2-arachidonoyl glycerol. Br. J. Pharmacol. 2002, 136, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, M.A.; Elie, R. Effects of marijuana and D-amphetamine on the appetite, food consumption and various cardio-respiratory variables in man. Union Med. Can. 1975, 104, 914–921. [Google Scholar] [PubMed]
- Chopra, R.N.; Chopra, G.S. The Present Position of Hemp-Drug Addiction in India. Indian Med. Res. Mem. 1939, 31, 1–119. [Google Scholar] [CrossRef]
- Bouquet, J. Cannabis. Bull. Nurc. 1951, 3, 22–45. [Google Scholar]
- Abel, E.L. Cannabis: Effects on hunger and thirst. Behav. Biol. 1975, 15, 255–281. [Google Scholar] [CrossRef]
- Clark, T.M.; Jones, J.M.; Hall, A.G.; Tabner, S.A.; Kmiec, R.L. Theoretical Explanation for Reduced Body Mass Index and Obesity Rates in Cannabis Users. Cannabis Cannabinoid Res. 2018, 3, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Ceccarini, J.; Kuepper, R.; Kemels, D.; van Os, J.; Henquet, C.; Van Laere, K. [18F]MK-9470 PET measurement of cannabinoid CB1 receptor availability in chronic cannabis users. Add. Biol. 2015, 20, 357–367. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, D.C.; Cortes-Briones, J.A.; Ranganathan, M.; Thurnauer, H.; Creatura, G.; Surti, T.; Planeta, B.; Neumeister, A.; Pittman, B.; Normandin, M.; et al. Rapid Changes in CB1 Receptor Availability in Cannabis Dependent Males after Abstinence from Cannabis. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2016, 1, 60–67. [Google Scholar] [CrossRef]
- Hirvonen, J.; Goodwin, R.S.; Li, C.T.; Terry, G.E.; Zoghbi, S.S.; Morse, C.; Pike, V.W.; Volkow, N.D.; Huestis, M.A.; Innis, R.B. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol. Psychiatry 2012, 17, 642–649. [Google Scholar] [CrossRef]
- Colombo, G.; Agabio, R.; Diaz, G.; Lobina, C.; Reali, R.; Gessa, G.L. Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sci. 1998, 63, PL113–PL117. [Google Scholar] [CrossRef]
- Arnone, M.; Maruani, J.; Chaperon, F.; Thiebot, M.H.; Poncelet, M.; Soubrie, P.; Le Fur, G. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology 1997, 132, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Ravinet Trillou, C.; Arnone, M.; Delgorge, C.; Gonalons, N.; Keane, P.; Maffrand, J.P.; Soubrie, P. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R345–353. [Google Scholar] [CrossRef] [PubMed]
- Simiand, J.; Keane, M.; Keane, P.E.; Soubrie, P. SR 141716, a CB1 cannabinoid receptor antagonist, selectively reduces sweet food intake in marmoset. Behav. Pharmacol. 1998, 9, 179–181. [Google Scholar] [PubMed]
- Freedland, C.S.; Poston, J.S.; Porrino, L.J. Effects of SR141716A, a central cannabinoid receptor antagonist, on food-maintained responding. Pharmacol. Biochem. Behav. 2000, 67, 265–270. [Google Scholar] [CrossRef]
- Ward, S.J.; Dykstra, L.A. The role of CB1 receptors in sweet versus fat reinforcement: Effect of CB1 receptor deletion, CB1 receptor antagonism (SR141716A) and CB1 receptor agonism (CP-55940). Behav. Pharmacol. 2005, 16, 381–388. [Google Scholar] [CrossRef]
- Cota, D.; Marsicano, G.; Tschop, M.; Grubler, Y.; Flachskamm, C.; Schubert, M.; Auer, D.; Yassouridis, A.; Thone-Reineke, C.; Ortmann, S.; et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J. Clin. Investig. 2003, 112, 423–431. [Google Scholar] [CrossRef]
- Despres, J.P.; Golay, A.; Sjostrom, L.; Rimonabant in Obesity-Lipids Study Group. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N. Engl. J. Med. 2005, 353, 2121–2134. [Google Scholar] [CrossRef]
- Pi-Sunyer, F.X.; Aronne, L.J.; Heshmati, H.M.; Devin, J.; Rosenstock, J.; RIO-North America Study Group. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: A randomized controlled trial. JAMA 2006, 295, 761–775. [Google Scholar] [CrossRef]
- Van Gaal, L.F.; Rissanen, A.M.; Scheen, A.J.; Ziegler, O.; Rossner, S.; Group, R.I.-E.S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 2005, 365, 1389–1397. [Google Scholar] [CrossRef]
- Scheen, A.J.; Finer, N.; Hollander, P.; Jensen, M.D.; Van Gaal, L.F. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: A randomised controlled study. Lancet 2006, 368, 1660–1672. [Google Scholar] [CrossRef]
- Despres, J.P.; Ross, R.; Boka, G.; Almeras, N.; Lemieux, I.; ADAGIO-Lipids Investigators. Effect of rimonabant on the high-triglyceride/low-HDL-cholesterol dyslipidemia, intraabdominal adiposity, and liver fat: The ADAGIO-Lipids trial. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Hollander, P.; Chevalier, S.; Iranmanesh, A. SERENADE: The Study Evaluating Rimonabant Efficacy in Drug-naive Diabetic Patients: Effects of monotherapy with rimonabant, the first selective CB1 receptor antagonist, on glycemic control, body weight, and lipid profile in drug-naive type 2 diabetes. Diabetes Care 2008, 31, 2169–2176. [Google Scholar] [CrossRef] [PubMed]
- Hollander, P.A.; Amod, A.; Litwak, L.E.; Chaudhari, U. Effect of rimonabant on glycemic control in insulin-treated type 2 diabetes: The ARPEGGIO trial. Diabetes Care 2010, 33, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.; Kristensen, P.K.; Bartels, E.M.; Bliddal, H.; Astrup, A. Efficacy and safety of the weight-loss drug rimonabant: A meta-analysis of randomised trials. Lancet 2007, 370, 1706–1713. [Google Scholar] [CrossRef]
- Jones, D. End of the line for cannabinoid receptor 1 as an anti-obesity target? Nat. Rev. Drug Discov. 2008, 7, 961–962. [Google Scholar] [CrossRef] [PubMed]
- Gomez, R.; Navarro, M.; Ferrer, B.; Trigo, J.M.; Bilbao, A.; Del Arco, I.; Cippitelli, A.; Nava, F.; Piomelli, D.; Rodriguez de Fonseca, F. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J. Neurosci. 2002, 22, 9612–9617. [Google Scholar] [CrossRef]
- Bensaid, M.; Gary-Bobo, M.; Esclangon, A.; Maffrand, J.P.; Le Fur, G.; Oury-Donat, F.; Soubrie, P. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol. Pharmacol. 2003, 63, 908–914. [Google Scholar] [CrossRef]
- Jourdan, T.; Djaouti, L.; Demizieux, L.; Gresti, J.; Verges, B.; Degrace, P. CB1 antagonism exerts specific molecular effects on visceral and subcutaneous fat and reverses liver steatosis in diet-induced obese mice. Diabetes 2010, 59, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Quarta, C.; Bellocchio, L.; Mancini, G.; Mazza, R.; Cervino, C.; Braulke, L.J.; Fekete, C.; Latorre, R.; Nanni, C.; Bucci, M.; et al. CB(1) signaling in forebrain and sympathetic neurons is a key determinant of endocannabinoid actions on energy balance. Cell Metab. 2010, 11, 273–285. [Google Scholar] [CrossRef]
- Osei-Hyiaman, D.; Liu, J.; Zhou, L.; Godlewski, G.; Harvey-White, J.; Jeong, W.I.; Batkai, S.; Marsicano, G.; Lutz, B.; Buettner, C.; et al. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J. Clin. Investig. 2008, 118, 3160–3169. [Google Scholar] [CrossRef]
- Pagotto, U.; Marsicano, G.; Cota, D.; Lutz, B.; Pasquali, R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr. Rev. 2006, 27, 73–100. [Google Scholar] [CrossRef] [PubMed]
- Udi, S.; Hinden, L.; Earley, B.; Drori, A.; Reuveni, N.; Hadar, R.; Cinar, R.; Nemirovski, A.; Tam, J. Proximal Tubular Cannabinoid-1 Receptor Regulates Obesity-Induced CKD. J. Am. Soc. Nephrol. 2017, 28, 3518–3532. [Google Scholar] [CrossRef]
- Jourdan, T.; Godlewski, G.; Cinar, R.; Bertola, A.; Szanda, G.; Liu, J.; Tam, J.; Han, T.; Mukhopadhyay, B.; Skarulis, M.C.; et al. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nat. Med. 2013, 19, 1132–1140. [Google Scholar] [CrossRef]
- Bordicchia, M.; Battistoni, I.; Mancinelli, L.; Giannini, E.; Refi, G.; Minardi, D.; Muzzonigro, G.; Mazzucchelli, R.; Montironi, R.; Piscitelli, F.; et al. Cannabinoid CB1 receptor expression in relation to visceral adipose depots, endocannabinoid levels, microvascular damage, and the presence of the Cnr1 A3813G variant in humans. Metabolism 2010, 59, 734–741. [Google Scholar] [CrossRef]
- Engeli, S.; Bohnke, J.; Feldpausch, M.; Gorzelniak, K.; Janke, J.; Batkai, S.; Pacher, P.; Harvey-White, J.; Luft, F.C.; Sharma, A.M.; et al. Activation of the peripheral endocannabinoid system in human obesity. Diabetes 2005, 54, 2838–2843. [Google Scholar] [CrossRef] [PubMed]
- Bluher, M.; Engeli, S.; Kloting, N.; Berndt, J.; Fasshauer, M.; Batkai, S.; Pacher, P.; Schon, M.R.; Jordan, J.; Stumvoll, M. Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes 2006, 55, 3053–3060. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Verrijken, A.; Hakkarainen, A.; Petrosino, S.; Mertens, I.; Lundbom, N.; Piscitelli, F.; Westerbacka, J.; Soro-Paavonen, A.; Matias, I.; et al. Role of insulin as a negative regulator of plasma endocannabinoid levels in obese and nonobese subjects. Eur. J. Endocrinol. 2009, 161, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Abdulnour, J.; Yasari, S.; Rabasa-Lhoret, R.; Faraj, M.; Petrosino, S.; Piscitelli, F.; Prud’ Homme, D.; Di Marzo, V. Circulating endocannabinoids in insulin sensitive vs. insulin resistant obese postmenopausal women. A MONET group study. Obesity (Silver Spring) 2014, 22, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Knani, I.; Earley, B.J.; Udi, S.; Nemirovski, A.; Hadar, R.; Gammal, A.; Cinar, R.; Hirsch, H.J.; Pollak, Y.; Gross, I.; et al. Targeting the endocannabinoid/CB1 receptor system for treating obesity in Prader-Willi syndrome. Mol. Metab. 2016, 5, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Azar, S.; Sherf-Dagan, S.; Nemirovski, A.; Webb, M.; Raziel, A.; Keidar, A.; Goitein, D.; Sakran, N.; Shibolet, O.; Tam, J.; et al. Circulating Endocannabinoids Are Reduced Following Bariatric Surgery and Associated with Improved Metabolic Homeostasis in Humans. Obes. Surg. 2019, 29, 268–276. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Azar, S.; Nemirovski, A.; Webb, M.; Halpern, Z.; Shibolet, O.; Tam, J. Serum levels of endocannabinoids are independently associated with nonalcoholic fatty liver disease. Obesity 2017, 25, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Ruiz de Azua, I.; Mancini, G.; Srivastava, R.K.; Rey, A.A.; Cardinal, P.; Tedesco, L.; Zingaretti, C.M.; Sassmann, A.; Quarta, C.; Schwitter, C.; et al. Adipocyte cannabinoid receptor CB1 regulates energy homeostasis and alternatively activated macrophages. J. Clin. Investig. 2017, 127, 4148–4162. [Google Scholar] [CrossRef]
- Gonzalez-Mariscal, I.; Montoro, R.A.; Doyle, M.E.; Liu, Q.R.; Rouse, M.; O’Connell, J.F.; Santa-Cruz Calvo, S.; Krzysik-Walker, S.M.; Ghosh, S.; Carlson, O.D.; et al. Absence of cannabinoid 1 receptor in beta cells protects against high-fat/high-sugar diet-induced beta cell dysfunction and inflammation in murine islets. Diabetologia 2018, 61, 1470–1483. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Mariscal, I.; Montoro, R.A.; O’Connell, J.F.; Kim, Y.; Gonzalez-Freire, M.; Liu, Q.R.; Alfaras, I.; Carlson, O.D.; Lehrmann, E.; Zhang, Y.; et al. Muscle cannabinoid 1 receptor regulates Il-6 and myostatin expression, governing physical performance and whole-body metabolism. FASEB J. 2019. [Google Scholar] [CrossRef] [PubMed]
- Drori, A.; Permyakova, A.; Hadar, R.; Udi, S.; Nemirovski, A.; Tam, J. Cannabinoid-1 receptor regulates mitochondrial dynamics and function in renal proximal tubular cells. Diabetes Obes. Metab. 2019, 21, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Hinden, L.; Udi, S.; Drori, A.; Gammal, A.; Nemirovski, A.; Hadar, R.; Baraghithy, S.; Permyakova, A.; Geron, M.; Cohen, M.; et al. Modulation of Renal GLUT2 by the Cannabinoid-1 Receptor: Implications for the Treatment of Diabetic Nephropathy. J. Am. Soc. Nephrol. JASN 2018, 29, 434–448. [Google Scholar] [CrossRef]
- Wager, T.T.; Chandrasekaran, R.Y.; Hou, X.; Troutman, M.D.; Verhoest, P.R.; Villalobos, A.; Will, Y. Defining desirable central nervous system drug space through the alignment of molecular properties, in vitro ADME, and safety attributes. ACS Chem. Neurosci. 2010, 1, 420–434. [Google Scholar] [CrossRef]
- Chorvat, R.J. Peripherally restricted CB1 receptor blockers. Bioorganic med. Chem. Lett. 2013, 23, 4751–4760. [Google Scholar] [CrossRef]
- Sharma, M.K.; Murumkar, P.R.; Kanhed, A.M.; Giridhar, R.; Yadav, M.R. Prospective therapeutic agents for obesity: Molecular modification approaches of centrally and peripherally acting selective cannabinoid 1 receptor antagonists. Eur. J. Med. Chem. 2014, 79, 298–339. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.K.; Murumkar, P.R.; Giridhar, R.; Yadav, M.R. Exploring structural requirements for peripherally acting 1,5-diaryl pyrazole-containing cannabinoid 1 receptor antagonists for the treatment of obesity. Mol. Divers. 2015, 19, 871–893. [Google Scholar] [CrossRef]
- Yadav, M.R.; Murumkar, P.R. Advances in patented CB1 receptor antagonists for obesity. Pharm. Patent Anal. 2018, 7, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.; Vemuri, V.K.; Liu, J.; Batkai, S.; Mukhopadhyay, B.; Godlewski, G.; Osei-Hyiaman, D.; Ohnuma, S.; Ambudkar, S.V.; Pickel, J.; et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J. Clin. Invest. 2010, 120, 2953–2966. [Google Scholar] [CrossRef] [PubMed]
- Cluny, N.L.; Vemuri, V.K.; Chambers, A.P.; Limebeer, C.L.; Bedard, H.; Wood, J.T.; Lutz, B.; Zimmer, A.; Parker, L.A.; Makriyannis, A.; et al. A novel peripherally restricted cannabinoid receptor antagonist, AM6545, reduces food intake and body weight, but does not cause malaise, in rodents. Br. J. Pharmacol. 2010, 161, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Argueta, D.A.; DiPatrizio, N.V. Peripheral endocannabinoid signaling controls hyperphagia in western diet-induced obesity. Physiol. Behav. 2017, 171, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Bowles, N.P.; Karatsoreos, I.N.; Li, X.; Vemuri, V.K.; Wood, J.A.; Li, Z.; Tamashiro, K.L.; Schwartz, G.J.; Makriyannis, A.M.; Kunos, G.; et al. A peripheral endocannabinoid mechanism contributes to glucocorticoid-mediated metabolic syndrome. Proc. Natl. Acad. Sci. USA 2015, 112, 285–290. [Google Scholar] [CrossRef]
- Boon, M.R.; Kooijman, S.; van Dam, A.D.; Pelgrom, L.R.; Berbee, J.F.; Visseren, C.A.; van Aggele, R.C.; van den Hoek, A.M.; Sips, H.C.; Lombes, M.; et al. Peripheral cannabinoid 1 receptor blockade activates brown adipose tissue and diminishes dyslipidemia and obesity. FASEB J. 2014, 28, 5361–5375. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zhang, G.; Mou, C.; Fu, X.; Chen, Y. Peripheral CB1 Receptor Neutral Antagonist, AM6545, Ameliorates Hypometabolic Obesity and Improves Adipokine Secretion in Monosodium Glutamate Induced Obese Mice. Front. Pharmacol. 2018, 9, 156. [Google Scholar] [CrossRef]
- Tam, J.; Cinar, R.; Liu, J.; Godlewski, G.; Wesley, D.; Jourdan, T.; Szanda, G.; Mukhopadhyay, B.; Chedester, L.; Liow, J.S.; et al. Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab. 2012, 16, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.; Szanda, G.; Drori, A.; Liu, Z.; Cinar, R.; Kashiwaya, Y.; Reitman, M.L.; Kunos, G. Peripheral cannabinoid-1 receptor blockade restores hypothalamic leptin signaling. Mol. Metab. 2017, 6, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Klumpers, L.E.; Fridberg, M.; de Kam, M.L.; Little, P.B.; Jensen, N.O.; Kleinloog, H.D.; Elling, C.E.; van Gerven, J.M. Peripheral selectivity of the novel cannabinoid receptor antagonist TM38837 in healthy subjects. Br. J. Clin. Pharmacol. 2013, 76, 846–857. [Google Scholar] [CrossRef]
- Takano, A.; Gulyas, B.; Varnas, K.; Little, P.B.; Noerregaard, P.K.; Jensen, N.O.; Elling, C.E.; Halldin, C. Low brain CB1 receptor occupancy by a second generation CB1 receptor antagonist TM38837 in comparison with rimonabant in nonhuman primates: A PET study. Synapse 2014, 68, 89–97. [Google Scholar] [CrossRef]
- Hung, M.S.; Chang, C.P.; Li, T.C.; Yeh, T.K.; Song, J.S.; Lin, Y.; Wu, C.H.; Kuo, P.C.; Amancha, P.K.; Wong, Y.C.; et al. Discovery of 1-(2,4-dichlorophenyl)-4-ethyl-5-(5-(2-(4-(trifluoromethyl)phenyl)ethynyl)thiophe n-2-yl)-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide as a potential peripheral cannabinoid-1 receptor inverse agonist. ChemMedChem 2010, 5, 1439–1443. [Google Scholar] [CrossRef]
- Ward, S.J.; Raffa, R.B. Rimonabant redux and strategies to improve the future outlook of CB1 receptor neutral-antagonist/inverse-agonist therapies. Obesity 2011, 19, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Noerregaard, P.K.; Fridberg, M.; Elling, C.E. TM38837—A novel second generation peripheral selective CB1 receptor antagonist with efficacy and potency in rodent obesity models equal to brain-penetrant CB1 antagonist rimonabant. In Proceedings of the 20th Annual Symposium of the International Cannabinoid Research Society, Lund, Sweden, 23–27 July 2010. [Google Scholar]
- Hsiao, W.C.; Shia, K.S.; Wang, Y.T.; Yeh, Y.N.; Chang, C.P.; Lin, Y.; Chen, P.H.; Wu, C.H.; Chao, Y.S.; Hung, M.S. A novel peripheral cannabinoid receptor 1 antagonist, BPR0912, reduces weight independently of food intake and modulates thermogenesis. Diabetes Obes. Metab. 2015, 17, 495–504. [Google Scholar] [CrossRef]
- Mastinu, A.; Pira, M.; Pinna, G.A.; Pisu, C.; Casu, M.A.; Reali, R.; Marcello, S.; Murineddu, G.; Lazzari, P. NESS06SM reduces body weight with an improved profile relative to SR141716A. Pharmacol. Res. 2013, 74, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, P.; Serra, V.; Marcello, S.; Pira, M.; Mastinu, A. Metabolic side effects induced by olanzapine treatment are neutralized by CB1 receptor antagonist compounds co-administration in female rats. Eur. Neuropsychopharmacol. 2017, 27, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Pavon, F.J.; Bilbao, A.; Hernandez-Folgado, L.; Cippitelli, A.; Jagerovic, N.; Abellan, G.; Rodriguez-Franco, M.A.; Serrano, A.; Macias, M.; Gomez, R.; et al. Antiobesity effects of the novel in vivo neutral cannabinoid receptor antagonist 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-3-hexyl-1H-1,2,4-triazole--LH 21. Neuropharmacology 2006, 51, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Pavon, F.J.; Serrano, A.; Perez-Valero, V.; Jagerovic, N.; Hernandez-Folgado, L.; Bermudez-Silva, F.J.; Macias, M.; Goya, P.; de Fonseca, F.R. Central versus peripheral antagonism of cannabinoid CB1 receptor in obesity: Effects of LH-21, a peripherally acting neutral cannabinoid receptor antagonist, in Zucker rats. J. Neuroendocrinol. 2008, 20 (Suppl. 1), 116–123. [Google Scholar] [CrossRef]
- Alonso, M.; Serrano, A.; Vida, M.; Crespillo, A.; Hernandez-Folgado, L.; Jagerovic, N.; Goya, P.; Reyes-Cabello, C.; Perez-Valero, V.; Decara, J.; et al. Anti-obesity efficacy of LH-21, a cannabinoid CB(1) receptor antagonist with poor brain penetration, in diet-induced obese rats. Br. J. Pharmacol. 2012, 165, 2274–2291. [Google Scholar] [CrossRef]
- Chen, R.Z.; Frassetto, A.; Lao, J.Z.; Huang, R.R.; Xiao, J.C.; Clements, M.J.; Walsh, T.F.; Hale, J.J.; Wang, J.; Tong, X.; et al. Pharmacological evaluation of LH-21, a newly discovered molecule that binds to cannabinoid CB1 receptor. Eur. J. Pharmacol. 2008, 584, 338–342. [Google Scholar] [CrossRef] [PubMed]
- LoVerme, J.; Duranti, A.; Tontini, A.; Spadoni, G.; Mor, M.; Rivara, S.; Stella, N.; Xu, C.; Tarzia, G.; Piomelli, D. Synthesis and characterization of a peripherally restricted CB1 cannabinoid antagonist, URB447, that reduces feeding and body-weight gain in mice. Bioorganic Med. Chem. Lett. 2009, 19, 639–643. [Google Scholar] [CrossRef]
- DiPatrizio, N.V.; Astarita, G.; Schwartz, G.; Li, X.; Piomelli, D. Endocannabinoid signal in the gut controls dietary fat intake. Proc. Natl. Acad. Sci. USA 2011, 108, 12904–12908. [Google Scholar] [CrossRef] [PubMed]
- DiPatrizio, N.V.; Joslin, A.; Jung, K.M.; Piomelli, D. Endocannabinoid signaling in the gut mediates preference for dietary unsaturated fats. FASEB J. 2013, 27, 2513–2520. [Google Scholar] [CrossRef]
- Son, M.H.; Kim, H.D.; Chae, Y.N.; Kim, M.K.; Shin, C.Y.; Ahn, G.J.; Choi, S.H.; Yang, E.K.; Park, K.J.; Chae, H.W.; et al. Peripherally acting CB1-receptor antagonist: The relative importance of central and peripheral CB1 receptors in adiposity control. Int. J. Obes. 2010, 34, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Receveur, J.M.; Murray, A.; Linget, J.M.; Norregaard, P.K.; Cooper, M.; Bjurling, E.; Nielsen, P.A.; Hogberg, T. Conversion of 4-cyanomethyl-pyrazole-3-carboxamides into CB1 antagonists with lowered propensity to pass the blood-brain-barrier. Bioorganic Med. Chem. Lett. 2010, 20, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Greco, M.N.; Macielag, M.J.; Teleha, C.A.; DesJarlais, R.L.; Tang, Y.; Ho, G.; Hou, C.; Chen, C.; Zhao, S.; et al. 6-Benzhydryl-4-amino-quinolin-2-ones as Potent Cannabinoid Type 1 (CB1) Receptor Inverse Agonists and Chemical Modifications for Peripheral Selectivity. J. Med. Chem. 2018, 61, 10276–10298. [Google Scholar] [CrossRef]
- Matthews, J.M.; McNally, J.J.; Connolly, P.J.; Xia, M.; Zhu, B.; Black, S.; Chen, C.; Hou, C.; Liang, Y.; Tang, Y.; et al. Tetrahydroindazole derivatives as potent and peripherally selective cannabinoid-1 (CB1) receptor inverse agonists. Bioorganic Med. Chem. Lett. 2016, 26, 5346–5349. [Google Scholar] [CrossRef]
- Chen, W.; Shui, F.; Liu, C.; Zhou, X.; Li, W.; Zheng, Z.; Fu, W.; Wang, L. Novel Peripherally Restricted Cannabinoid 1 Receptor Selective Antagonist TXX-522 with Prominent Weight-Loss Efficacy in Diet Induced Obese Mice. Front. Pharmacol. 2017, 8, 707. [Google Scholar] [CrossRef]
- Rover, S.; Andjelkovic, M.; Benardeau, A.; Chaput, E.; Guba, W.; Hebeisen, P.; Mohr, S.; Nettekoven, M.; Obst, U.; Richter, W.F.; et al. 6-Alkoxy-5-aryl-3-pyridinecarboxamides, a new series of bioavailable cannabinoid receptor type 1 (CB1) antagonists including peripherally selective compounds. J. Med. Chem. 2013, 56, 9874–9896. [Google Scholar] [CrossRef]
- Fulp, A.; Zhang, Y.; Bortoff, K.; Seltzman, H.; Snyder, R.; Wiethe, R.; Amato, G.; Maitra, R. Pyrazole antagonists of the CB1 receptor with reduced brain penetration. Bioorg Med. Chem. 2016, 24, 1063–1070. [Google Scholar] [CrossRef]
- Amato, G.S.; Manke, A.; Vasukuttan, V.; Wiethe, R.W.; Snyder, R.W.; Runyon, S.P.; Maitra, R. Synthesis and pharmacological characterization of functionalized 6-piperazin-1-yl-purines as cannabinoid receptor 1 (CB1) inverse agonists. Bioorg Med. Chem. 2018, 26, 4518–4531. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Shin, H.; Rho, J.G.; Kim, J.E.; Son, D.H.; Yoon, J.; Lee, Y.J.; Park, J.H.; Song, B.J.; Choi, C.S.; et al. Peripheral cannabinoid 1 receptor blockade mitigates adipose tissue inflammation via NLRP3 inflammasome in mouse models of obesity. Diabetes Obes. Metab 2018, 20, 2179–2189. [Google Scholar] [CrossRef]
- Han, J.H.; Shin, H.; Park, J.Y.; Rho, J.G.; Son, D.H.; Kim, K.W.; Seong, J.K.; Yoon, S.H.; Kim, W. A novel peripheral cannabinoid 1 receptor antagonist, AJ5012, improves metabolic outcomes and suppresses adipose tissue inflammation in obese mice. FASEB J. 2019, 33, 4314–4326. [Google Scholar] [CrossRef] [PubMed]
- Fulp, A.; Bortoff, K.; Seltzman, H.; Zhang, Y.; Mathews, J.; Snyder, R.; Fennell, T.; Maitra, R. Design and synthesis of cannabinoid receptor 1 antagonists for peripheral selectivity. J. Med. Chem. 2012, 55, 2820–2834. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Vazquez, E.; Ocampo-Montalban, H.; Ceron-Romero, L.; Cruz, M.; Gomez-Zamudio, J.; Hiriart-Valencia, G.; Villalobos-Molina, R.; Flores-Flores, A.; Estrada-Soto, S. Antidiabetic, antidyslipidemic and toxicity profile of ENV-2: A potent pyrazole derivative against diabetes and related diseases. Eur. J. Pharmacol. 2017, 803, 159–166. [Google Scholar] [CrossRef]
- Chen, W.; Liu, H.; Guan, H.; Xue, N.; Wang, L. Cannabinoid CB1 receptor inverse agonist MJ08 stimulates glucose production via hepatic sympathetic innervation in rats. Eur. J. Pharmacol. 2017, 814, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xu, C.; Liu, H.Y.; Long, L.; Zhang, W.; Zheng, Z.B.; Xie, Y.D.; Wang, L.L.; Li, S. Novel selective cannabinoid CB(1) receptor antagonist MJ08 with potent in vivo bioactivity and inverse agonistic effects. Acta Pharmacol. Sin. 2011, 32, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Seltzman, H.H.; Maitra, R.; Bortoff, K.; Henson, J.; Reggio, P.H.; Wesley, D.; Tam, J. Metabolic Profiling of CB1 Neutral Antagonists. Methods Enzymol. 2017, 593, 199–215. [Google Scholar]
- Hurst, D.; Umejiego, U.; Lynch, D.; Seltzman, H.; Hyatt, S.; Roche, M.; McAllister, S.; Fleischer, D.; Kapur, A.; Abood, M.; et al. Biarylpyrazole inverse agonists at the cannabinoid CB1 receptor: importance of the C-3 carboxamide oxygen/lysine3.28(192) interaction. J. Med. Chem. 2006, 49, 5969–5987. [Google Scholar] [CrossRef]
| Compound | CB1R Ki/EC50/IC50 | CB2R Ki/EC50/IC50 | Nature of Compound | cLogP/LogP | TPSA/PSA (Å2) | HBD | Animal Model | Efficacy | Brain/Plasma Ratio | Structure | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LH-21 | EC50 = 76.9 nM | EC50 = 6.56 µM | Neutral antagonist | N/A | N/A | N/A | Obese and lean Zucker rats | Reduces food intake, no change in lipid level and plasma glucose | N/A | 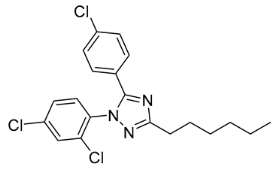 | [138,139,140,141] |
| URB447 | IC50 = 313 nM | IC50 = 41 nM | Neutral antagonist (CB1R)/ agonist (CB2R) | LogP = 6.39 | PSA = 48.02 | N/A | ob/ob mice | Reduces food intake and body weight gain | N/A | 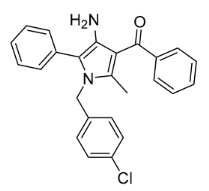 | [142,143,144] |
| AM6545 | Ki = 3.3 nM | CB1R/CB2R > 100 | Neutral antagonist | LogP = 3.3 | PSA = 116 | 1 | DIO C57BL/6 mice | Reduces body weight, hepatic triglyceride content, and hepatocellular damage; increases fat oxidation | 0.03 | 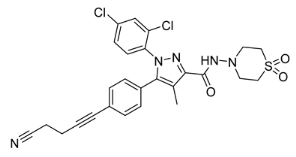 | [122,123,124,125,126,127] |
| Compound 1 | IC50 = 159 nM | >10 µM | Antagonist | N/A | N/A | N/A | DIO C57BL/6 mice | Reduces body weight and suppresses DIO-induced elevation in hepatic SREBP-1 expression | CLapp., uptake = 0.00228 | 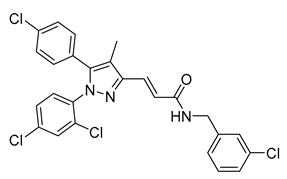 | [145] |
| Compound D4 | IC50 = 2.6 nM | CB1R/CB2R > 1000 nM | Antagonist | N/A | N/A | N/A | DIO C57BL/6 mice | Reduces body weight | 0.098 | 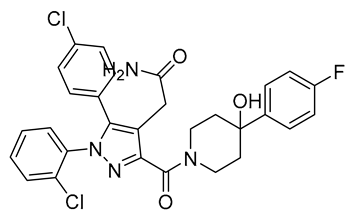 | [146] |
| TM38837 (BPR0912) | IC50 = 8.5 nM EC50 = 18.5 nM | IC50 = 605 nM | Antagonist | LogP = 8.91 | TPSA = 78 | 1 | DIO C57BL/6 mice | Decreases body weight and increases thermogenesis | 0.03 | 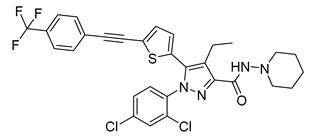 | [130,131,132,133,134,135] |
| JD5037 | Ki = 0.35 nM | CB1R/CB2R > 700 nM | Inverse agonist | cLogP = 6 | PSA = 117 | 3 | DIO C57BL/6 mice | Reduces food intake, body weight, and improves hormonal/ metabolic abnormalities | 0.02 | 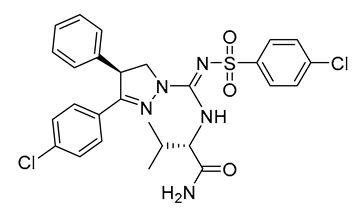 | [102,109,116,128,129] |
| Compound 14h | Ki = 5.1 nM | Ki > 10,000 nM | Antagonist | LogP = 3.7 | N/A | N/A | DIO Sprague−Dawley rats | No metabolic effect | 0.13 | 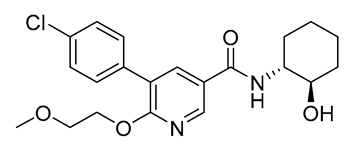 | [150] |
| NESS06SM | Ki = 10.25 nM | Ki > 5000 nM | Neutral antagonist | cLogP = 4.62 | TPSA = 59.39 | N/A | DIO C57BL/6 mice | Reduces body weight and visceral fat mass, improves blood glucose and dyslipidemia | logBB = −0.038 (low) | 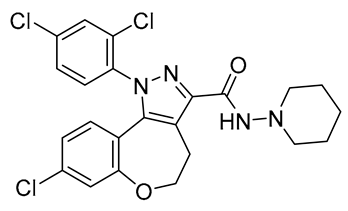 | [136,137] |
| Compound 2p | EC50 = 0.035 µM | EC50 = 2.0 µM | Inverse agonist | cLogP = 7.27 | TPSA = 59.8 | N/A | DIO C57BL/6 mice | Lowers plasma glucose levels | 0.05 |  | [148] |
| Compound 8c | Ki = 8.82 nM | Ki = 1545 nM | Inverse agonist | N/A | TPSA = 76 | N/A | N/A | N/A | 0.15 |  | [151] |
| TXX522 | IC50 = 10.33 nmol/L | IC50 > 10 µmol/L | Neutral antagonist | LogP = 7.95 | TPSA = 56.73 | 1 | DIO C57BL/6 mice | Reduces body weight and fat mass, decreases metabolic complications | 0.02 (Kp) | 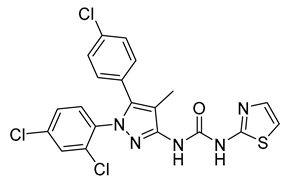 | [149] |
| Compound 6a | EC50 = 0.0082 µM | EC50 > 10 µM | Inverse agonist | cLogP = 6.15 | TPSA = 86.9 | 2 | DIO C57BL/6 mice | Reduces body weight, food intake, insulin level, liver fat, and cholesterol | 0.027 | 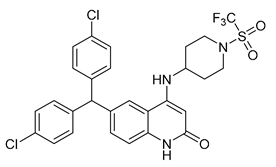 | [147] |
| Compound 65 | Ki = 4.0 nM | Ki > 10,000 nM | Inverse agonist | N/A | N/A | N/A | N/A | N/A | 0.18 | 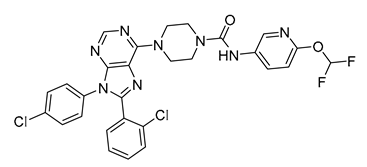 | [152] |
| AJ5018 | IC50 = 90.4 nM | N/A | Antagonist | N/A | N/A | N/A | DIO C57BL/6 and db/db mice | Reduces hyperglycemia, dyslipidemia, hepatic steatosis, energy expenditure, and insulin resistance | 0.1 | 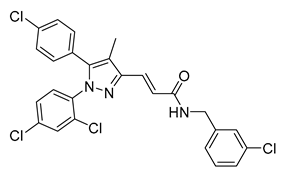 | [153] |
| AJ5012 | N/A | N/A | Antagonist | AlogP = 5.328 | PSA = 84.836 | N/A | DIO C57BL/6 and db/db mice | Reduces weight, increases energy expenditure; improves metabolic abnormalities, glycemic control, and insulin sensitivity | 0.2 | 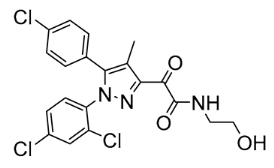 | [154] |
| Compound 17a | Ki = 47.1 nM | Ki = 20,000 nM | Antagonist | N/A | TPSA = 79 | Sprague Dawley rats | N/A | 0.0320 |  | [155] | |
| Compound 18a | Ki = 2.9 nM | Ki = 2510 nM | Antagonist | N/A | TPSA = 76 | Sprague Dawley rats | N/A | 0.0214 | 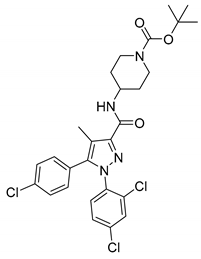 | [155] | |
| Compound 18f | Ki = 14.7 nM | Ki = 3349 nM | Antagonist | N/A | TPSA = 79 | Sprague Dawley rats | N/A | 0.379 | 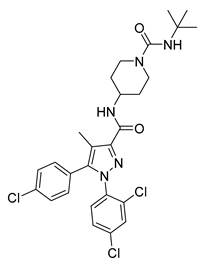 | [155] | |
| ENV-2 | N/A | N/A | Antagonist | N/A | N/A | N/A | Wistar rats | Reduces glycemia and dyslipidemia | N/A | 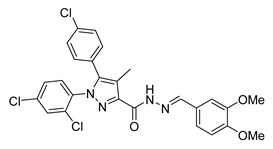 | [156] |
| MJ08 | Ki = 25.4 nM IC50 = 99.9 nmol/L | N/A | Inverse agonist | N/A | N/A | N/A | Wistar rats, DIO C57BL/6 mice | Stimulates hepatic glucose production | N/A | 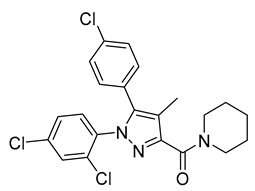 | [157,158] |
| PISMR | Ki = 57 nM | N/A | Antagonist | N/A | N/A | N/A | DIO C57Bl/6 mice | Reduces weight, food intake, and adiposity as well as improving glycemic control and lipid homeostasis | 0.24 | 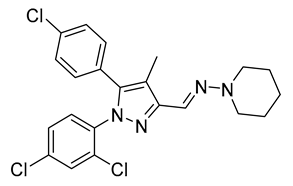 | [159,160] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirsch, S.; Tam, J. Cannabis: From a Plant That Modulates Feeding Behaviors toward Developing Selective Inhibitors of the Peripheral Endocannabinoid System for the Treatment of Obesity and Metabolic Syndrome. Toxins 2019, 11, 275. https://doi.org/10.3390/toxins11050275
Hirsch S, Tam J. Cannabis: From a Plant That Modulates Feeding Behaviors toward Developing Selective Inhibitors of the Peripheral Endocannabinoid System for the Treatment of Obesity and Metabolic Syndrome. Toxins. 2019; 11(5):275. https://doi.org/10.3390/toxins11050275
Chicago/Turabian StyleHirsch, Shira, and Joseph Tam. 2019. "Cannabis: From a Plant That Modulates Feeding Behaviors toward Developing Selective Inhibitors of the Peripheral Endocannabinoid System for the Treatment of Obesity and Metabolic Syndrome" Toxins 11, no. 5: 275. https://doi.org/10.3390/toxins11050275
APA StyleHirsch, S., & Tam, J. (2019). Cannabis: From a Plant That Modulates Feeding Behaviors toward Developing Selective Inhibitors of the Peripheral Endocannabinoid System for the Treatment of Obesity and Metabolic Syndrome. Toxins, 11(5), 275. https://doi.org/10.3390/toxins11050275






