Structurally Robust and Functionally Highly Versatile—C-Type Lectin (-Related) Proteins in Snake Venoms
Abstract
1. Introduction
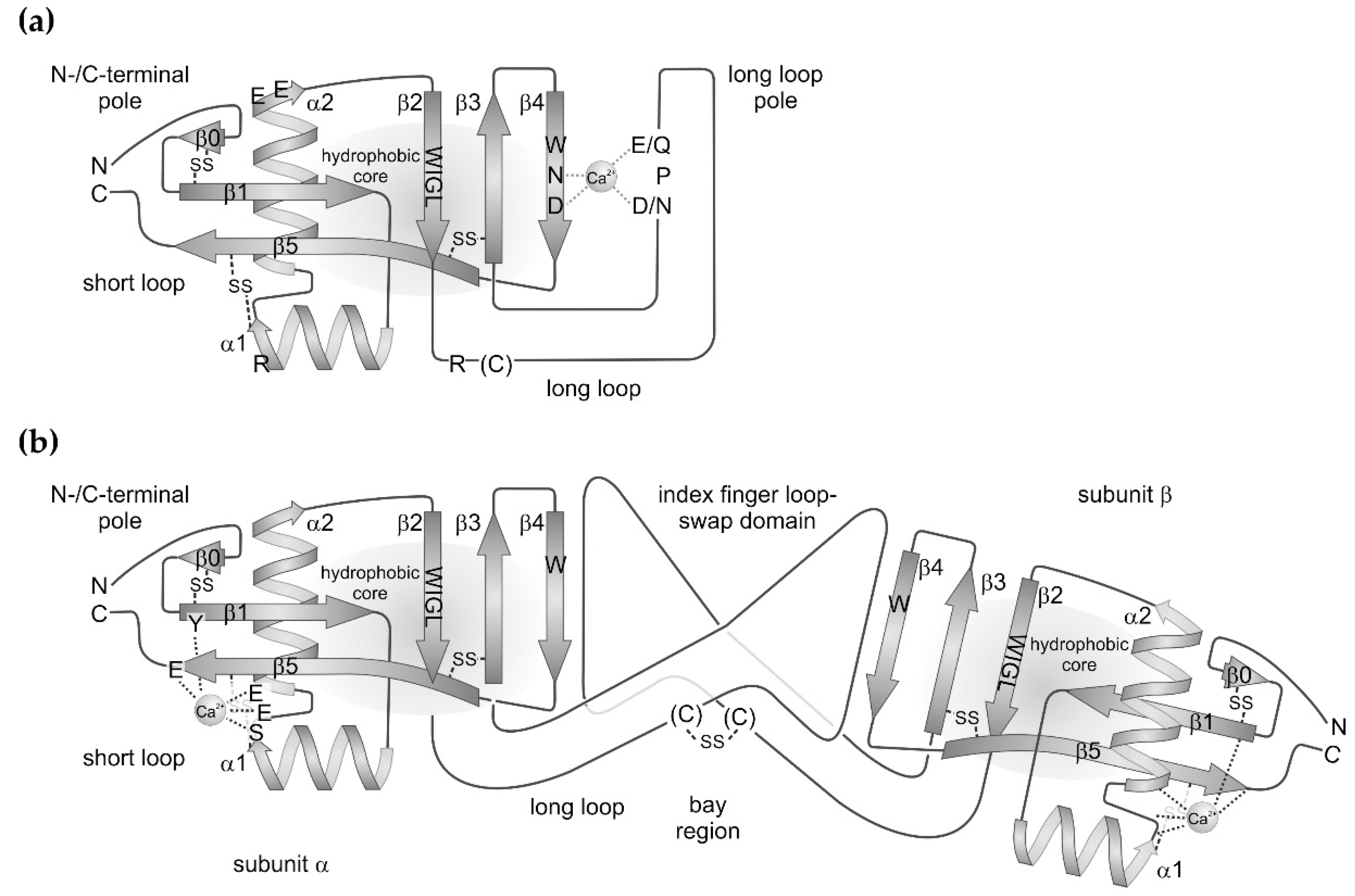
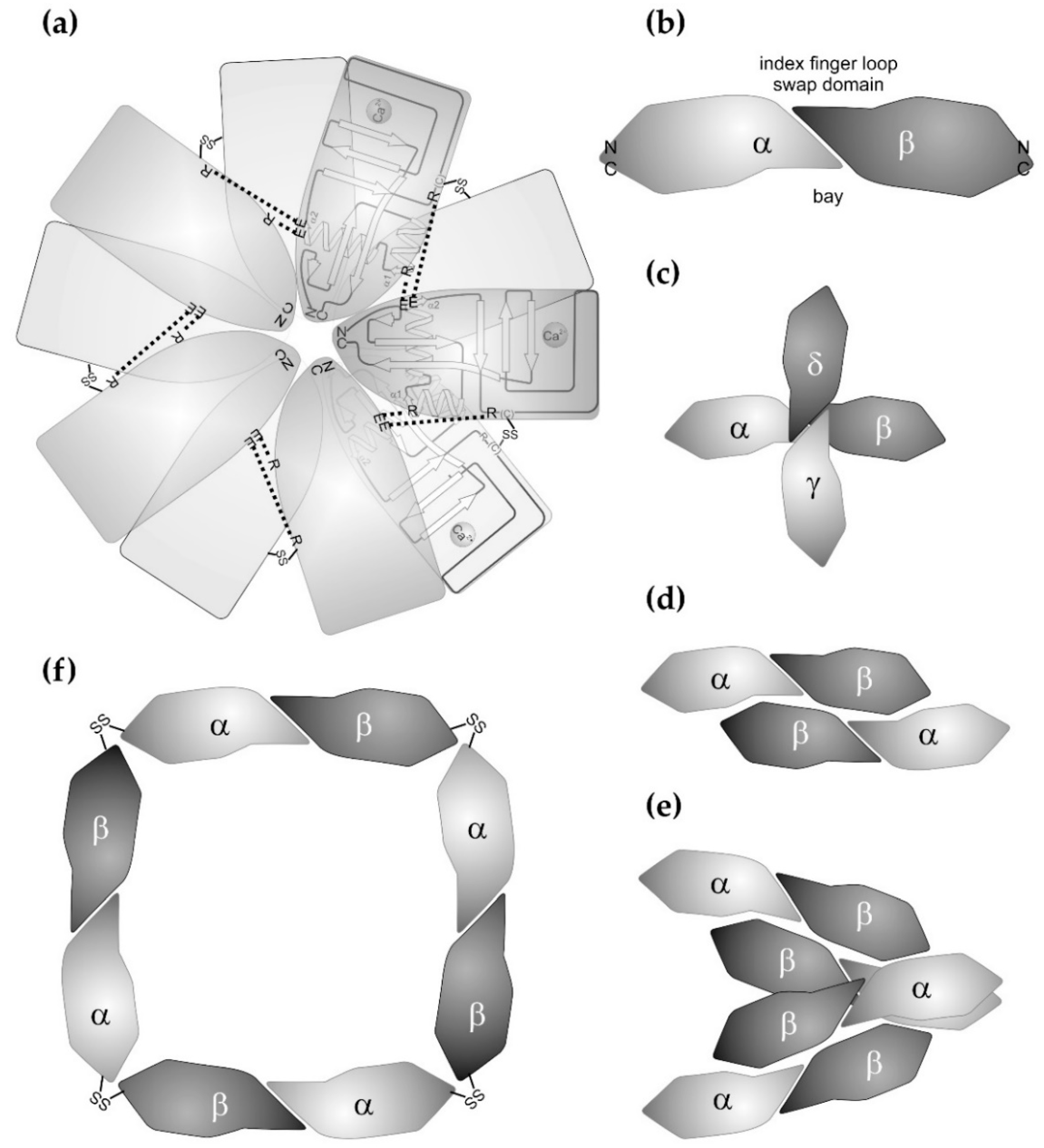
2. Structure of the CTLD and its Variations with Respect to Snake Venom CTLD-Containing Proteins
3. Functional Diversity of SV-CTLD
4. Sugar-Binding C-Type Lectins from Snake Venoms
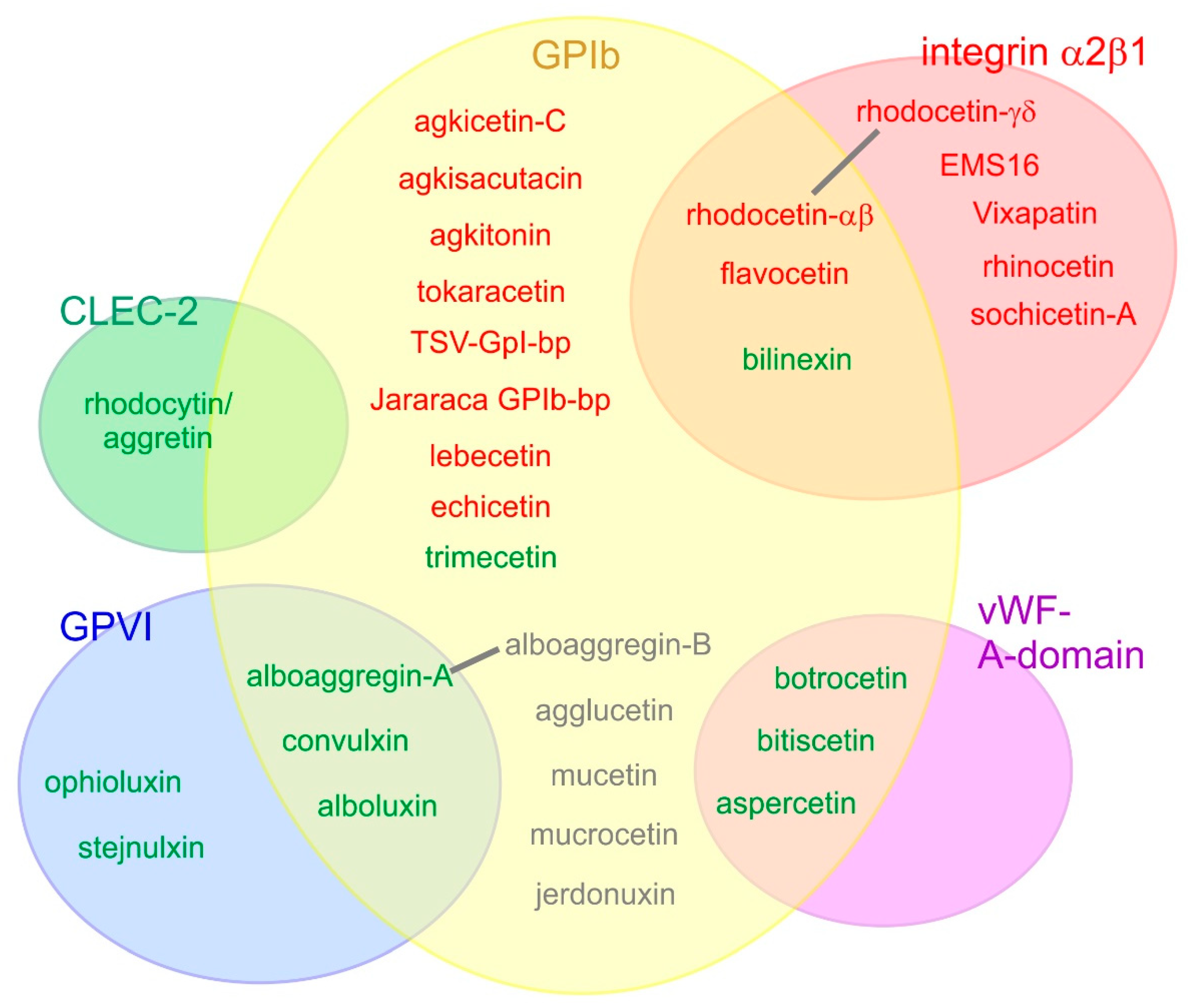
5. Snake Venom-C-Type Lectin-Related Protein (SV-CLRPs)/Snaclecs
5.1. SV-CLRPs Targeting Clotting Factors
5.2. SV-CLRPs Targeting Platelet Receptors
5.3. Novel Targets for SV-CLRPs on Endothelial Cells
6. Translational Potential of SV-CLRPs into Medicine and Perspectives
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations:
| CLRP | C-type lectin-related protein |
| CTLD | C-type lectin domain |
| GP | glycoprotein |
| vWF | von Willebrand factor |
References
- Kilpatrick, D.C. Animal lectins: A historical introduction and overview. Biochim. Biophys. Acta 2002, 1572, 187–197. [Google Scholar] [CrossRef]
- Zelensky, A.N.; Gready, J.E. The C-type lectin-like domain superfamily. FEBS J. 2005, 272, 6179–6217. [Google Scholar] [CrossRef] [PubMed]
- Drickamer, K. C-type lectin-like domains. Curr. Opin. Struct. Biol. 1999, 9, 585–590. [Google Scholar] [CrossRef]
- Zelensky, A.N.; Gready, J.E. Comparative analysis of structural properties of the C-type-lectin-like domain (CTLD). Proteins 2003, 52, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Kohda, D.; Morton, C.J.; Parkar, A.A.; Hatanaka, H.; Inagaki, F.M.; Campbell, I.D.; Day, A.J. Solution structure of the link module: A hyaluronan-binding domain involved in extracellular matrix stability and cell migration. Cell 1996, 86, 767–775. [Google Scholar] [CrossRef]
- Hamburger, Z.A.; Brown, M.S.; Isberg, R.R.; Bjorkman, P.J. Crystal structure of invasin: A bacterial integrin-binding protein. Science 1999, 286, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G.; Prasannan, S.; Daniell, S.; Fleming, K.; Frankel, G.; Dougan, G.; Connerton, I.; Matthews, S. Structure of the cell-adhesion fragment of intimin from enteropathogenic Escherichia coli. Nat. Struct. Biol. 1999, 6, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, R.N.; Wang, C. Bacterial invasin: Structure, function, and implication for targeted oral gene delivery. Curr. Drug Deliv. 2006, 3, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Chou, K.C. Knowledge-based model building of the tertiary structures for lectin domains of the selectin family. J. Protein Chem. 1996, 15, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Graves, B.J.; Crowther, R.L.; Chandran, C.; Rumberger, J.M.; Li, S.; Huang, K.S.; Presky, D.H.; Familletti, P.C.; Wolitzky, B.A.; Burns, D.K. Insight into E-selectin/ligand interaction from the crystal structure and mutagenesis of the lec/EGF domains. Nature 1994, 367, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Cummings, R.D.; Smith, D.F. The selectin family of carbohydrate-binding proteins: Structure and importance of carbohydrate ligands for cell adhesion. Bioessays 1992, 14, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Chijiwa, T.; Oda-Ueda, N.; Ohno, M. Molecular diversity and accelerated evolution of C-type lectin-like proteins from snake venom. Toxicon 2005, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H.; Fujimoto, Z.; Koizumi, M.; Kano, H.; Atoda, H.; Morita, T. Structure of coagulation factors IX/X-binding protein, a heterodimer of C-type lectin domains. Nat. Struct. Biol. 1997, 4, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Nobuhisa, I.; Deshimaru, M.; Chijiwa, T.; Nakashima, K.; Ogawa, T.; Shimohigashi, Y.; Fukumaki, Y.; Hattori, S.; Kihara, H.; Ohno, M. Structures of genes encoding phospholipase A2 inhibitors from the serum of Trimeresurus flavoviridis snake. Gene 1997, 191, 31–37. [Google Scholar] [CrossRef]
- Nobuhisa, I.; Inamasu, S.; Nakai, M.; Tatsui, A.; Mimori, T.; Ogawa, T.; Shimohigashi, Y.; Fukumaki, Y.; Hattori, S.; Kihara, H.; et al. Characterization and evolution of a gene encoding a Trimeresurus flavoviridis serum protein that inhibits basic phospholipase A2 isozymes in the snake’s venom. Eur. J. Biochem. 1997, 249, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Estevao-Costa, M.I.; Sanz-Soler, R.; Johanningmeier, B.; Eble, J.A. Snake venom components in medicine: From the symbolic rod of Asclepius to tangible medical research and application. Int. J. Biochem. Cell Biol. 2018, 104, 94–113. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Primers 2017, 3, 17063. [Google Scholar] [CrossRef] [PubMed]
- Clemetson, K.J. Snaclecs (snake C-type lectins) that inhibit or activate platelets by binding to receptors. Toxicon 2010, 56, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- Gartner, T.K.; Stocker, K.; Williams, D.C. Thrombolectin: A lectin isolated from Bothrops atrox venom. FEBS Lett. 1980, 117, 13–16. [Google Scholar] [CrossRef]
- Aranda-Souza, M.A.; Rossato, F.A.; Costa, R.A.; Figueira, T.R.; Castilho, R.F.; Guarniere, M.C.; Nunes, E.S.; Coelho, L.C.; Correia, M.T.; Vercesi, A.E. A lectin from Bothrops leucurus snake venom raises cytosolic calcium levels and promotes B16-F10 melanoma necrotic cell death via mitochondrial permeability transition. Toxicon 2014, 82, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, L.E.; Nunes, D.C.; Cardoso, T.M.; Santos Pde, S.; Goulart, L.R.; Rodrigues, R.S.; Richardson, M.; Borges, M.H.; Yoneyama, K.A.; Rodrigues, V.M. Biochemical and functional characterization of a C-type lectin (BpLec) from Bothrops pauloensis snake venom. Int. J. Biol. Macromol. 2013, 54, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Sanz, L.; Escolano, J.; Ferretti, M.; Biscoglio, M.J.; Rivera, E.; Crescenti, E.J.; Angulo, Y.; Lomonte, B.; Gutierrez, J.M.; Calvete, J.J. Snake venomics of the South and Central American Bushmasters. Comparison of the toxin composition of Lachesis muta gathered from proteomic versus transcriptomic analysis. J. Proteomics 2008, 71, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Hamako, J.; Suzuki, Y.; Hayashi, N.; Kimura, M.; Ozeki, Y.; Hashimoto, K.; Matsui, T. Amino acid sequence and characterization of C-type lectin purified from the snake venom of Crotalus ruber. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 146, 299–306. [Google Scholar] [CrossRef] [PubMed]
- De-Simone, S.G.; Netto, C.C.; Silva, F.P., Jr. Simple affinity chromatographic procedure to purify β-galactoside binding lectins. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2006, 838, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes-Gomes, V.; Oliveira-Carvalho, A.L.; Junqueira-de-Azevedo, I.L.; DL, S.D.; Pujol-Luz, M.; Castro, H.C.; Ho, P.L.; Zingali, R.B. Cloning, characterization, and structural analysis of a C-type lectin from Bothrops insularis (BiL) venom. Arch. Biochem. Biophys. 2004, 432, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, J.; Kusunoki, T.; Kasai, K. Complete primary structure of a galactose-specific lectin from the venom of the rattlesnake Crotalus atrox. Homologies with Ca2(+)-dependent-type lectins. J. Biol. Chem. 1991, 266, 2320–2326. [Google Scholar] [PubMed]
- Ogilvie, M.L.; Dockter, M.E.; Wenz, L.; Gartner, T.K. Isolation and characterization of lactose-binding lectins from the venoms of the snakes Lachesis muta and Dendroaspis jamesonii. J. Biochem. 1986, 100, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wu, X.F.; Xia, Q.C.; Wang, K.Y. Cloning of a galactose-binding lectin from the venom of Trimeresurus stejnegeri. Biochem. J. 1999, 341 Pt 3, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.R.; Nagar, B.; Young, N.M.; Hirama, T.; Rini, J.M. X-ray crystal structure of a galactose-specific C-type lectin possessing a novel decameric quaternary structure. Biochemistry 2004, 43, 3783–3792. [Google Scholar] [CrossRef] [PubMed]
- Sartim, M.A.; Pinheiro, M.P.; de Padua, R.A.P.; Sampaio, S.V.; Nonato, M.C. Structural and binding studies of a C-type galactose-binding lectin from Bothrops jararacussu snake venom. Toxicon 2017, 126, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Abreu, P.A.; Albuquerque, M.G.; Rodrigues, C.R.; Castro, H.C. Structure-function inferences based on molecular modeling, sequence-based methods and biological data analysis of snake venom lectins. Toxicon 2006, 48, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Zha, H.G.; Lee, W.H.; Zhang, Y. Cloning of cDNAs encoding C-type lectins from Elapidae snakes Bungarus fasciatus and Bungarus multicinctus. Toxicon 2001, 39, 1887–1892. [Google Scholar] [CrossRef]
- Aranda-Souza, M.A.; Lorena, V.M.B.; Correia, M.; Pereira-Neves, A.; Figueiredo, R. A C-type lectin from Bothrops leucurus snake venom forms amyloid-like aggregates in RPMI medium and are efficiently phagocytosed by peritoneal macrophages. Toxicon 2019, 157, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zeng, R.; Wu, X.F.; Wang, K.Y. Expression of isolated C-type carbohydrate recognition domains. Biochem. Biophys. Res. Commun. 2000, 271, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Komori, Y.; Nikai, T.; Tohkai, T.; Sugihara, H. Primary structure and biological activity of snake venom lectin (APL) from Agkistrodon p. piscivorus (Eastern cottonmouth). Toxicon 1999, 37, 1053–1064. [Google Scholar] [CrossRef]
- Ozeki, Y.; Matsui, T.; Hamako, J.; Suzuki, M.; Fujimura, Y.; Yoshida, E.; Nishida, S.; Titani, K. C-type galactoside-binding lectin from Bothrops jararaca venom: Comparison of its structure and function with those of botrocetin. Arch. Biochem. Biophys. 1994, 308, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Earl, S.T.; Robson, J.; Trabi, M.; de Jersey, J.; Masci, P.P.; Lavin, M.F. Characterisation of a mannose-binding C-type lectin from Oxyuranus scutellatus snake venom. Biochimie 2011, 93, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Ip, W.K.; Takahashi, K.; Ezekowitz, R.A.; Stuart, L.M. Mannose-binding lectin and innate immunity. Immunol. Rev. 2009, 230, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Van Asbeck, E.C.; Hoepelman, A.I.; Scharringa, J.; Herpers, B.L.; Verhoef, J. Mannose binding lectin plays a crucial role in innate immunity against yeast by enhanced complement activation and enhanced uptake of polymorphonuclear cells. BMC Microbiol. 2008, 8, 229. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Ezekowitz, R.A. The role of the mannose-binding lectin in innate immunity. Clin. Infect. Dis. 2005, 41 (Suppl. 7), S440–S444. [Google Scholar] [CrossRef] [PubMed]
- Pires, W.L.; de Castro, O.B.; Kayano, A.M.; da Silva Setubal, S.; Pontes, A.S.; Nery, N.M.; Paloschi, M.V.; Dos Santos Pereira, S.; Stabeli, R.G.; Fernandes, C.F.C.; et al. Effect of BjcuL, a lectin isolated from Bothrops jararacussu, on human peripheral blood mononuclear cells. Toxicol. In Vitro 2017, 41, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Elifio-Esposito, S.; Tomazeli, L.; Schwartz, C.; Gimenez, A.P.; Fugii, G.M.; Fernandes, L.C.; Zishler, L.F.; Stuelp-Campelo, P.M.; Moreno, A.N. Human neutrophil migration and activation by BJcuL, a galactose binding lectin purified from Bothrops jararacussu venom. BMC Immunol. 2011, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, L.E.; Lopes, D.S.; Gimenes, S.N.C.; Deconte, S.R.; Ferreira, B.A.; Alves, P.T.; Filho, L.R.G.; Tomiosso, T.C.; Rodrigues, R.S.; Yoneyama, K.A.G.; et al. Angiogenenic effects of BpLec, a C-type lectin isolated from Bothrops pauloensis snake venom. Int. J. Biol. Macromol. 2017, 102, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.C.; Fabres-Klein, M.H.; de Oliveira, L.L.; Feio, R.N.; Malouin, F.; Ribon Ade, O. A C-type lectin from Bothrops jararacussu venom disrupts Staphylococcal biofilms. PLoS ONE 2015, 10, e0120514. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Wuster, W. Assembling an arsenal: Origin and evolution of the snake venom proteome inferred from phylogenetic analysis of toxin sequences. Mol. Biol. Evol. 2004, 21, 870–883. [Google Scholar] [CrossRef] [PubMed]
- Junqueira-de-Azevedo, I.L.; Campos, P.F.; Ching, A.T.; Mackessy, S.P. Colubrid Venom Composition: An -Omics Perspective. Toxins 2016, 8, 230. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Navdaev, A.; Clemetson, J.M.; Clemetson, K.J. Snake venom C-type lectins interacting with platelet receptors. Structure-function relationships and effects on haemostasis. Toxicon 2005, 45, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Morita, T. Structures and functions of snake venom CLPs (C-type lectin-like proteins) with anticoagulant-, procoagulant-, and platelet-modulating activities. Toxicon 2005, 45, 1099–1114. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Mizuno, H.; Atoda, H.; Morita, T. Crystal structure of flavocetin-A, a platelet glycoprotein Ib-binding protein, reveals a novel cyclic tetramer of C-type lectin-like heterodimers. Biochemistry 2000, 39, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Ling, Q.D.; Liau, M.Y.; Huang, T.F. A tetrameric glycoprotein Ib-binding protein, agglucetin, from Formosan pit viper: Structure and interaction with human platelets. Thromb. Haemost. 2003, 90, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Huang, T.F. A novel tetrameric venom protein, agglucetin from Agkistrodon acutus, acts as a glycoprotein Ib agonist. Thromb. Haemost. 2001, 86, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.F.; Ko, T.P.; Hung, C.C.; Chu, J.; Wang, A.H.; Chiou, S.H. Crystal structure of a platelet-agglutinating factor isolated from the venom of Taiwan habu (Trimeresurus mucrosquamatus). Biochem. J. 2004, 378, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Horii, K.; Brooks, M.T.; Herr, A.B. Convulxin forms a dimer in solution and can bind eight copies of glycoprotein VI: Implications for platelet activation. Biochemistry 2009, 48, 2907–2914. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.M.; Wegner, S.V.; Martins Cavaco, A.C.; Estevao-Costa, M.I.; Sanz-Soler, R.; Niland, S.; Nosov, G.; Klingauf, J.; Spatz, J.P.; Eble, J.A. The spatial molecular pattern of integrin recognition sites and their immobilization to colloidal nanobeads determine α2β1 integrin-dependent platelet activation. Biomaterials 2018, 167, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, Y.; Suzuki-Inoue, K.; Inoue, O. Platelet receptors activated via mulitmerization: Glycoprotein VI, GPIb-IX-V, and CLEC-2. J. Thromb. Haemost. 2013, 11 (Suppl. 1), 330–339. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Furihata, K.; Cheli, Y.; Radis-Baptista, G.; Kunicki, T.J. Effect of multimer size and a natural dimorphism on the binding of convulxin to platelet glycoprotein (GP)VI. J. Thromb. Haemost. 2006, 4, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.T.; Zela, S.P.; Gava, L.M.; Michelan-Duarte, S.; Cintra, A.C.; Arni, R.K. Crystal structure of the platelet activator convulxin, a disulfide-linked α4β4 cyclic tetramer from the venom of Crotalus durissus terrificus. Biochem. Biophys. Res. Commun. 2003, 310, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Batuwangala, T.; Leduc, M.; Gibbins, J.M.; Bon, C.; Jones, E.Y. Structure of the snake-venom toxin convulxin. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Clemetson, K.J.; Lu, Q.; Clemetson, J.M. Snake C-type lectin-like proteins and platelet receptors. Pathophysiol. Haemost. Thromb. 2005, 34, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Clemetson, K.J.; Navdaev, A.; Dormann, D.; Du, X.Y.; Clemetson, J.M. Multifunctional snake C-type lectins affecting platelets. Haemostasis 2001, 31, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Morita, T. Structure-function relationships of C-type lectin-related proteins. Pathophysiol. Haemost. Thromb. 2005, 34, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Arlinghaus, F.T.; Eble, J.A. C-type lectin-like proteins from snake venoms. Toxicon 2012, 60, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Broos, K.; De Meyer, S.F.; Feys, H.B.; Vanhoorelbeke, K.; Deckmyn, H. Blood platelet biochemistry. Thromb. Res. 2012, 129, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Gale, A.J. Continuing education course #2: Current understanding of hemostasis. Toxicol. Pathol. 2011, 39, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Clemetson, J.M.; Clemetson, K.J. Snake venoms and hemostasis. J. Thromb. Haemost. 2005, 3, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Sajevic, T.; Leonardi, A.; Krizaj, I. Haemostatically active proteins in snake venoms. Toxicon 2011, 57, 627–645. [Google Scholar] [CrossRef] [PubMed]
- Escalante, T.; Rucavado, A.; Fox, J.W.; Gutierrez, J.M. Key events in microvascular damage induced by snake venom hemorrhagic metalloproteinases. J. Proteomics 2011, 74, 1781–1794. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.; Gutierrez, J.M.; Borkow, G.; Ovadia, M. Ultrastructural alterations in mouse capillary blood vessels after experimental injection of venom from the snake Bothrops asper (Terciopelo). Exp. Mol. Pathol. 1992, 57, 124–133. [Google Scholar] [CrossRef]
- Du, X.Y.; Sim, D.S.; Lee, W.H.; Zhang, Y. Blood cells as targets of snake toxins. Blood Cells Mol. Dis. 2006, 36, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Weisel, J.W.; Litvinov, R.I. Fibrin formation, structure and properties. Subcell. Biochem. 2017, 82, 405–456. [Google Scholar] [CrossRef] [PubMed]
- Markland, F.S., Jr.; Swenson, S. Snake venom metalloproteinases. Toxicon 2013, 62, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.M. The long road of research on snake venom serine proteinases. Toxicon 2013, 62, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S. ADAM and ADAMTS Family Proteins and Snake Venom Metalloproteinases: A Structural Overview. Toxins 2016, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Masood, R.; Ali, I.; Ullah, K.; Ali, H.; Akbar, H.; Betzel, C. Thrombin-like enzymes from snake venom: Structural characterization and mechanism of action. Int. J. Biol. Macromol. 2018, 114, 788–811. [Google Scholar] [CrossRef] [PubMed]
- Takeya, H.; Nishida, S.; Miyata, T.; Kawada, S.; Saisaka, Y.; Morita, T.; Iwanaga, S. Coagulation factor X activating enzyme from Russell’s viper venom (RVV-X). A novel metalloproteinase with disintegrin (platelet aggregation inhibitor)-like and C-type lectin-like domains. J. Biol. Chem. 1992, 267, 14109–14117. [Google Scholar] [PubMed]
- Yamada, D.; Sekiya, F.; Morita, T. Isolation and characterization of carinactivase, a novel prothrombin activator in Echis carinatus venom with a unique catalytic mechanism. J. Biol. Chem. 1996, 271, 5200–5207. [Google Scholar] [PubMed]
- Zingali, R.B.; Jandrot-Perrus, M.; Guillin, M.C.; Bon, C. Bothrojaracin, a new thrombin inhibitor isolated from Bothrops jararaca venom: Characterization and mechanism of thrombin inhibition. Biochemistry 1993, 32, 10794–10802. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.Q.; Raposo, J.G.; Wisner, A.; Guimaraes, J.A.; Bon, C.; Zingali, R.B. Allosteric changes of thrombin catalytic site induced by interaction of bothrojaracin with anion-binding exosites I and II. Biochem. Biophys. Res. Commun. 1999, 262, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.Q.; Zingali, R.B. Inhibition of prothrombin activation by bothrojaracin, a C-type lectin from Bothrops jararaca venom. Arch. Biochem. Biophys. 2000, 382, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Tsai, I.H. Functional and sequence characterization of coagulation factor IX/factor X-binding protein from the venom of Echis carinatus leucogaster. Biochemistry 1996, 35, 5264–5271. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Kumashiro, M.; Yamazaki, Y.; Atoda, H.; Morita, T. Anticoagulant mechanism of factor IX/factor X-binding protein isolated from the venom of Trimeresurus flavoviridis. J. Biochem. 2009, 145, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H.; Fujimoto, Z.; Atoda, H.; Morita, T. Crystal structure of an anticoagulant protein in complex with the Gla domain of factor X. Proc. Natl. Acad. Sci. USA 2001, 98, 7230–7234. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H.; Fujimoto, Z.; Koizumi, M.; Kano, H.; Atoda, H.; Morita, T. Crystal structure of coagulation factor IX-binding protein from habu snake venom at 2.6 A: Implication of central loop swapping based on deletion in the linker region. J. Mol. Biol. 1999, 289, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Morita, T. C-type lectin-related proteins from snake venoms. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2004, 4, 357–373. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, F.; Yamashita, T.; Morita, T. Role of calcium(II) ions in the recognition of coagulation factors IX and X by IX/X-bp, an anticoagulant from snake venom. Biochemistry 1995, 34, 10043–10047. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.H.; Sohn, Y.D.; Hwang, K.C.; Jang, Y.; Kim, D.S.; Chung, K.H. Characterization and cDNA cloning of halyxin, a heterogeneous three-chain anticoagulant protein from the venom of Agkistrodon halys brevicaudus. Toxicon 2002, 40, 947–957. [Google Scholar] [CrossRef]
- Atoda, H.; Ishikawa, M.; Mizuno, H.; Morita, T. Coagulation factor X-binding protein from Deinagkistrodon acutus venom is a Gla domain-binding protein. Biochemistry 1998, 37, 17361–17370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, X.; Shen, D.; Song, J.; Guo, M.; Yan, X. Anticoagulation factor I, a snaclec (snake C-type lectin) from Agkistrodon acutus venom binds to FIX as well as FX: Ca2+ induced binding data. Toxicon 2012, 59, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; Teng, M.; Niu, L. Purification, crystallization and preliminary crystallographic analysis of AHP IX-bp, a zinc ion and pH-dependent coagulation factor IX binding protein from Agkistrodon halys Pallas venom. Acta Crystallogr. D Biol. Crystallogr. 2003, 59, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Morita, T. Use of snake venom inhibitors in studies of the function and tertiary structure of coagulation factors. Int. J. Hematol. 2004, 79, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Marsh, N.A. Diagnostic uses of snake venom. Haemostasis 2001, 31, 211–217. [Google Scholar] [CrossRef] [PubMed]
- McCleary, R.J.; Kini, R.M. Non-enzymatic proteins from snake venoms: A gold mine of pharmacological tools and drug leads. Toxicon 2013, 62, 56–74. [Google Scholar] [CrossRef] [PubMed]
- Iwahashi, H.; Kimura, M.; Nakajima, K.; Yamada, D.; Morita, T. Determination of plasma prothrombin level by Ca2+-dependent prothrombin activator (CA-1) during warfarin anticoagulation. J. Heart Valve Dis. 2001, 10, 388–392. [Google Scholar] [PubMed]
- Yamada, D.; Morita, T. CA-1 method, a novel assay for quantification of normal prothrombin using a Ca2+ -dependent prothrombin activator, carinactivase-1. Thromb. Res. 1999, 94, 221–226. [Google Scholar] [CrossRef]
- Fukushima, S.; Tanaka, T.; Sato, T.; Shirakawa, Y.; Asayama, K.; Shirahata, A. Prothrombin levels in newborn infants by the carinactivase-1 method. Semin. Thromb. Hemost. 2002, 28, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Tans, G.; Rosing, J. Snake venom activators of factor X: An overview. Haemostasis 2001, 31, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Saboor, M.; Ayub, Q.; Samina Ilyas, M. Platelet receptors; an instrumental of platelet physiology. Pak. J. Med. Sci. 2013, 29, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Andrews, R.K.; Berndt, M.C. Platelet physiology and thrombosis. Thromb. Res. 2004, 114, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Lof, A.; Muller, J.P.; Brehm, M.A. A biophysical view on von Willebrand factor activation. J. Cell. Physiol. 2018, 233, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Yasar, S.J.; Abdullah, O.; Fay, W.; Balla, S. Von Willebrand factor revisited. J. Interv. Cardiol. 2018, 31, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Andrews, R.K.; Shen, Y.; Gardiner, E.E.; Berndt, M.C. Platelet adhesion receptors and (patho)physiological thrombus formation. Histol. Histopathol. 2001, 16, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Wijeyewickrema, L.C.; Berndt, M.C.; Andrews, R.K. Snake venom probes of platelet adhesion receptors and their ligands. Toxicon 2005, 45, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Andrews, R.K.; Gardiner, E.E.; Shen, Y.; Whisstock, J.C.; Berndt, M.C. Glycoprotein Ib-IX-V. Int. J. Biochem. Cell Biol. 2003, 35, 1170–1174. [Google Scholar] [CrossRef]
- Ozaki, Y.; Asazuma, N.; Suzuki-Inoue, K.; Berndt, M.C. Platelet GPIb-IX-V-dependent signaling. J. Thromb. Haemost. 2005, 3, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ge, H.; Chen, H.; Li, H.; Liu, Y.; Chen, L.; Li, X.; Liu, J.; Niu, L.; Teng, M. Crystal structure of agkisacucetin, a Gpib-binding snake C-type lectin that inhibits platelet adhesion and aggregation. Proteins 2012, 80, 1707–1711. [Google Scholar] [CrossRef] [PubMed]
- Li, T.T.; Fan, M.L.; Hou, S.X.; Li, X.Y.; Barry, D.M.; Jin, H.; Luo, S.Y.; Kong, F.; Lau, L.F.; Dai, X.R.; et al. A novel snake venom-derived GPIb antagonist, anfibatide, protects mice from acute experimental ischaemic stroke and reperfusion injury. Br. J. Pharmacol. 2015, 172, 3904–3916. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Tsai, I.H. Functional and sequence characterization of agkicetin, a new glycoprotein Ib antagonist isolated from Agkistrodon acutus venom. offf2p4. Biochem. Biophys. Res. Commun. 1995, 210, 472–477. [Google Scholar] [CrossRef]
- Xu, G.; Ulrichts, H.; Vauterin, S.; De Meyer, S.F.; Deckmyn, H.; Teng, M.; Niu, L. How does agkicetin-C bind on platelet glycoprotein Ibα and achieve its platelet effects? Toxicon 2005, 45, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Zha, X.D.; Liu, J.; Xu, K.S. cDNA cloning, sequence analysis, and recombinant expression of akitonin β, a C-type lectin-like protein from Agkistrodon acutus. Acta Pharmacol. Sin. 2004, 25, 372–377. [Google Scholar] [PubMed]
- Kawasaki, T.; Fujimura, Y.; Usami, Y.; Suzuki, M.; Miura, S.; Sakurai, Y.; Makita, K.; Taniuchi, Y.; Hirano, K.; Titani, K. Complete amino acid sequence and identification of the platelet glycoprotein Ib-binding site of jararaca GPIb-BP, a snake venom protein isolated from Bothrops jararaca. J. Biol. Chem. 1996, 271, 10635–10639. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Zhang, Y. Molecular cloning and characterization of a platelet glycoprotein Ib-binding protein from the venom of Trimeresurus stejnegeri. Toxicon 2003, 41, 885–892. [Google Scholar] [CrossRef]
- Taniuchi, Y.; Kawasaki, T.; Fujimura, Y.; Suzuki, M.; Titani, K.; Sakai, Y.; Kaku, S.; Hisamichi, N.; Satoh, N.; Takenaka, T.; et al. Flavocetin-A and -B, two high molecular mass glycoprotein Ib binding proteins with high affinity purified from Trimeresurus flavoviridis venom, inhibit platelet aggregation at high shear stress. Biochim. Biophys. Acta 1995, 1244, 331–338. [Google Scholar] [CrossRef]
- Fujimura, Y.; Ikeda, Y.; Miura, S.; Yoshida, E.; Shima, H.; Nishida, S.; Suzuki, M.; Titani, K.; Taniuchi, Y.; Kawasaki, T. Isolation and characterization of jararaca GPIb-BP, a snake venom antagonist specific to platelet glycoprotein Ib. Thromb. Haemost. 1995, 74, 743–750. [Google Scholar] [PubMed]
- Sarray, S.; Srairi, N.; Hatmi, M.; Luis, J.; Louzir, H.; Regaya, I.; Slema, H.; Marvaldi, J.; El Ayeb, M.; Marrakchi, N. Lebecetin, a potent antiplatelet C-type lectin from Macrovipera lebetina venom. Biochim. Biophys. Acta 2003, 1651, 30–40. [Google Scholar] [CrossRef]
- Navdaev, A.; Subramanian, H.; Petunin, A.; Clemetson, K.J.; Gambaryan, S.; Walter, U. Echicetin coated polystyrene beads: A novel tool to investigate GPIb-specific platelet activation and aggregation. PLoS ONE 2014, 9, e93569. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Holt, J.C.; Niewiarowski, S. Isolation, characterization and amino acid sequence of echicetin β subunit, a specific inhibitor of von Willebrand factor and thrombin interaction with glycoprotein Ib. Biochem. Biophys. Res. Commun. 1994, 205, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Lu, W.; Beviglia, L.; Niewiarowski, S.; Kirby, E.P. Echicetin: A snake venom protein that inhibits binding of von Willebrand factor and alboaggregins to platelet glycoprotein Ib. Blood 1993, 81, 2321–2328. [Google Scholar] [PubMed]
- Navdaev, A.; Lochnit, G.; Eble, J.A. The rhodocetin αβ subunit targets GPIb and inhibits von Willebrand factor induced platelet activation. Toxicon 2011, 57, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Arpijuntarangkoon, J.; Rojnuckarin, P.; Muanpasitporn, C.; Kaeothip, S.; Sangvanich, P.; Intragumtornchai, T. Molecular cloning and sequence analysis of alboaggregin B. Platelets 2007, 18, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, M.A.; Tan, L.; Holt, J.C.; Peng, M.; Karczewski, J.; Calvete, J.J.; Niewiarowski, S. Alboaggregins A and B. Structure and interaction with human platelets. Thromb. Haemost. 1998, 79, 609–613. [Google Scholar] [PubMed]
- Peng, M.; Lu, W.; Kirby, E.P. Alboaggregin-B: A new platelet agonist that binds to platelet membrane glycoprotein Ib. Biochemistry 1991, 30, 11529–11536. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Navdaev, A.; Clemetson, J.M.; Clemetson, K.J. GPIb is involved in platelet aggregation induced by mucetin, a snake C-type lectin protein from Chinese habu (Trimeresurus mucrosquamatus) venom. Thromb. Haemost. 2004, 91, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.M.; Wu, J.B.; Zhang, Y.; Yu, G.Y.; Lee, W.H.; Lu, Q.M.; Zhang, Y. Jerdonuxin, a novel snaclec (snake C-type lectin) with platelet aggregation activity from Trimeresurus jerdonii venom. Toxicon 2011, 57, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Doggett, T.; Laurenzi, I.J.; Liddington, R.C.; Diacovo, T.G. The snake venom protein botrocetin acts as a biological brace to promote dysfunctional platelet aggregation. Nat. Struct. Mol. Biol. 2005, 12, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Doggett, T.A.; Bankston, L.A.; Cruz, M.A.; Diacovo, T.G.; Liddington, R.C. Structural basis of von Willebrand factor activation by the snake toxin botrocetin. Structure 2002, 10, 943–950. [Google Scholar] [CrossRef]
- Maita, N.; Nishio, K.; Nishimoto, E.; Matsui, T.; Shikamoto, Y.; Morita, T.; Sadler, J.E.; Mizuno, H. Crystal structure of von Willebrand factor A1 domain complexed with snake venom, bitiscetin: Insight into glycoprotein Ibα binding mechanism induced by snake venom proteins. J. Biol. Chem. 2003, 278, 37777–37781. [Google Scholar] [CrossRef] [PubMed]
- Rucavado, A.; Soto, M.; Kamiguti, A.S.; Theakston, R.D.; Fox, J.W.; Escalante, T.; Gutierrez, J.M. Characterization of aspercetin, a platelet aggregating component from the venom of the snake Bothrops asper which induces thrombocytopenia and potentiates metalloproteinase-induced hemorrhage. Thromb. Haemost. 2001, 85, 710–715. [Google Scholar] [PubMed]
- Matsui, T.; Hamako, J. Structure and function of snake venom toxins interacting with human von Willebrand factor. Toxicon 2005, 45, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- Hirotsu, S.; Mizuno, H.; Fukuda, K.; Qi, M.C.; Matsui, T.; Hamako, J.; Morita, T.; Titani, K. Crystal structure of bitiscetin, a von Willebrand factor-dependent platelet aggregation inducer. Biochemistry 2001, 40, 13592–13597. [Google Scholar] [CrossRef] [PubMed]
- Madamanchi, A.; Santoro, S.A.; Zutter, M.M. α2β1 Integrin. Adv. Exp. Med. Biol. 2014, 819, 41–60. [Google Scholar] [CrossRef] [PubMed]
- Barczyk, M.; Carracedo, S.; Gullberg, D. Integrins. Cell Tissue Res. 2010, 339, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.D.; Humphries, M.J. Integrin structure, activation, and interactions. Cold Spring Harb. Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Eble, J.A. Collagen-binding integrins as pharmaceutical targets. Curr. Pharm. Des. 2005, 11, 867–880. [Google Scholar] [CrossRef] [PubMed]
- Humphries, J.D.; Byron, A.; Humphries, M.J. Integrin ligands at a glance. J. Cell Sci. 2006, 119, 3901–3903. [Google Scholar] [CrossRef] [PubMed]
- Humphries, J.D.; Chastney, M.R.; Askari, J.A.; Humphries, M.J. Signal transduction via integrin adhesion complexes. Curr. Opin. Cell Biol. 2018, 56, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.H.; Carman, C.V.; Springer, T.A. Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 2007, 25, 619–647. [Google Scholar] [CrossRef] [PubMed]
- Kanchanawong, P.; Shtengel, G.; Pasapera, A.M.; Ramko, E.B.; Davidson, M.W.; Hess, H.F.; Waterman, C.M. Nanoscale architecture of integrin-based cell adhesions. Nature 2010, 468, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Arlinghaus, F.T.; Momic, T.; Ammar, N.A.; Shai, E.; Spectre, G.; Varon, D.; Marcinkiewicz, C.; Heide, H.; Lazarovici, P.; Eble, J.A. Identification of α2β1 integrin inhibitor VP-i with anti-platelet properties in the venom of Vipera palaestinae. Toxicon 2013, 64, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J. The continuing saga of snake venom disintegrins. Toxicon 2013, 62, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Eble, J.A.; Beermann, B.; Hinz, H.J.; Schmidt-Hederich, A. α2β1 integrin is not recognized by rhodocytin but is the specific, high affinity target of rhodocetin, an RGD-independent disintegrin and potent inhibitor of cell adhesion to collagen. J. Biol. Chem. 2001, 276, 12274–12284. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Kini, R.M.; Chung, M.C. Rhodocetin, a novel platelet aggregation inhibitor from the venom of Calloselasma rhodostoma (Malayan pit viper): Synergistic and noncovalent interaction between its subunits. Biochemistry 1999, 38, 7584–7593. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz, C.; Lobb, R.R.; Marcinkiewicz, M.M.; Daniel, J.L.; Smith, J.B.; Dangelmaier, C.; Weinreb, P.H.; Beacham, D.A.; Niewiarowski, S. Isolation and characterization of EMS16, a C-lectin type protein from Echis multisquamatus venom, a potent and selective inhibitor of the α2β1 integrin. Biochemistry 2000, 39, 9859–9867. [Google Scholar] [CrossRef] [PubMed]
- Pilorget, A.; Conesa, M.; Sarray, S.; Michaud-Levesque, J.; Daoud, S.; Kim, K.S.; Demeule, M.; Marvaldi, J.; El Ayeb, M.; Marrakchi, N.; et al. Lebectin, a Macrovipera lebetina venom-derived C-type lectin, inhibits angiogenesis both in vitro and in vivo. J. Cell. Physiol. 2007, 211, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Momic, T.; Cohen, G.; Reich, R.; Arlinghaus, F.T.; Eble, J.A.; Marcinkiewicz, C.; Lazarovici, P. Vixapatin (VP12), a C-type lectin-protein from Vipera xantina palestinae venom: Characterization as a novel anti-angiogenic compound. Toxins 2012, 4, 862–877. [Google Scholar] [CrossRef] [PubMed]
- Vaiyapuri, S.; Hutchinson, E.G.; Ali, M.S.; Dannoura, A.; Stanley, R.G.; Harrison, R.A.; Bicknell, A.B.; Gibbins, J.M. Rhinocetin, a venom-derived integrin-specific antagonist inhibits collagen-induced platelet and endothelial cell functions. J. Biol. Chem. 2012, 287, 26235–26244. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, P.; Calvete, J.J.; Eble, J.A.; Lazarovici, P.; Marcinkiewicz, C. Identification of inhibitors of α2β1 integrin, members of C-lectin type proteins, in Echis sochureki venom. Toxicol. Appl. Pharmacol. 2013, 269, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Eble, J.A.; Niland, S.; Bracht, T.; Mormann, M.; Peter-Katalinic, J.; Pohlentz, G.; Stetefeld, J. The α2β1 integrin-specific antagonist rhodocetin is a cruciform, heterotetrameric molecule. FASEB J. 2009, 23, 2917–2927. [Google Scholar] [CrossRef] [PubMed]
- Bracht, T.; Figueiredo de Rezende, F.; Stetefeld, J.; Sorokin, L.M.; Eble, J.A. Monoclonal antibodies reveal the alteration of the rhodocetin structure upon α2β1 integrin binding. Biochem. J. 2011, 440, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Niland, S.; Ditkowski, B.; Parrandier, D.; Roth, L.; Augustin, H.; Eble, J.A. Rhodocetin-αβ-induced neuropilin-1-cMet association triggers restructuring of matrix contacts in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Eble, J.A.; McDougall, M.; Orriss, G.L.; Niland, S.; Johanningmeier, B.; Pohlentz, G.; Meier, M.; Karrasch, S.; Estevao-Costa, M.I.; Martins Lima, A.; et al. Dramatic and concerted conformational changes enable rhodocetin to block α2β1 integrin selectively. PLoS Biol. 2017, 15, e2001492. [Google Scholar] [CrossRef] [PubMed]
- Arlinghaus, F.T.; Eble, J.A. The collagen-binding integrin α2β1 is a novel interaction partner of the Trimeresurus flavoviridis venom protein flavocetin-A. J. Biol. Chem. 2013, 288, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Du, X.Y.; Navdaev, A.; Clemetson, J.M.; Magnenat, E.; Wells, T.N.; Clemetson, K.J. Bilinexin, a snake C-type lectin from Agkistrodon bilineatus venom agglutinates platelets via GPIb and α2β1. Thromb. Haemost. 2001, 86, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Dutting, S.; Bender, M.; Nieswandt, B. Platelet GPVI: A target for antithrombotic therapy?! Trends Pharmacol. Sci. 2012, 33, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Nieswandt, B.; Watson, S.P. Platelet-collagen interaction: Is GPVI the central receptor? Blood 2003, 102, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Rayes, J.; Watson, S.P.; Nieswandt, B. Functional significance of the platelet immune receptors GPVI and CLEC-2. J. Clin. Investig. 2019, 129, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Marjoram, R.J.; Li, Z.; He, L.; Tollefsen, D.M.; Kunicki, T.J.; Dickeson, S.K.; Santoro, S.A.; Zutter, M.M. α2β1 integrin, GPVI receptor, and common FcRγ chain on mouse platelets mediate distinct responses to collagen in models of thrombosis. PLoS ONE 2014, 9, e114035. [Google Scholar] [CrossRef] [PubMed]
- Pugh, N.; Simpson, A.M.; Smethurst, P.A.; de Groot, P.G.; Raynal, N.; Farndale, R.W. Synergism between platelet collagen receptors defined using receptor-specific collagen-mimetic peptide substrata in flowing blood. Blood 2010, 115, 5069–5079. [Google Scholar] [CrossRef] [PubMed]
- Du, X.Y.; Clemetson, J.M.; Navdaev, A.; Magnenat, E.M.; Wells, T.N.; Clemetson, K.J. Ophioluxin, a convulxin-like C-type lectin from Ophiophagus hannah (King cobra) is a powerful platelet activator via glycoprotein VI. J. Biol. Chem. 2002, 277, 35124–35132. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Du, X.Y.; Lu, Q.M.; Clemetson, K.J.; Zhang, Y. Stejnulxin, a novel snake C-type lectin-like protein from Trimeresurus stejnegeri venom is a potent platelet agonist acting specifically via GPVI. Thromb. Haemost. 2003, 90, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Jandrot-Perrus, M.; Lagrue, A.H.; Leduc, M.; Okuma, M.; Bon, C. Convulxin-induced platelet adhesion and aggregation: Involvement of glycoproteins VI and IaIIa. Platelets 1998, 9, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Polgar, J.; Clemetson, J.M.; Kehrel, B.E.; Wiedemann, M.; Magnenat, E.M.; Wells, T.N.; Clemetson, K.J. Platelet activation and signal transduction by convulxin, a C-type lectin from Crotalus durissus terrificus (tropical rattlesnake) venom via the p62/GPVI collagen receptor. J. Biol. Chem. 1997, 272, 13576–13583. [Google Scholar] [CrossRef] [PubMed]
- Du, X.-Y.; Magnenat, E.; Wells, T.N.; Clemetson, K.J. Alboluxin, a snake C-type lectin from Trimeresurus albolabris venom is a potent platelet agonist acting via GPIb and GPVI. Thromb. Haemost. 2002, 87, 692–698. [Google Scholar] [CrossRef]
- Andrews, R.K.; Kroll, M.H.; Ward, C.M.; Rose, J.W.; Scarborough, R.M.; Smith, A.I.; Lopez, J.A.; Berndt, M.C. Binding of a novel 50-kilodalton alboaggregin from Trimeresurus albolabris and related viper venom proteins to the platelet membrane glycoprotein Ib-IX-V complex. Effect on platelet aggregation and glycoprotein Ib-mediated platelet activation. Biochemistry 1996, 35, 12629–12639. [Google Scholar] [CrossRef] [PubMed]
- Dormann, D.; Clemetson, J.M.; Navdaev, A.; Kehrel, B.E.; Clemetson, K.J. Alboaggregin A activates platelets by a mechanism involving glycoprotein VI as well as glycoprotein Ib. Blood 2001, 97, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Niedergang, F.; Alcover, A.; Knight, C.G.; Farndale, R.W.; Barnes, M.J.; Francischetti, I.M.; Bon, C.; Leduc, M. Convulxin binding to platelet receptor GPVI: Competition with collagen related peptides. Biochem. Biophys. Res. Commun. 2000, 273, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Kanaji, S.; Kanaji, T.; Furihata, K.; Kato, K.; Ware, J.L.; Kunicki, T.J. Convulxin binds to native, human glycoprotein Ibα. J. Biol. Chem. 2003, 278, 39452–39460. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Karim, Z.A.; Whiteheart, S.W. Arf6 plays an early role in platelet activation by collagen and convulxin. Blood 2006, 107, 3145–3152. [Google Scholar] [CrossRef] [PubMed]
- Lagrue, A.H.; Francischetti, I.M.; Guimaraes, J.A.; Jandrot-Perrus, M. Phosphatidylinositol 3′-kinase and tyrosine-phosphatase activation positively modulate Convulxin-induced platelet activation. Comparison with collagen. FEBS Lett. 1999, 448, 95–100. [Google Scholar] [CrossRef]
- Bruserud, O. The snake venom rhodocytin from Calloselasma rhodostoma- a clinically important toxin and a useful experimental tool for studies of C-type lectin-like receptor 2 (CLEC-2). Toxins 2013, 5, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Samaridis, J.; Angman, L. Molecular characterization of two novel C-type lectin-like receptors, one of which is selectively expressed in human dendritic cells. Eur. J. Immunol. 2000, 30, 697–704. [Google Scholar] [CrossRef]
- Kato, Y.; Kaneko, M.K.; Kunita, A.; Ito, H.; Kameyama, A.; Ogasawara, S.; Matsuura, N.; Hasegawa, Y.; Suzuki-Inoue, K.; Inoue, O.; et al. Molecular analysis of the pathophysiological binding of the platelet aggregation-inducing factor podoplanin to the C-type lectin-like receptor CLEC-2. Cancer Sci. 2008, 99, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Suzuki-Inoue, K.; Fuller, G.L.; Garcia, A.; Eble, J.A.; Pohlmann, S.; Inoue, O.; Gartner, T.K.; Hughan, S.C.; Pearce, A.C.; Laing, G.D.; et al. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood 2006, 107, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Suzuki-Inoue, K.; Kato, Y.; Inoue, O.; Kaneko, M.K.; Mishima, K.; Yatomi, Y.; Yamazaki, Y.; Narimatsu, H.; Ozaki, Y. Involvement of the snake toxin receptor CLEC-2, in podoplanin-mediated platelet activation, by cancer cells. J. Biol. Chem. 2007, 282, 25993–26001. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.A.; Eble, J.A.; O’Callaghan, C.A. Crystal structure of rhodocytin, a ligand for the platelet-activating receptor CLEC-2. Protein Sci. 2008, 17, 1611–1616. [Google Scholar] [CrossRef] [PubMed]
- Hooley, E.; Papagrigoriou, E.; Navdaev, A.; Pandey, A.V.; Clemetson, J.M.; Clemetson, K.J.; Emsley, J. The crystal structure of the platelet activator aggretin reveals a novel (αβ)2 dimeric structure. Biochemistry 2008, 47, 7831–7837. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.A.; Christou, C.M.; James, J.R.; Fenton-May, A.E.; Moncayo, G.E.; Mistry, A.R.; Davis, S.J.; Gilbert, R.J.; Chakera, A.; O’Callaghan, C.A. The platelet receptor CLEC-2 is active as a dimer. Biochemistry 2009, 48, 10988–10996. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.E.; Pollitt, A.Y.; Mori, J.; Eble, J.A.; Tomlinson, M.G.; Hartwig, J.H.; O’Callaghan, C.A.; Futterer, K.; Watson, S.P. CLEC-2 activates Syk through dimerization. Blood 2010, 115, 2947–2955. [Google Scholar] [CrossRef] [PubMed]
- Pollitt, A.Y.; Grygielska, B.; Leblond, B.; Desire, L.; Eble, J.A.; Watson, S.P. Phosphorylation of CLEC-2 is dependent on lipid rafts, actin polymerization, secondary mediators, and Rac. Blood 2010, 115, 2938–2946. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.E.; Sinha, U.; Pandey, A.; Eble, J.A.; O’Callaghan, C.A.; Watson, S.P. Critical Role for an acidic amino acid region in platelet signaling by the HemITAM (hemi-immunoreceptor tyrosine-based activation motif) containing receptor CLEC-2 (C-type lectin receptor-2). J. Biol. Chem. 2013, 288, 5127–5135. [Google Scholar] [CrossRef] [PubMed]
- Severin, S.; Pollitt, A.Y.; Navarro-Nunez, L.; Nash, C.A.; Mourao-Sa, D.; Eble, J.A.; Senis, Y.A.; Watson, S.P. Syk-dependent phosphorylation of CLEC-2: A novel mechanism of hem-immunoreceptor tyrosine-based activation motif signaling. J. Biol. Chem. 2011, 286, 4107–4116. [Google Scholar] [CrossRef] [PubMed]
- Fuller, G.L.; Williams, J.A.; Tomlinson, M.G.; Eble, J.A.; Hanna, S.L.; Pohlmann, S.; Suzuki-Inoue, K.; Ozaki, Y.; Watson, S.P.; Pearce, A.C. The C-type lectin receptors CLEC-2 and Dectin-1, but not DC-SIGN, signal via a novel YXXL-dependent signaling cascade. J. Biol. Chem. 2007, 282, 12397–12409. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.A.; O’Callaghan, C.A. Molecular analysis of the interaction of the snake venom rhodocytin with the platelet receptor CLEC-2. Toxins 2011, 3, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Nagae, M.; Morita-Matsumoto, K.; Kato, M.; Kaneko, M.K.; Kato, Y.; Yamaguchi, Y. A platform of C-type lectin-like receptor CLEC-2 for binding O-glycosylated podoplanin and nonglycosylated rhodocytin. Structure 2014, 22, 1711–1721. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.A.; Brown, J.; Harlos, K.; Eble, J.A.; Walter, T.S.; O’Callaghan, C.A. The crystal structure and mutational binding analysis of the extracellular domain of the platelet-activating receptor CLEC-2. J. Biol. Chem. 2007, 282, 3165–3172. [Google Scholar] [CrossRef] [PubMed]
- Osada, M.; Inoue, O.; Ding, G.; Shirai, T.; Ichise, H.; Hirayama, K.; Takano, K.; Yatomi, Y.; Hirashima, M.; Fujii, H.; et al. Platelet activation receptor CLEC-2 regulates blood/lymphatic vessel separation by inhibiting proliferation, migration, and tube formation of lymphatic endothelial cells. J. Biol. Chem. 2012, 287, 22241–22252. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, Y.; Tamura, S.; Suzuki-Inoue, K. New horizon in platelet function: With special reference to a recently-found molecule, CLEC-2. Thromb. J. 2016, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Suzuki-Inoue, K.; Tsukiji, N.; Shirai, T.; Osada, M.; Inoue, O.; Ozaki, Y. Platelet CLEC-2: Roles Beyond Hemostasis. Semin. Thromb. Hemost. 2018, 44, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Suzuki-Inoue, K. Roles of the CLEC-2-podoplanin interaction in tumor progression. Platelets 2018, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shirai, T.; Inoue, O.; Tamura, S.; Tsukiji, N.; Sasaki, T.; Endo, H.; Satoh, K.; Osada, M.; Sato-Uchida, H.; Fujii, H.; et al. C-type lectin-like receptor 2 promotes hematogenous tumor metastasis and prothrombotic state in tumor-bearing mice. J. Thromb. Haemost. 2017, 15, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Erpenbeck, L.; Schön, M.P. Deadly allies: The fatal interplay between platelets and metastizing cancer cells. Blood 2010, 115, 3427–3436. [Google Scholar] [CrossRef] [PubMed]
- Camerer, E. Platelets, protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood 2004, 104, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Shirai, T.; Tsukiji, N.; Otake, S.; Tamura, S.; Ichikawa, J.; Osada, M.; Satoh, K.; Ozaki, Y.; Suzuki-Inoue, K. Functional characterization of recombinant snake venom rhodocytin: Rhodocytin mutant blocks CLEC-2/podoplanin-dependent platelet aggregation and lung metastasis. J. Thromb. Haemost. 2018, 16, 960–972. [Google Scholar] [CrossRef] [PubMed]
- Tsukiji, N.; Osada, M.; Sasaki, T.; Shirai, T.; Satoh, K.; Inoue, O.; Umetani, N.; Mochizuki, C.; Saito, T.; Kojima, S.; et al. Cobalt hematoporphyrin inhibits CLEC-2-podoplanin interaction, tumor metastasis, and arterial/venous thrombosis in mice. Blood Adv. 2018, 2, 2214–2225. [Google Scholar] [CrossRef] [PubMed]
- Koch, S. Neuropilin signalling in angiogenesis. Biochem. Soc. Trans. 2012, 40, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Niland, S.; Eble, J.A. Neuropilins in the context of tumor vasculature. Int. J. Mol. Sci. 2019, 20, 639. [Google Scholar] [CrossRef] [PubMed]
- Prud’homme, G.J.; Glinka, Y. Neuropilins are multifunctional coreceptors involved in tumor initiation, growth, metastasis and immunity. Oncotarget 2012, 3, 921–939. [Google Scholar] [CrossRef] [PubMed]
- Wild, J.R.; Staton, C.A.; Chapple, K.; Corfe, B.M. Neuropilins: Expression and roles in the epithelium. Int. J. Exp. Pathol. 2012, 93, 81–103. [Google Scholar] [CrossRef] [PubMed]
- Trusolino, L.; Bertotti, A.; Comoglio, P.M. MET signalling: Principles and functions in development, organ regeneration and cancer. Nat. Rev. Mol. Cell Biol. 2010, 11, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; NMartins Cavaco, A.C.; Seebach, J.; Niland, S.; Zimmermann, J.; Hanschmann, E.-M.; Hallmann, R.; Schillers, H.; Eble, J.A. Signals of the neuropilin-1-MET axis and cues of mechanical force exertion converge to elicit inflammmatory activation in coherent endothelial cells. J. Immunol. 2019, 202, 1559–1572. [Google Scholar] [CrossRef] [PubMed]
- Niland, S.; Komljenovic, D.; Macas, J.; Bracht, T.; Bauerle, T.; Liebner, S.; Eble, J.A. Rhodocetin-αβ selectively breaks the endothelial barrier of the tumor vasculature in HT1080 fibrosarcoma and A431 epidermoid carcinoma tumor models. Oncotarget 2018, 9, 22406–22422. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Waheed, H.; Moin, S.F.; Choudhary, M.I. Snake Venom: From Deadly Toxins to Life-saving Therapeutics. Curr. Med. Chem. 2017, 24, 1874–1891. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz, C. Applications of snake venom components to modulate integrin activities in cell-matrix interactions. Int. J. Biochem. Cell Biol. 2013, 45, 1974–1986. [Google Scholar] [CrossRef] [PubMed]
- Rosenow, F.; Ossig, R.; Thormeyer, D.; Gasmann, P.; Schluter, K.; Brunner, G.; Haier, J.; Eble, J.A. Integrins as antimetastatic targets of RGD-independent snake venom components in liver metastasis [corrected]. Neoplasia 2008, 10, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.R.; Carrim, N.; Neves, M.A.; McKeown, T.; Stratton, T.W.; Coelho, R.M.; Lei, X.; Chen, P.; Xu, J.; Dai, X.; et al. Platelets and platelet adhesion molecules: Novel mechanisms of thrombosis and anti-thrombotic therapies. Thromb. J. 2016, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Clemetson, K.J.; Lu, Q.; Clemetson, J.M. Snake venom proteins affecting platelets and their applications to anti-thrombotic research. Curr. Pharm. Des. 2007, 13, 2887–2892. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Reheman, A.; Hou, Y.; Zhou, H.; Wang, Y.; Marshall, A.H.; Liang, C.; Dai, X.; Li, B.X.; Vanhoorelbeke, K.; et al. Anfibatide, a novel GPIb complex antagonist, inhibits platelet adhesion and thrombus formation in vitro and in vivo in murine models of thrombosis. Thromb. Haemost. 2014, 111, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, T.; Zhao, Y.; Qian, Y.; Li, X.; Dai, X.; Huang, D.; Pan, T.; Zhou, L. Platelet glycoprotein receptor Ib blockade ameliorates experimental cerebral ischemia-reperfusion injury by strengthening the blood-brain barrier function and anti-thrombo-inflammatory property. Brain Behav. Immun. 2018, 69, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.Y.; Li, R.; Le, Z.Y.; Li, Q.L.; Chen, Z.W. Anfibatide protects against rat cerebral ischemia/reperfusion injury via TLR4/JNK/caspase-3 pathway. Eur. J. Pharmacol. 2017, 807, 127–137. [Google Scholar] [CrossRef] [PubMed]
- NIH U.S. National Library of Medicine, ClinicalTrials.gov. Anfibatide Phase Ib-IIa Clinical Trial, ClinicalTrials.gov Identifier: NCT01585259. Available online: https://clinicaltrials.gov/ct2/show/NCT01585259 (accessed on 29 December 2018).
- Dane, K.; Chaturvedi, S. Beyond plasma exchange: Novel therapies for thrombotic thrombocytopenic purpura. Hematol. Am. Soc. Hematol. Educ. Program. 2018, 2018, 539–547. [Google Scholar] [CrossRef]
- Zheng, L.; Mao, Y.; Abdelgawwad, M.S.; Kocher, N.K.; Li, M.; Dai, X.; Li, B.; Zheng, X.L. Therapeutic efficacy of the platelet glycoprotein Ib antagonist anfibatide in murine models of thrombotic thrombocytopenic purpura. Blood Adv. 2016, 1, 75–83. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eble, J.A. Structurally Robust and Functionally Highly Versatile—C-Type Lectin (-Related) Proteins in Snake Venoms. Toxins 2019, 11, 136. https://doi.org/10.3390/toxins11030136
Eble JA. Structurally Robust and Functionally Highly Versatile—C-Type Lectin (-Related) Proteins in Snake Venoms. Toxins. 2019; 11(3):136. https://doi.org/10.3390/toxins11030136
Chicago/Turabian StyleEble, Johannes A. 2019. "Structurally Robust and Functionally Highly Versatile—C-Type Lectin (-Related) Proteins in Snake Venoms" Toxins 11, no. 3: 136. https://doi.org/10.3390/toxins11030136
APA StyleEble, J. A. (2019). Structurally Robust and Functionally Highly Versatile—C-Type Lectin (-Related) Proteins in Snake Venoms. Toxins, 11(3), 136. https://doi.org/10.3390/toxins11030136




