The Roles of Vitamin C in Skin Health
Abstract
1. Introduction
2. Role of Nutrition in Skin Health
Nutrition Issues Specific to the Skin
3. Vitamin C Content of Skin
3.1. The Bioavailability and Uptake of Vitamin C into the Skin
3.1.1. The Sodium-Dependent Vitamin C Transporters
3.1.2. Bioavailability and Uptake
3.1.3. Topical Application of Vitamin C
3.1.4. Vitamin C Deficiency
4. Potential Functions of Vitamin C in the Skin
4.1. The Promotion of Collagen Formation
4.2. The Ability to Scavenge Free Radicals and Dispose of Toxic Oxidants
4.3. Inhibition of Melanogenesis
4.4. Interaction with Cell Signalling Pathways
4.5. Modulation of Epigenetic Pathways
5. Challenges to the Maintenance of Skin Health and Potential Protection by Vitamin C
- Deterioration due to normal aging, contributing to loss of elasticity and wrinkle formation.
- Exposure to the elements, leading to discolouration, dryness and accelerated wrinkling.
- Chemical insults including exposure to oxidising beauty and cleansing products (hair dyes, soaps, detergents, bleaches).
- Direct injury, as in wounding and burning.
5.1. Skin Aging
- Limiting exposure to environmental risk factors such as smoking, poor nutrition and chronic exposure to sunlight, which cause premature skin aging.
- Using treatments to potentially reverse skin damage, including topical or systemic treatments that help regenerate the elastic fibre system and collagen [126].
5.1.1. The Role of Vitamin C in the Prevention of Skin Aging
5.1.2. Nutritional Studies Linking Vitamin C with Skin Health
5.2. UV Radiation and Photoaging
Vitamin-C-Mediated Protection against Photoaging and UV Damage
5.3. Dry Skin
- A deficiency in the skin barrier lipids, the ceramides, has been identified. These lipids are the main intercellular lipids in the stratum corneum, accounting for 40 to 50 percent of total lipids [155].
- A reduction in substances known as the natural moisturising factor (NMF) [156,157] is also thought to be involved in dry skin. These substances are found in the stratum corneum within the corneocytes, where they bind water, allowing the corneocyte to remain hydrated despite the drying effects of the environment.
- More recently, a deficiency of the skin’s own moisture network in the epidermis, mediated by the newly discovered aquaporin water channels, has been suggested to play a role [131].
Potential for Vitamin C to Prevent Dry Skin Conditions
5.4. Wrinkles
The Effect of Vitamin C on Wrinkle Formation and Reversal
5.5. Wound Healing
Vitamin C and the Benefits for Wound Healing
5.6. Skin Inflammatory Conditions
Vitamin C and Skin Inflammation
6. Conclusions
- Skin fibroblasts have an absolute dependence on vitamin C for the synthesis of collagen, and for the regulation of the collagen/elastin balance in the dermis. There is ample in vitro data with cultured cells demonstrating this dependency. In addition, vitamin C supplementation of animals has shown improved collagen synthesis in vivo.
- Skin keratinocytes have the capacity to accumulate high concentrations of vitamin C, and this in association with vitamin E affords protection against UV irradiation. This information is available from in vitro studies with cultured cells, with supportive information from animal and human studies.
- Analysis of keratinocytes in culture has shown that vitamin C influences gene expression of antioxidant enzymes, the organisation and accumulation of phospholipids, and promotes the formation of the stratum corneum and the differentiation of the epithelium in general.
- Delivery of vitamin C into the skin via topical application remains challenging. Although some human studies have suggested a beneficial effect with respect to UV irradiation protection, most effective formulations contain both vitamins C and E, plus a delivery vehicle.
- Good skin health is positively associated with fruit and vegetable intake in a number of well-executed intervention studies. The active component in the fruit and vegetables responsible for the observed benefit is unidentified, and the effect is likely to be multi-factorial, although vitamin C status is closely aligned with fruit and vegetable intake.
- Signs of aging in human skin can be ameliorated through the provision of vitamin C. A number of studies support this, although measurement of skin changes is difficult. Some studies include objective measures of collagen deposition and wrinkle depth.
- The provision of vitamin C to the skin greatly assists wound healing and minimises raised scar formation. This has been demonstrated in numerous clinical studies in humans and animals.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Weller, R.H.; John, A.; Savin, J.; Dahl, M. The Function and Structure of Skin, 5th ed.; Wiley-Blackwell: Massachusetts, MA, USA, 2008. [Google Scholar]
- Patton, K.T.; Thibodeau, G.A. Anthony’s Textbook of Anatomy & Physiology; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Wickett, R.R.; Visscher, M.O. Structure and function of the epidermal barrier. Am. J. Infect. Control 2006, 34, 15. [Google Scholar] [CrossRef]
- Marks, R. The stratum corneum barrier: The final frontier. J. Nutr. 2004, 134, 2017–2021. [Google Scholar]
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Blume-Peytavi, U.; Kottner, J.; Sterry, W.; Hodin, M.W.; Griffiths, T.W.; Watson, R.E.; Hay, R.J.; Griffiths, C.E. Age-associated skin conditions and diseases: Current perspectives and future options. Gerontologist 2016, 56, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Park, K. Role of micronutrients in skin health and function. Biomol. Ther. 2015, 23, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Boelsma, E.; Van de Vijver, L.P.; Goldbohm, R.A.; Klopping-Ketelaars, I.A.; Hendriks, H.F.; Roza, L. Human skin condition and its associations with nutrient concentrations in serum and diet. Am. J. Clin. Nutr. 2003, 77, 348–355. [Google Scholar] [PubMed]
- Brescoll, J.; Daveluy, S. A review of vitamin B12 in dermatology. Am. J. Clin. Dermatol. 2015, 16, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Fedeles, F.; Murphy, M.; Rothe, M.J.; Grant-Kels, J.M. Nutrition and bullous skin diseases. Clin. Dermatol. 2010, 28, 627–643. [Google Scholar] [CrossRef] [PubMed]
- Sauberlich, H.E. A History of Scurvy and Vitamin C. In Vitamin C in Health and Disease, 1st ed.; Packer, L., Fuchs, J., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1997; pp. 1–24. [Google Scholar]
- Talarico, V.; Aloe, M.; Barreca, M.; Galati, M.C.; Raiola, G. Do you remember scurvy? Clin. Ther. 2014, 165, 253–256. [Google Scholar] [PubMed]
- Alqanatish, J.T.; Alqahtani, F.; Alsewairi, W.M.; Al-kenaizan, S. Childhood scurvy: An unusual cause of refusal to walk in a child. Pediatr. Rheumatol. 2015, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Peterkofsky, B. Ascorbate requirement for hydroxylation and secretion of procollagen: Relationship to inhibition of collagen synthesis in scurvy. Am. J. Clin. Nutr. 1991, 54, 1135–1140. [Google Scholar]
- Ellinger, S.; Stehle, P. Efficacy of vitamin supplementation in situations with wound healing disorders: Results from clinical intervention studies. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Benditt, E.P. Wound healing and collagen formation: II. Fine structure in experimental scurvy. J. Cell Biol. 1962, 12, 533–551. [Google Scholar] [CrossRef] [PubMed]
- Hodges, R.E.; Baker, E.M.; Hood, J.; Sauberlich, H.E.; March, S.C. Experimental scurvy in man. Am. J. Clin. Nutr. 1969, 22, 535–548. [Google Scholar] [PubMed]
- Hodges, R.E.; Hood, J.; Canham, J.E.; Sauberlich, H.E.; Baker, E.M. Clinical manifestations of ascorbic acid deficiency in man. Am. J. Clin. Nutr. 1971, 24, 432–443. [Google Scholar] [PubMed]
- Evans, J.R.; Lawrenson, J.G. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef]
- Placzek, M.; Gaube, S.; Kerkmann, U.; Gilbertz, K.P.; Herzinger, T.; Haen, E.; Przybilla, B. Ultraviolet B-induced DNA damage in human epidermis is modified by the antioxidants ascorbic acid and D-alpha-tocopherol. J. Investig. Dermatol 2005, 124, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.S.; Cameron, G.S.; Pence, B.C. Antioxidant nutrients protect against UVB-induced oxidative damage to DNA of mouse keratinocytes in culture. J. Investig. Dermatol. 1996, 106, 1086–1089. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L. Skin ageing and its treatment. J. Pathol. 2007, 211, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Zussman, J.; Ahdout, J.; Kim, J. Vitamins and photoaging: Do scientific data support their use? J. Am. Acad. Dermatol. 2010, 63, 507–525. [Google Scholar] [CrossRef] [PubMed]
- Langton, A.K.; Sherratt, M.J.; Griffiths, C.E.; Watson, R.E. A new wrinkle on old skin: The role of elastic fibres in skin ageing. Int. J. Cosmet. Sci. 2010, 32, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Rhie, G.; Shin, M.H.; Seo, J.Y.; Choi, W.W.; Cho, K.H.; Kim, K.H.; Park, K.C.; Eun, H.C.; Chung, J.H. Aging- and photoaging-dependent changes of enzymic and nonenzymic antioxidants in the epidermis and dermis of human skin in vivo. J. Investig. Dermatol. 2001, 117, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Shindo, Y.; Witt, E.; Han, D.; Epstein, W.; Packer, L. Enzymic and non-enzymic antioxidants in epidermis and dermis of human skin. J. Investig. Dermatol. 1994, 102, 122–124. [Google Scholar] [CrossRef] [PubMed]
- McArdle, F.; Rhodes, L.E.; Parslew, R.; Jack, C.I.; Friedmann, P.S.; Jackson, M.J. UVR-induced oxidative stress in human skin in vivo: Effects of oral vitamin C supplementation. Free Radic. Biol. Med. 2002, 33, 1355–1362. [Google Scholar] [CrossRef]
- Kirk, J.E. Vitamins and Hormones; Academic Press: New York, NY, USA, 1962; pp. 83–92. [Google Scholar]
- Schaus, R. The vitamin C content of human pituitary, cerebral cortex, heart, and skeletal muscle and its relation to age. Am. J. Clin. Nutr. 1957, 5, 3. [Google Scholar]
- Yavorsky, M.; Almaden, P.; King, C.G. The vitamin C content of human tissues. J. Biol. Chem. 1934, 106, 525–529. [Google Scholar]
- Lloyd, B.B.; Sinclair, H.M. Chapter 1, pp. 369–471. In Biochemistry and Physiology of Nutrition; Bourne, G.H., Kidder, G.W., Eds.; Academic Press: New York, NY, USA, 1953. [Google Scholar]
- Carr, A.C.; Bozonet, S.M.; Pullar, J.M.; Simcock, J.W.; Vissers, M.C. Human skeletal muscle ascorbate is highly responsive to changes in vitamin C intake and plasma concentrations. Am. J. Clin. Nutr. 2013, 97, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Shindo, Y.; Witt, E.; Han, D.; Packer, L. Dose-response effects of acute ultraviolet irradiation on antioxidants and molecular markers of oxidation in murine epidermis and dermis. J. Investig. Dermatol. 1994, 102, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Shindo, Y.; Witt, E.; Packer, L. Antioxidant defense mechanisms in murine epidermis and dermis and their responses to ultraviolet light. J. Investig. Dermatol. 1993, 100, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, L.A.; Aswad, A.; Connor, M.J.; Lowe, N. Depletion of cutaneous glutathione and the induction of inflammation by 8-methoxypsoralen plus UVA radiation. J. Investig. Dermatol. 1986, 87, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.U.; Thiele, J.J.; Cross, C.E.; Packer, L. Vitamin C, uric acid, and glutathione gradients in murine stratum corneum and their susceptibility to ozone exposure. J. Investig. Dermatol. 1999, 113, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Steiling, H.; Longet, K.; Moodycliffe, A.; Mansourian, R.; Bertschy, E.; Smola, H.; Mauch, C.; Williamson, G. Sodium-dependent vitamin C transporter isoforms in skin: Distribution, kinetics, and effect of UVB-induced oxidative stress. Free Radic. Biol. Med. 2007, 43, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Lauer, A.C.; Groth, N.; Haag, S.F.; Darvin, M.E.; Lademann, J.; Meinke, M.C. Dose-dependent vitamin C uptake and radical scavenging activity in human skin measured with in vivo electron paramagnetic resonance spectroscopy. Skin Pharmacol. Physiol. 2013, 26, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Mandl, J.; Szarka, A.; Banhegyi, G. Vitamin C: Update on physiology and pharmacology. Br. J. Pharmacol. 2009, 157, 1097–1110. [Google Scholar] [CrossRef] [PubMed]
- May, J.M. The SLC23 family of ascorbate transporters: Ensuring that you get and keep your daily dose of vitamin C. Br. J. Pharmacol. 2011, 164, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Savini, I.; Rossi, A.; Pierro, C.; Avigliano, L.; Catani, M.V. SVCT1 and SVCT2: Key proteins for vitamin C uptake. Amino Acids 2008, 34, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Rajan, D.P.; Huang, W.; Dutta, B.; Devoe, L.D.; Leibach, F.H.; Ganapathy, V.; Prasad, P.D. Human placental sodium-dependent vitamin C transporter (SVCT2): Molecular cloning and transport function. Biochem. Biophys. Res. Commun. 1999, 262, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.; Cantilena, C.C.; Dhariwal, K.R. In situ kinetics and ascorbic acid requirements. World Rev. Nutr. Diet 1993, 72, 114–127. [Google Scholar] [PubMed]
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.; Dhariwal, K.R.; Washko, P.; Welch, R.; Wang, Y.H.; Cantilena, C.C.; Yu, R. Ascorbic acid and reaction kinetics in situ: A new approach to vitamin requirements. J. Nutr. Sci. Vitaminol. 1992, 38, 169–172. [Google Scholar] [CrossRef]
- Levine, M.; Dhariwal, K.R.; Welch, R.W.; Wang, Y.; Park, J.B. Determination of optimal vitamin C requirements in humans. Am. J. Clin. Nutr. 1995, 62, 1347–1356. [Google Scholar]
- Carr, A.C.; Frei, B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am. J. Clin. Nutr. 1999, 69, 1086–1107. [Google Scholar] [PubMed]
- Carr, A.C.; Bozonet, S.M.; Pullar, J.M.; Simcock, J.W.; Vissers, M.C. A randomized steady-state bioavailability study of synthetic versus natural (kiwifruit-derived) vitamin C. Nutrients 2013, 5, 3684–3695. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Pereira, E.S.P.; Assumpção, E.C.; Dos Santos, F.B.C.; Ota, F.S.; De Oliveira Pereira, M.; Fidelis, M.C.; Fávaro, R.; Langen, S.S.B.; De Arruda, L.H.F.; et al. Assessment of clinical effects and safety of an oral supplement based on marine protein, vitamin C, grape seed extract, zinc, and tomato extract in the improvement of visible signs of skin aging in men. Clin. Cosmet. Investig. Dermatol. 2015, 8, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, J.; Kern, H. Modulation of UV-light-induced skin inflammation by d-alpha-tocopherol and l-ascorbic acid: A clinical study using solar simulated radiation. Free Radic. Biol. Med. 1998, 25, 1006–1012. [Google Scholar] [CrossRef]
- Nusgens, B.V.; Humbert, P.; Rougier, A.; Colige, A.C.; Haftek, M.; Lambert, C.A.; Richard, A.; Creidi, P.; Lapiere, C.M. Topically applied vitamin C enhances the mRNA level of collagens I and III, their processing enzymes and tissue inhibitor of matrix metalloproteinase 1 in the human dermis. J. Investig. Dermatol. 2001, 116, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Humbert, P.G.; Haftek, M.; Creidi, P.; Lapiere, C.; Nusgens, B.; Richard, A.; Schmitt, D.; Rougier, A.; Zahouani, H. Topical ascorbic acid on photoaged skin. Clinical, topographical and ultrastructural evaluation: Double-blind study vs. placebo. Exp. Dermatol. 2003, 12, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.R.; Shen, S.C.; Kuo-Hsien, W.; Hu, C.H.; Fang, J.Y. Lasers and microdermabrasion enhance and control topical delivery of vitamin C. J. Investig. Dermatol. 2003, 121, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Selim, M.A.; Shea, C.R.; Grichnik, J.M.; Omar, M.M.; Monteiro-Riviere, N.A.; Pinnell, S.R. UV photoprotection by combination topical antioxidants vitamin C and vitamin E. J. Am. Acad. Dermatol. 2003, 48, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Sauermann, K.; Jaspers, S.; Koop, U.; Wenck, H. Topically applied vitamin C increases the density of dermal papillae in aged human skin. BMC Dermatol. 2004, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Pinnell, S.R. Cutaneous photodamage, oxidative stress, and topical antioxidant protection. J. Am. Acad. Dermatol. 2003, 48, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Stamford, N.P.J. Stability, transdermal penetration, and cutaneous effects of ascorbic acid and its derivatives. J. Cosmet. Dermatol. 2012, 11, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Nayama, S.; Takehana, M.; Kanke, M.; Itoh, S.; Ogata, E.; Kobayashi, S. Protective effects of sodium-l-ascorbyl-2 phosphate on the development of UVB-induced damage in cultured mouse skin. Biol. Pharm. Bull. 1999, 22, 1301–1305. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Takehana, M.; Itoh, S.; Ogata, E. Protective effect of magnesium-l-ascorbyl-2 phosphate against skin damage induced by UVB irradiation. Photochem. Photobiol. 1996, 64, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Maia Campos, P.M.; Gaspar, L.R.; Goncalves, G.M.; Pereira, L.H.; Semprini, M.; Lopes, R.A. Comparative effects of retinoic acid or glycolic acid vehiculated in different topical formulations. Biomed. Res. Int. 2015, 2015, 650316. [Google Scholar] [CrossRef] [PubMed]
- Pinnell, S.R.; Yang, H.; Omar, M.; Monteiro-Riviere, N.; DeBuys, H.V.; Walker, L.C.; Wang, Y.; Levine, M. Topical l-ascorbic acid: Percutaneous absorption studies. Dermatol. Surg. 2001, 27, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, I.; Muto, N.; Murakami, K.; Akiyama, J. Collagen synthesis in human skin fibroblasts is stimulated by a stable form of ascorbate, 2-O-alpha-d-glucopyranosyl-l-ascorbic acid. J. Nutr. 1992, 122, 871–877. [Google Scholar] [PubMed]
- Yamamoto, I.; Suga, S.; Mitoh, Y.; Tanaka, M.; Muto, N. Antiscorbutic activity of l-ascorbic acid 2-glucoside and its availability as a vitamin C supplement in normal rats and guinea pigs. J. Pharmacobio-Dyn. 1990, 13, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Jurkovic, P.; Sentjurc, M.; Gasperlin, M.; Kristl, J.; Pecar, S. Skin protection against ultraviolet induced free radicals with ascorbyl palmitate in microemulsions. Eur. J. Pharm. Biopharm. 2003, 56, 59–66. [Google Scholar] [CrossRef]
- Wu, Y.; Zheng, X.; Xu, X.G.; Li, Y.H.; Wang, B.; Gao, X.H.; Chen, H.D.; Yatskayer, M.; Oresajo, C. Protective effects of a topical antioxidant complex containing vitamins C and E and ferulic acid against ultraviolet irradiation-induced photodamage in Chinese women. J. Drugs Dermatol. 2013, 12, 464–468. [Google Scholar] [PubMed]
- Xu, T.H.; Chen, J.Z.; Li, Y.H.; Wu, Y.; Luo, Y.J.; Gao, X.H.; Chen, H.D. Split-face study of topical 23.8% l-ascorbic acid serum in treating photo-aged skin. J. Drugs Dermatol. 2012, 11, 51–56. [Google Scholar] [PubMed]
- Serrano, G.; Almudever, P.; Serrano, J.M.; Milara, J.; Torrens, A.; Exposito, I.; Cortijo, J. Phosphatidylcholine liposomes as carriers to improve topical ascorbic acid treatment of skin disorders. Clin. Cosmet. Investig. Dermatol. 2015, 8, 591–599. [Google Scholar] [PubMed]
- Carr, A.C.; Vissers, M.C. Good nutrition matters: Hypovitaminosis C associated with depressed mood and poor wound healing. N. Z. Med. J. 2012, 125, 107–109. [Google Scholar]
- Hinek, A.; Kim, H.J.; Wang, Y.; Wang, A.; Mitts, T.F. Sodium l-ascorbate enhances elastic fibers deposition by fibroblasts from normal and pathologic human skin. J. Dermatol. Sci. 2014, 75, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, V.; Ivanova, S.; Kalinovsky, T.; Niedzwiecki, A.; Rath, M. Inhibition of collagen synthesis by select calcium and sodium channel blockers can be mitigated by ascorbic acid and ascorbyl palmitate. Am. J. Cardiovasc. Dis. 2016, 6, 26–35. [Google Scholar] [PubMed]
- Kivirikko, K.I.; Myllyla, R.; Pihlajaniemi, T. Protein hydroxylation: Prolyl 4-hydroxylase, an enzyme with four cosubstrates and a multifunctional subunit. FASEB J. 1989, 3, 1609–1617. [Google Scholar] [PubMed]
- May, J.M.; Harrison, F.E. Role of vitamin C in the function of the vascular endothelium. Antioxid. Redox Signal. 2013, 19, 2068–2083. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Saito, N.; Kurita, K.; Shimokado, K.; Maruyama, N.; Ishigami, A. Ascorbic acid enhances the expression of type 1 and type 4 collagen and SVCT2 in cultured human skin fibroblasts. Biochem. Biophys. Res. Commun. 2013, 430, 579–584. [Google Scholar] [CrossRef] [PubMed]
- May, J.M.; Qu, Z.C. Transport and intracellular accumulation of vitamin C in endothelial cells: Relevance to collagen synthesis. Arch. Biochem. Biophys. 2005, 434, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.L.; Elsas, L.J.; Priest, R.E. Ascorbate action on normal and mutant human lysyl hydroxylases from cultured dermal fibroblasts. J. Investig. Dermatol. 1979, 72, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Parsons, K.K.; Maeda, N.; Yamauchi, M.; Banes, A.J.; Koller, B.H. Ascorbic acid-independent synthesis of collagen in mice. Am. J. Physiol. Endocrinol. Metab. 2006, 290, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Pihlajaniemi, T.; Myllyla, R.; Kivirikko, K.I. Prolyl 4-hydroxylase and its role in collagen synthesis. J. Hepatol. 1991, 13, 2–7. [Google Scholar] [CrossRef]
- Duarte, T.L.; Cooke, M.S.; Jones, G.D. Gene expression profiling reveals new protective roles for vitamin C in human skin cells. Free Radic. Biol. Med. 2009, 46, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Takahashi, S.; Shiga, Y.; Yoshimi, T.; Miura, T. Hypoxic induction of prolyl 4-hydroxylase alpha (I) in cultured cells. J. Biol. Chem. 2000, 275, 14139–14146. [Google Scholar] [CrossRef] [PubMed]
- Geesin, J.C.; Darr, D.; Kaufman, R.; Murad, S.; Pinnell, S.R. Ascorbic acid specifically increases type I and type III procollagen messenger RNA levels in human skin fibroblast. J. Investig. Dermatol. 1988, 90, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.M.; LuValle, P.A.; Zoia, O.; Quaglino, D., Jr.; Giro, M. Ascorbate differentially regulates elastin and collagen biosynthesis in vascular smooth muscle cells and skin fibroblasts by pretranslational mechanisms. J. Biol. Chem. 1997, 272, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.L.; Combs, S.B.; Pinnell, S.R. Effects of ascorbic acid on proliferation and collagen synthesis in relation to the donor age of human dermal fibroblasts. J. Investig. Dermatol. 1994, 103, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Tajima, S.; Pinnell, S.R. Ascorbic acid preferentially enhances type I and III collagen gene transcription in human skin fibroblasts. J. Dermatol. Sci. 1996, 11, 250–253. [Google Scholar] [CrossRef]
- Agrawal, S.; Kumar, A.; Dhali, T.K.; Majhi, S.K. Comparison of oxidant-antioxidant status in patients with vitiligo and healthy population. Kathmandu Univ. Med. J. 2014, 12, 132–136. [Google Scholar] [CrossRef]
- Nagata, C.; Nakamura, K.; Wada, K.; Oba, S.; Hayashi, M.; Takeda, N.; Yasuda, K. Association of dietary fat, vegetables and antioxidant micronutrients with skin ageing in Japanese women. Br. J. Nutr. 2010, 103, 1493–1498. [Google Scholar] [CrossRef] [PubMed]
- Bissett, D.L.; Chatterjee, R.; Hannon, D.P. Photoprotective effect of superoxide-scavenging antioxidants against ultraviolet radiation-induced chronic skin damage in the hairless mouse. Photodermatol. Photoimmunol. Photomed. 1990, 7, 56–62. [Google Scholar] [PubMed]
- Shukla, A.; Rasik, A.M.; Patnaik, G.K. Depletion of reduced glutathione, ascorbic acid, vitamin E and antioxidant defence enzymes in a healing cutaneous wound. Free Radic. Res. 1997, 26, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Steenvoorden, D.P.; Van Henegouwen, G.M. The use of endogenous antioxidants to improve photoprotection. J. Photochem. Photobiol. B 1997, 41, 1–10. [Google Scholar] [CrossRef]
- Darr, D.; Dunston, S.; Faust, H.; Pinnell, S. Effectiveness of antioxidants (vitamin C and E) with and without sunscreens as topical photoprotectants. Acta Derm Venereol. 1996, 76, 264–268. [Google Scholar] [PubMed]
- DeBuys, H.V.; Levy, S.B.; Murray, J.C.; Madey, D.L.; Pinnell, S.R. Modern approaches to photoprotection. Dermatol. Clin. 2000, 18, 577–590. [Google Scholar] [CrossRef]
- Dreher, F.; Gabard, B.; Schwindt, D.A.; Maibach, H.I. Topical melatonin in combination with vitamins E and C protects skin from ultraviolet-induced erythema: A human study in vivo. Br. J. Dermatol. 1998, 139, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Mukai, K. Kinetic study of the reaction of vitamin C derivatives with tocopheroxyl (vitamin E radical) and substituted phenoxyl radicals in solution. Biochim. Biophys. Acta 1989, 993, 168–173. [Google Scholar] [CrossRef]
- Tanaka, K.; Hashimoto, T.; Tokumaru, S.; Iguchi, H.; Kojo, S. Interactions between vitamin C and vitamin E are observed in tissues of inherently scorbutic rats. J. Nutr. 1997, 127, 2060–2064. [Google Scholar] [PubMed]
- Kameyama, K.; Sakai, C.; Kondoh, S.; Yonemoto, K.; Nishiyama, S.; Tagawa, M.; Murata, T.; Ohnuma, T.; Quigley, J.; Dorsky, A.; et al. Inhibitory effect of magnesium l-ascorbyl-2-phosphate (VC-PMG) on melanogenesis in vitro and in vivo. J. Am. Acad. Dermatol. 1996, 34, 29–33. [Google Scholar] [CrossRef]
- Matsuda, S.; Shibayama, H.; Hisama, M.; Ohtsuki, M.; Iwaki, M. Inhibitory effects of a novel ascorbic derivative, disodium isostearyl 2-O-l-ascorbyl phosphate on melanogenesis. Chem. Pharm. Bull. 2008, 56, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Ebanks, J.P.; Wickett, R.R.; Boissy, R.E. Mechanisms regulating skin pigmentation: The rise and fall of complexion coloration. Int. J. Mol. Sci. 2009, 10, 4066–4087. [Google Scholar] [CrossRef] [PubMed]
- Pasonen-Seppanen, S.; Suhonen, T.M.; Kirjavainen, M.; Suihko, E.; Urtti, A.; Miettinen, M.; Hyttinen, M.; Tammi, M.; Tammi, R. Vitamin C enhances differentiation of a continuous keratinocyte cell line (REK) into epidermis with normal stratum corneum ultrastructure and functional permeability barrier. Histochem. Cell. Biol. 2001, 116, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Ponec, M.; Weerheim, A.; Kempenaar, J.; Mulder, A.; Gooris, G.S.; Bouwstra, J.; Mommaas, A.M. The formation of competent barrier lipids in reconstructed human epidermis requires the presence of vitamin C. J. Investig. Dermatol. 1997, 109, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Savini, I.; Catani, M.V.; Rossi, A.; Duranti, G.; Melino, G.; Avigliano, L. Characterization of keratinocyte differentiation induced by ascorbic acid: Protein kinase C involvement and vitamin C homeostasis. J. Investig. Dermatol. 2002, 118, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Behne, M.; Quiec, D.; Elias, P.M.; Holleran, W.M. Vitamin C stimulates sphingolipid production and markers of barrier formation in submerged human keratinocyte cultures. J. Investig. Dermatol. 2001, 117, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.P.; Shin, K.O.; Park, K.; Yun, H.J.; Mann, S.; Lee, Y.M.; Cho, Y. Vitamin C stimulates epidermal ceramide production by regulating its metabolic enzymes. Biomol. Ther. 2015, 23, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Marionnet, C.; Vioux-Chagnoleau, C.; Pierrard, C.; Sok, J.; Asselineau, D.; Bernerd, F. Morphogenesis of dermal-epidermal junction in a model of reconstructed skin: Beneficial effects of vitamin C. Exp. Dermatol. 2006, 15, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.C.; Gunningham, S.P.; Morrison, M.J.; Dachs, G.U.; Currie, M.J. Modulation of hypoxia-inducible factor-1 alpha in cultured primary cells by intracellular ascorbate. Free Radic. Biol. Med. 2007, 42, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.C.; Kuiper, C.; Dachs, G.U. Regulation of the 2-oxoglutarate-dependent dioxygenases and implications for cancer. Biochem. Soc. Trans. 2014, 42, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.C.; Lee, W.G.; Hampton, M.B. Regulation of apoptosis by vitamin C. Specific protection of the apoptotic machinery against exposure to chlorinated oxidants. J. Biol. Chem. 2001, 276, 46835–46840. [Google Scholar] [CrossRef] [PubMed]
- Kao, J.; Huey, G.; Kao, R.; Stern, R. Ascorbic acid stimulates production of glycosaminoglycans in cultured fibroblasts. Exp. Mol. Pathol. 1990, 53, 1–10. [Google Scholar] [CrossRef]
- Kang, J.S.; Kim, H.N.; Jung, D.J.; Kim, J.E.; Mun, G.H.; Kim, Y.S.; Cho, D.; Shin, D.H.; Hwang, Y.I.; Lee, W.J. Regulation of UVB-induced IL-8 and MCP-1 production in skin keratinocytes by increasing vitamin C uptake via the redistribution of SVCT-1 from the cytosol to the membrane. J. Investig. Dermatol. 2007, 127, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Savini, I.; D’Angelo, I.; Ranalli, M.; Melino, G.; Avigliano, L. Ascorbic acid maintenance in HaCaT cells prevents radical formation and apoptosis by UV-B. Free Radic. Biol. Med. 1999, 26, 1172–1180. [Google Scholar] [CrossRef]
- Tebbe, B.; Wu, S.; Geilen, C.C.; Eberle, J.; Kodelja, V.; Orfanos, C.E. l-Ascorbic acid inhibits UVA-induced lipid peroxidation and secretion of IL-1alpha and IL-6 in cultured human keratinocytes in vitro. J. Investig. Dermatol. 1997, 108, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Minor, E.A.; Court, B.L.; Young, J.I.; Wang, G. Ascorbate induces ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine. J. Biol. Chem. 2013, 288, 13669–13674. [Google Scholar] [CrossRef] [PubMed]
- Blaschke, K.; Ebata, K.T.; Karimi, M.M.; Zepeda-Martinez, J.A.; Goyal, P.; Mahapatra, S.; Tam, A.; Laird, D.J.; Hirst, M.; Rao, A.; et al. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature 2013, 500, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Mao, S.Q.; Zhao, B.; Chong, Z.; Yang, Y.; Zhao, C.; Zhang, D.; Huang, H.; Gao, J.; Li, Z.; et al. Ascorbic acid enhances Tet-mediated 5-methylcytosine oxidation and promotes DNA demethylation in mammals. J. Am. Chem. Soc. 2013, 135, 10396–10403. [Google Scholar] [CrossRef] [PubMed]
- Song, C.X.; He, C. Potential functional roles of DNA demethylation intermediates. Trends Biochem. Sci. 2013, 38, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Lian, C.G.; Xu, Y.; Ceol, C.; Wu, F.; Larson, A.; Dresser, K.; Xu, W.; Tan, L.; Hu, Y.; Zhan, Q.; et al. Loss of 5-hydroxymethylcytosine is an epigenetic hallmark of melanoma. Cell 2012, 150, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, C.B.; Yang, C.; Dickson, K.M.; Shao, H.; Van Booven, D.; Harbour, J.W.; Liu, Z.J.; Wang, G. Epigenetic reprogramming of melanoma cells by vitamin C treatment. Clin. Epigenet. 2015, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, C.; Vissers, M.C. Ascorbate as a co-factor for Fe- and 2-oxoglutarate dependent dioxygenases: Physiological activity in tumor growth and progression. Front. Oncol. 2014, 4, 359. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.R.; Qin, H.H.; Wu, W.Y.; He, S.J.; Xu, J.H. Vitamin C protects against UV irradiation-induced apoptosis through reactivating silenced tumor suppressor genes p21 and p16 in a Tet-dependent DNA demethylation manner in human skin cancer cells. Cancer Biother. Radiopharm. 2014, 29, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Sticozzi, C.; Belmonte, G.; Cervellati, F.; Demaude, J.; Chen, N.; Krol, Y.; Oresajo, C. Vitamin C compound mixtures prevent ozone-induced oxidative damage in human keratinocytes as initial assessment of pollution protection. PLoS ONE 2015, 10, e0131097. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Muresan, X.M.; Sticozzi, C.; Belmonte, G.; Pecorelli, A.; Cervellati, F.; Demaude, J.; Krol, Y.; Oresajo, C. Ozone-induced damage in 3D-kkin model is prevented by topical vitamin C and vitamin E compound mixtures application. J. Dermatol. Sci. 2016, 82, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Puizina-Ivic, N. Skin aging. Acta Dermatovenerol. Alp. Pannonica Adriat. 2008, 17, 47–54. [Google Scholar] [PubMed]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Fenske, N.A.; Lober, C.W. Structural and functional changes of normal aging skin. J. Am. Acad. Dermatol. 1986, 15, 571–585. [Google Scholar] [CrossRef]
- Kang, S.; Fisher, G.J.; Voorhees, J.J. Photoaging: Pathogenesis, prevention, and treatment. Clin. Geriatr. Med. 2001, 17, 643–659. [Google Scholar] [CrossRef]
- El-Domyati, M.; Attia, S.; Saleh, F.; Brown, D.; Birk, D.E.; Gasparro, F.; Ahmad, H.; Uitto, J. Intrinsic aging vs. photoaging: A comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp. Dermatol. 2002, 11, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Torres, M.; Shindo, Y.; Packer, L. Effect of age on antioxidants and molecular markers of oxidative damage in murine epidermis and dermis. J. Investig. Dermatol. 1994, 102, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Naylor, E.C.; Watson, R.E.; Sherratt, M.J. Molecular aspects of skin ageing. Maturitas 2011, 69, 249–256. [Google Scholar] [CrossRef] [PubMed]
- White-Chu, E.F.; Reddy, M. Dry skin in the elderly: Complexities of a common problem. Clin. Dermatol. 2011, 29, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Derm.-Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Monnat, R.J., Jr. “...Rewritten in the skin”: Clues to skin biology and aging from inherited disease. J. Investig. Dermatol. 2015, 135, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D. Aging skin: The role of diet: Facts and controversies. Clin. Dermatol. 2013, 31, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Marini, A. Beauty from the inside. Does it really work? Hautarzt 2011, 62, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, M.C.; Franco, O.H.; Granger, S.P.; Murray, P.G.; Mayes, A.E. Dietary nutrient intakes and skin-aging appearance among middle-aged American women. Am. J. Clin. Nutr. 2007, 86, 1225–1231. [Google Scholar] [PubMed]
- Pezdirc, K.; Hutchesson, M.; Whitehead, R.; Ozakinci, G.; Perrett, D.; Collins, C.E. Can dietary intake influence perception of and measured appearance? A systematic review. Nutr. Res. 2015, 35, 175–197. [Google Scholar] [CrossRef] [PubMed]
- Bertuccelli, G.; Zerbinati, N.; Marcellino, M.; Nanda Kumar, N.S.; He, F.; Tsepakolenko, V.; Cervi, J.; Lorenzetti, A.; Marotta, F. Effect of a quality-controlled fermented nutraceutical on skin aging markers: An antioxidant-control, double-blind study. Exp. Ther. Med. 2016, 11, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zheng, X.; Zhong, X.; Shetty, A.K.; Elias, P.M.; Bollag, W.B. Aquaporin-3 in keratinocytes and skin: Its role and interaction with phospholipase D2. Arch. Biochem. Biophys. 2011, 508, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Podda, M.; Traber, M.G.; Weber, C.; Yan, L.J.; Packer, L. UV-irradiation depletes antioxidants and causes oxidative damage in a model of human skin. Free Radic. Biol. Med. 1998, 24, 55–65. [Google Scholar] [CrossRef]
- Buettner, G.R.; Motten, A.G.; Chignell, C.E. ESR detection of endogenous ascorbyl free radical in mouse skin: Enhancement of radical production during UV irradiation following topical application of chlorpromazine. Photochem. Photobiol. 1987, 46, 161–162. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Green, A.C. Dietary antioxidants and melanoma: Evidence from cohort and intervention studies. Nutr. Cancer 2015, 67, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Vile, G.F.; Tyrrell, R.M. UVA radiation-induced oxidative damage to lipids and proteins in vitro and in human skin fibroblasts is dependent on iron and singlet oxygen. Free Radic. Biol. Med. 1995, 18, 721–730. [Google Scholar] [CrossRef]
- Sander, C.S.; Chang, H.; Salzmann, S.; Muller, C.S.; Ekanayake-Mudiyanselage, S.; Elsner, P.; Thiele, J.J. Photoaging is associated with protein oxidation in human skin in vivo. J. Investig. Dermatol. 2002, 118, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Berneburg, M.; Plettenberg, H.; Krutmann, J. Photoaging of human skin. Photodermatol. Photoimmunol. Photomed. 2000, 16, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Kligman, L.H.; Kligman, A.M. The nature of photoaging: Its prevention and repair. Photo-Dermatology 1986, 3, 215–227. [Google Scholar] [PubMed]
- Trojahn, C.; Dobos, G.; Blume-Peytavi, U.; Kottner, J. The skin barrier function: Differences between intrinsic and extrinsic aging. G. Ital. Dermatol. Venereol. 2015, 150, 687–692. [Google Scholar] [PubMed]
- Darr, D.; Combs, S.; Dunston, S.; Manning, T.; Pinnell, S. Topical vitamin C protects porcine skin from ultraviolet radiation-induced damage. Br. J. Dermatol. 1992, 127, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Mikirova, N.A.; Ichim, T.E.; Riordan, N.H. Anti-angiogenic effect of high doses of ascorbic acid. J. Transl. Med. 2008, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Pinnell, S.R.; Darr, D.; Kurimoto, I.; Itami, S.; Yoshikawa, K.; Streilein, J.W. Vitamin C abrogates the deleterious effects of UVB radiation on cutaneous immunity by a mechanism that does not depend on TNF-alpha. J. Investig. Dermatol. 1997, 109, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Eberlein-Konig, B.; Placzek, M.; Przybilla, B. Protective effect against sunburn of combined systemic ascorbic acid (vitamin C) and d-alpha-tocopherol (vitamin E). J. Am. Acad. Dermatol. 1998, 38, 45–48. [Google Scholar] [CrossRef]
- Crisan, D.; Roman, I.; Crisan, M.; Scharffetter-Kochanek, K.; Badea, R. The role of vitamin C in pushing back the boundaries of skin aging: An ultrasonographic approach. Clin. Cosmet. Investig. Dermatol. 2015, 8, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.C.; Burch, J.A.; Streilein, R.D.; Iannacchione, M.A.; Hall, R.P.; Pinnell, S.R. A topical antioxidant solution containing vitamins C and E stabilized by ferulic acid provides protection for human skin against damage caused by ultraviolet irradiation. J. Am. Acad. Dermatol. 2008, 59, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Amber, K.T.; Shiman, M.I.; Badiavas, E.V. The use of antioxidants in radiotherapy-induced skin toxicity. Integr. Cancer Ther. 2014, 13, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.H.; Lin, J.Y.; Gupta, R.D.; Tournas, J.A.; Burch, J.A.; Selim, M.A.; Monteiro-Riviere, N.A.; Grichnik, J.M.; Zielinski, J.; Pinnell, S.R. Ferulic acid stabilizes a solution of vitamins C and E and doubles its photoprotection of skin. J. Investig. Dermatol. 2005, 125, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Grether-Beck, S.; Marini, A.; Jaenicke, T.; Krutmann, J. Effective photoprotection of human skin against infrared A radiation by topically applied antioxidants: Results from a vehicle controlled, double-blind, randomized study. Photochem. Photobiol. 2015, 91, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Traikovich, S.S. Use of topical ascorbic acid and its effects on photodamaged skin topography. Arch. Otolaryngol. Head Neck Surg. 1999, 125, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Jungersted, J.M.; Hellgren, L.I.; Jemec, G.B.; Agner, T. Lipids and skin barrier function--A clinical perspective. Contact Dermat. 2008, 58, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, A.V.; Scott, I.R.; Harding, C.R.; Bowser, P.A. Stratum corneum moisturization at the molecular level. J. Investig. Dermatol. 1994, 103, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Boyce, S.T.; Supp, A.P.; Swope, V.B.; Warden, G.D. Vitamin C regulates keratinocyte viability, epidermal barrier, and basement membrane in vitro, and reduces wound contraction after grafting of cultured skin substitutes. J. Investig. Dermatol. 2002, 118, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Craven, N.M.; Watson, R.E.; Jones, C.J.; Shuttleworth, C.A.; Kielty, C.M.; Griffiths, C.E. Clinical features of photodamaged human skin are associated with a reduction in collagen VII. Br. J. Dermatol. 1997, 137, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Sachs, D.L.; Rittie, L.; Chubb, H.A.; Orringer, J.; Fisher, G.; Voorhees, J.J. Hypo-collagenesis in photoaged skin predicts response to anti-aging cosmeceuticals. J. Cosmet. Dermatol. 2013, 12, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Contet-Audonneau, J.L.; Jeanmaire, C.; Pauly, G. A histological study of human wrinkle structures: Comparison between sun-exposed areas of the face, with or without wrinkles, and sun-protected areas. Br. J. Dermatol. 1999, 140, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.R.; Dixon, T.K.; Bhattacharyya, T.K. Effects of topicals on the aging skin process. Facial Plast. Surg. Clin. North Am. 2013, 21, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, L.T.; Toft, B.G.; Rygaard, J.; Ladelund, S.; Paddon, M.; James, T.; Taylor, R.; Gottrup, F. Effect of smoking, smoking cessation, and nicotine patch on wound dimension, vitamin C, and systemic markers of collagen metabolism. Surgery 2010, 148, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Rodero, M.P.; Khosrotehrani, K. Skin wound healing modulation by macrophages. Int. J. Clin. Exp. Pathol. 2010, 3, 643–653. [Google Scholar] [PubMed]
- Ilina, O.; Friedl, P. Mechanisms of collective cell migration at a glance. J. Cell Sci. 2009, 122, 3203–3208. [Google Scholar] [CrossRef] [PubMed]
- Yun, I.S.; Yoo, H.S.; Kim, Y.O.; Rah, D.K. Improved scar appearance with combined use of silicone gel and vitamin C for Asian patients: A comparative case series. Aesthet. Plast. Surg. 2013, 37, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.; Fuhrman, M.P. Nutrients and wound healing: Still searching for the magic bullet. Nutr. Clin. Pract. 2005, 20, 331–347. [Google Scholar] [CrossRef] [PubMed]
- Young, M.E. Malnutrition and wound healing. Heart Lung 1988, 17, 60–67. [Google Scholar] [PubMed]
- Lund, C.C.; Crandon, J.H. Ascorbic acid and human wound healing. Ann. Surg. 1941, 114, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Basketter, D.A.; White, I.R.; Kullavanijaya, P.; Tresukosol, P.; Wichaidit, M.; McFadden, J.P. Influence of vitamin C on the elicitation of allergic contact dermatitis to p-phenylenediamine. Contact Dermat. 2016, 74, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, E.; Faintuch, J.; Machado Moreira, E.A.; Goncalves da Silva, V.R.; Lopes Pereima, M.J.; Martins Fagundes, R.L.; Filho, D.W. Supplementation of vitamin E, vitamin C, and zinc attenuates oxidative stress in burned children: A randomized, double-blind, placebo-controlled pilot study. J. Burn Care Res. 2009, 30, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Ubbink, D.T.; Santema, T.B.; Stoekenbroek, R.M. Systemic wound care: A meta-review of cochrane systematic reviews. Surg. Technol. Int. 2014, 24, 99–111. [Google Scholar] [PubMed]
- Furue, M.; Kadono, T. “Inflammatory skin march” in atopic dermatitis and psoriasis. Inflamm. Res. 2017. [Google Scholar] [CrossRef]
- Han, H.; Roan, F.; Ziegler, S.F. The atopic march: Current insights into skin barrier dysfunction and epithelial cell-derived cytokines. Immunol. Rev. 2017, 278, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Liakou, A.I.; Theodorakis, M.J.; Melnik, B.C.; Pappas, A.; Zouboulis, C.C. Nutritional clinical studies in dermatology. J. Drugs Dermatol. 2013, 12, 1104–1109. [Google Scholar] [PubMed]
- Rackett, S.C.; Rothe, M.J.; Grant-Kels, J.M. Diet and dermatology. The role of dietary manipulation in the prevention and treatment of cutaneous disorders. J. Am. Acad. Dermatol. 1993, 29, 447–461. [Google Scholar] [CrossRef]
- Pappas, A.; Liakou, A.; Zouboulis, C.C. Nutrition and skin. Rev. Endocr. Metab. Disord. 2016, 17, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Leveque, N.; Robin, S.; Muret, P.; Mac-Mary, S.; Makki, S.; Berthelot, A.; Kantelip, J.P.; Humbert, P. In vivo assessment of iron and ascorbic acid in psoriatic dermis. Acta Derm. Venereol. 2004, 84, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Kim, Y.J.; Kwon, O.; Kim, N.I.; Cho, Y. Associations among plasma vitamin C, epidermal ceramide and clinical severity of atopic dermatitis. Nutr. Res. Pract. 2016, 10, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Kallner, A.B.; Hartmann, D.; Hornig, D.H. On the requirements of ascorbic acid in man: Steady-state turnover and body pool in smokers. Am. J. Clin. Nutr. 1981, 34, 1347–1355. [Google Scholar] [PubMed]
- Evans-Olders, R.; Eintracht, S.; Hoffer, L.J. Metabolic origin of hypovitaminosis C in acutely hospitalized patients. Nutrition 2010, 26, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.; Eintracht, S.; Hoffer, L.J. Vitamin C deficiency in a university teaching hospital. J. Am. Coll. Nutr. 2008, 27, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Findik, R.B.; Ilkaya, F.; Guresci, S.; Guzel, H.; Karabulut, S.; Karakaya, J. Effect of vitamin C on collagen structure of cardinal and uterosacral ligaments during pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 201, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.J.; Landsman, A.S. The effects of a moderate and high dose of vitamin C on wound healing in a controlled guinea pig model. J. Foot Ankle Surg. 1999, 38, 333–338. [Google Scholar] [CrossRef]
- Quevedo, W.C., Jr.; Holstein, T.J.; Dyckman, J.; McDonald, C.J.; Isaacson, E.L. Inhibition of UVR-induced tanning and immunosuppression by topical applications of vitamins C and E to the skin of hairless (hr/hr) mice. Pigment Cell Res. 2000, 13, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, H.; Kim, S.; Kim, M.; Kang, H.; Kim, N.; An, S.; Koh, J.; Jung, H. Evaluation of the anti-wrinkle effect of an ascorbic acid-loaded dissolving microneedle patch via a double-blind, placebo-controlled clinical study. Int. J. Cosmet. Sci. 2016, 38, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Pecorelli, A.; Belmonte, G.; Pambianchi, E.; Cervellati, F.; Lynch, S.; Krol, Y.; Oresajo, C. Protective Effects of Topical Vitamin C Compound Mixtures against Ozone-Induced Damage in Human Skin. J. Investig. Dermatol. 2017, 137, 1373–1375. [Google Scholar] [CrossRef] [PubMed]
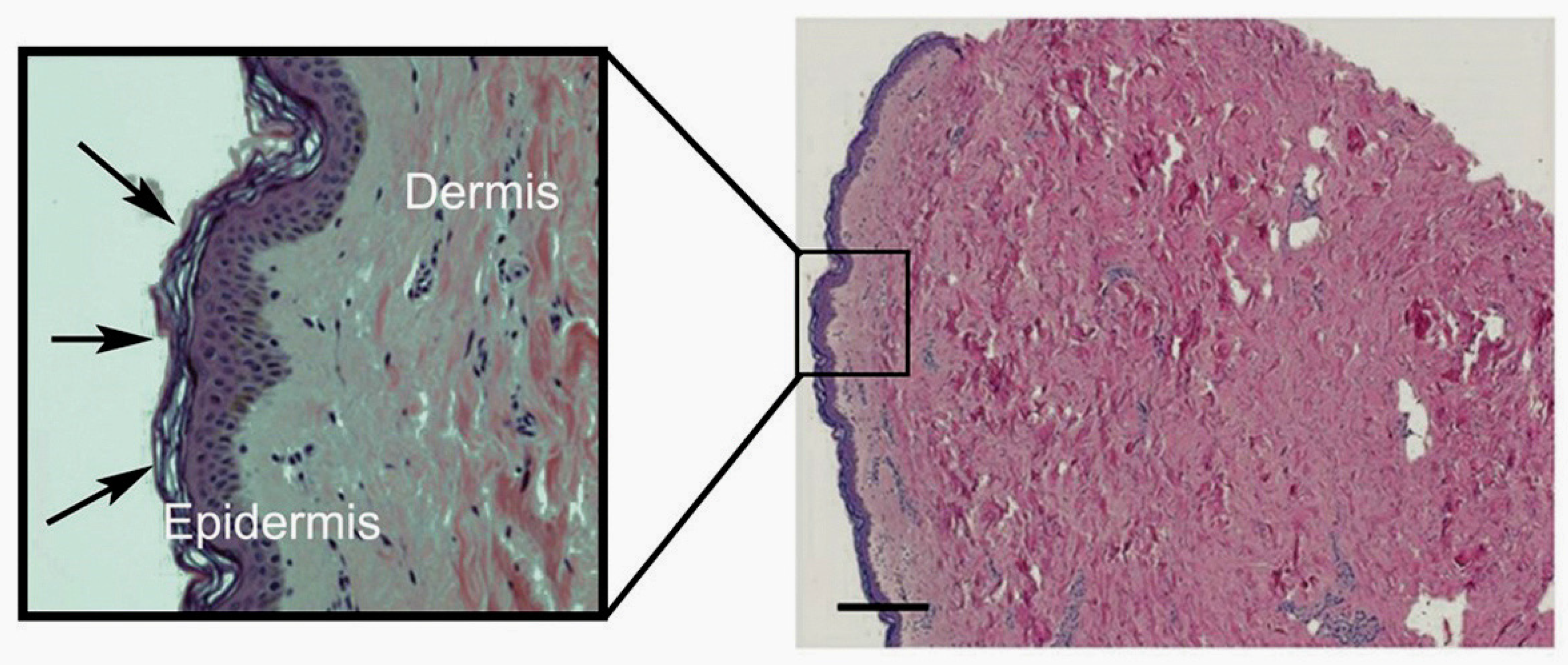
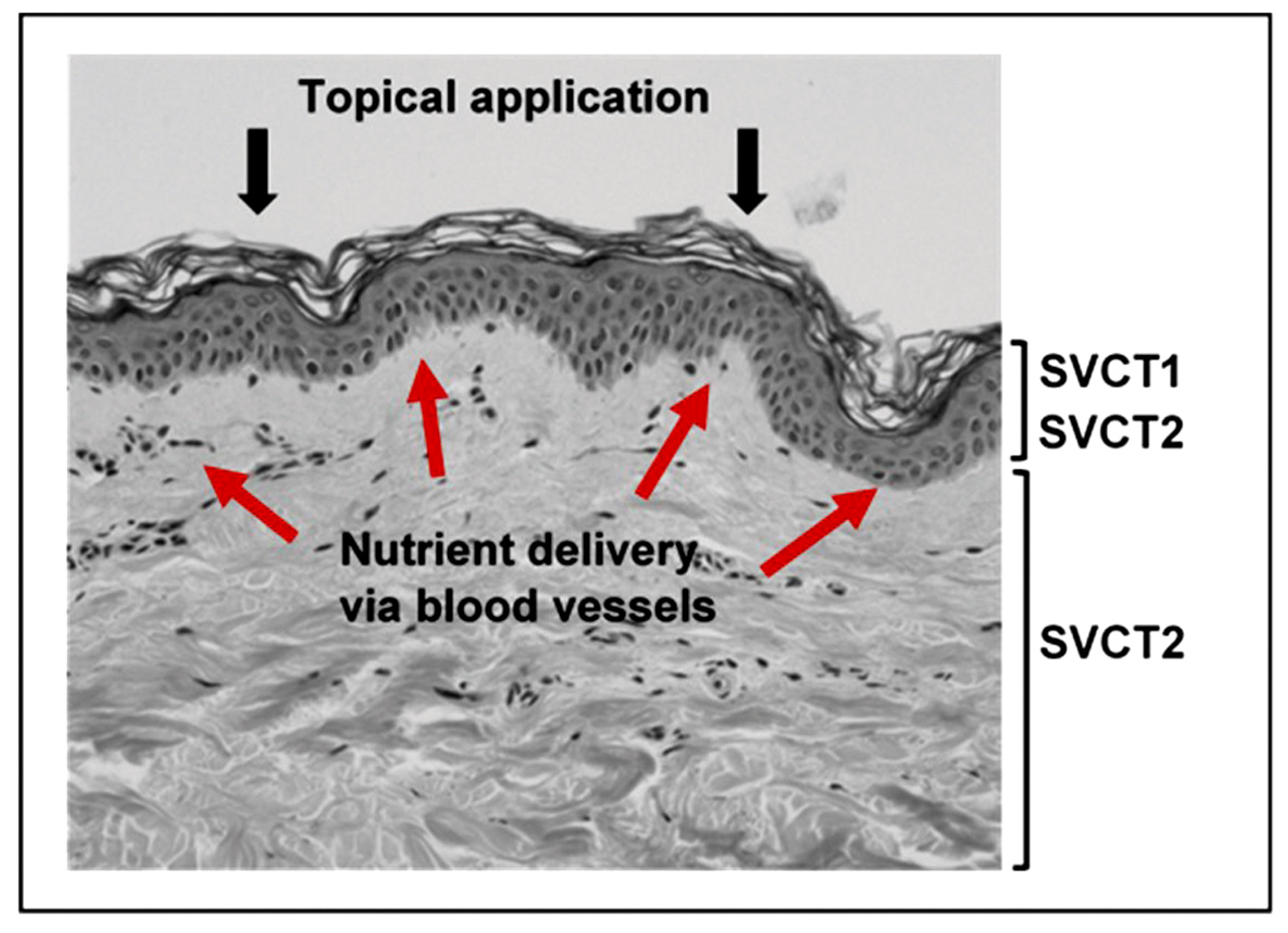
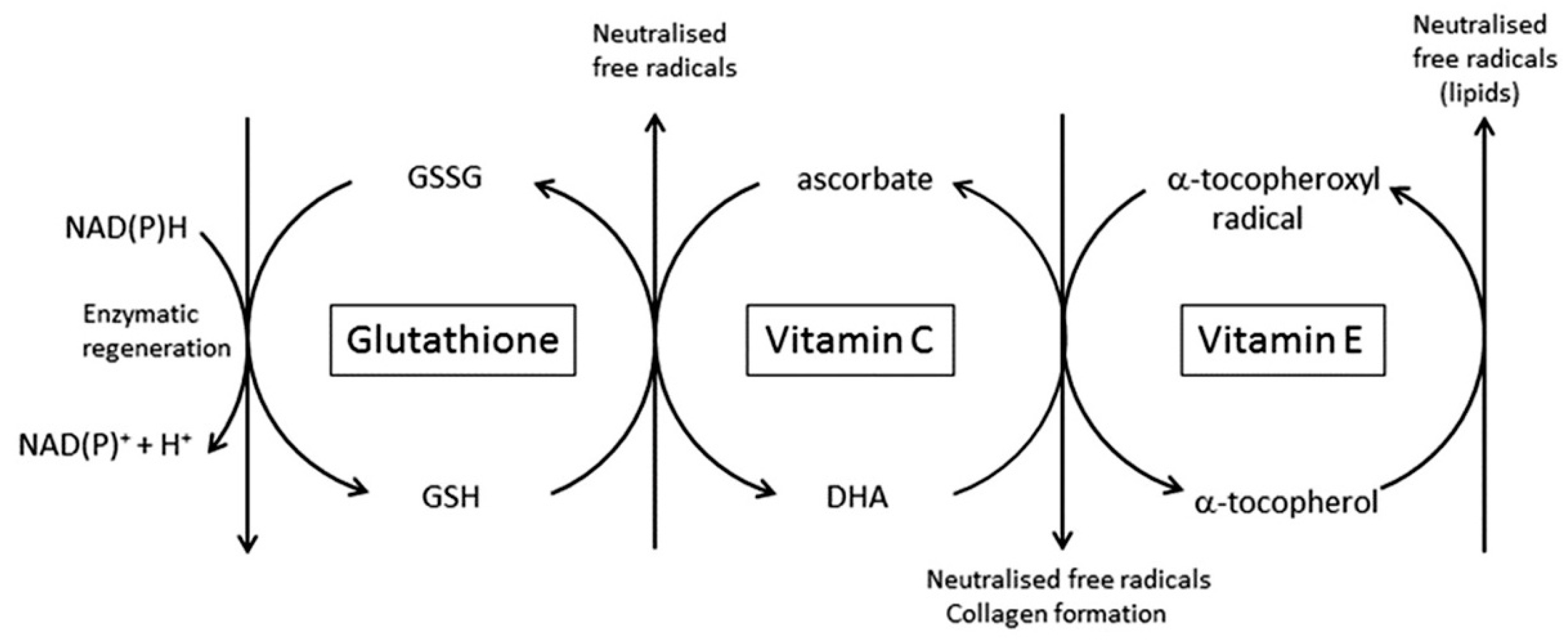
| Tissue | Vitamin C Content (mg/100 g Wet Weight) | References |
|---|---|---|
| Adrenal glands | 30–40 | [28] |
| Pituitary glands | 40–50 | [29] |
| Liver | 10–16 | [28,30] |
| Spleen | 10–15 | [28,31] |
| Lungs | 7 | [28] |
| Kidneys | 5–15 | [30] |
| Heart muscle | 5–15 | [28,29,31] |
| Skeletal muscle | 3–4 | [29,32] |
| Brain | 13–15 | [28] |
| Skin-epidermis | 6–64 | [25,26,27] |
| Skin-dermis | 3–13 | [25,26,27] |
| Study Description | Measured Parameters | Outcome and Comment | Reference |
|---|---|---|---|
| Effects on collagen and elastin synthesis | |||
| Vit. C effects on collagen and elastin synthesis in human skin fibroblasts and vascular smooth muscle cells. | Monitored vit. C time of exposure and dose on collagen synthesis and gene expression, and elastin synthesis and gene regulation. | Vit. C exposure increased collagen, decreased elastin. Stabilization of collagen mRNA, lesser stability of elastin mRNA, and repression of elastin gene transcription. | [81] |
| Effect of vit. C on collagen synthesis and SVCT2 expression in human skin fibroblasts. Vit. C added to culture medium for 5 days. | Vit. C uptake measured into cells, collagen I and IV measured with RT-PCR and ELISA, and RT-PCR for SVCT2. | Vit. C increased collagen I and IV, and increased SVCT2 expression. | [73] |
| Effect of vit. C on elastin generation by fibroblasts from normal human skin, stretch-marked skin, keloids and dermal fat. | Immunohistochemistry and western blotting for detection of elastin and precursors. | 50 and 200 µM vit. C increased elastin production, 800 µM inhibited. No measures of vit. C uptake into cells. | [69] |
| Effects on morphology, differentiation and gene expression | |||
| Vit. C addition to cultures of rat keratinocytes (REK). | Effect on differentiation and stratum corneum formation. | Morphology showed enhanced stratum corneum structure, increased keratohyalin granules and organization of intercellular lipid lamellae in the interstices of the stratum corneum. Increased profilaggrin and filaggrin. | [97] |
| Effect of vit. C on human keratinocyte (HaCaT) cell line differentiation in vitro. | Measured development of cornified envelope (CE), gene expression. | CE formation and keratinocyte differentiation induced by vit. C, suggesting a role in formation of stratum corneum and barrier formation in vivo. | [99] |
| Effect of vit. C supplementation on gene expression in human skin fibroblasts. | Total RNA nano assay, for genetic profiling, with and without vit. C in culture medium. | Increased gene expression for DNA replication and repair and cell cycle progression. Increased mitogenic stimulation and cell motility in the context of wound healing. Faster repair of damaged DNA bases. | [78] |
| Effect of vit. C on dermal epidermal junction in skin model (keratinocytes and fibroblasts). | Keratinocyte organisation, fibroblast number, basement membrane protein deposition and mRNA expression. | Vit. C improved keratinocyte and basement membrane organisation. Increased fibroblast number, saw deposition of basement membrane proteins. | [102] |
| Effect of vit. C on cultured skin models—combined human epidermal keratinocytes and dermal fibroblasts. | Monitored morphology, lipid composition. | Vit. C, but not vit. E, improved epidermal morphology, ceramide production and phospholipid layer formation. | [98] |
| Protective effects against UV irradiation | |||
| Effect of vit. C on UVA irradiation of primary cultures of human keratinocytes. | Vit. C added in low concentrations, monitored MDA, TBA, GSH, cell viability, IL-1, IL-6 generation. | Vit. C improved resistance to UVA, decreased MDA and TBA levels, increased GSH levels, decreased IL-1 and IL-6 levels. | [109] |
| Effect of vit. C uptake into human keratinocyte (HaCaT) cell line on outcome to UV irradiation. | Accumulation of vit. C in keratinocytes, antioxidant capacity by DHDCF and apoptosis induction by UV irradiation. | Keratinocytes accumulated mM levels of vit. C, increasing antioxidant status and protecting against apoptosis. | [108] |
| Effect of UVB on vit. C uptake into human keratinocyte cell line (HaCaT) and effects on inflammatory gene expression. | Cellular vit. C measured by HPLC, mRNA expression for chemokines, western blotting for SVCT localisation. | Vit. C uptake was increased with UVB irradiation, chemokine expression decreased with vit. C uptake. | [107] |
| Protective effects against ozone exposure | |||
| Effect of antioxidant mixtures of vit. C, vit. E and ferulic acid on exposure of cultured normal human keratinocytes to ozone. | Cell viability, proliferation, HNE, protein carbonyls, Nrf2, NFkappaB activation, IL-8 generation. | Vit. C-containing mixtures inhibited toxicity. The presence of vit. E provided additional protection against HNE and protein carbonyls. | [118] |
| Protection of cultured skin cells against ozone exposure with vit. C, vit. E, and resveratrol. 3-D culture of human dermis—fibroblasts with collagen I + III. | Cell death, HNE levels, expression of transcription factors Nrf-2 and NfkappaB | Extensive protection against cell damage with mixtures containing vit. C. Increased expression of antioxidant proteins. Additional effect of vit. E + C. No effect with Vit. E alone. | [119] |
| Type of Skin Damage | Cause | Skin Structure Affected | Evidence of Protection by Vitamin C | References |
|---|---|---|---|---|
| Sunburn | Acute and excessive UV exposure. | Cell death of all skin cells, with associated inflammation. | Improving skin vitamin C and vitamin E levels can improve resistance to UV exposure. | [21,50,86,90,107,150,151,152] |
| Photoaging, oxidant-induced damage | Chronic UV overexposure, cigarette smoking. | Damaged collagen and elastin matrix, thinning of the epidermal layer. | Decreased signs of aging with higher fruit and vegetable intake. Protection inferred from studies with acute UV exposure. | [27,54,89,139,145,146,147,148] |
| Hyperpigmentation | Chronic UV exposure and environmental stresses. | Excessive pigment formation and propagation of melanocytes in the epidermis. | Nutrition studies showing improved skin colour with higher fruit and vegetable intake. | Reviewed in [134,135] |
| Wrinkle formation | Natural aging, oxidative stress, UV exposure, smoking, medical treatments. | Dermal layer changes, deterioration of collagen and elastic fibres. | Lessening of wrinkle depth following vitamin C supplementation. Increased collagen formation by fibroblasts in cell culture. | [69,73,79,80,81,82,135,149] |
| Skin sagging | Natural aging, oxidative stress damage, extreme weight loss. | Loss of elastin and collagen fibres, thinning of skin layers, loss of muscle tone. | Improved skin tightness in individuals with higher fruit and vegetable intake. | Reviewed in [134,135] |
| Loss of colour | Natural aging, UV exposure, illness. | Thinning of skin layers, loss of melanocytes or decreased melanin formation, loss of vasculature in dermis. | Improved skin tone with high fruit and vegetable intake. | Reviewed in [94,95,134,135] |
| Surface roughness | Chemical and UV exposure, physical abrasion, allergy and inflammation. | Stratum corneum, loss of skin moisture barrier function. | Vitamin C enhances production of barrier lipids in cell culture. | [98,99,100,101,102,157] |
| Dry skin | Medications, illness, extreme temperature, low humidity and wind exposure. | Stratum corneum, loss of skin barrier lipids and natural moisturising factor. | Vitamin C enhances production of barrier lipids in cell culture. | [98,99,100,101,102,157] |
| Excessive scar formation, generation of keloids | Ineffective wound healing. | Fibroblast function, collagen and elastin formation. | Supplementation improves wound healing, prevents keloid formation in vivo, enhances collagen formation by fibroblasts in vitro. | [73,79,80,81,82,166,167] |
| Poor wound healing, thickening rough skin | Vitamin C deficiency. | All skin cell functions, collagen formation. | Direct association Vitamin C deficiency prevents wound healing. | [162,166,169] |
| Inflammatory skin lesions | Allergic and auto-inflammation. | Skin barrier integrity, underlying inflammation and swelling. | Nutrition support, decreased levels associated with loss of barrier lipid ceramide. | [179] |
| Study Description | Measured Parameters | Outcome and Comment | References |
|---|---|---|---|
| Animal Studies | |||
| Oral Supplementation | |||
| Dietary supplementation of pregnant female rats. Addition of 1.25 mg/mL vitamin C to drinking water for duration of gestation. | Monitored collagen and elastin content of uterosacral ligaments by histology staining and subjective assessment. | Increased collagen production in vit.- C-supplemented rats, decreased elastin loss. Implied prevention of pelvic organ prolapse and stress urinary incontinence. | [183] |
| Wound healing in guinea pigs following supplementation with moderate and high-dose vit. C. | Dorsal wound healing rate and strength of repair monitored. | Increased vit. C associated with faster wound recovery and strength of skin integrity. Small sample size limited stats. | [184] |
| Topical application | |||
| Topical application of vit. C and vit. E-containing cream to nude mice, followed by UV irradiation. | Measured melanocyte differentiation post-irradiation. Change of skin colour—tanning, inflammation. | UVR-induced proliferation and melanogenesis of melanocytes were reduced by vit. C and E. Melanocyte population and confluence reduced when vit. C present. | [185] |
| Cultured skin—human keratinocytes and fibroblasts attached to collagen-glycosamino-glycan substrates, incubated for five weeks ± 0.1 mM vit. C, and then grafted to athymic mice. | Collagen IV, collagen VII and laminin 5 synthesis, epidermal barrier formation and skin graft take in athymic nude mice. | Increased cell viability and basement membrane development in vitro, better graft ability in vivo. | [157] |
| Human Studies | |||
| Oral supplementation | |||
| 90-day oral supplementation with a fermented papaya preparation or an antioxidant cocktail (10 mg trans-resveratrol, 60 μg selenium, 10 mg vitamin E, 50 mg vitamin C) in 60 healthy non-smoker males and females aged 40–65 years, all with clinical signs of skin aging. | Skin surface, brown spots, skin evenness, skin moisture, elasticity (face), lipid peroxidation, superoxide dismutase levels, nitric oxide (NO) generation, and the expression levels of key genes (outer forearm sample). | Improved skin elasticity, moisture and antioxidant capacity with both fermented papaya and antioxidant cocktail. Increased effect of papaya extract and on gene expression. No baseline measures in study population. Antioxidant components of the fermented papaya unknown and direct link with vit. C not available. | [135] |
| Intervention with 47 men aged 30–45 given oral supplement of 54 mg or 22 mg of vit. C, 28 mg tomato extract, 27 mg grape seed extract, 210 mg of marine complex, 4 mg zinc gluconate for 180 days. | Subjective assessment of appearance and objective measures of collagen and elastin (histology and measurement in biopsy material). | Improvement in erythema, hydration, radiance, and overall appearance. Decreased intensity of general skin spots, UV spots, and brown spots, improved skin texture and appearance of pores. Increased collagen (43%–57%) and elastin (20%–31%). | [49] |
| Supplementation of 33 healthy men and women (aged 22–50), with placebo, 100 mg vit. C or 180 mg vit. C daily for four weeks. | EPR measurement of TEMPO scavenging in skin on arm. Raman resonance spectroscopy for skin carotenoids. | Improved oxygen radical scavenging with vit. C supplementation, dose dependency indicated and rapid response (obvious within two weeks). | [38] |
| Three month supplementation of 12 males and six females (21–77 y) with 2 g vit. C and 1000 IU D-alpha-tocopherol. | Measured blood vitamin levels before and after, skin resilience to UVB, detection of DNA crosslinks in skin biopsy. | Serum vit. C and vit. E doubled during intervention (implies sub-saturation at baseline). Minimal erythema dose increased with supplementation, DNA damage halved. | [20] |
| Investigation of antioxidant capacity in human skin before and after UV irradiation; effect of supplementation with 500 mg vit. C per day. | Measurement of erythema and antioxidant levels following UVB irradiation. | Vit. C and E levels increased, but levels not realistic (plasma vit. C 21 µM before and 26 µM after 500 mg daily). Skin MDA and glutathione content lowered, no effect on MED. | [27] |
| Topical application | |||
| Topical application of vit. C cream in advance of application of hair dye product p-phenylenediamine. | Visual assessment of allergic reaction following patch application on volunteer skin (on back). | Decreased or ablation of dermatitis and allergic response due to local antioxidant action of vit. C in cream. | [170] |
| Clinical study applying vit. C in liposomes to human skin (abdomen), then exposure to UV irradiation. | Measured penetration through skin layers, delivery of vit. C, loss of Trolox, TNFalpha and Il-1beta. | Increased vit. C levels in epidermis and dermis with liposomes. Protection against UV increased over liposomes alone. | [67] |
| Microneedle skin patches to deliver vit. C into the skin assessed on areas of slight wrinkle formation (around eyes). | Global Photodamage Score by visual inspection. Skin replica analysis and skin assessment by visiometer. | Slightly improved photodamage score and lessening of wrinkles after 12 weeks of treatment with vit. C-loaded patches. | [186] |
| Vit. C-based solution containing Rosa moschata oil rich in vitamins A, C, E, essential fatty acids /placebo moisturizer cream applied to facial skin of 60 healthy female subjects for 40–60 days. | Ultrasound monitoring thickness of the epidermis and dermis, and low (LEP), medium (MEP), high echogenic pixels (HEP), reflecting hydration, inflammatory processes, elastin and collagen degeneration (LEP), and structure of collagen, elastin and microfibrils (MEP and LEP). | Data suggest epidermis but not the dermis increased in thickness. Increase in MEP and HEP (collagen and elastin synthesis) and decreased LEP (inflammation and collagen degeneration). No vit. C status measurements in skin of individuals. | [149] |
| In vivo study with 30 healthy adults. Protective effect of SPF30 sunscreen with and without anti-oxidants (vit. E, grape seed extract, ubiquinone and vit. C) against Infra-Red A irradiation on previously unexposed skin (buttock). | Skin biopsy analysis; mRNA and RT-PCR for matrix metalloprotein-1 (MMP-1) expression 24 h post irradiation. | Sunscreen plus antioxidants protected skin against MMP-1 increase, sunscreen alone did not. No indication of levels of antioxidants, or whether they were able to penetrate into skin layers. Multi-component antioxidant mix. | [153] |
| In vivo study of 15 healthy adults. Protective effect of vitamin C mixtures (vit. C, vit. E, ferulic acid OR vitamin C, phoretin, ferulic acid) on ozone exposure on forearms. | Skin biopsy analysis; 4-HNE and 8-iso prostaglandin levels, immunofluorescence for NF-kB p65, cyclooxygenase-2, matrix metalloprotein-9 (MMP-9), type III collagen. After 5 days of 0.8 ppm ozone for 3h/d. | Vitamin C mixture reduced ozone induced elevation in lipid peroxidation products, NF-kB p65, cyclooxygenase-2 expression and completely prevented MMP-9 induction by ozone. No indication of levels of antioxidants, or whether they were able to penetrate into skin layers. Multi-component antioxidant mix. | [187] |
| Test of topical silicone gel with vit. C on scar formation in a population of 80 Asian people. Gel applied for six months after operation. | Scar formation monitored by modified Vancouver Scar Scale (VSS) as well as erythema and melanin indices by spectrophotometer. | Vit. C decreased scar elevation and erythema, decreased melanin index. Improved wound healing (stitch removal). | [166] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. https://doi.org/10.3390/nu9080866
Pullar JM, Carr AC, Vissers MCM. The Roles of Vitamin C in Skin Health. Nutrients. 2017; 9(8):866. https://doi.org/10.3390/nu9080866
Chicago/Turabian StylePullar, Juliet M., Anitra C. Carr, and Margreet C. M. Vissers. 2017. "The Roles of Vitamin C in Skin Health" Nutrients 9, no. 8: 866. https://doi.org/10.3390/nu9080866
APA StylePullar, J. M., Carr, A. C., & Vissers, M. C. M. (2017). The Roles of Vitamin C in Skin Health. Nutrients, 9(8), 866. https://doi.org/10.3390/nu9080866







