PREVIEW: Prevention of Diabetes through Lifestyle Intervention and Population Studies in Europe and around the World. Design, Methods, and Baseline Participant Description of an Adult Cohort Enrolled into a Three-Year Randomised Clinical Trial
Abstract
:1. Introduction
2. Methods
2.1. Aims of the Study
2.2. Primary and Secondary Endpoints
2.3. Study Setting and Design
2.4. Participants, Recruitment, and Randomisation
2.5. Description of Interventions
2.5.1. Low-Calorie Diet (LCD)
2.5.2. Weight Maintenance Phase: Intervention Diets
2.5.3. Weight Maintenance Phase: Physical Activity Programmes
2.5.4. Group Visits and the Behavioural Modification Program
2.6. Collection of Data and Description of Analyses
2.7. Data Management
2.8. Governance and Quality Management
2.9. Statistical Power and Basic Analyses
2.10. Ethical Issues
3. Results
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care 2011, 34 (Suppl. 1), S11–S61. [Google Scholar]
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, T.; Rosenbauer, J.; Wild, S.H.; Spijkerman, A.M.W.; Baan, C.; Forouhi, N.G.; Herder, C.; Rathmann, W. Diabetes in Europe: An update. Diabetes Res. Clin. Pract. 2014, 103, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Schembre, S.M.; Steinbrecher, A.; Erber, E.; Pagano, I.; Grandinetti, A.; Kolonel, L.N.; Maskarinec, G. Ethnic differences in weight gain and diabetes risk: The Multiethnic Cohort Study. Diabetes Metab. 2011, 37, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Norat, T.; Leitzmann, M.; Tonstad, S.; Vatten, L.J. Physical activity and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis. Eur. J. Epidemiol. 2015, 30, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Bhupathiraju, S.N.; Tobias, D.K.; Malik, V.S.; Pan, A.; Hruby, A.; Manson, J.E.; Willett, W.C.; Hu, F.B. Glycemic index, glycemic load, and risk of type 2 diabetes: Results from 3 large US cohorts and an updated meta-analysis. Am. J. Clin. Nutr. 2014, 100, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef]
- Li, G.; Zhang, P.; Wang, J.; An, Y.; Gong, Q.; Gregg, E.W.; Yang, W.; Zhang, B.; Shuai, Y.; Hong, J.; et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: A 23-year follow-up study. Lancet Diabetes Endocrinol. 2014, 2, 474–480. [Google Scholar] [CrossRef]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [PubMed]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.Z.; O’Dea, K.; Gomez, M.; Girgis, S.; Colagiuri, R. Diet and exercise in the prevention of diabetes. J. Hum. Nutr. Diet. 2010, 23, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Davis, E.J.; Sparks, K.C.; Hirst, K.; Costacou, T.; Lovejoy, J.C.; Regensteiner, J.G.; Hoskin, M.A.; Kriska, A.M.; Bray, G.A.; Diabetes Prevention Program Research Group. Dietary intake in the diabetes prevention program cohort: Baseline and 1-year post randomization. Ann. Epidemiol. 2004, 14, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.; Lindström, J.; Valle, T.; Aunola, S.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Lauhkonen, M.; Lehto, P.; et al. Prevention of Type II diabetes in subjects with impaired glucose tolerance: The Diabetes Prevention Study (DPS) in Finland. Study design and 1-year interim report on the feasibility of the lifestyle intervention programme. Diabetologia 1999, 42, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.R.; Li, G.W.; Hu, Y.H.; Wang, J.X.; Yang, W.Y.; An, Z.X.; Hu, Z.X.; Lin, J.; Xiao, J.Z.; Cao, H.B.; et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997, 20, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.I.; De Leeuw, I.; Hermansen, K.; Karamanos, B.; Karlström, B.; Katsilambros, N.; Riccardi, G.; Rivellese, A.A.; Rizkalla, S.; Slama, G.; et al. Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2004, 14, 373–394. [Google Scholar] [CrossRef]
- Ajala, O.; English, P.; Pinkney, J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am. J. Clin. Nutr. 2013, 97, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Buyken, A.E.; Mitchell, P.; Ceriello, A.; Brand-Miller, J. Optimal dietary approaches for prevention of type 2 diabetes: A life-course perspective. Diabetologia 2010, 53, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.M.; Dalskov, S.-M.; van Baak, M.; Jebb, S.A.; Papadaki, A.; Pfeiffer, A.F.H.; Martinez, J.A.; Handjieva-Darlenska, T.; Kunešová, M.; Pihlsgård, M.; et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N. Engl. J. Med. 2010, 363, 2102–2113. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A.; Raben, A.; Geiker, N. The role of higher protein diets in weight control and obesity-related comorbidities. Int. J. Obes. 2015, 39, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Gastaldelli, A.; Horowitz, J.F.; Endert, E.; Wolfe, R.R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. 1993, 265 Pt 1, E380–E391. [Google Scholar] [PubMed]
- Houmard, J.A.; Tanner, C.J.; Slentz, C.A.; Duscha, B.D.; McCartney, J.S.; Kraus, W.E. Effect of the volume and intensity of exercise training on insulin sensitivity. J. Appl. Physiol. 2004, 96, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Fogelholm, M.; Anderssen, S.; Gunnarsdottir, I.; Lahti-Koski, M. Dietary macronutrients and food consumption as determinants of long-term weight change in adult populations: A systematic literature review. Food Nutr. Res. 2012, 56. [Google Scholar] [CrossRef] [PubMed]
- Aller, E.E.J.G.; Larsen, T.M.; Claus, H.; Lindroos, A.K.; Kafatos, A.; Pfeiffer, A.; Martinez, J.A.; Handjieva-Darlenska, T.; Kunesova, M.; Stender, S.; et al. Weight loss maintenance in overweight subjects on ad libitum diets with high or low protein content and glycemic index: The DIOGENES trial 12-month results. Int. J. Obes. 2014, 38, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Watt, M.J.; Heigenhauser, G.J.; Spriet, L.L. Effects of dynamic exercise intensity on the activation of hormone-sensitive lipase in human skeletal muscle. J. Physiol. 2003, 547 Pt 1, 301–308. [Google Scholar] [CrossRef] [PubMed]
- WHO. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia. Available online: http://www.who.int/diabetes/publications/diagnosis_diabetes2006/en/ (accessed on 19 July 2017).
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Silventoinen, K.; Pankow, J.; Lindström, J.; Jousilahti, P.; Hu, G.; Tuomilehto, J. The validity of the Finnish Diabetes Risk Score for the prediction of the incidence of coronary heart disease and stroke, and total mortality. Eur. J. Cardiovasc. Prev. Rehabil. 2005, 12, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- 2008 Physical Activity Guidelines for Americans. Available online: http://www.health.gov/paguidelines/guidelines/ (accessed on 19 July 2017).
- Kahlert, D.; Unyi-Reicherz, A.; Stratton, G.; Meinert Larsen, T.; Fogelholm, M.; Raben, A.; Schlicht, W. PREVIEW Behavior Modification Intervention Toolbox (PREMIT): A Study Protocol for a Psychological Element of a Multicenter Project. Front. Psychol. 2016, 7, 1136. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; Abraham, C.; Whittington, C.; McAteer, J.; Gupta, S. Effective techniques in healthy eating and physical activity interventions: A meta-regression. Health Psychol. 2009, 28, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Greaves, .C.J.; Sheppard, K.E.; Abraham, C.; Hardeman, W.; Roden, M.; Evans, P.H.; Schwarz, P.; IMAGE Study Group. Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health 2011, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Olander, E.K.; Fletcher, H.; Williams, S.; Atkinson, L.; Turner, A.; French, D.P. What are the most effective techniques in changing obese individuals’ physical activity self-efficacy and behaviour: A systematic review and meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2013, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; Richardson, M.; Johnston, M.; Abraham, C.; Francis, J.; Hardeman, W.; Eccles, M.P.; Cane, J.; Wood, C.E. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: Building an international consensus for the reporting of behavior change interventions. Ann. Behav. Med. 2013, 46, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.O.; DiClemente, C.C. Stages of change in the modification of problem behaviors. Prog. Behav. Modif. 1992, 28, 183–218. [Google Scholar] [PubMed]
- PREVIEW. Available online: http://previewstudy.com (accessed on 19 July 2017).
- The Diabetes Prevention Program: Baseline characteristics of the randomized cohort. The Diabetes Prevention Program Research Group. Diabetes Care 2000, 23, 1619–1629.
- Edelstein, S.L.; Knowler, W.C.; Bain, R.P.; Andres, R.; Barrett-Connor, E.L.; Dowse, G.K.; Haffner, S.M.; Pettitt, D.J.; Sorkin, J.D.; Muller, D.C.; et al. Predictors of progression from impaired glucose tolerance to NIDDM: An analysis of six prospective studies. Diabetes 1997, 46, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Saudek, C.D.; Herman, W.H.; Sacks, D.B.; Bergenstal, R.M.; Edelman, D.; Davidson, M.B. A new look at screening and diagnosing diabetes mellitus. J. Clin. Endocrinol. Metab. 2008, 93, 2447–2453. [Google Scholar] [CrossRef] [PubMed]
- Malkani, S.; Mordes, J.P. Implications of using hemoglobin A1C for diagnosing diabetes mellitus. Am. J. Med. 2011, 124, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Nordic Nutrition Recommendations Project Group. Nordic Nutrition Recommendations 2012. Integrating Nutrition and Physical Activity, 5th ed.; Nordic Council of Ministers: Copenhagen, Denmark, 2014. [Google Scholar]
- Dietary Guidelines for Americans. Available online: http://www.cnpp.usda.gov/DietaryGuidelines (accessed on 19 July 2017).
- Dhurandhar, N.V.; Schoeller, D.; Brown, A.W.; Heymsfield, S.B.; Thomas, D.; Sørensen, T.I.A.; Speakman, J.R.; Jeansonne, M.; Allison, D.B.; Energy Balance Measurement Working Group. Energy balance measurement: When something is not better than nothing. Int. J. Obes. 2015, 39, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Corella, D.; Ordovás, J.M. Biomarkers: Background, classification and guidelines for applications in nutritional epidemiology. Nutr. Hosp. 2015, 31 (Suppl. 3), 177–188. [Google Scholar] [PubMed]
- Brand-Miller, J.C.; Stockmann, K.; Atkinson, F.; Petocz, P.; Denyer, G. Glycemic index, postprandial glycemia, and the shape of the curve in healthy subjects: Analysis of a database of more than 1000 foods. Am. J. Clin. Nutr. 2009, 89, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Whelan, W.J.; Hollar, D.; Agatston, A.; Dodson, H.J.; Tahal, D.S. The glycemic response is a personal attribute. IUBMB Life 2010, 62, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.S.; Dalleck, L.C.; Tjonna, A.E.; Beetham, K.S.; Coombes, J.S. The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: A systematic review and meta-analysis. Sports Med. 2015, 45, 679–692. [Google Scholar] [CrossRef] [PubMed]
| Higher Protein (25 E% a) Moderate Carbohydrate (45 E%) Low GI b (≤50) Diet | Moderate Protein (15 E%) Higher Carbohydrate (55 E%) Medium GI (≥56) Diet | |
|---|---|---|
| Comparison between the groups |
|
|
| Food items with increased use (relative to the other group) |
|
|
| Similar use |
| |
| High-Intensity Physical Activity (HI) | Moderate-Intensity Physical Activity (MI) | |
|---|---|---|
| Heart rate |
|
|
| Examples of activities (these may vary depending on the fitness level of the participant) |
|
|
| Weekly duration (in total) |
|
|
| Recommended weekly frequency |
|
|
| Daily duration (guideline) |
|
|
| Additional exercises |
| |
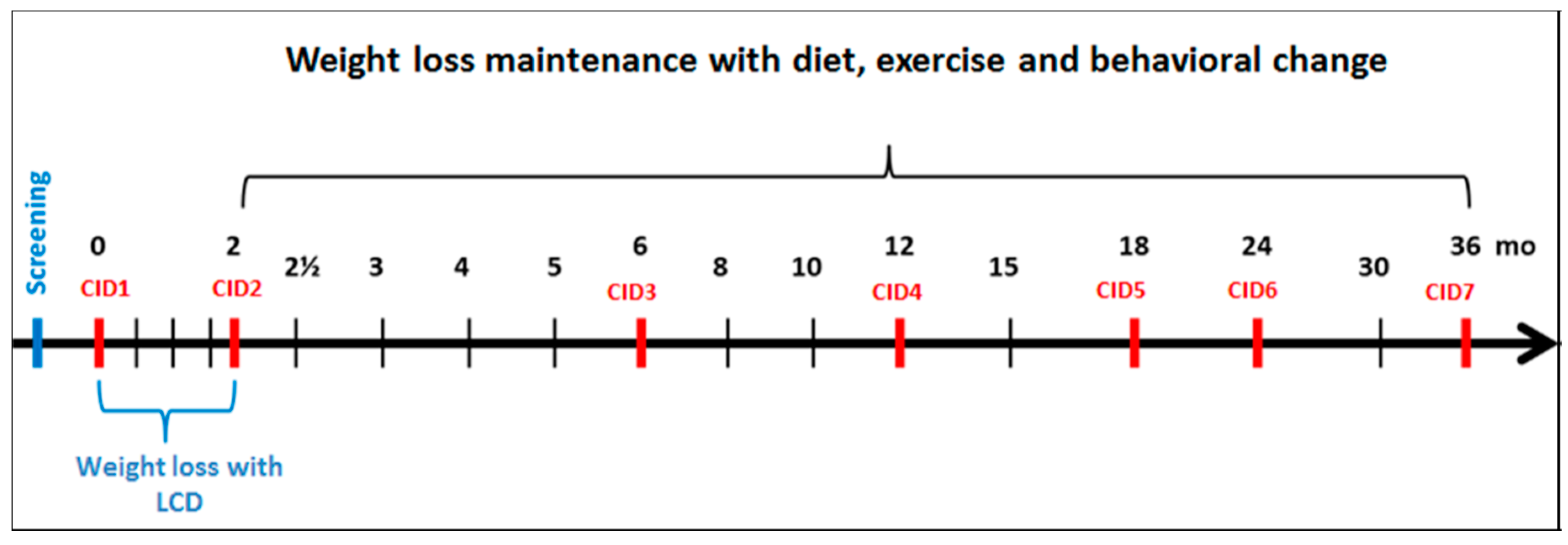
| Outcome | Data Collection Method | Assessment Time-Points (Month) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 6 | 12 | 18 | 24 | 36 | ||
| CID1 | CID2 | CID3 | CID4 | CID5 | CID6 | CID7 | ||
| Glucose tolerance/diagnosis of T2D | 75 g oral glucose tolerance test | × | × | × | × | × | ||
| Blood chemistry (lipid metabolism, glucose metabolism, inflammation markers, etc.) | Fasting venous blood specimen | × | × | × | × | × | × | × |
| Urinary nitrogen | 24-h urine collection | × | × | × | × | × | ||
| Risk markers for colon cancer (e.g., Short Chain Fatty Acids) | 3-day faecal collection a | × | × | |||||
| Gut microbiota | Faecal spot sample a | × | × | |||||
| Weight, height, BMI and anthropometrics | Weight; height (week 0 and 156); waist and hip circumference | × | × | × | × | × | × | × |
| Body composition | Body composition by DXA, BodPod or Bioelectrical impedance (BIA) | × | × | × | × | × | × | |
| Blood pressure and resting heart rate | Resting blood pressure and heart rate | × | × | × | × | × | × | × |
| Nutrient intakes, dietary GI and food consumption | 4-day food record | × | × | × | × | × | ||
| Physical activity | 7-day accelerometer, 7-day physical activity log, Baecke questionnaire | × | × | × | × | × | ||
| Maximal oxygen uptake | VO2 max test by ergometer or treadmill b | × | × | × | ||||
| Psycho-social mediators and moderators health behaviour | Several questionnaires (listed with references in Supplementary Table S2) | × | × | × | × | × | × | |
| Eating behaviour | Three Factor Eating Questionnaire (TFEQ) | × | × | × | × | × | × | |
| Sleeping | Epworth Sleepiness Scale (ESS), Pittsburgh Sleep Quality Index (PSQI) | × | × | × | × | × | × | |
| Stress and mood | Perceived Stress Scale (PSS), Profile of Mood Scale (POMS) | × | × | × | × | × | × | |
| Quality of life | WHO Quality of Life questionnaire | x | x | x | x | |||
| Work ability | Work Ability Index questionnaire | x | x | x | x | |||
| Cost-effectiveness | Questionnaire designed by the PREVIEW research group | x | x | x | x | |||
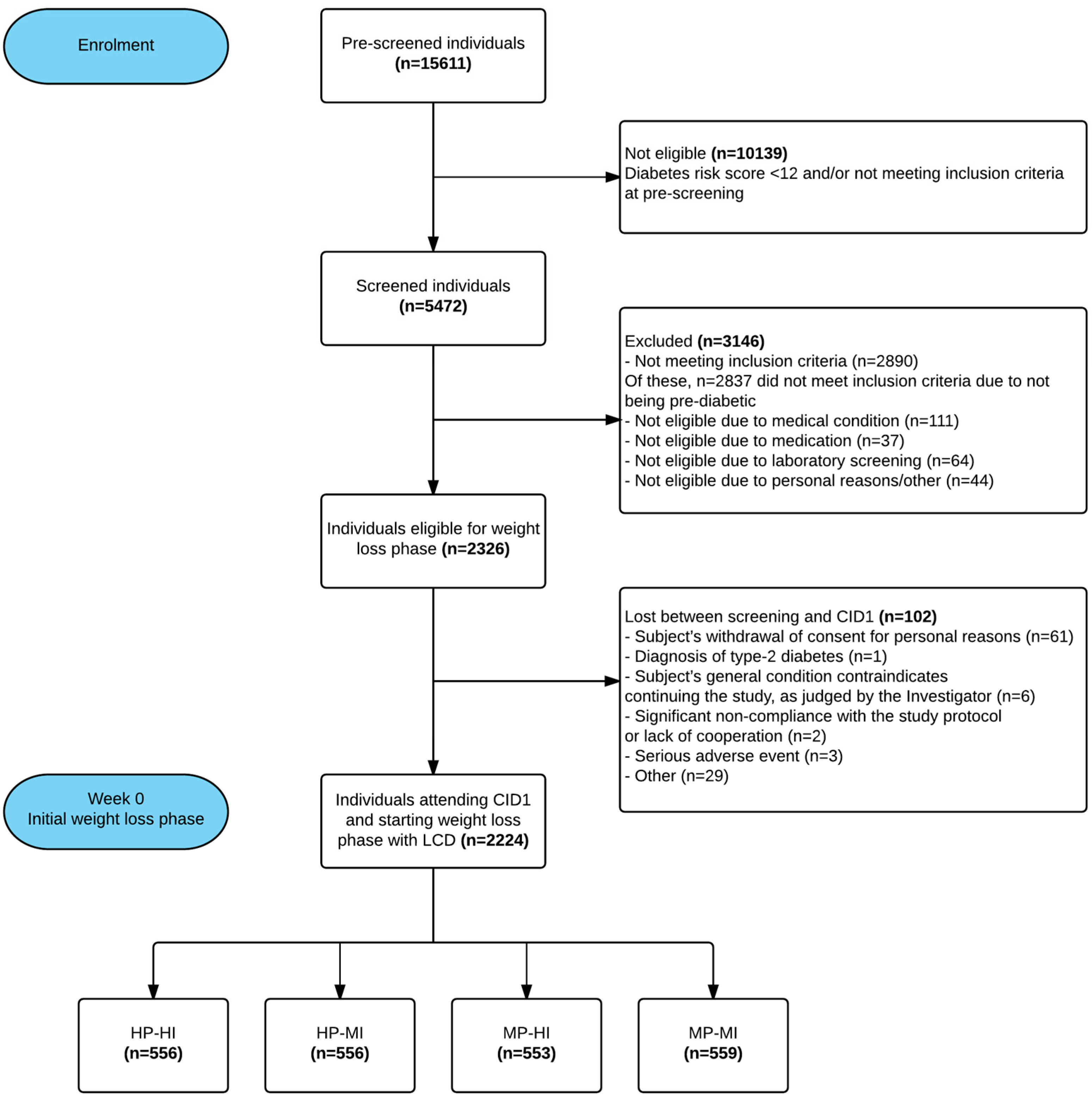
| Site | Pre-Screened | Screened | Randomised (n) | Men (n) | Women (n) | Age 25–45 Years (n) | Age 46–54 Years (n) | Age 55–70 Years (n) | Mean Age Years (SD) |
|---|---|---|---|---|---|---|---|---|---|
| UCPH | 2061 | 908 | 379 | 159 | 220 | 86 | 62 | 233 | 54.2 (10.9) |
| HEL | 1269 | 633 | 289 | 88 | 201 | 39 | 33 | 221 | 58.2 (8.9) |
| UM | 675 | 553 | 203 | 94 | 109 | 42 | 17 | 145 | 56.6 (10.0) |
| UNOTT | 3914 | 979 | 264 | 102 | 162 | 95 | 42 | 133 | 51.6 (12.0) |
| UNAV | 1740 | 732 | 307 | 93 | 214 | 145 | 82 | 93 | 47.5 (10.6) |
| MU | 1190 | 488 | 368 | 87 | 281 | 190 | 7 | 158 | 47.8 (12.0) |
| UNSYD | 3108 | 595 | 195 | 56 | 139 | 59 | 36 | 102 | 53.0 (10.8) |
| UOA | 1654 | 584 | 321 | 77 | 244 | 156 | 47 | 103 | 47.0 (11.4) |
| Total | 15,611 | 5472 | 2326 | 756 | 1570 | 812 | 326 | 1188 | 51.6 (11.6) |
| HP: Higher Protein (25 E%) Moderate Carbohydrate (45 E%) Low GI (≤50) Diet | MP: Moderate Protein (15 E%) Higher Carbohydrate (55 E%) Medium GI (≥56) Diet | |||
|---|---|---|---|---|
| Moderate-Intensity Physical Activity | High-Intensity Physical Activity | Moderate-Intensity Physical Activity | High-Intensity Physical Activity | |
| No. (men/women) | 556 (184/372) | 556 (177/379) | 559 (180/379) | 553 (179/374) |
| Age, years | 51.6 ± 11.5 | 51.8 ± 11.7 | 51.4 ± 11.2 | 51.4 ± 11.8 |
| Anthropometrics | ||||
| Height, cm | 168 ± 9 | 168 ± 9 | 168 ± 9 | 168 ± 10 |
| Weight, kg | 99.3 ± 20.8 | 100.6 ± 21.0 | 101.6 ± 22.6 | 98.7 ± 20.9 |
| Body Mass Index, kg/m2 | 35.1 ± 6.5 | 35.6 ± 6.7 | 35.7 ± 6.6 | 35.0 ± 6.4 |
| Waist circumference, cm | 109.6 ± 15.2 | 111.0 ± 15.3 | 111.1 ± 15.4 | 109.6 ± 14.5 |
| Hip circumference, cm | 117.6 ± 14.5 | 118.8 ± 14.8 | 119.2 ± 13.9 | 117.8 ± 13.8 |
| Body fat (% of weight) | 43.0 ± 7.5 | 43.5 ± 7.5 | 43.5 ± 7.9 | 43.1 ± 7.8 |
| Blood chemistry and blood pressure | ||||
| f P-glucose, mmol/L | 6.2 ± 0.8 | 6.2 ± 0.6 | 6.2 ± 0.7 | 6.2 ± 0.8 |
| 2hP-glucose, mmol/L | 7.8 ± 2.3 | 7.7 ± 2.25 | 7.5 ± 2.2 | 7.7 ± 2.1 |
| fP-insulin, mU/L | 13.6 ± 7.9 | 14.0 ± 8.7 | 13.2 ± 7.7 | 13.1 ± 7.2 |
| HbA1c, mmol/mol | 36.6 ± 3.9 | 36.8 ± 3.9 | 36.7 ± 4.2 | 36.7 ± 4.0 |
| Cholesterol, mmol/L | 5.2 ± 1.0 | 5.1 ± 1.0 | 5.3 ± 1.0 | 5.1 ± 1.0 |
| LDL cholesterol, mmol/L | 3.3 ± 0.9 | 3.2 ± 0.8 | 3.3 ± 0.9 | 3.2 ± 0.8 |
| HDL cholesterol, mmol/L | 1.3 ± 0.3 | 1.3 ± 0.3 | 1.3 ± 0.3 | 1.3 ± 0.3 |
| Triglycerides, mmol/L | 1.5 ± 0.8 | 1.5 ± 0.7 | 1.5 ± 0.9 | 1.5 ± 0.8 |
| CRP, mg/L | 5.3 ± 6.3 | 6.0 ± 8.7 | 5.2 ± 5.1 | 5.1 ± 7.5 |
| Systolic BP, mmHg | 128.5 ± 15.6 | 129.7 ± 16.2 | 128.7 ± 16.3 | 129.3 ± 15.5 |
| Diastolic BP, mmHg | 78.2 ± 10.9 | 77.6 ± 11.2 | 78.4 ± 11.5 | 78.3 ± 10.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fogelholm, M.; Larsen, T.M.; Westerterp-Plantenga, M.; Macdonald, I.; Martinez, J.A.; Boyadjieva, N.; Poppitt, S.; Schlicht, W.; Stratton, G.; Sundvall, J.; et al. PREVIEW: Prevention of Diabetes through Lifestyle Intervention and Population Studies in Europe and around the World. Design, Methods, and Baseline Participant Description of an Adult Cohort Enrolled into a Three-Year Randomised Clinical Trial. Nutrients 2017, 9, 632. https://doi.org/10.3390/nu9060632
Fogelholm M, Larsen TM, Westerterp-Plantenga M, Macdonald I, Martinez JA, Boyadjieva N, Poppitt S, Schlicht W, Stratton G, Sundvall J, et al. PREVIEW: Prevention of Diabetes through Lifestyle Intervention and Population Studies in Europe and around the World. Design, Methods, and Baseline Participant Description of an Adult Cohort Enrolled into a Three-Year Randomised Clinical Trial. Nutrients. 2017; 9(6):632. https://doi.org/10.3390/nu9060632
Chicago/Turabian StyleFogelholm, Mikael, Thomas Meinert Larsen, Margriet Westerterp-Plantenga, Ian Macdonald, J. Alfredo Martinez, Nadka Boyadjieva, Sally Poppitt, Wolfgang Schlicht, Gareth Stratton, Jouko Sundvall, and et al. 2017. "PREVIEW: Prevention of Diabetes through Lifestyle Intervention and Population Studies in Europe and around the World. Design, Methods, and Baseline Participant Description of an Adult Cohort Enrolled into a Three-Year Randomised Clinical Trial" Nutrients 9, no. 6: 632. https://doi.org/10.3390/nu9060632
APA StyleFogelholm, M., Larsen, T. M., Westerterp-Plantenga, M., Macdonald, I., Martinez, J. A., Boyadjieva, N., Poppitt, S., Schlicht, W., Stratton, G., Sundvall, J., Lam, T., Jalo, E., Christensen, P., Drummen, M., Simpson, E., Navas-Carretero, S., Handjieva-Darlenska, T., Muirhead, R., Silvestre, M. P., ... Raben, A. (2017). PREVIEW: Prevention of Diabetes through Lifestyle Intervention and Population Studies in Europe and around the World. Design, Methods, and Baseline Participant Description of an Adult Cohort Enrolled into a Three-Year Randomised Clinical Trial. Nutrients, 9(6), 632. https://doi.org/10.3390/nu9060632










