Consumption of Dairy Yogurt Containing Lactobacillus paracasei ssp. paracasei, Bifidobacterium animalis ssp. lactis and Heat-Treated Lactobacillus plantarum Improves Immune Function Including Natural Killer Cell Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Study Design and Intervention
2.3. Anthropometric Parameters and Blood Pressure
2.4. Serum Glucose and Lipid Profiles
2.5. Serum Albumin, White Blood Cell, High-Sensitivity C-Reactive Protein and Immunoglobulin G Levels
2.6. Cytokine Assays
2.7. Isolation of PBMCs
2.8. Cytotoxic Activity of NK Cells
2.9. Daily Energy Intake and Physical Activity Measurements
2.10. Statistical Analysis
3. Results
3.1. Effects on Clinical Characteristics Following Twelve Weeks of Consuming Dairy Yogurt Containing L. paracasei, B. lactis and Heat-Treated L. plantarum
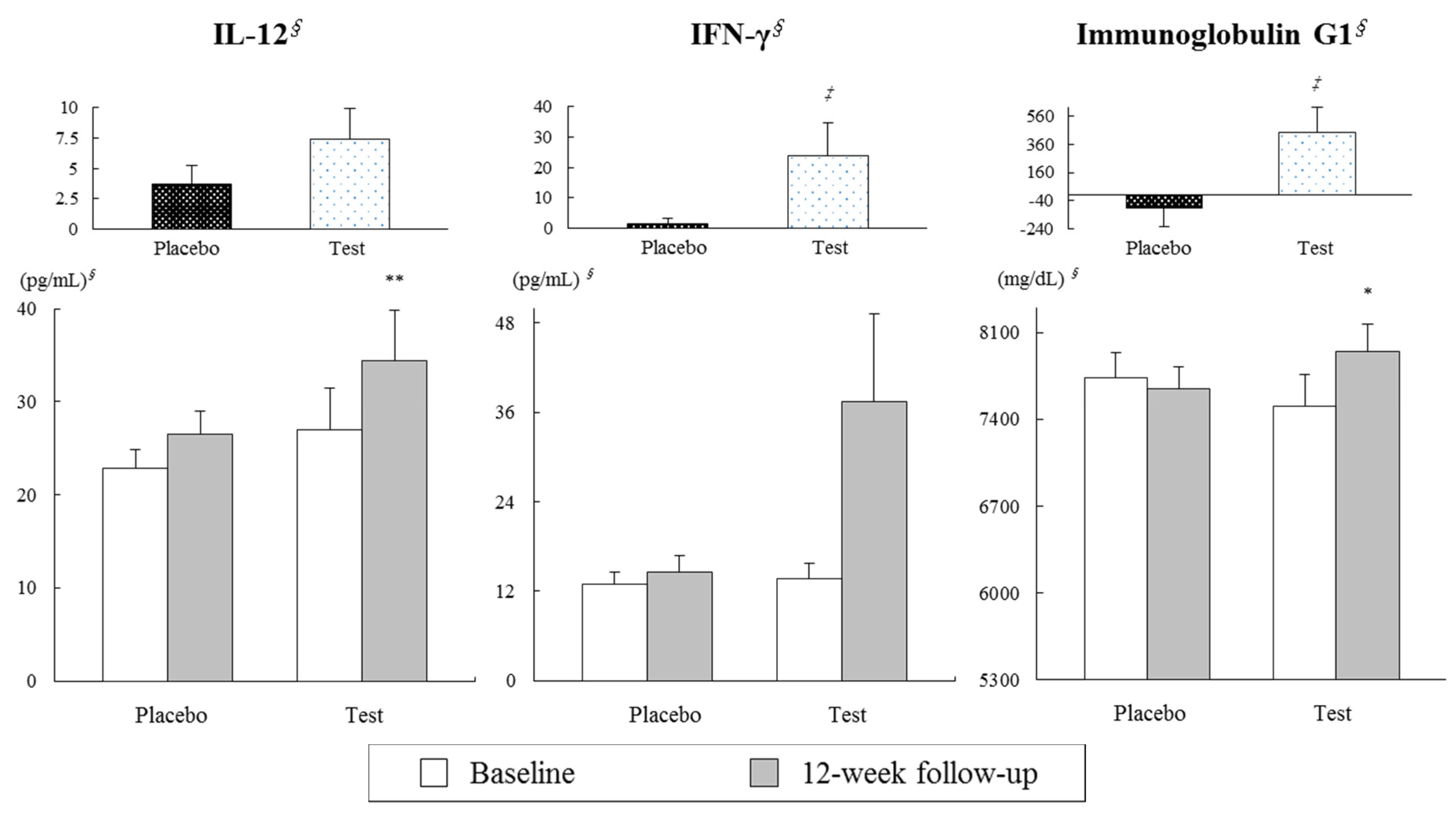
3.2. Effects on Serum Cytokine and Immunoglobulin Concentrations Following Twelve Weeks of Consuming Dairy Yogurt Containing L. paracasei, B. lactis and Heat-Treated L. plantarum
3.3. Effects on NK Cell Activity Following Twelve Weeks of Consuming Dairy Yogurt Containing L. paracasei, B. lactis and Heat-Treated L. plantarum
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gavazzi, G.; Krause, K.H. Ageing and infection. Lancet Infect. Dis. 2002, 2, 659–666. [Google Scholar] [CrossRef]
- Ben-Yehuda, A.; Weksler, M.E. Immune senescence: Mechanisms and clinical implications. Cancer Investig. 1992, 10, 525–531. [Google Scholar] [CrossRef]
- Makinodan, T.; James, S.J.; Inamizu, T.; Chang, M.P. Immunologic basis for susceptibility to infection in the aged. Gerontology 1984, 30, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Adolfsson, O.; Meydani, S.N.; Russell, R.M. Yogurt and gut function. Am. J. Clin. Nutr. 2004, 80, 245–256. [Google Scholar] [PubMed]
- El-Abbadi, N.H.; Dao, M.C.; Meydani, S.N. Yogurt: Role in healthy and active aging. Am. J. Clin. Nutr. 2014, 99, 1263S–1270S. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, R.; Shah, N.P. Immune system stimulation by probiotic microorganisms. Crit. Rev. Food Sci. Nutr. 2014, 54, 938–956. [Google Scholar] [CrossRef] [PubMed]
- Dallal, M.M.S.; Yazdi, M.H.; Holakuyee, M.; Hassan, Z.M.; Abolhassani, M.; Mahdavi, M. Lactobacillus casei ssp. casei induced Th1 cytokine profile and natural killer cells activity in invasive ductal carcinoma bearing mice. Iran. J. Allergy Asthma Immunol. 2012, 11, 183–189. [Google Scholar]
- Kawashima, T.; Hayashi, K.; Kosaka, A.; Kawashima, M.; Igarashi, T.; Tsutsui, H.; Obata, A. Lactobacillus plantarum strain YU from fermented foods activates Th1 and protective immune responses. Int. Immunopharmacol. 2011, 11, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Makino, S.; Ikegami, S.; Kume, A.; Horiuchi, H.; Sasaki, H.; Orii, N. Reducing the risk of infection in the elderly by dietary intake of yoghurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. Br. J. Nutr. 2010, 104, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Rizzardini, G.; Eskesen, D.; Calder, P.C.; Capetti, A.; Jespersen, L.; Clerici, M. Evaluation of the immune benefits of two probiotic strains Bifidobacterium animalis ssp. lactis, BB-12® and Lactobacillus paracasei ssp. paracasei, L. casei 431® in an influenza vaccination model: A randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2012, 107, 876–884. [Google Scholar]
- Tobita, K.; Yanaka, H.; Otani, H. Heat-treated Lactobacillus crispatus KT strains reduce allergic symptoms in mice. J. Agric. Food Chem. 2009, 57, 5586–5590. [Google Scholar] [CrossRef] [PubMed]
- Murosaki, S.; Yamamoto, Y.; Ito, K.; Inokuchi, T.; Kusaka, H.; Ikeda, H.; Yoshikai, Y. Heat-killed Lactobacillus plantarum L-137 suppresses naturally fed antigen-specific IgE production by stimulation of IL-12 production in mice. J. Allergy Clin. Immunol. 1998, 102, 57–64. [Google Scholar] [CrossRef]
- Hirose, Y.; Murosaki, S.; Yamamoto, Y.; Yoshikai, Y.; Tsuru, T. Daily intake of heat-killed Lactobacillus plantarum L-137 augments acquired immunity in healthy adults. J. Nutr. 2006, 136, 3069–3073. [Google Scholar] [PubMed]
- Lee, H.A.; Kim, H.; Lee, K.W.; Park, K.Y. Dead nano-sized Lactobacillus plantarum inhibits azoxymethane/dextran sulfate sodium-induced colon cancer in Balb/c mice. J. Med. Food 2015, 18, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Okumura, K. Effects of a fermented milk drink containing Lactobacillus casei strain Shirota on the human NK-cell activity. J. Nutr. 2007, 137, 791S–793S. [Google Scholar] [PubMed]
- Makino, S.; Ikegami, S.; Kano, H.; Sashihara, T.; Sugano, H.; Horiuchi, H.; Oda, M. Immunomodulatory effects of polysaccharides produced by Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. J. Dairy Sci. 2006, 89, 2873–2881. [Google Scholar] [CrossRef]
- Vidal, S.M.; Khakoo, S.I.; Biron, C.A. Natural killer cell responses during viral infections: Flexibility and conditioning of innate immunity by experience. Curr. Opin. Virol. 2011, 1, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Ogata, K.; An, E.; Shioi, Y.; Nakamura, K.; Luo, S.; Yokose, N.; Dan, K. Association between natural killer cell activity and infection in immunologically normal elderly people. Clin. Exp. Immunol. 2001, 124, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Heremans, H.; Dillen, C.; van Damme, J.; Billiau, A. Essential role for natural killer cells in the lethal lipopolysaccharide-induced Shwartzman-like reaction in mice. Eur. J. Immunol. 1994, 24, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-γ: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef] [PubMed]
- Sayers, T.J.; Mason, L.H.; Wiltrout, T.A. Trafficking and activation of murine natural killer cells: Differing roles for IFN-γ and IL-2. Cell. Immunol. 1990, 127, 311–326. [Google Scholar] [CrossRef]
- Harris, D.P.; Haynes, L.; Sayles, P.C.; Duso, D.K.; Eaton, S.M.; Lepak, N.M.; Lund, F.E. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat. Immunol. 2000, 1, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.P.; Kandaswami, C.; Mahajan, S.; Chadha, K.C.; Chawda, R.; Nair, H.; Schwartz, S.A. The flavonoid, quercetin, differentially regulates Th-1 (IFNγ) and Th-2 (IL4) cytokine gene expression by normal peripheral blood mononuclear cells. Biochim. Biophys. Acta 2002, 1593, 29–36. [Google Scholar] [CrossRef]
- Ertel, W.; Keel, M.; Neidhardt, R.; Steckholzer, U.; Kremer, J.P.; Ungethuem, U.; Trentz, O. Inhibition of the defense system stimulating interleukin-12 interferon-γ pathway during critical illness. Blood 1997, 89, 1612–1620. [Google Scholar] [PubMed]
- Trinchieri, G. Proinflammatory and immunoregulatory functions of interleukin-12. Int. Rev. Immunol. 1998, 16, 365–396. [Google Scholar] [CrossRef] [PubMed]
- Klein-Franke, A.; Anderer, F.A. IL-12-mediated activation of MHC-unrestricted cytotoxicity of human PBMC subpopulations: Synergic action of a plant rhamnogalacturonan. Anticancer Res. 1995, 15, 2511–2516. [Google Scholar] [PubMed]
- Kalia, V.; Sarkar, S.; Gourley, T.S.; Rouse, B.T.; Ahmed, R. Differentiation of memory B and T cells. Curr. Opin. Immunol. 2006, 18, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Rimm, E.B.; Giovannucci, E.L.; Stampfer, M.J.; Colditz, G.A.; Litin, L.B.; Willett, W.C. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am. J. Epidemiol. 1992, 135, 1114–1126. [Google Scholar] [CrossRef] [PubMed]
| Total Subjects (n = 152) | pa | pb | pc | ||||
|---|---|---|---|---|---|---|---|
| Placebo (n = 79) | Test (n = 73) | ||||||
| Baseline | Follow-up | Baseline | Follow-up | ||||
| Age (year) | 65.7 ± 0.56 | 65.7 ± 0.50 | 0.988 | ||||
| Male/Female n, (%) | 24 (30.4)/55 (69.6) | 21 (28.8)/52 (71.2) | 0.828 | ||||
| Current smoker n, (%) | 4 (5.1) | 7 (9.6) | 0.282 | ||||
| Current drinker n, (%) | 32 (40.5) | 25 (34.2) | 0.426 | ||||
| BMI (kg/m2) | 23.7 ± 0.31 | 23.7 ± 0.33 | 23.6 ± 0.25 | 23.7 ± 0.26 | 0.856 | 0.895 | |
| Change | −0.03 ± 0.06 | 0.09 ± 0.06 | 0.131 | ||||
| Systolic BP (mmHg) | 125.7 ± 1.73 | 123.4 ± 1.72 | 124.3 ± 1.94 | 122.9 ± 1.94 | 0.601 | 0.832 | |
| Change | −2.23 ± 1.50 | −1.42 ± 1.61 | 0.713 | ||||
| Diastolic BP (mmHg) | 77.1 ± 0.93 | 76.9 ± 1.22 | 76.8 ± 1.19 | 75.4 ± 1.42 | 0.822 | 0.408 | |
| Change | −0.20 ± 0.99 | −1.41 ± 1.00 | 0.394 | ||||
| Glucose (mg/dL) ∮ | 88.3 ± 1.19 | 89.0 ± 1.27 | 87.8 ± 0.93 | 88.9 ± 1.35 | 0.863 | 0.955 | |
| Change | 0.73 ± 0.85 | 1.10 ± 1.01 | 0.783 | ||||
| Triglyceride (mg/dL) ∮ | 122.6 ± 6.31 | 126.3 ± 6.25 | 123.0 ± 8.41 | 138.0 ± 7.39 ** | 0.816 | 0.230 | |
| Change | 3.66 ± 5.49 | 15.0 ± 8.11 | 0.243 | ||||
| Total cholesterol (mg/dL) ∮ | 206.7 ± 4.38 | 204.8 ± 4.35 | 209.2 ± 4.10 | 208.3 ± 4.07 | 0.585 | 0.488 | |
| Change | −1.86 ± 3.00 | −0.93 ± 2.32 | 0.807 | ||||
| HDL-cholesterol (mg/dL) ∮ | 54.8 ± 1.54 | 54.0 ± 1.63 | 54.8 ± 1.64 | 54.7 ± 1.71 | 0.920 | 0.810 | |
| Change | −0.75 ± 1.15 | −0.11 ± 0.88 | 0.662 | ||||
| LDL-cholesterol (mg/dL) ∮ | 127.4 ± 4.39 | 125.6 ± 4.09 | 129.8 ± 4.02 | 126.0 ± 3.64 | 0.529 | 0.747 | |
| Change | −1.85 ± 2.71 | −3.82 ± 2.49 | 0.595 | ||||
| Serum albumin (mg/dL) ∮ | 4.55 ± 0.02 | 4.55 ± 0.03 | 4.55 ± 0.02 | 4.52 ± 0.03 | 0.939 | 0.497 | |
| Change | −0.01 ± 0.02 | −0.03 ± 0.02 | 0.361 | ||||
| White blood cells (×103/μL) ∮ | 5.33 ± 0.12 | 5.29 ± 0.14 | 5.61 ± 0.13 | 5.71 ± 0.18 | 0.089 | 0.070 | |
| Change | −0.03 ± 0.12 | 0.10 ± 0.13 | 0.424 | ||||
| hs-CRP (mg/L) ∮ | 0.80 ± 0.07 | 2.01 ± 0.71 * | 1.24 ± 0.26 | 1.77 ± 0.50 | 0.449 | 0.781 | |
| Change | 1.21 ± 0.72 | 0.53 ± 0.55 | 0.460 | ||||
| TNF-α (pg/mL) ∮ | 22.5 ± 4.93 | 22.9 ± 5.58 | 23.1 ± 4.46 | 21.4 ± 3.13 | 0.798 | 0.754 | |
| Change | 0.39 ± 2.23 | −1.77 ± 2.62 | 0.529 | ||||
| Immunoglobulin G3 (mg/dL) ∮ | 265.6 ± 18.1 | 266.2 ± 18.6 | 256.8 ± 20.4 | 246.0 ± 18.5 | 0.565 | 0.506 | |
| Change | 0.66 ± 14.6 | −10.8 ± 11.9 | 0.548 | ||||
| Total Subjects (n = 152) | pa | pb | pc | pd | ||||
|---|---|---|---|---|---|---|---|---|
| Placebo (n = 79) | Test (n = 73) | |||||||
| Baseline | Follow-up | Baseline | Follow-up | |||||
| NK cell activity 10:1 (%) ∮ | 23.9 ± 1.72 | 26.1 ± 1.79 | 22.0 ± 1.77 | 35.1 ± 2.16 *** | 0.395 | <0.001 | ||
| Change | 2.26 ± 1.82 | 13.2 ± 2.00 | <0.001 | <0.001 | ||||
| NK cell activity 5:1 (%) ∮ | 17.3 ± 1.34 | 18.0 ± 1.26 | 14.6 ± 1.34 | 25.2 ± 1.87 *** | 0.349 | 0.002 | ||
| Change | 0.69 ± 1.55 | 10.6 ± 1.78 | <0.001 | <0.001 | ||||
| NK cell activity 2.5:1 (%) ∮ | 12.4 ± 0.94 | 13.1 ± 1.09 | 12.0 ± 1.19 | 20.4 ± 1.54 *** | 0.157 | <0.001 | ||
| Change | 0.73 ± 1.22 | 8.33 ± 1.55 | <0.001 | <0.001 | ||||
| NK cell activity 1.25:1 (%) ∮ | 10.5 ± 1.01 | 11.6 ± 1.15 | 9.78 ± 1.14 | 16.9 ± 1.61 *** | 0.190 | 0.001 | ||
| Change | 1.11 ± 1.32 | 7.08 ± 1.60 | 0.004 | 0.004 | ||||
| NK cell activity 0.625:1 (%) ∮ | 10.2 ± 1.15 | 9.57 ± 0.95 | 8.48 ± 1.20 | 14.7 ± 1.75 *** | 0.059 | 0.019 | ||
| Change | −0.65 ± 1.36 | 6.23 ± 1.66 | 0.002 | 0.002 | ||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, A.; Lee, Y.J.; Yoo, H.J.; Kim, M.; Chang, Y.; Lee, D.S.; Lee, J.H. Consumption of Dairy Yogurt Containing Lactobacillus paracasei ssp. paracasei, Bifidobacterium animalis ssp. lactis and Heat-Treated Lactobacillus plantarum Improves Immune Function Including Natural Killer Cell Activity. Nutrients 2017, 9, 558. https://doi.org/10.3390/nu9060558
Lee A, Lee YJ, Yoo HJ, Kim M, Chang Y, Lee DS, Lee JH. Consumption of Dairy Yogurt Containing Lactobacillus paracasei ssp. paracasei, Bifidobacterium animalis ssp. lactis and Heat-Treated Lactobacillus plantarum Improves Immune Function Including Natural Killer Cell Activity. Nutrients. 2017; 9(6):558. https://doi.org/10.3390/nu9060558
Chicago/Turabian StyleLee, Ayoung, Young Ju Lee, Hye Jin Yoo, Minkyung Kim, Yeeun Chang, Dong Seog Lee, and Jong Ho Lee. 2017. "Consumption of Dairy Yogurt Containing Lactobacillus paracasei ssp. paracasei, Bifidobacterium animalis ssp. lactis and Heat-Treated Lactobacillus plantarum Improves Immune Function Including Natural Killer Cell Activity" Nutrients 9, no. 6: 558. https://doi.org/10.3390/nu9060558
APA StyleLee, A., Lee, Y. J., Yoo, H. J., Kim, M., Chang, Y., Lee, D. S., & Lee, J. H. (2017). Consumption of Dairy Yogurt Containing Lactobacillus paracasei ssp. paracasei, Bifidobacterium animalis ssp. lactis and Heat-Treated Lactobacillus plantarum Improves Immune Function Including Natural Killer Cell Activity. Nutrients, 9(6), 558. https://doi.org/10.3390/nu9060558




