Abstract
Insomnia is a serious worldwide health threat, affecting nearly one third of the general population. Melatonin has been reported to improve sleep efficiency and it was found that eating melatonin-rich foods could assist sleep. During the last decades, melatonin has been widely identified and qualified in various foods from fungi to animals and plants. Eggs and fish are higher melatonin-containing food groups in animal foods, whereas in plant foods, nuts are with the highest content of melatonin. Some kinds of mushrooms, cereals and germinated legumes or seeds are also good dietary sources of melatonin. It has been proved that the melatonin concentration in human serum could significantly increase after the consumption of melatonin containing food. Furthermore, studies show that melatonin exhibits many bioactivities, such as antioxidant activity, anti-inflammatory characteristics, boosting immunity, anticancer activity, cardiovascular protection, anti-diabetic, anti-obese, neuroprotective and anti-aging activity. This review summaries the dietary sources and bioactivities of melatonin, with special attention paid to the mechanisms of action.
1. Introduction
Melatonin, N-acetyl-5-methoxy tryptamine, was first isolated from bovine pineal gland [1]. As the biological roles of melatonin were widely studied, the recognized therapeutical effects and the health benefits of melatonin could cover a broad range. Melatonin could regulate human physiological rhythm, alleviate related disorders like jet lag [2] and insomnia [3], scavenge free radical species [4], enhance the immune system [5], show anti-aging [6] and anti-inflammatory effects [7] and perform anticancer activities [8]. Moreover, melatonin could also exhibit neuroprotective effects [9], facilitate the control of chronic diseases, such as cardiovascular diseases [10], diabetes [11] and obesity [12]. In addition, melatonin could even regulate the mood [13], sexual maturation [14] and body temperature [15]. Recent research revealed the promising therapeutical application of melatonin in periodontology [16].
After being long considered as a hormone exclusively produced in the pineal gland of animals (Figure 1), melatonin has been identified in plants [17], insects [18], fungi [19] and bacteria [20]. Given the potent health effects of melatonin, many foods have been tested in the past decades and melatonin was identified and quantified in both animal foods and edible plants [21,22]. Huge differences of melatonin concentrations were reported among various food species and/or organs, ranging from pg/g to mg/g [22,23]. Additionally, it was well documented that the consumption of melatonin-rich foods may induce the potential health impacts by significantly increasing the serum melatonin concentration and antioxidant capacity in human beings [24]. Therefore, those foods containing melatonin are now popular and regarded as promising nutraceuticals [25,26,27].
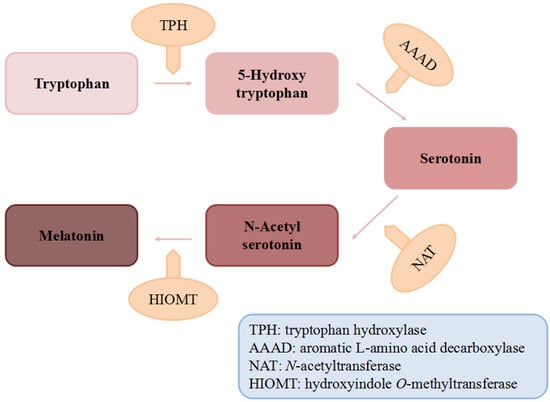
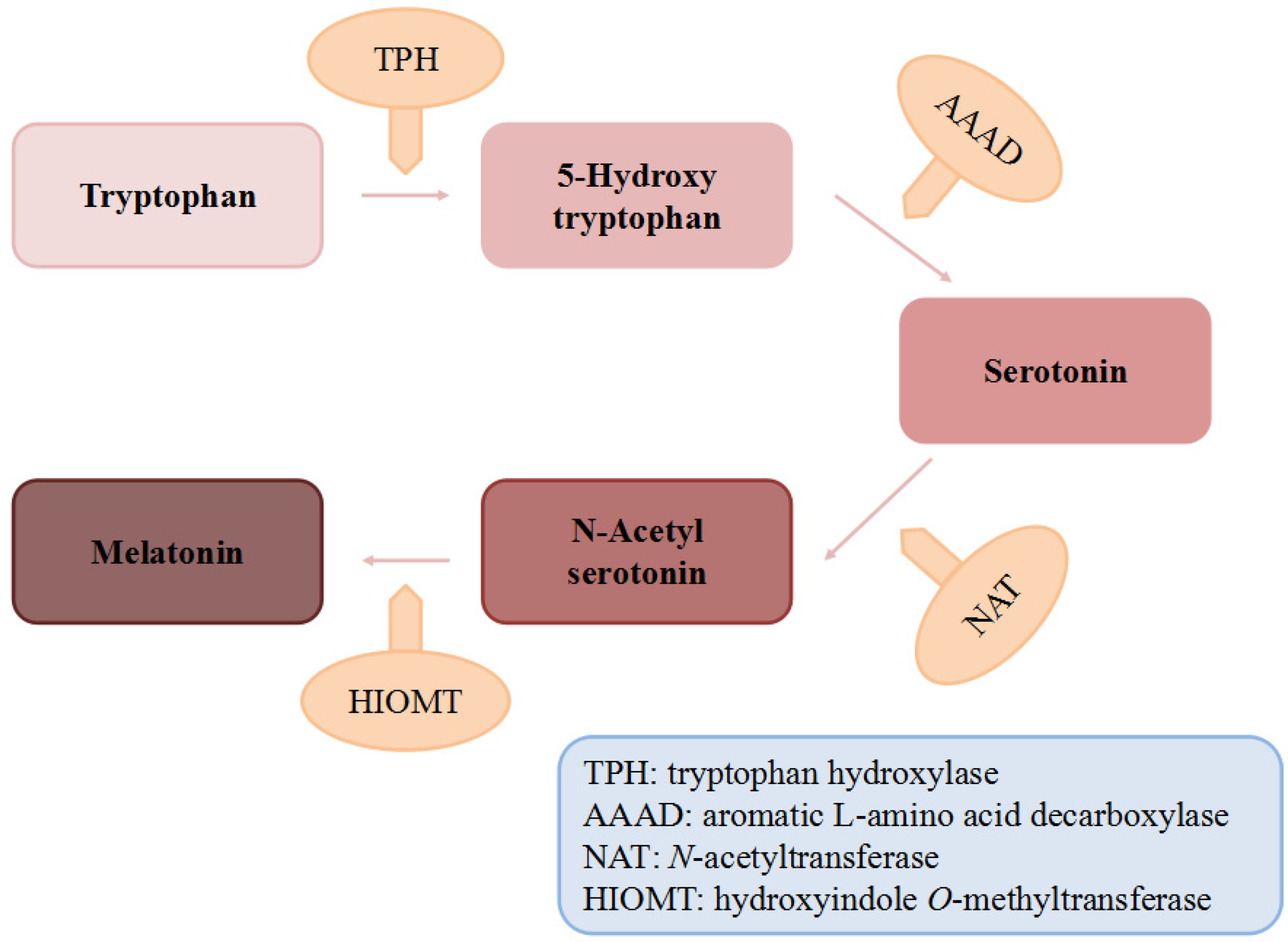
Figure 1.
The biosynthesis of melatonin.
This review outlines the dietary sources, summarizes the bioactivities of melatonin, and special attention was paid to the mechanisms of action. A search of PubMed and Web of Science was conducted, and the related peer-reviewed articles published in English within 10 years were included because of the huge number of studies.
2. Dietary Sources of Melatonin
Melatonin exists widely in many kinds of food stuffs (Table 1). However, the content of melatonin in foods exhibits huge differences from species to species. Much higher melatonin was observed in nuts and medical herbs [28,29]. For the same species, it varies in different cultivars [30]. A study qualified the melatonin contents in 58 cultivars of corns, which ranged 10–2034 ng/g dry weight (DW) [31]. Additionally, in both animal foods and plant foods, melatonin could distribute unevenly in one individual animal or plant because of the different biophysical dynamic features in organs [22,32]. In edible plants, fruits generally present the lowest melatonin content, while the seeds and leaves have the highest one [33,34]. Furthermore, the melatonin concentration in the plant food products is also associated with the environment, in which the plants are cultured, including the temperature, sunlight exposing duration, ripening process, agrochemical treatment etc. [35,36]. Therefore, it is challenging and promising to clarify the factors influencing melatonin concentration in foods so as to select the effective approaches, such as germination of seeds, to increase the melatonin content in foods.

Table 1.
Concentration of melatonin in food.
2.1. Animal Foods
In animal foods, melatonin concentrations were found higher in eggs and fish than those in meat [22]. Melatonin was detected in breast milk of human beings and also in the milk provided by other animals [22,37]. Similar as the fluctuation in plasma, i.e., relatively low during daytime and relatively high at night, melatonin levels in milk also showed a circadian rhythm, indicating that night milking might increase the benefits from milk as the melatonin concentration was approximately ten times compared to that in the daytime [38]. Furthermore, melatonin was found in colostrum with the comparable concentration as it in plasma [39], which could benefit the newborns that lack the established rhythmic excretion of melatonin in the first couple of weeks in their lives [40]. Neither the artificial formulas nor the fermented milk drink were tested with detectable melatonin [41,42].
2.2. Plant Foods
2.2.1. Cereals
Cereals are largely consumed all around the world, in which melatonin contents were also investigated. Wang et al. studied melatonin contents in 58 cultivars of corns and 25 of rice, finding huge variety due to genotypes: 0–2034 ng/g (mean: 96.5 ng/g) for corns and 0–264 ng/g (mean: 16 ng/g) for rice [31]. Additionally, they also discovered the pigmented rice contained higher melatonin contents, which was consistent with the findings from Setyaningsih et al. [17]. Moreover, Setyaningsih et al. reported that the level of melatonin in nonglutinous black rice was almost twice as that in the glutinous type and the melatonin concentration was 1/3 less in polished rice compared to that in the whole ones. For other cereals such as wheat, barley and oats, melatonin was found relatively high, e.g., 124.7 ± 14.9 ng/g fresh weight (FW) in wheat, 82.3 ± 6.0 ng/g FW in barley and 90.6 ± 7.7 ng/g FW in oats, respectively [34]. In terms of bread, crumb was found with higher melatonin levels than the crust [20].
2.2.2. Fruits
Generally, melatonin was found in many commonly consumed fruits. Grapes [43], cherries [30] and strawberries [44] were the most popular ones in the investigation of melatonin contents, which also showed the difference between cultivars. The highest melatonin in three kinds of fruits was reported in the range of 8.9–158.9 ng/g DW in the skin of grapes (Vitis vinifera L. cv. Malbec) [45], 13.46 ± 1.10 ng/g FW in tart cherries (Prunus cerasus L. cv. Balaton) [46], and 11.26 ± 0.13 ng/g FW in strawberry (Fragaria ananassa L. cv. Festival) [44]. Other fruits contained melatonin at a relatively low level.
2.2.3. Vegetables
Melatonin exists in lots of common vegetables, although it remains undetectable in potatoes and very low in beetroots [47,48]. Tomatoes and peppers were the most studied vegetables, and they showed relatively high melatonin concentrations in the vegetable group, i.e., 11.9 ng/g FW or 93.4 ng/g DW in pepper (Capsicum annuum L. cv. F26) and 14.77 ng/g FW or 249.98 ng/g DW in tomato (Solanum lycopersicum L. cv. Optima) [49], and 23.87 ± 2.02 ng/g FW in tomato (Lycopersicon esculentum cv. Bonda) [44].
Mushrooms also contain melatonin, e.g., 12,900 ± 770 ng/g DW in Basidiomycota (Lactarius deliciosus), 6800 ± 60 ng/g DW in Basidiomycota (Boletus edulis) [42], and 4300–6400 ng/g DW in Agaricus bisporus [50].
2.2.4. Legumes and Seeds (Raw and Germinated)
Melatonin occurred in many legumes and seeds, and in some particular seeds, such as white and black mustard seeds, melatonin was as high as 189 ng/g DW and 129 ng/g DW, respectively [51]. Moreover, the germination process of legumes and seeds was proved to be able to increase the melatonin levels significantly [52,53]. For example, in germinated soybean seeds, the melatonin level reached a peak of 1.89 ± 0.11 ng/g DW during germination, which was 400% increase than that in raw soybean seeds [52]. Similarly, melatonin was observed more than 11 folds in the germinated mung bean seeds as it was in the raw ones [53].
2.2.5. Nuts
Melatonin was found in various nuts, and pistachio (Pistacia vera L.) was reported with the highest content (233,000 ng/g DW) to date [28].
2.2.6. Juices and Beverages
The popular alcoholic drinks like beer [42,54] and wine [55,56], were tested for melatonin, with 0.09 ± 0.01 ng/mL in beer [42] and up to 129.5 ± 3.5 ng/mL in wine [42,54]. Besides the melatonin originated from the main ingredients, it was also considered to be synthesized during the fermentation process due to the yeast growth [54,57].
As for the other drinks, coffee usually contains high concentration of melatonin because coffee beans contained melatonin at a very high level and even much higher in roasted beans [58]. Melatonin also existed in juices [43], cacao [42] and balsamic vinegars [59]. However, it was not found in concentrates [60] or in tea [42], including green tea and black tea.
2.2.7. Medical Herbs
Many medical herbs were analyzed and some of them contained melatonin at a level of more than 1000 ng/g DW [61,62]. In Huang-qin (Scutellaria biacalensis) melatonin was significantly high as 7110 ng/g DW, and in St. John’s Wort (Hypericum perforatum) melatonin concentration was 4490 ng/g DW in flowers and 1750 ng/g DW in leaves [29]. Besides, 108 Chinese medical herbs were quantified, and melatonin was more than 10 ng/g DW in 64 tested herbs [61].
2.2.8. Edible Oils
Melatonin ranged 0.03–0.29 ng/g in the tested edible oils, among which refined linseed presented 0.29 ng/g and virgin soybean 0.19 ng/g [63,64]. De la Puerta et al. also demonstrated that generally the melatonin levels in the refined olive and sunflower oil were about half of those in the extra virgin olive oil [63].
2.2.9. Yeast
Melatonin was found ranging 2.2 ± 0.14 ng/g in yeast, particularly Saccharomyces cerevisae, which is widely used in bread baking and alcohol industry [22].
Collectively, melatonin has been found in lots of foods. In animal foods, eggs and fish are relatively rich in melatonin, whereas in plant foods, nuts contains the highest melatonin contents, and some cereals and germinated legumes or seeds are also with high contents of melatonin. Mushrooms are also high-melatonin foods. However, it should be pointed out that the data on content of melatonin in foods in Table 1 were obtained by different analytical methods. Thus, quality of these data depended on the pretreatment method of sample and the analytical method itself. For example, in terms of radioimmunoassay (RIA) and enzyme-linked immunoabsorbent assay (ELISA), melatonin content could be over-estimated as some other components in the foods might also react with the related antibodies and enzymes, resulting in the lower accuracy. In addition, high-performance liquid chromatograph with ultraviolet detector (HPLC-UV) has low sensitivity and selectivity. Although high-performance liquid chromatograph with fluorescence detector (HPLC-FD) has high sensitivity, its selectivity is also low. Thus, melatonin content could be over-estimated by HPLC-UV or HPLC-FD method. Generally, gas chromatography-mass spectrometry (GC-MS) and high-performance liquid chromatography-mass spectrometry (HPLC-MS) have high resolution and sensitivity. Therefore, the data obtained by GC-MS or HPLC-MS are more accurate than those obtained by other methods [65].
2.3. Other Issues about Melatonin Intake
2.3.1. The Alternation of Endogenous Melatonin along Life Cycle
It has been well-documented that the secretion and/or serum levels of melatonin alter through a human lifetime [66,67,68]. Specifically, the serum levels of endogenous melatonin during the day and at night were measured in 367 volunteers (210 males and 157 females), aged 3 days to 90 years [67]. The results of this study demonstrated that nighttime serum melatonin concentration was low during the first 6 months of life [27.3 ± 5.4 (±standard error, SE) pg/mL], then reached a peak (329.5 ± 42.0 pg/mL) at 1–3 years of age, followed by a huge drop to 62.5 ± 9.0 pg/mL in the group of aged 15–20 years, probably due to the body size expansion during childhood and puberty without an increase in the rate of secretion. Then an additional decline followed in adulthood until 70–90 year of age (29.2 ± 6.1 pg/mL), attributing to a degenerative pineal gland associated with aging. Overall, the alternations of nocturnal serum melatonin during lifetime showed a fragmentary and inconsistent manner. Meanwhile, daytime serum melatonin levels were low and not age-related.
2.3.2. Bioavailability of Exogenous Melatonin
Melatonin has been known and used to assist sleep disorders [8] or jet lag [69] as a dietary supplement or as a drug, and recently it has also been reported to synergize other drugs as an adjuvant to improve their efficiency or to attenuate their side effects [70]. Herein, the bioavailability of melatonin has been studied by some researchers. In a study in vivo among 12 healthy volunteers, melatonin was administrated intravenously (i.v.) by 2 mg and orally by 2 and 4 mg, and in neither of these two phases the difference in serum half-life was found [71]. This study revealed the poor absolute bioavailability of oral melatonin tablets in both dosages, which was approximately 15%, possibly because of the poor oral absorption, large first-pass metabolism, or combined. Moreover, another study was conducted with 12 young healthy volunteers (6 males, 6 females), with 250 μg oral solution of D7 melatonin, a molecule in which seven hydrogen atoms are replaced by seven deuterium atoms. It was reported that the absolute bioavailability of melatonin ranged from 1% to 37% and higher in women (16.8% ± 12.7%) than in men (8.6% ± 3.9%). It was also observed that the apparent terminal half-life values were 36 ± 2 and 41 ± 10 min for males and females respectively, indicating the fast-release property of melatonin [72]. Besides, 7 healthy male volunteers (ages 31.1 ± 1.1) were given an ingestion of 3 mg melatonin, and a marked increase in serum melatonin (3561 ± 1201 pg/mL) was found within 20 min, followed by a gradual decrease, but the level still remained higher than the basal level at 240 min after the ingestion [73].
2.3.3. Benefits of Consuming Melatonin-Containing Foods
Since the secretion of endogenous melatonin decreases after childhood, increasing dietary consumption could be a good option. The studies showed that intake of the food rich in melatonin may gain health impacts by increasing circulating melatonin [24,74,75,76,77]. For instance, when the rats were fed with walnuts (Juglans regia L.) containing melatonin concentrations of 3.5 ± 1.0 ng/g, the increased blood melatonin concentrations and total antioxidant capacity of blood were observed, indicating walnuts could provide beneficial effects as a good food source of melatonin [78]. Additionally, in a study conducted with young, middle-aged and elderly participants (20 ± 10 year-old, 45 ± 10 year-old and 75 ± 10 year-old, respectively), the total antioxidant capacity were reported to significantly increase in the three groups of individuals after the intake of the experimental juice of grape (Vitis vinifera cv. Tempranillo), 200 mL twice a day (as the lunch and dinner desserts) for 5 days [76] as well as the urinary 6-sulfatoxymelatonin, a major metabolite of melatonin commonly used as a biomarker indicating its bioavailability [79]. Moreover, it was found in vivo in 12 healthy male volunteers that the consumption of tropical fruit (banana) or fruit juices (orange and pineapple) significantly increased the serum melatonin concentration and the highest value was observed at 120 min after intake, i.e., compared with before consumption, pineapple with 146 pg/mL versus 48 pg/mL (p = 0.002), orange with 151 pg/mL versus 40 pg/mL (p = 0.005), and banana with 140 pg/mL versus 32 pg/mL (p = 0.008), respectively. Besides, the antioxidant capacity in the serum also markedly increased, suggested by the significant increases in two indicators, i.e., ferric reducing antioxidant power (FRAP) assay and oxygen radical antioxidant capacity (ORAC) [24]. Furthermore, as the germination of legumes significantly increased the melatonin content, it was reported that in Sprague-Dawley rats, the melatonin concentrations in plasma increased by 16% (p < 0.05) after the administration of kidney bean sprout extract via gavage, which correspondingly led to the increase of urinary 6-sulfatoxymelatonin content (p < 0.01), antioxidant capacities did not show significant variation though [80].
2.3.4. Guidance on Regulating Dietary Supplement of Melatonin
Dietary supplement of melatonin could be another option to recompense the physiologically declined activity of pineal gland. Different authorities have published their guidance on regulating the dietary supplements including melatonin. For instance, European Food Safety Authority published the Scientific Opinion on the substantiation of a health claim related to melatonin and reduction of sleep onset latency (ID 1698, 1780, 4080) pursuant to Article 13(1) of Regulation (EC) No. 1924/20061 in 2010. The Panel on Dietetic Products, Nutrition and Allergies considers that “melatonin is sufficiently characterized” and “reduction of sleep onset latency might be a beneficial physiological effect”. In addition, it was also concluded that “a cause and effect relationship has been established between the consumption of melatonin” and “The target population is assumed to be the general population”. Moreover, the dose was also suggested as “In order to obtain the claimed effect, 1 mg of melatonin should be consumed close to bedtime” [81]. While according to the latest updated data (on 22 March 2013) from Food Standards Australia New Zealand, melatonin was listed in the original table 3 on their official website [82], which presents the foods or properties of food from food-health relationships derived from 20 EU approved health claims that will not be added to Standard 1.2.7 and the rationale for exclusion “The claim refers to a dietary supplement.” Additionally, in each of the databases, named “AUSNUT 2011-13 Australian Health Survey (AHS) Dietary Supplement Details” and “AUSNUT 2011-13 Australian National Nutrition Physical Activity Survey (NNPAS) Dietary Supplement Nutrient Database”, only one dietary supplement of melatonin was listed with the same information, i.e., dietary supplement name: Nature’s Care Melatonin; ID 122196; 3 mg, Tablet, uncoated; classification code 33505 and classification sub-group, other supplements [82].
3. Bioactivities of Melatonin
Melatonin has been well known as a potent antioxidant, anti-inflammatory factor and immune modulator. It also contributes to regulating the circadian system [100], possesses tumor inhibitory properties [7], and shows its benefits on cardiovascular functions [101], blood pressure [101], and lipid and glucose metabolism [102] (Table 2).

Table 2.
Bioactivities and potential mechanisms of melatonin.
3.1. Antioxidant Activities
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) play an important role in a variety of physiological processes, such as regulating vascular tone, controlling ventilation, producing erythropoietin and transducing signals [103,104]. Importantly, at a desirable level they can promote cell survival, proliferation and differentiation [105]. However, excessive free radicals could result in DNA and RNA damage, protein denaturation, lipid peroxidation, leading to cell apoptosis or necrosis. Consequently, a series of health problems occur, including aging [106], inflammation [107], cancer [108], chronic metabolic disease [109,110], neurodegenerative disorders [9] and sepsis [111]. Many natural products have shown antioxidant activities and free radical scavenging capabilities [112,113,114,115,116], which could be used to prevent and treat the diseases induced by oxidative stress [117,118,119,120,121].
As a powerful endogenous radical scavenger, melatonin can directly remove the excessive free radicals. In addition, melatonin at 10 mg/kg was found to increase the efficiency of electron transport chain in mitochondria in old mice to lower electron leakage and reduce free radical generation [122]. Therefore, melatonin is essential to keep a stable physiological status in human body. Moreover, it could effectively play a role by modulating and acting synergistically with other reducing molecules like reductases [123] and some non-enzyme reductants [124], all of which work together to maintain normal homeostasis.
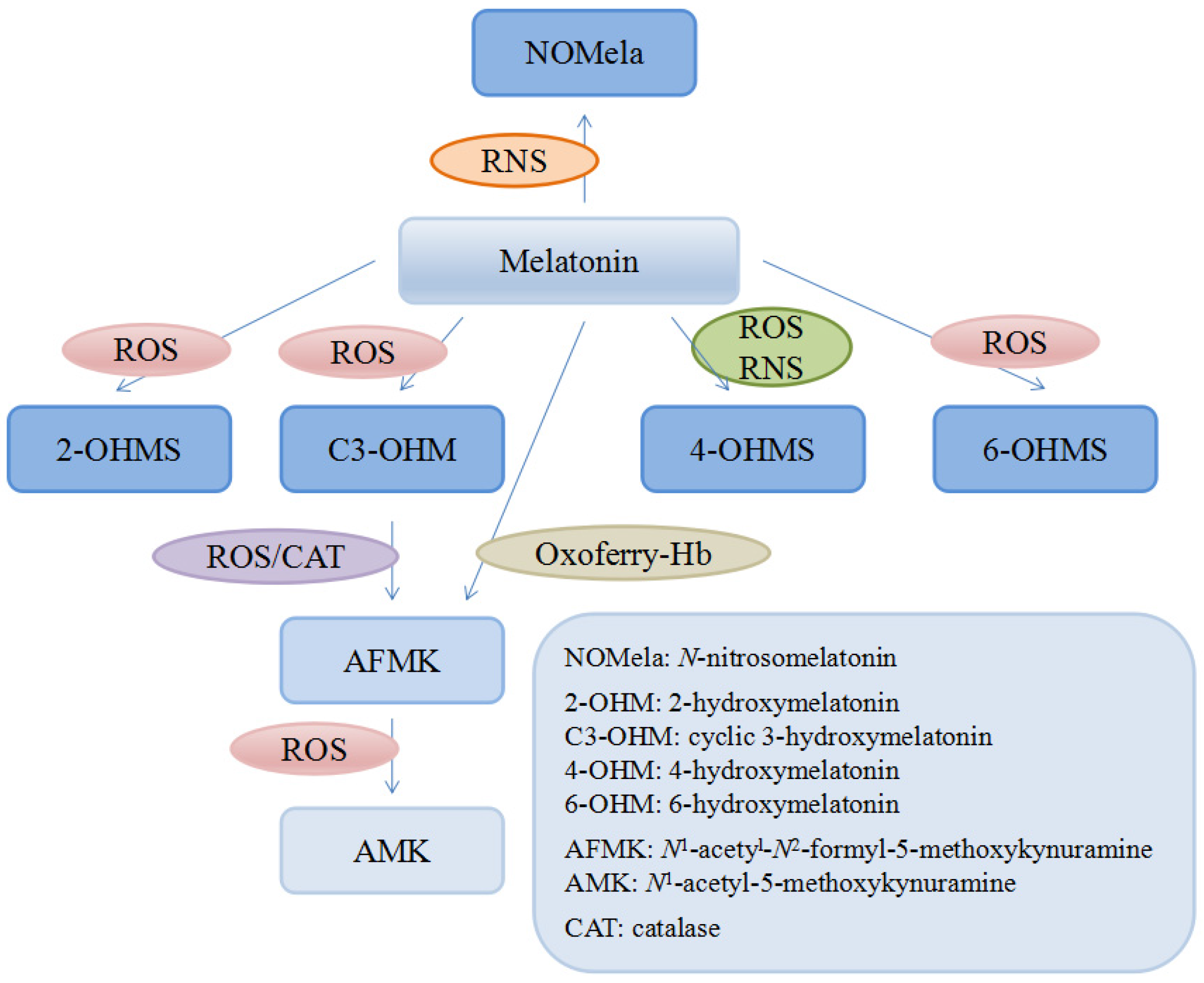
There are many kinds of reductants in human body like glutathione, NADH and vitamin C or E, compared to which melatonin was documented as a stronger antioxidant in eliminating some free radicals both in vitro and in vivo [123,125]. For instance, it was reported that in vitro the ability of melatonin to scavenge the hydroxyl radical (∙OH) was much higher compared with that of vitamin E [123], glutathione and mannitol [126]. Another in vitro study showed that melatonin could significantly inhibit the vasoconstriction induced by H2O2 in the human umbilical artery in a dose-dependent manner [127]. Moreover, it was found that in rats, melatonin was several times more powerful than vitamin C and E in protecting tissues from injuries induced by oxidative stress [125,128]. In addition, quite different from the other antioxidants, the metabolites of melatonin are also actively involved in scavenging free radicals, referred as cascade, though they are the intermediates in the process of reactions against radicals [129] (Figure 2). Interestingly, some of the metabolites are even more potent than its precursor. A good example is that the capacity of 3-hydroxymelatonin (C3-OHM) to reduce hypervalent hemoglobin was higher than that of its basic form and another example was N1-acetyl-5-methoxykynuramine (AMK) showing stronger capability of scavenging ROS and preventing protein oxidation than its precursor [130,131]. Such a profile greatly increases the effectiveness of melatonin as a powerful free radical scavenger. Considering the cascade effects of the metabolites, a melatonin molecule could scavenge up to 10 ROS/RNS molecules [131].
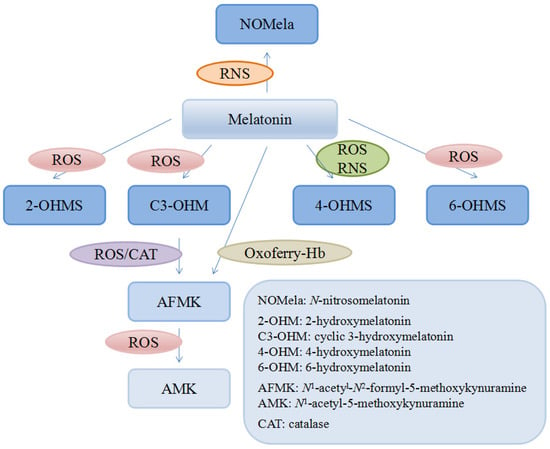
Figure 2.
Melatonin and its metabolites.
Numerous evidence supports that melatonin is a broad-spectrum free radical scavenger [132,133,134]. In addition to ROS/RNS, many other molecules could be modulated or scavenged by melatonin and its metabolites, such as hemoglobin-derived oxoferryl radicals [135]. Furthermore, in vitro and/or in vivo, melatonin is able to chelate toxic metals such as cadmium [136], mercury [137], arsenic [138], lead [139], aluminum [140], chromium [141], which are involved in the generation of free radicals. Moreover, melatonin and its metabolites were also documented to exhibit free radical avoidance properties, by downregulating pro-oxidative enzymes like inducible nitric oxide synthase (iNOS) both in vitro (dose-dependent) and in vivo as well as inhibiting the mRNA expression of cyclo-oxygenase 1 (COX-1) and COX-2 in human breast cancer cells (MCF-7) [142,143].
Melatonin also stimulates the synthesis of other antioxidants. For instance, melatonin was found to induce the expression of gamma-glutamylcysteine synthetase (γ-GCS), the rate-limiting enzyme of GSH synthesis, in human vascular endothelial cells (ECV304) [124]. It was also observed that in 2 neuronal cell lines, melatonin could regulate the expression of antioxidant enzymes (AOEs) at 1 nM under normal conditions, showing the mRNA increase of superoxide dismutase (SOD), glutathione peroxidase (GPx) [144]. Moreover, in vivo and/or in vitro melatonin prevents antioxidant enzymes from oxidative stress, and increases the activities of other redox enzymes, such as catalase (CAT) [145], glutathione reductase (GSH-Rd) [146], and glucose-6-phosphate dehydrogenase (G6PD) [147]. Besides, in vitro melatonin could combine with other antioxidants including vitamin C, vitamin E, glutathione, at the concentration of 2.5–1600 μM, leading to the markedly enhanced protective effects of removing radicals synergistically [148].
3.2. Anti-Inflammatory Activities
Inflammation can commonly occur locally and systematically, caused by many external or intrinsic factors. It has been well documented that the complex interaction of oxidative stress and inflammation results in many diseases. Melatonin could not only reduce the oxidative levels as stated, but also effectively fight against inflammation via different mechanisms such as essential signaling pathways [70,149], the modulation of related genes [150] and the activation membrane receptors [151], as discussed in detail below.
3.2.1. NF-κB Signaling Pathway Involved Mechanisms
Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) is distributed in nearly all the types of animal cells and it is well known to control DNA transcription, cytokine production and cell survival [152,153]. It is an important pro-inflammatory factor that plays a pivotal role in oxidative stress-induced inflammation. In numerous research, melatonin was found to exert anti-inflammatory activity by suppressing NF-κB signaling pathways [70,154,155,156,157]. Melatonin and its metabolites such as AFMK and AMK were able to modulate NF-κB and its downstream pro-inflammatory target genes such as iNOS [70], COX-1 [158], COX-2 [156], tumor necrosis factor α (TNF-α) [159], glia and fibrillary acidic protein (GFAP) [160], which contributed to the pathophysiology of many diseases. In vitro study, melatonin exerted cytoprotective and anti-inflammatory effects on oxidative stress-stimulated human chondrocytes by blocking the activated NF-κB as well as the phosphorylation of phosphatidyl inositol 3-kinase (PI3K)/Akt, p38, extracellular signal-regulated kinase (ERK), Jun N-terminal kinase (JNK), and mitogen-activated protein kinase (MAPK) in a dose- and time-dependent manner [161]. Another in vitro study showed melatonin at 1 nM also performed its anti-inflammatory functions by downregulating chemokine expression via the inhibition of NF-κB, signal transducer and activator of transcription (STAT)1/3 phosphorylation, and gamma-activated sequence (GAS)-driven transcriptional activity in lipopolysaccharide (LPS)-stimulated BV2 murine microglial cell line [162]. Additionally, the protective effect of melatonin on cigarette smoke-induced restenosis in rat carotid arteries after balloon injury was also observed as melatonin inhibited the inflammatory reaction via NF-κB signaling pathways [163]. Moreover, in mice with LPS-induced mastitis, besides the suppression of LPS-induced NF-κB activation, activating peroxisome proliferator-activated receptor gamma (PPAR-γ) was regarded as another underlying anti-inflammatory mechanism of melatonin [164]. A recent study indicated that exogenous melatonin could inhibit inhibitor of nuclear factor kappa-B kinase (IKK)/NF-κB signal transduction pathway in stimulated mast cells (RBL-2H3) to prevent inflammation and the effects were directly related with melatonin concentration used at 100 nM and 1 mM, based on which melatonin was implied to be used for the treatment of allergic inflammatory diseases [165].
3.2.2. SIRT1 Pathway Involved Mechanisms
It was suggested that human sirtuins may function as intracellular regulatory proteins with mono-ADP-ribosyl transferase activity, and sirtuin 1 (SIRT1), also known as NAD-dependent deacetylase, was reported to improve insulin sensitivity, as it could affect the activity of both the estrogen-related receptor alpha (ERR-α) and the peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α), which are essential metabolic regulatory transcription factors [149]. Specifically, SIRT1 was able to independently enhance ERR-α and modulate the effects of PGC-1α repression of glycolytic genes [166,167]. In a rabbit model with osteoarthritis (OA), intra-articular injection of melatonin at 20 mg/kg significantly reduced cartilage degradation, which was reversed by sirtinol, indicating SIRT1 pathway was involved [161]. Additionally, both in vitro and in vivo studies showed that melatonin reduced LPS-induced oxidative stress damage, acute neuroinflammation, and apoptotic neurodegeneration via SIRT1/Nrf2 (nuclear factor-erythroid 2-related factor 2) signaling pathway activation [168].
In addition to the pathways mentioned above, the anti-inflammatory effects of melatonin were also observed as it regulated the expression of some pro-inflammatory genes [150]. Moreover, it was found that melatonin could inhibit the expression of inflammatory chemokines/cytokines, i.e., chemokine (C-X-C motif) ligand 1 (CXCL1), chemokine (C-C motif) ligand 20 (CCL20), and interleukin 6 (IL-6) that was mediated by IL-17 and enhanced by increased insulin and insulin-like growth factor 1 (IGF-1) in the prostatic tissues of obese mouse through a glycogen synthase kinase 3β (GSK3β)-dependent mechanism [169]. Another study found that melatonin at 10 μM could show its anti-inflammatory impacts time-dependently by inducing temporal up-regulation of gene expression related to ubiquitin/proteasome system (UPS) in the human malaria parasite Plasmodium falciparum [170]. Additionally, it was reported that melatonin (10 mg/kg, intraperitoneally, i.p.) attenuated colitis with sleep deprivation in mice by downregulating mRNA of E2F transcription factor (E2F2) and histocompatibility class II antigen A, beta 1 (H2-Aβ1), indicating its clinical potential for patients with inflammatory bowel disease, particularly those suffering from sleep disturbances [171]. Furthermore, melatonin was reported to reduce intestinal ischemia-reperfusion-induced lung injury in rats dose-dependently by activating the expression of N-myc downstream-regulated gene 2 (NDRG2), which was involved in cellular differentiation, development, anti-apoptosis, anti-inflammatory cytokine, and antioxidant [172]. However, it should be pointed out that those results were from different animal models. If the same animal model was used, different results might be observed.
Besides, it has been reported that melatonin receptors were involved in its anti-inflammatory mechanism. It was found that melatonin played a role in maintaining the pro- and anti- inflammatory balance during infection by influencing leukocyte migration and apoptosis in carp possibly mediated by melatonin MT1 receptors in/on leukocytes [151].
3.3. Enhancing Immune Activities
Recently, numerous experimental evidence has shown that melatonin is involved in the interaction between the nervous, endocrine, and immune systems on the basis of the research on surgical or functional pinealectomy as well as the association between melatonin production and circadian and seasonal rhythms in the immune system [173,174,175]. In these three interactive systems, the reciprocal regulation exists. For instance, the immunological parameters such as TNF-α [176], IL-12 [177], interferon gamma (IFN-γ) [178], granulocyte colony-stimulating factor (G-CSF) and granulocyte–macrophage colony-stimulating factor (GM-CSF) [179] can affect the function of pineal gland. In addition, NF-κB could be active during pathogen invasion and melatonin synthesis could be promoted in macrophages while inhibited in pinealocytes. Therefore, immune-pineal axis has been defined as the shift in the melatonin production from pinealocytes to immune competent cells mediated by NF-κB [180,181]. Furthermore, melatonin exerted the neuroimmunomodulatory effect on the immune system via its membrane receptors, which have been identified in immune organs, tissues, bone marrow mononuclear cells (BMMNCs) and leukocytes, and even subcellular compartments [180,182]. With MT1 and MT2 receptors, melatonin was found to inhibit the production of forskolin-stimulated cyclic AMP (cAMP), cyclic GMP (cGMP) and diacylglycerol (DAG), leading to the improved immunity [183,184]. Besides, the specific nuclear melatonin receptors have been identified in Jurkat cells [185], lymphocytes [186], thymus [175] and spleen [187], which belong to the RZR/ROR subfamily of nuclear receptors.
It was observed that melatonin could reverse the weight loss of thymuses [175] and spleens [188] in different pinealectomized animal models and melatonin could increase tonsillar size [189], indicating the protective effects of melatonin on the immune organs. Besides, melatonin and its metabolites like AFMK were found to improve the proliferation, increase the activity and inhibit apoptosis of immune competent cells such as monocyte [190], natural killer (NK) cells [191] and neutrophils [192]. Melatonin could act on the membrane receptors MT1 and MT2 and increase the sensitivity of the immune cells to some cytokines such as TNF-α and IFN-γ in vivo at the dose of 10 mg/kg orally administrated [193]. Furthermore, Ghosh et al. found that melatonin could restored the suppressed immunity of T-cell culture in vitro, indicating melatonin might be valuable in regulating immunity via the functional interactions with gonadal steroid by developing some hormonal microcircuit (gonadal steroid and melatonin) in lymphatic organs [194]. Moreover, melatonin was able to modulate immune mediator production, e.g., increased IL-2, IFN-γ and IL-6 in monocytes [195] in cultured human mononuclear cells, decreased IL-8 and TNF-α in neutrophils [192], decreased IL-1β, IL-6, IL-8, IL-10 and TNF-α in macrophages in RAW264.7 cells [196]. This profile is of great importance as some cytokines have been shown to interact with immune cells and promote their growth, differentiation, activation, and survival. Besides the endocrine actions from the pineal melatonin, the melatonin synthesized in immune system could exhibit direct immunomodulatory effects by means of nonendocrine actions, including intra-, auto-, and/or paracrine actions via its membrane and/or nuclear receptors [197], which were crucial for human lymphocytes to generate an accurate response by modulating the IL-2/IL-2R system [198].
Melatonin was reported to regulate the ROS production in the essential immune cells such as monocytes [199] and neutrophils [200]. Moreover, melatonin was found to augment the general immunity by attenuating oxidative load associated with age in hamsters by 25 μg/100 g body weight for 30 days [201]. It was reported that melatonin could alleviate oxidative damage and suppress the immune status induced by stressful factors via its membrane receptor expression MT1 and MT2 in wild birds [202]. Due to its anti-inflammatory properties, melatonin could suppress systemic innate immune activation during sepsis in mice both in vivo and in vitro by blocking the NF-κB/NOD-like receptor P3 (NLRP3) connection through a sirtuin1-dependent pathway [154].
Collectively, it has been well documented that melatonin played a fundamental role in resisting harmful invasion and enhancing immunity as a member of the complex neuro–endocrine–immunological system.
3.4. Improving Circadian Rhythm and Sleep
It was estimated that about one-third of the general population is suffering from sleep disorders, mainly insomnia, and there is an increasing trend because of the more stressful working conditions and the progressive aging of society [203]. Circadian rhythms are common in nature and have been widely observed in cyanobacteria, fungi, plants, and animals [204]. Disturbance of circadian rhythms can cause many diseases like cancers [205], metabolic syndromes [206], reproductive diseases [207] etc. Disruption of circadian rhythms and sleep disorders are prevalent, which can do harm to patients’ overall health, worsen existing comorbidities and result in impaired quality of life, whereas melatonin could modulate circadian rhythm and improve sleep disorders [100,208,209,210].
In mammals, most of the physiological processes and behaviors are regulated by a network of circadian clocks. The circadian system consists of a central rhythm generator, the suprachiasmatic nucleus (SCN), and several peripheral oscillators [211]. Besides, the central clock can control the production of melatonin, which could modify the peripheral clocks and inversely alter the expression of circadian clock genes. Johnston et al. [212] found that rising melatonin levels could reset circadian rhythms in the mammalian pars tuberalis. Melatonin has long been known to help prevent and treat jet lag, a typical example of disrupted circadian rhythms often caused by travelling [2].
The animal models of melatonin-proficient (C3H) and melatonin-deficient (C57BL) mice are frequently used to study the role of melatonin on circadian rhythms. In a study, research on three clock gene proteins PER1, BMAL1 and CRY2 in the murine adrenal cortex and medulla was conducted and the results showed that in C3H mice, PER1 and CRY2 maximized in the middle of the light phase, whereas BMAL1 reached its peak in the dark phase and these three clock gene proteins levels displayed day/night variation in both the adrenal cortex and medulla. Similar patterns were revealed in the adrenal medulla of C57BL mice, but in the adrenal cortex of C57BL mice, clock gene protein levels were consistently lower than in C3H mice and did not change with time [211]. In another study, the modulatory effects on clock gene expression of melatonin was investigated in the retina of those two groups of mice, and the results demonstrated that melatonin functioned via post-transcriptional mechanisms and also played a role in rhythmic regulation of phosphorylated cAMP response element-binding protein (pCREB) levels in the mammalian retina [213].
As an external trigger of melatonin production, light, blue-enriched light in particular, was observed to significantly suppress the nocturnal increase in endogenous melatonin levels in human, indicating that a clock gene polymorphism could modulate light sensitivity in humans, especially in the individuals, who are homozygous for the PER3 5/5 allele [214]. Additionally, it was observed that melatonin activated Npas4, which drove the clock gene CRY1 responses to melatonin in vivo [215]. Furthermore, Bracci et al. [216] reported that alterations in peripheral clock gene expression, i.e., a significantly higher expression of BMAL1, CLOCK, NPAS2, PER1, PER2, and REVERBα and a lower expression of PER3, CRY1 and CRY2, were found at the beginning of the morning shift after a day off in rotating shift work nurses as well as significantly higher 17-β-estradiol levels compared to daytime nurses. Another study reported that both Period1 and BMAL1 expression increased in the hippocampus of Siberian hamsters after acute melatonin treatment of 20 μg/day, accompanied by the alterations of dendritic morphology, indicating melatonin could act as a signal to coordinate the circadian rhythm in neuronal remodeling [217]. Additionally, melatonin was found to adjust the expression pattern of clock genes (per1, per2, bmal1 and clock) in the SCN mainly by increasing amplitude in their expressional rhythms without inducing robust phase shifts in them. In addition, melatonin could alter the expression of genes of serotonergic neurotransmission (tph2, sert, vmat2 and 5ht1a) in the dorsal raphe and serotonin contents in the amygdala, and improve the depression-like behavior in C57BL/6J mice with seasonal affective disorder [218].
Melatonin has been known as the ‘hormone of darkness’ as its synthesis and secretion are controlled by light/dark cycles, i.e., its production decreases during daytime and increases at night. The decreased nocturnal plasma melatonin levels were found in patients with long-lasting insomniac complaints, indicating circadian rhythm dysfunctions [219]. Additionally, it was reported that sleep parameters were positively correlated with melatonin secretion in patients with insomnia in coronary care unit [220]. Furthermore, melatonin was found to regulate sleep by activating receptor MT1, MT2 and enhancing the excitability of medial lateral habenula (MLHb) neurons in rats [221].
Besides, a number of studies have shown that melatonin and melatoninergic agents were effective in the treatment of insomnia, as it could accelerate sleep initiation, increase sleep duration and slightly alter sleep architecture [222,223]. In addition, due to the short half-life of melatonin in circulation, a prolonged-release melatonin medicine (Circadin®) [224], melatonin derivatives (e.g., ramelteon) [203], another melatonin agonists (e.g., agomelatine) [225,226] have been approved by authorities in the EU and USA to treat insomnia and resynchronize circadian rhythms.
3.5. Anticancer Activities
According to the World Cancer Report 2014 from WHO, cancers are among the leading causes of morbidity and mortality all around the world, with approximately 14 million new cases and 8.2 million cancer related deaths in 2012 and the number of new cases is expected to rise by about 70% over the next 2 decades [227]. Many foods were found to contain natural components with strong anticancer activities, indicating the potential for the prevention and treatment of different cancers [7,228,229,230,231].
Melatonin has been proved to be highly involved in the etiology, development, metabolism, metastasis, and therapy of different subsets of tumors. The mechanisms include inhibiting tumor cell growth and proliferation [232], modulating the metabolism of tumor cells [233], promoting apoptosis [234], exerting antimetastatic and antiangiogenic effects [235], enhancing the sensitivities to the anticancer drugs, attenuating the side effects of radio- and chemotherapies [157]. In addition, mechanisms of melatonin impacts on cancers comprise the actions of melatonin receptors MT1 and MT2, the regulation of relevant genes expression and the modulation of some signaling pathways [236,237].
3.5.1. Effects on Tumor Cell Cycle, in Terms of Growth, Proliferation, Metabolism and Apoptosis
A study conducted by Wu et al. [232] demonstrated that both in vitro and in vivo, melatonin inhibited gastric tumor growth and peritoneal dissemination through the activation of endoplasmic reticulum (ER) stress and the inhibition of epithelial mesenchymal transition (EMT) via calpain-mediated C/enhancer-binding protein beta (EBPβ) and NF-κB cleavage. Another study demonstrated that melatonin could suppress breast cancer cell proliferation by exhibiting an anti-aromatase effect on hormonal positive breast cancer cells through the selective estrogen enzyme modulators (SEEMs) mechanism [238]. Furthermore, Hevia et al. reported that melatonin (1 mM) reduced glucose uptake and modified the expression of GLUT1 transporter in prostate cancer cells, resulting the attenuated glucose-induced tumor progression and the prolonged lifespan of tumor-bearing mice [233]. Moreover, Sohn et al. reported that the antiangiogenic properties of melatonin (1 mM) in hypoxic PC3 prostate cancer cells were mediated by enhancing the expression of miRNA3195 and miRNA374b [239]. Melatonin at pharmacological concentrations was able to significantly induce apoptosis of colorectal cancer LoVo cells in a dose-dependent manner via histone deacetylase 4 (HDAC4) nuclear import and decreasing H3 acetylation on Bcl-2 promoter, resulting in reduced Bcl-2 expression, which were mediated by inactivating Ca2+/calmodulin-dependent protein kinase II alpha (CaMKIIα) [240]. Besides, melatonin was found to enhance arsenic trioxide-induced apoptotic cell death at the dose of 2 mM by sustainably upregulating Redd1 expression and inhibiting mTORC1 upstream of the activated p38/JNK pathways in human breast cancer cells [234].
3.5.2. Effects on Invasion and Metastasis of Tumor Cells
Melatonin (1 mM) could modulate motility and invasiveness of HepG2 cell in vitro through the upregulation of tissue inhibitor of metalloproteinases 1 (TIMP-1) and the attenuation of matrix metalloproteinase-9 (MMP-9) expression and activity by inhibiting NF-κB signaling pathway [241]. Melatonin was found to suppress the transactivation of MMP-9 and the metastasis of renal cell carcinoma (the most lethal of all urological malignant tumors with the potent metastasis potential) via the inhibition of Akt-MAPKs pathway and NF-κB DNA-binding activity [242]. Additionally, melatonin showed its oncostatic, antimetastatic and antiangiogenic effects in breast cancer both in vitro and in vivo by blocking proliferation of tumor cells and inhibiting the expression of Rho-associated kinase protein (ROCK-1), one of the regulatory and effector molecules in charge of migration/invasion, which could promote tumor growth and metastasis when its expression was increased [235].
3.5.3. Therapy Adjunct in Tumor Treatment
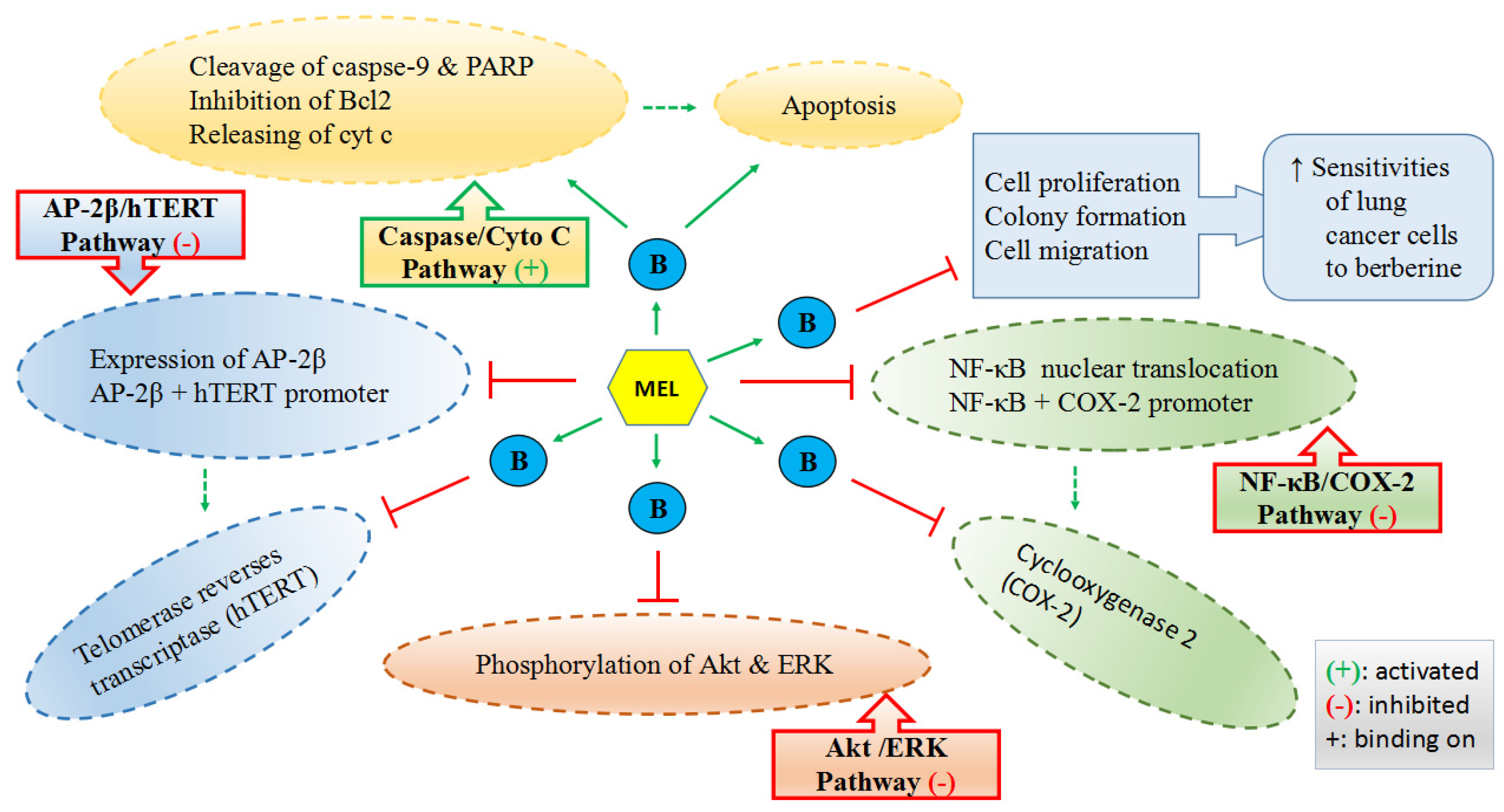
Lu et al. reported that melatonin could enhance the antitumor activity of berberine (B in Figure 3) in lung cancer cells by activating caspase/cytochrome c (cyt c) and inhibiting activator protein 2β (AP-2β)/human telomerase reserve transcriptase (hTERT), NF-κB/COX-2 and Akt/ERK signaling pathways as well as increasing the sensitivities of lung cancer cells to berberine (Figure 3) [157]. Alonso-Gonzalez et al. pointed out that the pretreatment of 1 nM melatonin before radiation could elevate the sensitivity of human breast cancer cells to radiotherapy by reducing the active estrogens levels in cancer cells through the increased p53 expression [243]. It was also found that melatonin-based creams could significantly lower the occurrence of acute radiation dermatitis in a double-blind randomized trial [244]. It was revealed that melatonin, as a powerful antioxidant, played a protective role at 10 mg/kg in rats to alleviate the testicular dysfunction induced by chemotherapy against testicular cancer [245], which is one of the most common cancers in men of reproductive age with a steadily increasing incidence [227]. Another in vivo study reported that melatonin (25 mg/kg) could synergize the chemotherapeutic effect of 5-fluorouracil (one of the most commonly used chemotherapeutic agents to treat colon cancer) in mice with colon cancer, by promoting the activation of the caspase/poly-ADP-ribose polymerase (PARP)-dependent apoptosis pathway, inhibiting PI3K/AKT and NF-κB/iNOS signaling pathways. The results of the same study also demonstrated that melatonin significantly enhanced the 5-fluorouracil-mediated inhibition of cell growth and metastasis of colon cancer cells [70]. Additionally, a meta-analysis of randomized controlled trials reported that melatonin, as an adjuvant, could substantially reduce the side effects caused by radiochemotherapy, presenting the improved tumor remission and the increased 1-year survival [246].
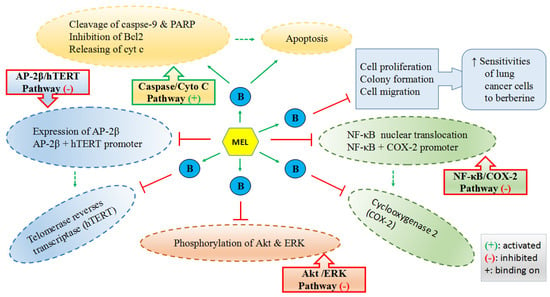
Figure 3.
Mechanisms of melatonin enhancing the sensitivities of lung cancer cells to berberine (B).
It was also observed that MT1 and MT2 mRNA expression levels increased markedly in vitro and in samples from cancer patients, indicating that the receptor-mediated mechanisms were involved in the anticancer activities of melatonin [236,237].
3.6. Cardiovascular Protection
Cardiovascular diseases (CVDs) are the No. 1 cause of death worldwide, which accounted for 31% of all global deaths in 2012, i.e., an estimated 17.5 million people died from CVDs [247]. It has been found that melatonin is involved in the pathophysiological process of CVDs, such as plaque formation, atherosclerosis, and infarction. Melatonin was reported to regulate platelet physiology [248], protect the vascular endothelium [249], regulate lipid and glucose metabolism and modulate blood pressure [250], by reducing atherosclerosis, inhibiting thrombosis, which contribute to prevent CVDs or facilitate the restoration of morphology and functions of heart and vessels after injury based on reducing oxidative stress, inhibiting inflammation, decreasing apoptosis and preventing postinfarction remodeling of cardiomyocyte [251].
It was reported that melatonin could reduce heart rate and blood pressure, regulate the cardiac rhythm and the vascular tone via neurohumoral regulation in which the antioxidant capacity was involved [252,253]. Moreover, melatonin has been reported to protect cardiomyocytes and blood vessels against fibrosis necrosis and vasculitis in radiation-induced heart disease in rats when administrated at 50 mg/kg [254]. Furthermore, melatonin was demonstrated to dose-dependently reduce flow shear stress-induced injury in bone marrow mesenchymal stem cells (BM-MSCs) through the activation of melatonin receptors and adenosine monophosphate activated protein kinase (AMPK)/ACC (acetyl-CoA carboxylase) signaling, which could benefit the patients with valvular heart disease [255].
Melatonin was proved to increase the survival of adipose-derived mesenchymal stem cells in infarcted heart in mice at 20 mg/kg by activating SIRT1 signaling [256]. Besides, melatonin was found to significantly reduce the infarct size in myocardial ischemia-reperfusion injury (IRI) and decrease apoptosis through the activation of Janus kinase 2 (JAK2)/STAT3 signaling and PI3K/Akt signaling [257,258]. Additionally, it was reported that melatonin at 5 μM could improve the cardiac regularity in Drosophila melanogaster without affecting heart rate, possibly via a specific G-protein-coupled receptor which is encoded by the CG 4313 gene and considered to be a candidate melatonin receptor, indicating melatonin might play an essential role in cardiac pacemaking [259]. Besides, melatonin was closely related with reverse remodeling after cardiac resynchronization therapy in patients with heart failure and ventricular dyssynchrony [260]. Furthermore, it was observed that melatonin could markedly improve cardiac dysfunction, mitigate cardiac remodeling after myocardial infarction, increase the level of autophagy, attenuate apoptosis, regulate the integrity and restore the function of mitochondria through the inhibition of mammalian Ste20-like kinase 1 (Mst1) and the promotion of SIRT1, indicating the Mst1/SIRT1 signaling was involved [261].
Melatonin has also been found to modulate blood pressure and protect the organs and tissues damaged by hypertension. As reported by WHO, hypertension is one of the leading risk factors for CVDs and it caused 9.4 million deaths in 2010. The worldwide prevalence of hypertension in adults aged 18 years and over was around 22% in 2014 [262]. Melatonin could reduce blood pressure, pulsatility index in the internal carotid artery and catecholamines, indicating melatonin was involved in the renin-angiotensin-aldosterone system, which plays an essential role in blood pressure regulation [250]. In addition, it has been demonstrated that melatonin could regulate blood pressure both experimentally and clinically [263,264]. Hung et al. reported that melatonin was able to improve endothelial dysfunction, suppress vascular inflammation, and ameliorate systemic hypertension in rats on the basis of antioxidant and anti-inflammatory properties [265]. In addition, it was observed that melatonin could mitigate renal injury induced by hypertension by reducing oxidative stress [266,267]. It was demonstrated that melatonin could attenuate intracranial hypertension by reducing cerebral edema via the anti-inflammatory mechanism [268]. Besides, melatonin exerted the regulatory effects on blood pressure due to the interaction with both cardiovascular system and the central nerve system (CNS), by restoring the balance between the sympathetic and parasympathetic vegetative system in favor with the latter [267]. Furthermore, melatonin could even reduce fetal blood pressure in ovine fetus via the membrane receptors MT1 and MT2, possibly by releasing endothelin [269].
Several studies showed that melatonin could reduce the levels of blood lipid, another risk factor for CVDs. Dyslipidemia is commonly accompanied with other disorders related to metabolic syndromes. Melatonin has been observed to effectively improve dysplipidemia by reducing LDL cholesterol levels, total cholesterol and triglycerides, but increase HDL-C, glucose tolerance and antioxidant potency due to its potent antioxidant effects [270,271,272].
3.7. Anti-Diabetic Activities
According to the WHO, an estimated 1.5 million deaths were directly caused by diabetes and another 2.2 million deaths were attributable to high blood glucose in 2012. Diabetes prevalence has been rising more rapidly, and there were 422 million diabetic people in 2014. Diabetes can cause severe complications leading to disability and death, and diabetes will be the 7th leading cause of death in 2030, as estimated [273]. It has been investigated that melatonin was able to prevent the complications by attenuating glucotoxicity to the organs and tissues mainly because of its antioxidant, anti-inflammatory and antiapoptotic effects.
Melatonin was found to increase the inhibited activity of catalase in rat liver cells and restore the dysfunctional mitochondria related to diabetes at the dose of 10 mg/kg, indicating melatonin could be a beneficial option to treat diabetes [274]. It was also shown that melatonin could improve dysglycemia in rats through the inhibition of hepatic gluconeogenesis and the activation of hypothalamic Akt via membrane receptors MT1 and MT2 [275]. Another study in rats with 10 mg/kg revealed that chronic melatonin administration at pharmacological doses was able to increases Ca2+ levels in lots of organs and tissues, such as liver, pancreas, muscle and white adipose tissues, resulting in the improved insulin sensitivity and secretion, indicating the potential clinical use of melatonin against type 2 diabetes [11]. In addition, melatonin showed its protective capability on myocardial cells in type 2 diabetic rats by reducing oxidative stress and ER stress (induced by the elevated blood glucose) through the activation of SIRT1 signaling pathway and inactivation of protein kinase-like endoplasmic reticulum kinase (PERK)/eukaryotic translation initiation factor 2 (eIF2α)/ATF4 (activating transcription factor 4) signaling pathway [276]. Besides, melatonin could prevent pancreatic islet failure caused by β-cell loss and dysfunction in type 2 diabetes, by attenuating β-cell apoptosis, improving its function, and prolonging its survival through the activation of β-cell melatonin signaling [277]. Recently, melatonin could improve the impaired memory caused by diabetes at 10 mg/kg in rats by improving neurogenesis, synaptogenesis in hippocampi, increasing the receptors of melatonin and insulin, and restoring the downstream signaling pathway for insulin [278]. Additionally, it was found that melatonin (250 μg, i.p.) could accelerate bone healing in rats with diabetes due to its antioxidant properties [279]. Furthermore, melatonin was also observed to restore the endothelial dysfunction and improve vascular responses in diabetic rats with 10 mg/kg administration [271].
3.8. Anti-Obese Activities
Data from WHO shows that more than 39% of adults (1.9 billion) were overweight and over 13% (600 million) were obese in 2014; meanwhile, 41 million children under the age of 5 were overweight or obese [280]. Obesity is also a strong risk factor for other metabolic diseases. Although obesity is a result of imbalance of energy intake and expenditure, many other factors are involved in and contribute to the obese conditions, such as chronic inflammation, oxidative stress, circadian disruption and sleep deprivation [281]. Melatonin has been reported to attenuate the damage caused by obesity.
Melatonin could play an important role in energy metabolism in obese mice based on gene modulatory effects, antioxidant and anti-inflammatory capacity. An in vivo study demonstrated that melatonin at 10 mg/kg could induce white adipose tissue browning in rats with obesity-related type 2 diabetes by increasing the uncoupling protein 1 (UCP1) and PGC-1α, the thermogenic proteins, which possibly provided the reasons why melatonin was considered as a contributor to control body weight without effects on food intake and physical activity levels [282]. Additionally, melatonin was observed to benefit homeostasis of renal glutathione which was overproduced due to oxidative stress in obese rats [283]. Moreover, melatonin could regulate the cytokines such as IL-8, IL-10, IFN-γ, and inducible protein 10, which were considered as predictors of overweight and obesity [284]. It was also observed that insulin, insulin-like growth factor 1 (IGF1), and IL-17 increased in obesity. In addition, IL-17 alone or combined with insulin could promote CXCL1 and CCL20 expression, which could be suppressed by melatonin at 10 nM through inhibiting Akt activation and increasing GSK3β activity [169]. Since the adipocytes produce adipokines, the increase of TNF-α, resistin, and visfatin was found under obese conditions in mice, which melatonin could suppress to some degree when used at 100 mg/kg, indicating the beneficial impact of melatonin to control obesity [285].
It has been reported that that both clock genes [286] and metabolic genes [287] were identified in adipose tissues, which could regulate lipid generation and metabolism, adipocyte proliferation and differentiation, and adipose endocrine functions. Moreover, a cohort study of women aged 16 and above in the UK revealed that light at night (LAN) was significantly associated with obesity, indicating melatonin was involved in the development of obesity due to its regulatory effects on sleep and circadian rhythms [288]. Furthermore, melatonin could promote circadian rhythm-mediated proliferation in adipose tissue in mice at 20 mg/kg by increasing the expression of adipocyte proliferation genes via a complex of Clock/histone deacetylase 3 (HDAC3)/c-Myc [206].
Melatonin was also reported to prevent the harmful effects induced by obesity on the main functional organs to reduce the complications of obesity. It was found that melatonin at 4 mg/kg could exhibit the cardioprotective effects in rats with diet-induced obesity as shown by the decreased myocardial infarct sizes and insulin resistant, and the increased serum adiponectin, protein kinase B (PKB)/Akt, ERK42/44, GSK-3β and STAT3 without affecting the body weight or visceral adiposity [289]. Melatonin could also prevent renal failure at the dose of 100 mg/kg through improving the morphology and the functions of renal convoluted tubules in obese mice, by increasing mitofusin-2 expression, an apoptosis modulator [290]. In addition, when used at 10 mg/kg, melatonin was proved to markedly improve non-alcoholic liver steatosis in mice with obesity induced by high fat diet, presenting the decreased TNF-α, IL-1β, IL-6, and phosphorylation of p38 and JNK1/2, indicating the MAPK-JNK/p38 signaling pathway was involved [291].
3.9. Neuroprotective Activities
Melatonin was found to exhibit the neuroprotective effects on CNS [292]. In a mouse model of traumatic brain injury (TBI), melatonin was administered by 10 mg/kg i.v. at 0, 1, 2, 3, and 4 h post-TBI, the pathological alternations were significantly ameliorated, such as cortical neuronal degeneration and brain edema, mainly due to its antioxidant property through the Nrf2-ARE (antioxidant responsive element) pathway [293]. It was also reported that melatonin could prevent the adult mice brain from IRI at the dose of 10 mg/kg, administrated intraperitoneally, twice (immediately after induction of ischemia and at reperfusion onset). The suggested underling mechanism was to restore the function of mitochondrial by activating SIRT1 signaling pathway [294]. Another research in rats with early brain injury following a hemorrhagic stroke, in particular subarachnoid hemorrhage (SAH), found that melatonin (150 mg/kg, i.p.) could markedly alleviate brain edema, restore the integrity of blood-brain barrier (BBB) by suppressing cortical expressions of proinflammatory cytokines (e.g., IL-1β, IL-6, and TNF-α) [295]. Besides, it was observed in mice that lower dose of melatonin (5 mg/kg, i.p.) could also reduce the injuries in both gray and white matter and better the neurobehavioral outcomes induced by transient focal cerebral ischemia as a potent radical scavenger [296]. Furthermore, melatonin (10 mg/kg) was found to exhibit the neuroprotective and anti-apoptotic effects on oxidative brain damage induced by hemolytic hyperbilirubinemia in newborn Sprague-Dawley rats by reversing the increased plasma TNF-α, IL-1β levels, as well as the decreased brain derived neurotrophic factor (BDNF), S100 calcium-binding protein B (S100B) and IL-10 values [297].
The benefits that melatonin could provide to the peripheral nerve have also been widely investigated. It was found that the diclofenac sodium (DS) exposure during pregnancy could damage the fetal peripheral nerve system in rats, which could be improved by melatonin at doses of 10 and 50 mg/kg, showing the significant increase in the axon numbers of right sciatic nerve in male newborn rats with both of the doses administrated. Moreover, the significant increase in the diameter of the axons was found only with high dose of melatonin [298]. Additionally, optic neuritis (ON) might induce permanent vision loss, characterized by inflammation, demyelination, and neurodegeneration of the optic nerve. Melatonin showed its preventive effects by subcutaneous pellet of 20 mg in adult male Wistar rats with ON induced by LPS. The mechanism might be preventing the decrease in visual evoked potentials (VEPs) and pupil light reflex (PLR), inhibiting microglial reactivity, astrocytosis, demyelination, and axon and retinal ganglion cell loss and preserving anterograde transport of cholera toxin β-subunit from the retina to the superior colliculus. In the same study, the therapeutical effects of melatonin on ON was also observed as it reversed the decrease in VEPs and PLR completely when the pellet was implanted at 4 days postinjection of LPS [299].
Since reduced melatonin concentrations were identified in chronic neurodegenerative disease, such as Alzheimer’s disease (AD) and Parkinson’s disease (PD), exogenous melatonin treatment showed excellent neuroprotective effects as melatonin is a member of neuroimmuno-endocrine regulator family, which is involved in circadian rhythm, immune and redox actions and also attributing to its potent capacity of anti-apoptosis, and anti-inflammation [300,301]. It was found that the decreased melatonin concentrations in blood were significantly correlated to the loss of hypothalamic gray matter volume and disease severity in PD patients [302]. In addition, it was reported that the melatonin at 4 mM could improve sleep disorders and synaptic dysfunction in Drosophila caused by human leucine-rich repeat kinase 2 (hLRRK2), the most common genetic factor of PD [303]. For AD, amyloid-beta (Aβ) is one of the key pathological factors, which could initiate of the cognitive symptoms of dementia and its aggregation could result in synaptic loss, inflammation and cell death [304]. It was reported that melatonin administration at 10 mg/kg could significantly restore mRNA and protein levels of β-APP-cleaving enzyme 1 (BACE1) and presenilin 1 (PS1) in aged mice [305]. In addition, melatonin (500 mg/kg in 25% ethanol, i.p.) was found to improve the injured spatial learning and memory induced by Aβ1–42, restore the synaptic plasticity, and attenuate astrogliosis in rat. In addition, the in vitro study in primary hippocampal neuron demonstrated that melatonin (50 μM) exhibited the neuroprotective effects against Aβ1–42 through the Musashi1/Notch1/Hairy and enhancer of split 1(Hes1) signaling pathway [301].
Besides, it is quite common that nerve injuries can also be induced by medications, toxic chemicals, irradiation or other causes. Oxaliplatin (Oxa) can cause neurotoxicity as a third-era platinum-based chemotherapy against colorectal cancers. The outcomes of 10 mg/kg i.p. melatonin pretreament in rats followed by Oxa injections (4 mg/kg, i.p.) showed that the motor activity and muscular strength were improved, presenting that the inactivation of Bcl-2, caspase 3 apoptotic protein and cyt c release were modulated as well as the non-enzymatic, enzymatic antioxidants and complex enzymes of mitochondria [306]. Additionally, melatonin was reported to prevent neurotoxicity induced by cadmium in mouse neuroblastoma cells, via the activation of transcription factor EB-dependent autophagy-lysosome [136]. Moreover, the beneficial effects of melatonin against irradiation was observed after melatonin was administered at 100 mg/kg in male Sprague-Dawley rats, due to the caspase-3 inhibition [307]. In addition, melatonin (50 mg/kg) was found to prevent the neuronal damage in the hippocampus in pregnant rats caused by 900 MHz electromagnetic fields (EMF), which might be associated with the use of mobile phones [308]. Furthermore, it has been suggested that if the N-acetyl group of melatonin was removed, its antioxidant and neuroprotective properties would be improved at the expense of toxic methamphetamine-like effects in several cell lines such as HT22 cells [309].
3.10. Other Bioactivities
Melatonin and its receptors are widely distributed in the body, which were identified in numerous organs, tissues, cells and subcellular compartment, such as brain, lung, muscles, bones, renal cells, reproductive cells, mitochondria and nuclei. It has been well documented that melatonin exerts beneficial effects on a large variety of diseases [310]. In terms of bones and muscles, it was suggested that melatonin supplementation of 10 mg/kg could counteract age-related bone loss in rats by improving the microstructure and biomechanical properties of aged bones [311]. Melatonin was also observed to improve muscle function in the animal model of Duchenne muscular dystrophy by causing the dystrophic muscle to contract and relax faster via decreasing plasma creatine kinase activity, providing a better redox status of the muscle [312], and normalizing plasma pro-inflammatory cytokines [150]. In addition, some in vitro study suggested that melatonin could represent a potential therapeutic impact on chronic respiratory diseases, e.g., asthma and chronic obstructive pulmonary diseases, in a dose-dependent manner, by inhibiting mucin 5AC production via the suppression of MAPK signaling in human airway epithelial cells [313]. Besides, a number of studies using rat models showed that melatonin could not only provide protection against acute kidney injury and partially reverse it [314], but also exert its protective effects on chronic kidney disease by scavenging free radicals [315], attenuating the chronic inflammation and limiting apoptosis [316]. Furthermore, an in vitro study found that melatonin pretreatment (100 μM) of human adipose tissue-derived mesenchymal stromal cells could improve their renoprotective and prosurvival effects on human kidney cells [317]. Moreover, it was found that the melatonin levels during pregnancy and labor were elevated and significantly higher than those after birth [318], and studies on pregnant rats indicated that melatonin interacted with other hormones and played a role as a triggering labor contributor [319]. Additionally, melatonin was able to attenuate the inhibitory effects of ROS on fertilization in both men and women [320,321]. It was also demonstrated in vivo that melatonin was closely associated with the health of oral cavity, presenting reducing damage caused by oxidative stress [322], suppressing inflammation, stimulating the proliferation of collagen and osseous tissue, improving wound healing [323] and preventing oral cancer [16]. Furthermore, several researches have shown that melatonin could contribute to a healthy aging and an extended lifespan, and a recent review has summarized the studies regarding the antiaging properties of melatonin [324].
In general, bioactivities of melatonin are commonly investigated with high purity in laboratory. For human beings, as the endogenous melatonin alters all through the life, the dietary intake might partially recompense the declined endogenous melatonin after puberty. While for the aged people, supplements might be more suitable, and even medicine could be applied for patients with sleep disorders.
3.11. Adverse Effects
No adverse effects has been observed by the consumption of melatonin in foods or drinks. In addition, 1–10 mg/kg is usually considered as standard dose for assisting sleep and there were no toxicological effects found at a dose of 10 mg melatonin (orally) in a 28-day randomized, double-blind clinical trial [326,327]. Furthermore, no toxic effects were observed with high-dose melatonin in pregnant animal models [328].
Nevertheless, some adverse effects, such as dizziness, headache, nausea and sleepiness, have been reported by administration of high-dose melatonin, which was used as treatment of some diseases [329,330]. In a case of melatonin overdose (oral administration), lethargy and disorientation occurred to a man of 66 years old caused by 24 mg/kg melatonin for relaxation and sleep before operation, though he recovered completely afterwards [331]. In addition, the results of research on intravenous administration of melatonin appeared not consistent. Some studies found no side effects when high-dose melatonin (i.v.) was used repeatedly to treat pain [332], sepsis [333], surgical procedures [334] and lung disease [335]. Other studies reported that minor signs of sedation, impaired psychomotor and disorientation could possibly be associated with melatonin (i.v.) [336,337]. Although melatonin shows its protective and pro-fertilization effects on reproductive organs in both men and women, puberty seems like a sensitive period for melatonin administration regardless of sex. As the exogenous melatonin was given to male rats, reduced testis size and suppressed sperm production were observed, which were reversed by the treatment with exogenous gonadotropins [338]. For female rats, delayed puberty onset were observed with exogenous melatonin as a result of luteinizing hormone and prolactin reduction [14,339]. Those results were because of the inhibitory effects of melatonin on gonadotropin-releasing hormone neurons in hypothalamus [340].
4. Conclusions
Melatonin has been identified and qualified in a large number of foods. The content of melatonin is higher in eggs and fish than that in meat in animal foods, while in plant foods, the highest contents of melatonin was found in nuts, and some cereals and germinated legumes or seeds are also rich in melatonin. Mushrooms are also good dietary sources of melatonin. In addition, the intake of melatonin containing foods could significantly increase the melatonin concentration in human serum, indicating melatonin could provide beneficial effects on health through foods. Studies have shown that melatonin has many bioactivities, such as antioxidant, anti-inflammatory, enhancing immunity, anticancer, improving circadian cycle, cardiovascular protecting, anti-diabetic, anti-obese, anti-aging and neuroprotection. Therefore, the consumption of foods rich in melatonin could not only improve insomnia, which affects one third of the general population worldwide, but also provide other health benefits. In the future, the content of melatonin is worth testing and evaluating in more foods to find new natural sources of melatonin, and the mechanisms of action are needed to be investigated more comprehensively. In addition, more clinical trials are necessary to be conducted to clarify the effects of melatonin on human beings. Meanwhile, some foods with extremely high content of melatonin are of great value to be developed into functional foods, which would contribute to the prevention and treatment of various diseases.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81372976), Key Project of Guangdong Provincial Science and Technology Program (No. 2014B020205002), and the Hundred-Talents Scheme of Sun Yat-Sen University.
Author Contributions
Xiao Meng, Sha Li and Hua-Bin Li conceived this paper; Xiao Meng, Ya Li, Yue Zhou and Dong-Ping Xu wrote this paper; Sha Li, Ren-You Gan and Hua-Bin Li revised the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 2-OHM | 2-hydroxymelatonin |
| 4-OHM | 4-hydroxymelatonin |
| 6-OHM | 6-hydroxymelatonin |
| AAAD | aromatic L-amino acid decarboxylase |
| Aβ | amyloid-beta |
| ACC | acetyl-CoA carboxylase |
| AD | Alzheimer’s disease |
| AD-MSCs | adipose-derived mesenchymal stem cells |
| AFMK | N1-acetyl-N2-formyl-5-methoxykynuramine |
| AMK | N1-acetyl-5-methoxykynuramine |
| AMPK | adenosine monophosphate-activated protein kinase |
| AP-2β | activator protein 2β |
| ARE | antioxidant responsive element |
| ATF4 | activating transcription factor 4 |
| BACE1 | β-APP-cleaving enzyme 1 |
| Bax | Bcl-2-associated X protein |
| BBB | blood-brain barrier |
| Bcl-2 | B-cell lymphoma 2 |
| BDNF | brain derived neurotrophic factor |
| bFGF | basic fibroblast growth factor |
| BMMNCs | bone marrow mononuclear cells |
| BM-MSCs | bone marrow mesenchymal stem cells |
| C3-OHM | cyclic 3-hydroxymelatonin |
| CaMKIIα | calmodulin dependent protein kinase II alpha |
| cAMP | cyclic AMP |
| CAT | catalase |
| CCL20 | chemokine C-C motif ligand 20 |
| cGMP | cyclic GMP |
| CNS | central nerve system |
| COX-2 | cyclo-oxygenase 2 |
| CVDs | cardiovascular diseases |
| CXCL1 | chemokine C-X-C motif ligand 1 |
| cyt c | cytochrome c |
| DAG | diacylglycerol |
| DS | diclofenac sodium |
| DW | dry weight |
| EBPβ | enhancer-binding protein beta |
| eIF2α | eukaryotic initiation factor 2α |
| EMF | electromagnetic fields |
| EMT | epithelial mesenchymal transition |
| eNOS | endothelial nitric oxide synthase |
| ER | endoplasmic reticulum |
| ERK | extracellular signal-regulated kinase |
| ERR-α | estrogen-related receptor alpha |
| FRAP | ferric reducing antioxidant power |
| FSS | flow shear stress |
| FW | fresh weight |
| G6PD | glucose-6-phosphate dehydrogenase |
| GAS | gamma-activated sequence |
| γ-GCS | gamma-glutamylcysteine synthetase |
| G-CSF | granulocyte colony-stimulating factor |
| GFAP | glia and fibrillary acidic protein |
| GM-CSF | granulocyte–macrophage colony-stimulating factor |
| GPx | glutathione peroxidase |
| GSH-Rd | glutathione reductase |
| GSK3β | glycogen synthase kinase 3β |
| H2-Ab1 | histocompatibility class II antigen A, beta 1 |
| HDAC | histone deacetylase |
| Hes1 | hairy and enhancer of split 1 |
| HIOMT | hydroxyindole O-methyltransferase |
| HOMA-IR index | homeostasis model assessment of insulin resistance index |
| hTERT | human telomerase reserve transcriptase |
| i.v. | intravenously |
| i.p. | intraperitoneally |
| IFN-γ | interferon gamma |
| IGF-1 | insulin-like growth factor 1 |
| IKK | inhibitor of nuclear factor kappa-B kinase |
| IL-6 | interleukin 6 |
| iNOS | inducible nitric oxide synthase |
| IRI | ischemia/reperfusion injury |
| JAK2 | Janus kinase 2 |
| JNK | Jun N-terminal kinase |
| LAN | light at night |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| MDH | malondialdehyde |
| MI | myocardial infarction |
| MLHb | medial lateral habenula |
| MMP-9 | matrix metalloproteinase-9 |
| Mst1 | mammalian Ste20-like kinase 1 |
| MT1, MT2 | melatonin receptors |
| NAD | Nicotinamide adenine dinucleotide |
| NAT | N-acetyltransferase |
| NDRG2 | N-myc downstream-regulated gene 2 |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NK | natural killer |
| NLRP3 | NOD-like receptor P3 |
| NO | nitric oxide |
| NOMela | N-nitrosomelatonin |
| Nrf2 | nuclear factor-erythroid 2-related factor 2 |
| OA | osteoarthritis |
| ON | optic neuritis |
| ORAC | oxygen radical antioxidant capacity |
| Oxa | oxaliplatin |
| p-Akt | phosphorylated protein kinase B |
| PARP | poly-ADP-ribose polymerase |
| pCREB | phosphorylated cAMP response element-binding protein |
| PD | Parkinson’s disease |
| PDGF | platelet-derived growth factor |
| PGC-1α | peroxisome proliferator-activated receptor gamma coactivator-1 alpha |
| PI3K | phosphatidyl inositol 3-kinase |
| PKB | protein kinase B |
| PLR | pupil light reflex |
| PPAR-γ | peroxisome proliferator-activated receptor gamma |
| PRDX1 | peroxiredoxin 1 |
| PS1 | presenilin 1 |
| PUMA | p53 upregulated modulator of apoptosis |
| RNS | reactive nitrogen species |
| ROCK-1 | Rho-associated kinase protein |
| ROS | reactive oxygen species |
| S100B | S100 calcium-binding protein B |
| SAH | subarachnoid hemorrhage |
| SCN | suprachiasmatic nucleus |
| SE | standard error |
| SEEMs | selective estrogen enzyme modulators |
| SIRT1 | sirtuin 1 |
| SOD | superoxide dismutase |
| STAT | signal transducer and activator of transcription |
| TBI | traumatic brain injury |
| TGF-β | transforming growth factor β |
| TIMP-1 | tissue inhibitor of metalloproteinases 1 |
| TNF-α | tumor necrosis factor α |
| TPH | tryptophan hydroxylase |
| UCP1 | uncoupling protein 1 |
| UPS | ubiquitin/proteasome system |
| VEGF | vascular endothelial growth factor |
| VEP | visual evoked potentials |
References
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, a pineal factor that lightens melanocytes. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Brown, G.M.; Pandi-Perumal, S.R.; Trakht, I.; Cardinali, D.P. Melatonin and its relevance to jet lag. Travel Med. Infect. Dis. 2009, 7, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumal, S.R.; Srinivasan, V.; Poeggeler, B.; Hardeland, R.; Cardinali, D.P. Drug Insight: The use of melatonergic agonists for the treatment of insomnia-focus on ramelteon. Nat. Clin. Pract. Neurol. 2007, 3, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Luo, X.; Li, L.; Peng, Q.; Yang, Y.; Zhao, L.; Ma, M.; Hou, Z. The protective effects of melatonin against oxidative stress and inflammation induced by acute cadmium exposure in mice testis. Biol. Trace Elem. Res. 2016, 170, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Huang, S.H.; Chen, J.W.; Wang, K.C.; Yang, Y.R.; Liu, P.F.; Lin, G.J.; Sytwu, H.K. Melatonin enhances interleukin-10 expression and suppresses chemotaxis to inhibit inflammation in situ and reduce the severity of experimental autoimmune encephalomyelitis. Int. Immunopharmacol. 2016, 31, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Oxenkrug, G.; Requintina, P.; Bachurin, S. Antioxidant and antiaging activity of N-acetylserotonin and melatonin in the in vivo models. Ann. N. Y. Acad. Sci. 2001, 939, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, S.; Li, H.B.; Deng, G.F.; Ling, W.H.; Wu, S.; Xu, X.R.; Chen, F. Antiproliferative activity of peels, pulps and seeds of 61 fruits. J. Funct. Foods 2013, 5, 1298–1309. [Google Scholar] [CrossRef]
- Anisimov, V.N.; Popovich, I.G.; Zabezhinski, M.A.; Anisimov, S.V.; Vesnushkin, G.M.; Vinogradova, I.A. Melatonin as antioxidant, geroprotector and anticarcinogen. Biochim. Biophys. Acta 2006, 1757, 573–589. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumal, S.R.; BaHammam, A.S.; Brown, G.M.; Spence, D.W.; Bharti, V.K.; Kaur, C.; Hardeland, R.; Cardinali, D.P. Melatonin antioxidative defense: Therapeutical implications for aging and neurodegenerative processes. Neurotox. Res. 2013, 23, 267–300. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumal, S.R.; Zisapel, N.; Srinivasan, V.; Cardinali, D.P. Melatonin and sleep in aging population. Exp. Gerontol. 2005, 40, 911–925. [Google Scholar] [CrossRef] [PubMed]
- Agil, A.; Elmahallawy, E.K.; Rodriguez-Ferrer, J.M.; Adem, A.; Bastaki, S.M.; Al-Abbadi, I.; Fino Solano, Y.A.; Navarro-Alarcon, M. Melatonin increases intracellular calcium in the liver, muscle, white adipose tissues and pancreas of diabetic obese rats. Food Funct. 2015, 6, 2671–2678. [Google Scholar] [CrossRef] [PubMed]
- Agil, A.; El-Hammadi, M.; Jimenez-Aranda, A.; Tassi, M.; Abdo, W.; Fernandez-Vazquez, G.; Reiter, R.J. Melatonin reduces hepatic mitochondrial dysfunction in diabetic obese rats. J. Pineal Res. 2015, 59, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Chenevard, R.; Suter, Y.; Erne, P. Effects of the heart-lung machine on melatonin metabolism and mood disturbances. Eur. J. Cardiothorac. Surg. 2008, 34, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Esquifino, A.I.; Villanua, M.A.; Agrasal, C. Effect of neonatal melatonin administration on sexual development in the rat. J. Steroid Biochem. 1987, 27, 1089–1093. [Google Scholar] [CrossRef]
- Agilli, M.; Aydin, F.N.; Cayci, T. The effect of body temperature, melatonin and cortisol on obesity in women: A biochemical evaluation? Clin. Nutr. 2015, 34, 332. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, S.; Khurshid, Z.; Zohaib, S.; Zafar, M.S. Therapeutic potential of melatonin in oral medicine and periodontology. Kaohsiung J. Med. Sci. 2016, 32, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Setyaningsih, W.; Saputro, I.E.; Barbero, G.F.; Palma, M.; Garcia Barroso, C. Determination of melatonin in rice (Oryza sativa) grains by pressurized liquid extraction. J. Agric. Food Chem. 2015, 63, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Escriva, L.; Manyes, L.; Barbera, M.; Martinez-Torres, D.; Meca, G. Determination of melatonin in Acyrthosiphon pisum aphids by liquid chromatography-tandem mass spectrometry. J. Insect Physiol. 2016, 86, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Muszynska, B.; Sulkowska-Ziaja, K. Analysis of indole compounds in edible Basidiomycota species after thermal processing. Food Chem. 2012, 132, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, C.; Kocadagli, T.; Gokmen, V. Formation of melatonin and its isomer during bread dough fermentation and effect of baking. J. Agric. Food Chem. 2014, 62, 2900–2905. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X. Melatonin: An antioxidant in edible plants. Ann. N. Y. Acad. Sci. 2002, 957, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Zanghi, B.M.; Manchester, L.C.; Reiter, R.J. Melatonin identified in meats and other food stuffs: Potentially nutritional impact. J. Pineal Res. 2014, 57, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, Y.; Herrera, T.; Benitez, V.; Arribas, S.M.; Lopez De Pablo, A.L.; Esteban, R.M.; Martin-Cabrejas, M.A. Estimation of scavenging capacity of melatonin and other antioxidants: Contribution and evaluation in germinated seeds. Food Chem. 2015, 170, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Sae-Teaw, M.; Johns, J.; Johns, N.P.; Subongkot, S. Serum melatonin levels and antioxidant capacities after consumption of pineapple, orange, or banana by healthy male volunteers. J. Pineal Res. 2013, 55, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.; Terron, M.P.; Garrido, M.; Pariente, J.A.; Barriga, C.; Rodriguez, A.B.; Paredes, S.D. Diets enriched with a Jerte Valley cherry-based nutraceutical product reinforce nocturnal behaviour in young and old animals of nocturnal (Rattus norvegicus) and diurnal (Streptopelia risoria) chronotypes. J. Anim. Physiol. Anim. Nutr. 2013, 97, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Iriti, M.; Faoro, F. Grape phytochemicals: A bouquet of old and new nutraceuticals for human health. Med. Hypotheses 2006, 67, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, C.K. Functional foods, herbs and nutraceuticals: Towards biochemical mechanisms of healthy aging. Biogerontology 2004, 5, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Oladi, E.; Mohamadi, M.; Shamspur, T.; Mostafavi, A. Spectrofluorimetric determination of melatonin in kernels of four different Pistacia varieties after ultrasound-assisted solid-liquid extraction. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 132, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Murch, S.J.; Simmons, C.B.; Saxena, P.K. Melatonin in feverfew and other medicinal plants. Lancet 1997, 350, 1598–1599. [Google Scholar] [CrossRef]
- González-Gómez, D.; Lozano, M.; Fernández-León, M.F.; Ayuso, M.C.; Bernalte, M.J.; Rodríguez, A.B. Detection and quantification of melatonin and serotonin in eight Sweet Cherry cultivars (Prunus avium L.). Eur. Food Res. Technol. 2009, 229, 223–229. [Google Scholar] [CrossRef]
- Wang, J.; Liang, C.; Li, S.; Zheng, J. Study on analysis method of melatonin and melatonin content in corn & rice seeds. Chin. Agric. Sci. Bull. 2009, 25, 20–24. [Google Scholar]
- Hernandez-Ruiz, J.; Arnao, M.B. Distribution of melatonin in different zones of lupin and barley plants at different ages in the presence and absence of light. J. Agric. Food Chem. 2008, 56, 10567–10573. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Burkhardt, S.; Manchester, L.C. Melatonin in plants. Nutr. Rev. 2001, 59, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Pandi-Perumal, S.R.; Cardinali, D.P. Melatonin. Int. J. Biochem. Cell Biol. 2006, 38, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Iriti, M.; Rossoni, M.; Faoro, F. Melatonin content in grape: Myth or panacea. J. Sci. Food Agric. 2006, 86, 1432–1438. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.Y.; Shi, X.Y.; Xiao, H.; Kang, K.; Liu, X.Y.; Zhan, J.C.; Huang, W.D. Effect of cultivar, temperature, and environmental conditions on the dynamic change of melatonin in mulberry fruit development and wine fermentation. J. Food Sci. 2016, 81, M958–M967. [Google Scholar] [CrossRef] [PubMed]
- Karunanithi, D.; Radhakrishna, A.; Sivaraman, K.P.; Biju, V.M. Quantitative determination of melatonin in milk by LC-MS/MS. J. Food Sci. Technol. 2014, 51, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Milagres, M.P.; Minim, V.P.; Minim, L.A.; Simiqueli, A.A.; Moraes, L.E.; Martino, H.S. Night milking adds value to cow’s milk. J. Sci. Food Agric. 2014, 94, 1688–1692. [Google Scholar] [CrossRef] [PubMed]
- Berthelot, X.; Laurentie, M.; Ravault, J.P.; Ferney, J.; Toutain, P.L. Circadian profile and production rate of melatonin in the cow. Domest. Anim. Endocrinol. 1990, 7, 315–322. [Google Scholar] [CrossRef]
- Kennaway, D.J.; Stamp, G.E.; Goble, F.C. Development of melatonin production in infants and the impact of prematurity. J. Clin. Endocrinol. Metab. 1992, 75, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Cohen Engler, A.; Hadash, A.; Shehadeh, N.; Pillar, G. Breastfeeding may improve nocturnal sleep and reduce infantile colic: Potential role of breast milk melatonin. Eur. J. Pediatr. 2012, 171, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Kocadagli, T.; Yilmaz, C.; Gokmen, V. Determination of melatonin and its isomer in foods by liquid chromatography tandem mass spectrometry. Food Chem. 2014, 153, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Mercolini, L.; Mandrioli, R.; Raggi, M.A. Content of melatonin and other antioxidants in grape-related foodstuffs: Measurement using a MEPS-HPLC-F method. J. Pineal Res. 2012, 53, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Sturtz, M.; Cerezo, A.B.; Cantos-Villar, E.; Garcia-Parrilla, M.C. Determination of the melatonin content of different varieties of tomatoes (Lycopersicon esculentum) and strawberries (Fragariaananassa). Food Chem. 2011, 127, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Boccalandro, H.E.; Gonzalez, C.V.; Wunderlin, D.A.; Silva, M.F. Melatonin levels, determined by LC-ESI-MS/MS, fluctuate during the day/night cycle in Vitis vinifera cv. Malbec: Evidence of its antioxidant role in fruits. J. Pineal Res. 2011, 51, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, S.; Tan, D.X.; Manchester, L.C.; Hardeland, R.; Reiter, R.J. Detection and quantification of the antioxidant melatonin in Montmorency and Balaton tart cherries (Prunus cerasus). J. Agric. Food Chem. 2001, 49, 4898–4902. [Google Scholar] [CrossRef] [PubMed]
- Badria, F.A. Melatonin, serotonin, and tryptamine in some Egyptian food and medicinal plants. J. Med. Food 2002, 5, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Riga, P.; Medina, S.; Garcia-Flores, L.A.; Gil-Izquierdo, A. Melatonin content of pepper and tomato fruits: Effects of cultivar and solar radiation. Food Chem. 2014, 156, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Muszynska, B.; Kala, K.; Sulkowska-Ziaja, K.; Krakowska, A.; Opoka, W. Agaricus bisporus and its in vitro culture as a source of indole compounds released into artificial digestive juices. Food Chem. 2016, 199, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Manchester, L.C.; Tan, D.X.; Reiter, R.J.; Park, W.; Monis, K.; Qi, W. High levels of melatonin in the seeds of edible plants: Possible function in germ tissue protection. Life Sci. 2000, 67, 3023–3029. [Google Scholar] [CrossRef]
- Zielin Ski, H.; Lewczuk, B.; Przybylska-Gornowicz, B.; Kozlowska, H. Melatonin in germinated legume seeds as a potentially significant agent for health. In Biologically-Active phytochemicals in Food: Analysis, Metabolism, Bioavailability and Function, Norwich, UK, 2001; Pfannhauser, W., Fenwick, G.R., Khokhar, S.R., Eds.; Royal Society of Chemistry: Cambridge, UK, 2001. [Google Scholar]
- Aguilera, Y.; Herrera, T.; Liebana, R.; Rebollo-Hernanz, M.; Sanchez-Puelles, C.; Martin-Cabrejas, M.A. Impact of melatonin enrichment during germination of legumes on bioactive compounds and antioxidant activity. J. Agric. Food Chem. 2015, 63, 7967–7974. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Moreno, H.; Calvo, J.R.; Maldonado, M.D. High levels of melatonin generated during the brewing process. J. Pineal Res. 2013, 55, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Naranjo, M.I.; Gil-Izquierdo, A.; Troncoso, A.M.; Cantos, E.; Garcia-Parrilla, M.C. Melatonin: A new bioactive compound in wine. J. Food Compos. Anal. 2011, 24, 603–608. [Google Scholar] [CrossRef]
- Stege, P.W.; Sombra, L.L.; Messina, G.; Martinez, L.D.; Silva, M.F. Determination of melatonin in wine and plant extracts by capillary electrochromatography with immobilized carboxylic multi-walled carbon nanotubes as stationary phase. Electrophoresis 2010, 31, 2242–2248. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Naranjo, M.I.; Gil-Izquierdo, A.; Troncoso, A.M.; Cantos-Villar, E.; Garcia-Parrilla, M.C. Melatonin is synthesised by yeast during alcoholic fermentation in wines. Food Chem. 2011, 126, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, A.; Giridhar, P.; Sankar, K.U.; Ravishankar, G.A. Melatonin and serotonin profiles in beans of Coffea species. J. Pineal Res. 2012, 52, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Vitalini, S.; Gardana, C.; Simonetti, P.; Fico, G.; Iriti, M. Melatonin, melatonin isomers and stilbenes in Italian traditional grape products and their antiradical capacity. J. Pineal Res. 2013, 54, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Kirakosyan, A.; Seymour, E.M.; Llanes, D.E.U.; Kaufman, P.B.; Bolling, S.F. Chemical profile and antioxidant capacities of tart cherry products. Food Chem. 2009, 115, 20–25. [Google Scholar] [CrossRef]
- Chen, G.; Huo, Y.; Tan, D.X.; Liang, Z.; Zhang, W.; Zhang, Y. Melatonin in Chinese medicinal herbs. Life Sci. 2003, 73, 19–26. [Google Scholar] [CrossRef]
- Padumanonda, T.; Johns, J.; Sangkasat, A.; Tiyaworanant, S. Determination of melatonin content in traditional Thai herbal remedies used as sleeping aids. Daru 2014, 22, 6. [Google Scholar] [CrossRef] [PubMed]
- de la Puerta, C.; Carrascosa-Salmoral, M.P.; García-Luna, P.P.; Lardone, P.J.; Herrera, J.L.; Fernández-Montesinos, R.; Guerrero, J.M.; Pozo, D. Melatonin is a phytochemical in olive oil. Food Chem. 2007, 104, 609–612. [Google Scholar] [CrossRef]
- Venegas, C.; Cabrera-Vique, C.; Garcia-Corzo, L.; Escames, G.; Acuna-Castroviejo, D.; Lopez, L.C. Determination of coenzyme Q10, coenzyme Q9, and melatonin contents in virgin argan oils: Comparison with other edible vegetable oils. J. Agric. Food Chem. 2011, 59, 12102–12108. [Google Scholar] [CrossRef] [PubMed]
- Reinholds, I.; Pugajeva, I.; Radenkovs, V.; Rjabova, J.; Bartkevics, V. Development and validation of new ultra-high-performance liquid chromatography-hybrid quadrupole-orbitrap mass spectrometry method for determination of melatonin in fruits. J. Chromatogr. Sci. 2016, 54, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Vakkuri, O.; Kivela, A.; Leppaluoto, J.; Valtonen, M.; Kauppila, A. Decrease in melatonin precedes follicle-stimulating hormone increase during perimenopause. Eur. J. Endocrinol. 1996, 135, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Waldhauser, F.; Weiszenbacher, G.; Tatzer, E.; Gisinger, B.; Waldhauser, M.; Schemper, M.; Frisch, H. Alterations in nocturnal serum melatonin levels in humans with growth and aging. J. Clin. Endocrinol. Metab. 1988, 66, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Palacios-Bois, J.; Schwartz, G.; Iskandar, H.; Thakur, M.; Quirion, R.; Nair, N.P. Circadian rhythms of melatonin and cortisol in aging. Biol. Psychiatry 1989, 25, 305–319. [Google Scholar] [CrossRef]
- Singh, M.; Jadhav, H.R. Melatonin: Functions and ligands. Drug Discov. Today 2014, 19, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xiao, X.; Zhang, C.; Yu, W.; Guo, W.; Zhang, Z.; Li, Z.; Feng, X.; Hao, J.; Zhang, K.; et al. Melatonin synergizes the chemotherapeutic effect of 5-fluorouracil in colon cancer by suppressing PI3K/AKT and NF-kappaB/iNOS signaling pathways. J. Pineal Res. 2017, 62. [Google Scholar] [CrossRef] [PubMed]
- DeMuro, R.L.; Nafziger, A.N.; Blask, D.E.; Menhinick, A.M.; Bertino, J.J. The absolute bioavailability of oral melatonin. J. Clin. Pharmacol. 2000, 40, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Fourtillan, J.B.; Brisson, A.M.; Gobin, P.; Ingrand, I.; Decourt, J.P.; Girault, J. Bioavailability of melatonin in humans after day-time administration of D7 melatonin. Biopharm. Drug Dispos. 2000, 21, 15–22. [Google Scholar] [CrossRef]
- Shirakawa, S.; Tsuchiya, S.; Tsutsumi, Y.; Kotorii, T.; Uchimura, N.; Sakamoto, T.; Yamada, S. Time course of saliva and serum melatonin levels after ingestion of melatonin. Psychiatry Clin. Neurosci. 1998, 52, 266–267. [Google Scholar] [CrossRef] [PubMed]
- Oba, S.; Nakamura, K.; Sahashi, Y.; Hattori, A.; Nagata, C. Consumption of vegetables alters morning urinary 6-sulfatoxymelatonin concentration. J. Pineal Res. 2008, 45, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, M.D.; Moreno, H.; Calvo, J.R. Melatonin present in beer contributes to increase the levels of melatonin and antioxidant capacity of the human serum. Clin. Nutr. 2009, 28, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Flores, D.; Gamero, E.; Garrido, M.; Ramirez, R.; Moreno, D.; Delgado, J.; Valdes, E.; Barriga, C.; Rodriguez, A.B.; Paredes, S.D. Urinary 6-sulfatoxymelatonin and total antioxidant capacity increase after the intake of a grape juice cv. Tempranillo stabilized with HHP. Food Funct. 2012, 3, 34–39. [Google Scholar] [CrossRef] [PubMed]
- González-Flores, D.; Velardo, B.; Garrido, M.; González-Gómez, D.; Lozano, M.; Ayuso, M.C.; Barriga, C.; Paredes, S.D.; Rodríguez, A.B. Ingestion of Japanese plums (Prunus salicina Lindl. cv. Crimson Globe) increases the urinary 6-sulfatoxymelatonin and total antioxidant capacity levels in young, middle-aged and elderly humans: nutritional and functional characterization of their content. J. Food Nutr. Res. 2011, 50, 229. [Google Scholar]
- Reiter, R.J.; Manchester, L.C.; Tan, D.X. Melatonin in walnuts: Influence on levels of melatonin and total antioxidant capacity of blood. Nutrition 2005, 21, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Schernhammer, E.S.; Feskanich, D.; Niu, C.; Dopfel, R.; Holmes, M.D.; Hankinson, S.E. Dietary correlates of urinary 6-sulfatoxymelatonin concentrations in the Nurses’ Health Study cohorts. Am. J. Clin. Nutr. 2009, 90, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, Y.; Rebollo-Hernanz, M.; Herrera, T.; Cayuelas, L.T.; Rodriguez-Rodriguez, P.; de Pablo, A.L.; Arribas, S.M.; Martin-Cabrejas, M.A. Intake of bean sprouts influences melatonin and antioxidant capacity biomarker levels in rats. Food Funct. 2016, 7, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Tetens, I. Scientific Opinion on the Substantiation of Health Claims Related to Melatonin and Alleviation of Subjective Feelings of Jet Lag (ID 1953), and Reduction of Sleep onset Latency, and Improvement of Sleep Quality (ID 1953) Pursuant to Article 13(1) of Regulation (EC) No 1924/2006; European Food Safety Authority: Parma, Itlay, 2010. [Google Scholar]
- Food Standards Australia New Zealand (FSANZ). Supporting document 9: Consideration of EU Approved Health Claims, P293—Nutrition, Health & Related Claim; Food Standards Australia New Zealand: Kingston, Australia, Wellington, Newzealand, 2013. [Google Scholar]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar] [PubMed]
- Byeon, Y.; Back, K. An increase in melatonin in transgenic rice causes pleiotropic phenotypes, including enhanced seedling growth, delayed flowering, and low grain yield. J. Pineal Res. 2014, 56, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ruiz, J.; Cano, A.; Arnao, M.B. Melatonin acts as a growth-stimulating compound in some monocot species. J. Pineal Res. 2005, 39, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A. New hypotheses for the health-protective mechanisms of whole-grain cereals: What is beyond fibre? Nutr. Res. Rev. 2010, 23, 65–134. [Google Scholar] [CrossRef] [PubMed]
- Hosseinian, F.S.; Li, W.; Beta, T. Measurement of anthocyanins and other phytochemicals in purple wheat. Food Chem. 2008, 109, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Wang, L.; Tan, D.X.; Zhao, Y.; Zheng, X.D.; Chen, H.; Li, Q.T.; Zuo, B.X.; Kong, J. Identification of genes for melatonin synthetic enzymes in ‘Red Fuji’ apple (Malus domestica Borkh. cv. Red) and their expression and melatonin production during fruit development. J. Pineal Res. 2013, 55, 443–451. [Google Scholar] [PubMed]
- Zhao, Y.; Tan, D.X.; Lei, Q.; Chen, H.; Wang, L.; Li, Q.T.; Gao, Y.; Kong, J. Melatonin and its potential biological functions in the fruits of sweet cherry. J. Pineal Res. 2013, 55, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Vitalini, S.; Gardana, C.; Zanzotto, A.; Simonetti, P.; Faoro, F.; Fico, G.; Iriti, M. The presence of melatonin in grapevine (Vitis vinifera L.) berry tissues. J. Pineal Res. 2011, 51, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.N.; Turi, C.E.; Shipley, P.R.; Murch, S.J. Comparisons of large (Vaccinium macrocarpon Ait.) and small (Vaccinium oxycoccos L., Vaccinium vitis-idaea L.) cranberry in British Columbia by phytochemical determination, antioxidant potential, and metabolomic profiling with chemometric analysis. Planta Med. 2012, 78, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P.; Tan, D.X.; Manchester, L.C.; Reiter, R.J. Purslane: A plant source of omega-3 fatty acids and melatonin. J. Pineal Res. 2005, 39, 331–332. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, M.; Ezura, H. Profiling of melatonin in the model tomato (Solanum lycopersicum L.) cultivar Micro-Tom. J. Pineal Res. 2009, 46, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, Y.; Reiter, R.J.; He, C.; Liu, G.; Lei, Q.; Zuo, B.; Zheng, X.D.; Li, Q.; Kong, J. Changes in melatonin levels in transgenic ‘Micro-Tom’ tomato overexpressing ovine AANAT and ovine HIOMT genes. J. Pineal Res. 2014, 56, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Zhang, N.; Yang, R.C.; Wang, L.; Sun, Q.Q.; Li, D.B.; Cao, Y.Y.; Weeda, S.; Zhao, B.; Ren, S.; Guo, Y.D. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA(4) interaction in cucumber (Cucumis sativus L.). J. Pineal Res. 2014, 57, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Byeon, Y.; Park, S.; Lee, H.Y.; Kim, Y.S.; Back, K. Elevated production of melatonin in transgenic rice seeds expressing rice tryptophan decarboxylase. J. Pineal Res. 2014, 56, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Tapia, M.I.; Sánchez-Morgado, J.R.; García-Parra, J.; Ramírez, R.; Hernández, T.; González-Gómez, D. Comparative study of the nutritional and bioactive compounds content of four walnut (Juglans regia L.) cultivars. J. Food Compos. Anal. 2013, 31, 232–237. [Google Scholar] [CrossRef]
- Fernandez-Pachon, M.S.; Medina, S.; Herrero-Martin, G.; Cerrillo, I.; Berna, G.; Escudero-Lopez, B.; Ferreres, F.; Martin, F.; Garcia-Parrilla, M.C.; Gil-Izquierdo, A. Alcoholic fermentation induces melatonin synthesis in orange juice. J. Pineal Res. 2014, 56, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Pothinuch, P.; Tongchitpakdee, S. Melatonin contents in mulberry (Morus spp.) leaves: Effects of sample preparation, cultivar, leaf age and tea processing. Food Chem. 2011, 128, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Wehr, T.A.; Duncan, W.C., Jr.; Sher, L.; Aeschbach, D.; Schwartz, P.J.; Turner, E.H.; Postolache, T.T.; Rosenthal, N.E. A circadian signal of change of season in patients with seasonal affective disorder. Arch. Gen. Psychiatry 2001, 58, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Rexhaj, E.; Pireva, A.; Paoloni-Giacobino, A.; Allemann, Y.; Cerny, D.; Dessen, P.; Sartori, C.; Scherrer, U.; Rimoldi, S.F. Prevention of vascular dysfunction and arterial hypertension in mice generated by assisted reproductive technologies by addition of melatonin to culture media. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1151–H1156. [Google Scholar] [CrossRef] [PubMed]
- Michurina, S.V.; Ishchenko, I.Y.; Arkhipov, S.A.; Klimontov, V.V.; Rachkovskaya, L.N.; Konenkov, V.I.; Zavyalov, E.L. Effects of melatonin, aluminum oxide, and polymethylsiloxane complex on the expression of LYVE-1 in the liver of mice with obesity and type 2 diabetes mellitus. Bull. Exp. Biol. Med. 2016, 162, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Bielli, A.; Scioli, M.G.; Mazzaglia, D.; Doldo, E.; Orlandi, A. Antioxidants and vascular health. Life Sci. 2015, 143, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Barton, S.K.; Tolcos, M.; Miller, S.L.; Christoph-Roehr, C.; Schmolzer, G.M.; Moss, T.J.; Hooper, S.B.; Wallace, E.M.; Polglase, G.R. Ventilation-induced brain injury in preterm neonates: A review of potential therapies. Neonatology 2016, 110, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Haldar, C. Age dependent nitro-oxidative load and melatonin receptor expression in the spleen and immunity of goat Capra hircus. Exp. Gerontol. 2014, 60, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Aktoz, T.; Aydogdu, N.; Alagol, B.; Yalcin, O.; Huseyinova, G.; Atakan, I.H. The protective effects of melatonin and vitamin E against renal ischemia-reperfusion injury in rats. Ren. Fail. 2007, 29, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Lissoni, P.; Chilelli, M.; Villa, S.; Cerizza, L.; Tancini, G. Five years survival in metastatic non-small cell lung cancer patients treated with chemotherapy alone or chemotherapy and melatonin: A randomized trial. J. Pineal Res. 2003, 35, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Paredes, S.D.; Forman, K.A.; Garcia, C.; Vara, E.; Escames, G.; Tresguerres, J.A. Protective actions of melatonin and growth hormone on the aged cardiovascular system. Horm. Mol. Biol. Clin. Investig. 2014, 18, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Zephy, D.; Ahmad, J. Type 2 diabetes mellitus: Role of melatonin and oxidative stress. Diabetes Metab. Syndr. 2015, 9, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Lin, K.C.; Wallace, C.G.; Chen, Y.T.; Yang, C.C.; Leu, S.; Chen, Y.C.; Sun, C.K.; Tsai, T.H.; Chen, Y.L.; et al. Additional benefit of combined therapy with melatonin and apoptotic adipose-derived mesenchymal stem cell against sepsis-induced kidney injury. J. Pineal Res. 2014, 57, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.F.; Lin, X.; Xu, X.R.; Gao, L.L.; Xie, J.F.; Li, H.B. Antioxidant capacities and total phenolic contents of 56 vegetables. J. Funct. Foods 2013, 5, 260–266. [Google Scholar] [CrossRef]
- Fu, L.; Xu, B.T.; Xu, X.R.; Qin, X.S.; Gan, R.Y.; Li, H.B. Antioxidant capacities and total phenolic contents of 56 wild fruits from South China. Molecules 2010, 15, 8602–8617. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.J.; Deng, G.F.; Xu, X.R.; Wu, S.; Li, S.; Xia, E.Q.; Li, F.; Chen, F.; Ling, W.H.; Li, H.B. Antioxidant capacities, phenolic compounds and polysaccharide contents of 49 edible macro-fungi. Food Funct. 2012, 3, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Li, A.N.; Li, S.; Li, H.B.; Xu, D.P.; Xu, X.R.; Chen, F. Total phenolic contents and antioxidant capacities of 51 edible and wild flowers. J. Funct. Foods 2014, 6, 319–330. [Google Scholar] [CrossRef]
- Li, S.; Li, S.K.; Gan, R.Y.; Song, F.L.; Kuang, L.; Li, H.B. Antioxidant capacities and total phenolic contents of infusions from 223 medicinal plants. Ind. Crops Prod. 2013, 51, 289–298. [Google Scholar] [CrossRef]
- Deng, G.F.; Xu, X.R.; Zhang, Y.; Li, D.; Gan, R.Y.; Li, H.B. Phenolic compounds and bioactivities of pigmented rice. Crit. Rev. Food Sci. 2013, 53, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.J.; Xu, D.P.; Zhou, T.; Zhou, Y.; Li, S.; Li, H.B. Bioactivities and health benefits of wild fruits. Int. J. Mol. Sci. 2016, 17, 1258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Li, Y.; Zhou, T.; Xu, D.P.; Zhang, P.; Li, S.; Li, H.B. Bioactivities and health benefits of mushrooms mainly from China. Molecules 2016, 21, 938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Okatani, Y.; Wakatsuki, A.; Reiter, R.J.; Miyahara, Y. Acutely administered melatonin restores hepatic mitochondrial physiology in old mice. Int. J. Biochem. Cell Biol. 2003, 35, 367–375. [Google Scholar] [CrossRef]
- Khaldy, H.; Escames, G.; Leon, J.; Vives, F.; Luna, J.D.; Acuna-Castroviejo, D. Comparative effects of melatonin, L-deprenyl, Trolox and ascorbate in the suppression of hydroxyl radical formation during dopamine autoxidation in vitro. J. Pineal Res. 2000, 29, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Urata, Y.; Honma, S.; Goto, S.; Todoroki, S.; Iida, T.; Cho, S.; Honma, K.; Kondo, T. Melatonin induces gamma-glutamylcysteine synthetase mediated by activator protein-1 in human vascular endothelial cells. Free Radic. Biol. Med. 1999, 27, 838–847. [Google Scholar] [CrossRef]
- Montilla, P.; Cruz, A.; Padillo, F.J.; Tunez, I.; Gascon, F.; Munoz, M.C.; Gomez, M.; Pera, C. Melatonin versus vitamin E as protective treatment against oxidative stress after extra-hepatic bile duct ligation in rats. J. Pineal Res. 2001, 31, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Poeggeler, B.; Reiter, R.J.; Tan, D.X.; Chen, L.D.; Manchester, L.C. Melatonin: A potent, endogenous hydroxyl radical scavenger. J. Pineal Res. 1993, 14, 57–60. [Google Scholar]
- Okatani, Y.; Watanabe, K.; Hayashi, K.; Wakatsuki, A.; Sagara, Y. Melatonin inhibits vasospastic action of hydrogen peroxide in human umbilical artery. J. Pineal Res. 1997, 22, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; Han, B.; Liu, M.; Yeh, C.; Casida, J.E. Phosphine-induced oxidative damage in rats: Attenuation by melatonin. Free Radic. Biol. Med. 2000, 28, 636–642. [Google Scholar] [CrossRef]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Poeggeler, B.; Lopez-Burillo, S.; Mayo, J.C.; Sainz, R.M.; Reiter, R.J. Mechanistic and comparative studies of melatonin and classic antioxidants in terms of their interactions with the ABTS cation radical. J. Pineal Res. 2003, 34, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Ressmeyer, A.R.; Mayo, J.C.; Zelosko, V.; Sainz, R.M.; Tan, D.X.; Poeggeler, B.; Antolin, I.; Zsizsik, B.K.; Reiter, R.J.; Hardeland, R. Antioxidant properties of the melatonin metabolite N1-acetyl-5-methoxykynuramine (AMK): Scavenging of free radicals and prevention of protein destruction. Redox Rep. 2003, 8, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Escames, G.; Lopez, L.C.; Ortiz, F.; Ros, E.; Acuna-Castroviejo, D. Age-dependent lipopolysaccharide-induced iNOS expression and multiorgan failure in rats: Effects of melatonin treatment. Exp. Gerontol. 2006, 41, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, M.F.; Narin, F.; Akkus, D.; Ozdamar, S. Effect of melatonin and vitamin C on expression of endothelial NOS in heart of chronic alcoholic rats. Toxicol. Ind. Health 2009, 25, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Buldak, R.J.; Pilc-Gumula, K.; Buldak, L.; Witkowska, D.; Kukla, M.; Polaniak, R.; Zwirska-Korczala, K. Effects of ghrelin, leptin and melatonin on the levels of reactive oxygen species, antioxidant enzyme activity and viability of the HCT 116 human colorectal carcinoma cell line. Mol. Med. Rep. 2015, 12, 2275–2282. [Google Scholar] [PubMed]
- Tesoriere, L.; Allegra, M.; D’Arpa, D.; Butera, D.; Livrea, M.A. Reaction of melatonin with hemoglobin-derived oxoferryl radicals and inhibition of the hydroperoxide-induced hemoglobin denaturation in red blood cells. J. Pineal Res. 2001, 31, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Pi, H.; Yang, Z.; Reiter, R.J.; Xu, S.; Chen, X.; Chen, C.; Zhang, L.; Yang, M.; Li, Y.; et al. Melatonin antagonizes cadmium-induced neurotoxicity by activating the transcription factor EB-dependent autophagy-lysosome machinery in mouse neuroblastoma cells. J. Pineal Res. 2016, 61, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.V.; Chhunchha, B. Protective role of melatonin against the mercury induced oxidative stress in the rat thyroid. Food Chem. Toxicol. 2010, 48, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, Z.; Liu, W.; Wang, J.; He, X.; Huang, H.; Zhang, J.; Yang, Z. Melatonin protects against arsenic trioxide-induced liver injury by the upregulation of Nrf2 expression through the activation of PI3K/AKT pathway. Oncotarget 2017, 8, 3773–3780. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Plata, E.; Quiroz-Compean, F.; Ramirez-Garcia, G.; Barrientos, E.Y.; Rodriguez-Morales, N.M.; Flores, A.; Wrobel, K.; Wrobel, K.; Mendez, I.; Diaz-Munoz, M.; et al. Melatonin reduces lead levels in blood, brain and bone and increases lead excretion in rats subjected to subacute lead treatment. Toxicol. Lett. 2015, 233, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Karabulut-Bulan, O.; Bayrak, B.B.; Arda-Pirincci, P.; Sarikaya-Unal, G.; Us, H.; Yanardag, R. Role of exogenous melatonin on cell proliferation and oxidant/antioxidant system in aluminum-induced renal toxicity. Biol. Trace Elem. Res. 2015, 168, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Alarcon, M.; Ruiz-Ojeda, F.J.; Blanca-Herrera, R.M.; Kaki, A.; Adem, A.; Agil, A. Melatonin administration in diabetes: Regulation of plasma Cr, V, and Mg in young male Zucker diabetic fatty rats. Food Funct. 2014, 5, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Leon, J.; Escames, G.; Rodriguez, M.I.; Lopez, L.C.; Tapias, V.; Entrena, A.; Camacho, E.; Carrion, M.D.; Gallo, M.A.; Espinosa, A.; et al. Inhibition of neuronal nitric oxide synthase activity by N1-acetyl-5-methoxykynuramine, a brain metabolite of melatonin. J. Neurochem. 2006, 98, 2023–2033. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Campa, C.; Gonzalez, A.; Mediavilla, M.D.; Alonso-Gonzalez, C.; Alvarez-Garcia, V.; Sanchez-Barcelo, E.J.; Cos, S. Melatonin inhibits aromatase promoter expression by regulating cyclooxygenases expression and activity in breast cancer cells. Br. J. Cancer 2009, 101, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.C.; Sainz, R.M.; Antoli, I.; Herrera, F.; Martin, V.; Rodriguez, C. Melatonin regulation of antioxidant enzyme gene expression. Cell. Mol. Life Sci. 2002, 59, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Mehrzadi, S.; Safa, M.; Kamrava, S.K.; Darabi, R.; Hayat, P.; Motevalian, M. Protective mechanisms of melatonin against hydrogen peroxide induced toxicity in human bone-marrow derived mesenchymal stem cells. Can. J. Physiol. Pharmacol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Bilici, D.; Suleyman, H.; Banoglu, Z.N.; Kiziltunc, A.; Avci, B.; Ciftcioglu, A.; Bilici, S. Melatonin prevents ethanol-induced gastric mucosal damage possibly due to its antioxidant effect. Dig. Dis. Sci. 2002, 47, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, M.; Bilici, D.; Kufrevioglu, O.I. Effects of melatonin on enzyme activities of glucose-6-phosphate dehydrogenase from human erythrocytes in vitro and from rat erythrocytes in vivo. Pharmacol. Res. 2001, 44, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Gitto, E.; Tan, D.X.; Reiter, R.J.; Karbownik, M.; Manchester, L.C.; Cuzzocrea, S.; Fulia, F.; Barberi, I. Individual and synergistic antioxidative actions of melatonin: Studies with vitamin E, vitamin C, glutathione and desferrioxamine (desferoxamine) in rat liver homogenates. J. Pharm. Pharmacol. 2001, 53, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, F.; Ge, X.; Yan, T.; Chen, X.; Shi, X.; Zhai, Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007, 6, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Chahbouni, M.; Escames, G.; Venegas, C.; Sevilla, B.; Garcia, J.A.; Lopez, L.C.; Munoz-Hoyos, A.; Molina-Carballo, A.; Acuna-Castroviejo, D. Melatonin treatment normalizes plasma pro-inflammatory cytokines and nitrosative/oxidative stress in patients suffering from Duchenne muscular dystrophy. J. Pineal Res. 2010, 48, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Kepka, M.; Szwejser, E.; Pijanowski, L.; Verburg-van Kemenade, B.M.; Chadzinska, M. A role for melatonin in maintaining the pro- and anti-inflammatory balance by influencing leukocyte migration and apoptosis in carp. Dev. Comp. Immunol. 2015, 53, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Perkins, N.D. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat. Rev. Mol. Cell Biol. 2007, 8, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, T.D. Introduction to NF-kappaB: Players, pathways, perspectives. Oncogene 2006, 25, 6680–6684. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.A.; Volt, H.; Venegas, C.; Doerrier, C.; Escames, G.; Lopez, L.C.; Acuna-Castroviejo, D. Disruption of the NF-kappaB/NLRP3 connection by melatonin requires retinoid-related orphan receptor-alpha and blocks the septic response in mice. FASEB J. 2015, 29, 3863–3875. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.P.; Fang, X.L.; Fang, N.; Wang, X.B.; Qian, H.Y.; Cao, Z.; Cheng, Y.; Wang, B.N.; Wang, Y. Melatonin ameliorates vascular endothelial dysfunction, inflammation, and atherosclerosis by suppressing the TLR4/NF-kappaB system in high-fat-fed rabbits. J. Pineal Res. 2013, 55, 388–398. [Google Scholar] [PubMed]
- Yi, C.; Zhang, Y.; Yu, Z.; Xiao, Y.; Wang, J.; Qiu, H.; Yu, W.; Tang, R.; Yuan, Y.; Guo, W.; et al. Melatonin enhances the anti-tumor effect of fisetin by inhibiting COX-2/iNOS and NF-kappaB/p300 signaling pathways. PLoS ONE 2014, 9, e99943. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.J.; Fu, L.; Tang, Z.; Zhang, C.; Qin, L.; Wang, J.; Yu, Z.; Shi, D.; Xiao, X.; Xie, F.; et al. Melatonin inhibits AP-2beta/hTERT, NF-kappaB/COX-2 and Akt/ERK and activates caspase/Cyto C signaling to enhance the antitumor activity of berberine in lung cancer cells. Oncotarget 2016, 7, 2985–3001. [Google Scholar] [PubMed]
- Mayo, J.C.; Sainz, R.M.; Tan, D.X.; Hardeland, R.; Leon, J.; Rodriguez, C.; Reiter, R.J. Anti-inflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophages. J. Neuroimmunol. 2005, 165, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Permpoonputtana, K.; Govitrapong, P. The anti-inflammatory effect of melatonin on methamphetamine-induced proinflammatory mediators in human neuroblastoma dopamine SH-SY5Y cell lines. Neurotox. Res. 2013, 23, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, R.; Nath, C.; Shukla, R. Melatonin attenuated mediators of neuroinflammation and alpha-7 nicotinic acetylcholine receptor mRNA expression in lipopolysaccharide (LPS) stimulated rat astrocytoma cells, C6. Free Radic. Res. 2012, 46, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.D.; Kim, Y.S.; Ko, S.H.; Yoon, I.J.; Cho, S.G.; Chun, Y.H.; Choi, B.J.; Kim, E.C. Cytoprotective and anti-inflammatory effects of melatonin in hydrogen peroxide-stimulated CHON-001 human chondrocyte cell line and rabbit model of osteoarthritis via the SIRT1 pathway. J. Pineal Res. 2012, 53, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Min, K.J.; Jang, J.H.; Kwon, T.K. Inhibitory effects of melatonin on the lipopolysaccharide-induced CC chemokine expression in BV2 murine microglial cells are mediated by suppression of Akt-induced NF-kappaB and STAT/GAS activity. J. Pineal Res. 2012, 52, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.H.; Li, Y.C.; Wang, Z.Q.; Liu, B.; Ye, W.; Ni, L.; Zeng, R.; Miao, S.Y.; Wang, L.F.; Liu, C.W. Protective effect of melatonin on cigarette smoke-induced restenosis in rat carotid arteries after balloon injury. J. Pineal Res. 2014, 57, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Shao, G.; Tian, Y.; Wang, H.; Liu, F.; Xie, G. Protective effects of melatonin on lipopolysaccharide-induced mastitis in mice. Int. Immunopharmacol. 2015, 29, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, M.D.; Garcia-Moreno, H.; Gonzalez-Yanes, C.; Calvo, J.R. Possible involvement of the inhibition of NF-kappaB factor in anti-inflammatory actions that melatonin exerts on mast cells. J. Cell Biochem. 2016, 117, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.J.; Tremblay, A.M.; Deblois, G.; Sylvain-Drolet, G.; Giguere, V. An acetylation switch modulates the transcriptional activity of estrogen-related receptor alpha. Mol. Endocrinol. 2010, 24, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Khan, M.; Jo, M.H.; Jo, M.G.; Amin, F.U.; Kim, M.O. Melatonin stimulates the SIRT1/Nrf2 signaling pathway counteracting lipopolysaccharide (LPS)-induced oxidative stress to rescue postnatal rat brain. CNS Neurosci. Ther. 2016. [Google Scholar] [CrossRef] [PubMed]
- Ge, D.; Dauchy, R.T.; Liu, S.; Zhang, Q.; Mao, L.; Dauchy, E.M.; Blask, D.E.; Hill, S.M.; Rowan, B.G.; Brainard, G.C.; et al. Insulin and IGF1 enhance IL-17-induced chemokine expression through a GSK3B-dependent mechanism: A new target for melatonin’s anti-inflammatory action. J. Pineal Res. 2013, 55, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Koyama, F.C.; Azevedo, M.F.; Budu, A.; Chakrabarti, D.; Garcia, C.R. Melatonin-induced temporal up-regulation of gene expression related to ubiquitin/proteasome system (UPS) in the human malaria parasite Plasmodium falciparum. Int. J. Mol. Sci. 2014, 15, 22320–22330. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Park, Y.S.; Kim, O.S.; Kim, J.H.; Baik, H.W.; Hong, Y.O.; Kim, S.S.; Shin, J.H.; Jun, J.H.; Jo, Y.; et al. Melatonin attenuates dextran sodium sulfate induced colitis with sleep deprivation: Possible mechanism by microarray analysis. Dig. Dis. Sci. 2014, 59, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Ni, Y.F.; Wang, W.C.; Du, H.Y.; Zhang, H.; Zhang, L.; Zhang, W.D.; Jiang, T. Melatonin attenuates intestinal ischemia—Reperfusion-induced lung injury in rats by upregulating N-myc downstream-regulated gene 2. J. Surg. Res. 2015, 194, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Ghosh, S.; Basu, P.; Haldar, C. Daily variation in melatonin level, antioxidant activity and general immune response of peripheral blood mononuclear cells and lymphoid tissues of Indian goat Capra hircus during summer and winter. Indian J. Exp. Biol. 2014, 52, 467–477. [Google Scholar] [PubMed]
- Zhang, M.; Wang, T.; Chen, H.M.; Chen, Y.Q.; Deng, Y.C.; Li, Y.T. Serum levels of interleukin-1 beta, interleukin-6 and melatonin over summer and winter in kidney deficiency syndrome in Bizheng rats. Chin. Med. Sci. J. 2014, 29, 107–111. [Google Scholar] [CrossRef]
- Provinciali, M.; Di Stefano, G.; Bulian, D.; Tibaldi, A.; Fabris, N. Effect of melatonin and pineal grafting on thymocyte apoptosis in aging mice. Mech. Ageing Dev. 1996, 90, 1–19. [Google Scholar] [CrossRef]
- Fernandes, P.A.; Cecon, E.; Markus, R.P.; Ferreira, Z.S. Effect of TNF-alpha on the melatonin synthetic pathway in the rat pineal gland: Basis for a ‘feedback’ of the immune response on circadian timing. J. Pineal Res. 2006, 41, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Lissoni, P.; Rovelli, F.; Giani, L.; Fumagalli, L.; Mandala, M. Immunomodulatory effects of IL-12 in relation to the pineal endocrine function in metastatic cancer patients. Nat. Immun. 1998, 16, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Withyachumnarnkul, B.; Nonaka, K.O.; Santana, C.; Attia, A.M.; Reiter, R.J. Interferon-gamma modulates melatonin production in rat pineal glands in organ culture. J. Interferon Res. 1990, 10, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J. CSF generation by pineal gland results in a robust melatonin circadian rhythm in the third ventricle as an unique light/dark signal. Med. Hypotheses 2016, 86, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Markus, R.P.; Cecon, E.; Pires-Lapa, M.A. Immune-pineal axis: Nuclear factor kappaB (NF-êB) mediates the shift in the melatonin source from pinealocytes to immune competent cells. Int. J. Mol. Sci. 2013, 14, 10979–10997. [Google Scholar] [CrossRef] [PubMed]
- Pontes, G.N.; Cardoso, E.C.; Carneiro-Sampaio, M.M.; Markus, R.P. Injury switches melatonin production source from endocrine (pineal) to paracrine (phagocytes)—Melatonin in human colostrum and colostrum phagocytes. J. Pineal Res. 2006, 41, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Haldar, C. Photoperiodic regulation of MT1 and MT2 melatonin receptor expression in spleen and thymus of a tropical rodent Funambulus pennanti during reproductively active and inactive phases. Chronobiol. Int. 2010, 27, 446–462. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Perganeda, A.; Pozo, D.; Guerrero, J.M.; Calvo, J.R. Signal transduction for melatonin in human lymphocytes: Involvement of a pertussis toxin-sensitive G protein. J. Immunol. 1997, 159, 3774–3781. [Google Scholar] [PubMed]
- Vishwas, D.K.; Haldar, C. Photoperiodic induced melatonin regulates immunity and expression pattern of melatonin receptor MT1 in spleen and bone marrow mononuclear cells of male golden hamster. J. Photochem. Photobiol. B 2013, 128, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Lardone, P.J.; Rubio, A.; Cerrillo, I.; Gomez-Corvera, A.; Carrillo-Vico, A.; Sanchez-Hidalgo, M.; Guerrero, J.M.; Fernandez-Riejos, P.; Sanchez-Margalet, V.; Molinero, P. Blocking of melatonin synthesis and MT(1) receptor impairs the activation of Jurkat T cells. Cell Mol. Life Sci. 2010, 67, 3163–3172. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, M.; Mihandoost, E.; Shirazi, A.; Sepehrizadeh, Z.; Bazzaz, J.T.; Ghazi-khansari, M. Melatonin may play a role in modulation of bax and bcl-2 expression levels to protect rat peripheral blood lymphocytes from gamma irradiation-induced apoptosis. Mutat. Res. 2012, 738–739, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Dong, Y.; Cao, J.; Wang, Z.; Zhang, Z.; Chen, Y. Developmental changes of melatonin receptor expression in the spleen of the chicken, Gallus domesticus. Acta Histochem. 2015, 117, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, M.K.; Hubbard, G.B.; Champney, T.H.; Vaughan, G.M.; Little, J.C.; Reiter, R.J. Splenic hypertrophy and extramedullary hematopoiesis induced in male Syrian hamsters by short photoperiod or melatonin injections and reversed by melatonin pellets or pinealectomy. Am. J. Anat. 1987, 179, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Lopez Gonzalez, M.A.; Guerrero, J.M.; Ceballo Pedraja, J.M.; Delgado Moreno, F. Melatonin in palate tonsils with recurrent acute tonsillitis and tonsillar hypertrophy. Acta Otorrinolaringol. Esp. 1998, 49, 625–628. [Google Scholar] [PubMed]
- Barjavel, M.J.; Mamdouh, Z.; Raghbate, N.; Bakouche, O. Differential expression of the melatonin receptor in human monocytes. J. Immunol. 1998, 160, 1191–1197. [Google Scholar] [PubMed]
- Kim, Y.O.; Ahn, Y.K.; Kim, J.H. Influence of melatonin on immunotoxicity of cadmium. Int. J. Immunopharmacol. 2000, 22, 275–284. [Google Scholar] [CrossRef]
- Silva, S.O.; Rodrigues, M.R.; Ximenes, V.F.; Bueno-da-Silva, A.E.; Amarante-Mendes, G.P.; Campa, A. Neutrophils as a specific target for melatonin and kynuramines: Effects on cytokine release. J. Neuroimmunol. 2004, 156, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Suke, S.G.; Pathak, R.; Ahmed, R.S.; Tripathi, A.K.; Banerjee, B.D. Melatonin treatment prevents modulation of cell-mediated immune response induced by propoxur in rats. Indian J. Biochem. Biophys. 2008, 45, 278–281. [Google Scholar] [PubMed]
- Ghosh, S.; Singh, A.K.; Haldar, C. Seasonal modulation of immunity by melatonin and gonadal steroids in a short day breeder goat Capra hircus. Theriogenology 2014, 82, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Maurino, S.; Pozo, D.; Carrillo-Vico, A.; Calvo, J.R.; Guerrero, J.M. Melatonin activates Th1 lymphocytes by increasing IL-12 production. Life Sci. 1999, 65, 2143–2150. [Google Scholar] [CrossRef]
- Xia, M.Z.; Liang, Y.L.; Wang, H.; Chen, X.; Huang, Y.Y.; Zhang, Z.H.; Chen, Y.H.; Zhang, C.; Zhao, M.; Xu, D.X.; et al. Melatonin modulates TLR4-mediated inflammatory genes through MyD88- and TRIF-dependent signaling pathways in lipopolysaccharide-stimulated RAW264.7 cells. J. Pineal Res. 2012, 53, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Vico, A.; Reiter, R.J.; Lardone, P.J.; Herrera, J.L.; Fernandez-Montesinos, R.; Guerrero, J.M.; Pozo, D. The modulatory role of melatonin on immune responsiveness. Curr. Opin. Investig. Drugs 2006, 7, 423–431. [Google Scholar] [PubMed]
- Carrillo-Vico, A.; Lardone, P.J.; Fernandez-Santos, J.M.; Martin-Lacave, I.; Calvo, J.R.; Karasek, M.; Guerrero, J.M. Human lymphocyte-synthesized melatonin is involved in the regulation of the interleukin-2/interleukin-2 receptor system. J. Clin. Endocrinol. Metab. 2005, 90, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Morrey, K.M.; McLachlan, J.A.; Serkin, C.D.; Bakouche, O. Activation of human monocytes by the pineal hormone melatonin. J. Immunol. 1994, 153, 2671–2680. [Google Scholar] [PubMed]
- Bouhafs, R.K.; Jarstrand, C. Effects of antioxidants on surfactant peroxidation by stimulated human polymorphonuclear leukocytes. Free Radic. Res. 2002, 36, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Vishwas, D.K.; Mukherjee, A.; Haldar, C.; Dash, D.; Nayak, M.K. Improvement of oxidative stress and immunity by melatonin: An age dependent study in golden hamster. Exp. Gerontol. 2013, 48, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Haldar, C. Experimentally induced stress, oxidative load and changes in immunity in a tropical wild bird, Perdicula asiatica: Involvement of melatonin and glucocorticoid receptors. Zoology 2014, 117, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, M. Pharmacology of ramelteon, a selective MT1/MT2 receptor agonist: A novel therapeutic drug for sleep disorders. CNS Neurosci. Ther. 2009, 15, 32–51. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.S.; Green, E.W.; Zhao, Y.; van Ooijen, G.; Olmedo, M.; Qin, X.; Xu, Y.; Pan, M.; Valekunja, U.K.; Feeney, K.A.; et al. Peroxiredoxins are conserved markers of circadian rhythms. Nature 2012, 485, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, S.R.; Luisi, N.; Balasubramanian, R.; Reeves, K.W. Sleep duration and endometrial cancer risk. Cancer Causes Control 2012, 23, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gan, L.; Luo, D.; Sun, C. Melatonin promotes circadian rhythm-induced proliferation through interaction of Clock/HDAC3/c-Myc in mice adipose tissue. J. Pineal Res. 2016. [Google Scholar] [CrossRef]
- Tamura, H.; Takasaki, A.; Taketani, T.; Tanabe, M.; Lee, L.; Tamura, I.; Maekawa, R.; Aasada, H.; Yamagata, Y.; Sugino, N. Melatonin and female reproduction. J. Obstet. Gynaecol. Res. 2014, 40, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lundmark, P.O.; Pandi-Perumal, S.R.; Srinivasan, V.; Cardinali, D.P.; Rosenstein, R.E. Melatonin in the eye: Implications for glaucoma. Exp. Eye Res. 2007, 84, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Espino, J.; Pariente, J.A.; Rodriguez, A.B. Role of melatonin on diabetes-related metabolic disorders. World J. Diabetes 2011, 2, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Neurobiology, pathophysiology, and treatment of melatonin deficiency and dysfunction. Sci. World J. 2012, 2012, 640389. [Google Scholar] [CrossRef] [PubMed]
- Torres-Farfan, C.; Seron-Ferre, M.; Dinet, V.; Korf, H.W. Immunocytochemical demonstration of day/night changes of clock gene protein levels in the murine adrenal gland: Differences between melatonin-proficient (C3H) and melatonin-deficient (C57BL) mice. J. Pineal Res. 2006, 40, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.D.; Tournier, B.B.; Andersson, H.; Masson-Pevet, M.; Lincoln, G.A.; Hazlerigg, D.G. Multiple effects of melatonin on rhythmic clock gene expression in the mammalian pars tuberalis. Endocrinology 2006, 147, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Dinet, V.; Ansari, N.; Torres-Farfan, C.; Korf, H.W. Clock gene expression in the retina of melatonin-proficient (C3H) and melatonin-deficient (C57BL) mice. J. Pineal Res. 2007, 42, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Chellappa, S.L.; Viola, A.U.; Schmidt, C.; Bachmann, V.; Gabel, V.; Maire, M.; Reichert, C.F.; Valomon, A.; Gotz, T.; Landolt, H.P.; et al. Human melatonin and alerting response to blue-enriched light depend on a polymorphism in the clock gene PER3. J. Clin. Endocrinol. Metab. 2012, 97, E433–E437. [Google Scholar] [CrossRef] [PubMed]
- West, A.; Dupre, S.M.; Yu, L.; Paton, I.R.; Miedzinska, K.; McNeilly, A.S.; Davis, J.R.; Burt, D.W.; Loudon, A.S. Npas4 is activated by melatonin, and drives the clock gene Cry1 in the ovine pars tuberalis. Mol. Endocrinol. 2013, 27, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Bracci, M.; Manzella, N.; Copertaro, A.; Staffolani, S.; Strafella, E.; Barbaresi, M.; Copertaro, B.; Rapisarda, V.; Valentino, M.; Santarelli, L. Rotating-shift nurses after a day off: Peripheral clock gene expression, urinary melatonin, and serum 17-beta-estradiol levels. Scand. J. Work Environ. Health 2014, 40, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Ikeno, T.; Nelson, R.J. Acute melatonin treatment alters dendritic morphology and circadian clock gene expression in the hippocampus of Siberian hamsters. Hippocampus 2015, 25, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.D.; Iwamoto, A.; Kawai, M.; Goda, R.; Matsuo, H.; Otsuka, T.; Nagasawa, M.; Furuse, M.; Yasuo, S. Melatonin adjusts the expression pattern of clock genes in the suprachiasmatic nucleus and induces antidepressant-like effect in a mouse model of seasonal affective disorder. Chronobiol. Int. 2015, 32, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Rodenbeck, A.; Hajak, G. Neuroendocrine dysregulation in primary insomnia. Rev. Neurol. 2001, 157, S57–S61. [Google Scholar] [PubMed]
- Takaesu, Y.; Futenma, K.; Kobayashi, M.; Komada, Y.; Tanaka, N.; Yamashina, A.; Inoue, Y. A preliminary study on the relationships between diurnal melatonin secretion profile and sleep variables in patients emergently admitted to the coronary care unit. Chronobiol. Int. 2015, 32, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Evely, K.M.; Hudson, R.L.; Dubocovich, M.L.; Haj-Dahmane, S. Melatonin receptor activation increases glutamatergic synaptic transmission in the rat medial lateral habenula. Synapse 2016, 70, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Waldhauser, F.; Kovacs, J.; Reiter, E. Age-related changes in melatonin levels in humans and its potential consequences for sleep disorders. Exp. Gerontol. 1998, 33, 759–772. [Google Scholar] [CrossRef]
- Carpentieri, A.; Diaz De Barboza, G.; Areco, V.; Peralta Lopez, M.; Tolosa De Talamoni, N. New perspectives in melatonin uses. Pharmacol. Res. 2012, 65, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, P.; Zisapel, N. Prolonged-release formulation of melatonin (Circadin) for the treatment of insomnia. Expert Opin. Pharmacother. 2012, 13, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Pandi-Perumal, S.R.; Trahkt, I.; Spence, D.W.; Poeggeler, B.; Hardeland, R.; Cardinali, D.P. Melatonin and melatonergic drugs on sleep: Possible mechanisms of action. Int. J. Neurosci. 2009, 119, 821–846. [Google Scholar] [CrossRef] [PubMed]
- Vimala, P.V.; Bhutada, P.S.; Patel, F.R. Therapeutic potential of agomelatine in epilepsy and epileptic complications. Med. Hypotheses 2014, 82, 105–110. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). Cancer Fact Sheet No. 297. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=2&ved=0ahUKEwjT_cjhzYfTAhVIqJQKHYFWCRgQFggjMAE&url=http%3A%2F%2Fwww.afro.who.int%2Findex.php%3Foption%3Dcom_docman%26task%3Ddoc_download%26gid%3D6139&usg=AFQjCNE1HISbfHk2ijLPCzy3VxfgMAbK3Q (accessed on 20 November 2016).
- Li, F.; Li, S.; Li, H.B.; Deng, G.F.; Ling, W.H.; Xu, X.R. Antiproliferative activities of tea and herbal infusions. Food Funct. 2013, 4, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhou, Y.; Li, Y.; Xu, D.P.; Li, S.; Li, H.B. Spices for prevention and treatment of cancers. Nutrients 2016, 8, 495. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Y.; Zhou, T.; Zheng, J.; Li, S.; Li, H.B. Dietary natural products for prevention and treatment of liver cancer. Nutrients 2016, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural polyphenols for prevention and treatment of cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.M.; Lin, W.Y.; Shen, C.C.; Pan, H.C.; Keh-Bin, W.; Chen, Y.C.; Jan, Y.J.; Lai, D.W.; Tang, S.C.; Tien, H.R.; et al. Melatonin set out to ER stress signaling thwarts epithelial mesenchymal transition and peritoneal dissemination via calpain-mediated C/EBPbeta and NFkappaB cleavage. J. Pineal Res. 2016, 60, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Hevia, D.; Gonzalez-Menendez, P.; Quiros-Gonzalez, I.; Miar, A.; Rodriguez-Garcia, A.; Tan, D.X.; Reiter, R.J.; Mayo, J.C.; Sainz, R.M. Melatonin uptake through glucose transporters: A new target for melatonin inhibition of cancer. J. Pineal Res. 2015, 58, 234–250. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.M.; Woo, S.H.; Oh, S.T.; Hong, S.E.; Choe, T.B.; Ye, S.K.; Kim, E.K.; Seong, M.K.; Kim, H.A.; Noh, W.C.; et al. Melatonin enhances arsenic trioxide-induced cell death via sustained upregulation of Redd1 expression in breast cancer cells. Mol. Cell Endocrinol. 2016, 422, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Borin, T.F.; Arbab, A.S.; Gelaleti, G.B.; Ferreira, L.C.; Moschetta, M.G.; Jardim-Perassi, B.V.; Iskander, A.S.; Varma, N.R.; Shankar, A.; Coimbra, V.B.; et al. Melatonin decreases breast cancer metastasis by modulating Rho-associated kinase protein-1 expression. J. Pineal Res. 2016, 60, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Ziolko, E.; Kokot, T.; Skubis, A.; Sikora, B.; Szota-Czyz, J.; Kruszniewska-Rajs, C.; Wierzgon, J.; Mazurek, U.; Grochowska-Niedworok, E.; Muc-Wierzgon, M. The profile of melatonin receptors gene expression and genes associated with their activity in colorectal cancer: A preliminary report. J. Biol. Regul. Homeost. Agents 2015, 29, 823–828. [Google Scholar] [PubMed]
- Watanabe, M.; Kobayashi, Y.; Takahashi, N.; Kiguchi, K.; Ishizuka, B. Expression of melatonin receptor (MT1) and interaction between melatonin and estrogen in endometrial cancer cell line. J. Obstet. Gynaecol. Res. 2008, 34, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Chottanapund, S.; Van Duursen, M.B.; Navasumrit, P.; Hunsonti, P.; Timtavorn, S.; Ruchirawat, M.; Van den Berg, M. Anti-aromatase effect of resveratrol and melatonin on hormonal positive breast cancer cells co-cultured with breast adipose fibroblasts. Toxicol. In Vitro 2014, 28, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E.J.; Won, G.; Lee, J.; Lee, S.; Kim, S.H. Upregulation of miRNA3195 and miRNA374b Mediates the anti-angiogenic properties of melatonin in Hypoxic PC-3 prostate cancer cells. J. Cancer 2015, 6, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.Y.; Li, W.M.; Zhou, L.L.; Lu, Q.N.; He, W. Melatonin induces apoptosis of colorectal cancer cells through HDAC4 nuclear import mediated by CaMKII inactivation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 58, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, R.; Carbajo-Pescador, S.; Prieto-Dominguez, N.; Garcia-Palomo, A.; Gonzalez-Gallego, J.; Mauriz, J.L. Inhibition of matrix metalloproteinase-9 and nuclear factor kappa B contribute to melatonin prevention of motility and invasiveness in HepG2 liver cancer cells. J. Pineal Res. 2014, 56, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.W.; Lee, L.M.; Lee, W.J.; Chu, C.Y.; Tan, P.; Yang, Y.C.; Chen, W.Y.; Yang, S.F.; Hsiao, M.; Chien, M.H. Melatonin inhibits MMP-9 transactivation and renal cell carcinoma metastasis by suppressing Akt-MAPKs pathway and NF-kappaB DNA-binding activity. J. Pineal Res. 2016, 60, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Gonzalez, C.; Gonzalez, A.; Martinez-Campa, C.; Menendez-Menendez, J.; Gomez-Arozamena, J.; Garcia-Vidal, A.; Cos, S. Melatonin enhancement of the radiosensitivity of human breast cancer cells is associated with the modulation of proteins involved in estrogen biosynthesis. Cancer Lett. 2016, 370, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, M.A.; Elkayam, R.; Gelernter, I.; Pfeffer, R.M. Melatonin for prevention of breast radiation dermatitis: A phase II, prospective, double-blind rendomized trial. Isr. Med. Assoc. J. 2016, 18, 188–192. [Google Scholar] [PubMed]
- Madhu, P.; Reddy, K.P.; Reddy, P.S. Role of melatonin in mitigating chemotherapy-induced testicular dysfunction in Wistar rats. Drug Chem. Toxicol. 2016, 39, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Jin, B.Z.; Ai, F.; Duan, C.H.; Lu, Y.Z.; Dong, T.F.; Fu, Q.L. The efficacy and safety of melatonin in concurrent chemotherapy or radiotherapy for solid tumors: A meta-analysis of randomized controlled trials. Cancer Chemother. Pharmacol. 2012, 69, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cardiovascular Diseases. Available online: http://www.who.int/mediacentre/factsheets/fs317/en/ (accessed on 8 December 2016).
- Wolf, K.; Braun, A.; Haining, E.J.; Tseng, Y.L.; Kraft, P.; Schuhmann, M.K.; Gotru, S.K.; Chen, W.; Hermanns, H.M.; Stoll, G.; et al. Partially defective store operated calcium entry and Hem(ITAM) signaling in platelets of serotonin transporter deficient Mice. PLoS ONE 2016, 11, e147664. [Google Scholar] [CrossRef] [PubMed]
- Grossman, E.; Laudon, M.; Yalcin, R.; Zengil, H.; Peleg, E.; Sharabi, Y.; Kamari, Y.; Shen-Orr, Z.; Zisapel, N. Melatonin reduces night blood pressure in patients with nocturnal hypertension. Am. J. Med. 2006, 119, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Arangino, S.; Cagnacci, A.; Angiolucci, M.; Vacca, A.M.; Longu, G.; Volpe, A.; Melis, G.B. Effects of melatonin on vascular reactivity, catecholamine levels, and blood pressure in healthy men. Am. J. Cardiol. 1999, 83, 1417–1419. [Google Scholar] [CrossRef]
- Wu, Q.; Jing, Y.; Yuan, X.; Zhang, X.; Li, B.; Liu, M.; Wang, B.; Li, H.; Liu, S.; Xiu, R. Melatonin treatment protects against acute spinal cord injury-induced disruption of blood spinal cord barrier in mice. J. Mol. Neurosci. 2014, 54, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Girouard, H.; Denault, C.; Chulak, C.; de Champlain, J. Treatment by N-acetylcysteine and melatonin increases cardiac baroreflex and improves antioxidant reserve. Am. J. Hypertens. 2004, 17, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Girouard, H.; Chulak, C.; LeJossec, M.; Lamontagne, D.; de Champlain, J. Chronic antioxidant treatment improves sympathetic functions and beta-adrenergic pathway in the spontaneously hypertensive rats. J. Hypertens. 2003, 21, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Gurses, I.; Ozeren, M.; Serin, M.; Yucel, N.; Erkal, H.S. Histopathological evaluation of melatonin as a protective agent in heart injury induced by radiation in a rat model. Pathol. Res. Pract. 2014, 210, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fan, C.; Deng, C.; Zhao, L.; Hu, W.; Di, S.; Ma, Z.; Zhang, Y.; Qin, Z.; Jin, Z.; et al. Melatonin reverses flow shear stress-induced injury in bone marrow mesenchymal stem cells via activation of AMP-activated protein kinase signaling. J. Pineal Res. 2016, 60, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Huang, W.; Li, X.; Gao, L.; Su, T.; Li, X.; Ma, S.; Liu, T.; Li, C.; Chen, J.; et al. Melatonin facilitates adipose-derived mesenchymal stem cells to repair the murine infarcted heart via the SIRT1 signaling pathway. J. Pineal Res. 2016, 60, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Duan, W.; Jin, Z.; Yi, W.; Yan, J.; Zhang, S.; Wang, N.; Liang, Z.; Li, Y.; Chen, W.; et al. JAK2/STAT3 activation by melatonin attenuates the mitochondrial oxidative damage induced by myocardial ischemia/reperfusion injury. J. Pineal Res. 2013, 55, 275–286. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Zhao, L.; Xi, C.; Li, H.; Shen, G.; Liu, H.; Zhang, S.; Sun, L. Melatonin attenuates sepsis-induced cardiac dysfunction via a PI3K/Akt-dependent mechanism. Basic Res. Cardiol. 2016, 111, 8. [Google Scholar] [CrossRef] [PubMed]
- VanKirk, T.; Powers, E.; Dowse, H.B. Melatonin increases the regularity of cardiac rhythmicity in the Drosophila heart in both wild-type and strains bearing pathogenic mutations. J. Comp. Physiol. B 2017, 187, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Rodriguez, A.; Abreu-Gonzalez, P.; Piccolo, R.; Galasso, G.; Reiter, R.J. Melatonin is associated with reverse remodeling after cardiac resynchronization therapy in patients with heart failure and ventricular dyssynchrony. Int. J. Cardiol. 2016, 221, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, L.; Yang, Y.; Guo, Y.; Fan, Y.; Zhang, M.; Man, W.; Gao, E.; Hu, W.; Reiter, R.J.; et al. Melatonin alleviates postinfarction cardiac remodeling and dysfunction by inhibiting Mst1. J. Pineal Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Status Report on Noncommunicable Diseases 2014. Available online: http://www.who.int/nmh/publications/ncd-status-report-2014/en/ (accessed on 9 December 2016).
- Simko, F.; Paulis, L. Melatonin as a potential antihypertensive treatment. J. Pineal Res. 2007, 42, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Leon, J.; Kilic, U.; Kilic, E. When melatonin gets on your nerves: Its beneficial actions in experimental models of stroke. Exp. Biol. Med. 2005, 230, 104–117. [Google Scholar]
- Hung, M.W.; Kravtsov, G.M.; Lau, C.F.; Poon, A.M.; Tipoe, G.L.; Fung, M.L. Melatonin ameliorates endothelial dysfunction, vascular inflammation, and systemic hypertension in rats with chronic intermittent hypoxia. J. Pineal Res. 2013, 55, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.F.; Guo, W.J.; Li, L.; Shao, S.; Qiao, X.; Shao, J.J.; Zhang, Q.; Li, R.S.; Wang, L.H. Melatonin attenuates hypertension-induced renal injury partially through inhibiting oxidative stress in rats. Mol. Med. Rep. 2016, 13, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Pechanova, O.; Paulis, L.; Simko, F. Peripheral and central effects of melatonin on blood pressure regulation. Int. J. Mol. Sci. 2014, 15, 17920–17937. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.Y.; Shen, F.C.; Xie, D.; Han, Q.P.; Fang, M.; Chen, C.B.; Zeng, H.K. Progress in drug treatment of cerebral edema. Mini Rev. Med. Chem. 2016, 16, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Lv, J.; Li, W.; Zhang, P.; Mao, C.; Xu, Z. Exogenous melatonin reduced blood pressure in late-term ovine fetus via MT1/MT2 receptor pathways. Reprod. Biol. 2016, 16, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Butun, I.; Ekmekci, H.; Ciftci, O.; Sonmez, H.; Caner, M.; Altug, T.; Kokoglu, E. The effects of different doses of melatonin on lipid peroxidation in diet-induced hypercholesterolemic rats. Bratisl. Lek. Listy 2013, 114, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Salmanoglu, D.S.; Gurpinar, T.; Vural, K.; Ekerbicer, N.; Dariverenli, E.; Var, A. Melatonin and L-carnitin improves endothelial disfunction and oxidative stress in Type 2 diabetic rats. Redox Biol. 2016, 8, 199–204. [Google Scholar] [CrossRef] [PubMed]
- She, M.; Hu, X.; Su, Z.; Zhang, C.; Yang, S.; Ding, L.; Laudon, M.; Yin, W. Piromelatine, a novel melatonin receptor agonist, stabilizes metabolic profiles and ameliorates insulin resistance in chronic sleep restricted rats. Eur. J. Pharmacol. 2014, 727, 60–65. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Report on Diabetes. Available online: http://www.who.int/mediacentre/factsheets/fs312/en/ (accessed on 18 December 2016).
- Zavodnik, I.B.; Lapshina, E.A.; Cheshchevik, V.T.; Dremza, I.K.; Kujawa, J.; Zabrodskaya, S.V.; Reiter, R.J. Melatonin and succinate reduce rat liver mitochondrial dysfunction in diabetes. J. Physiol. Pharmacol. 2011, 62, 421–427. [Google Scholar] [PubMed]
- Faria, J.A.; Kinote, A.; Ignacio-Souza, L.M.; de Araujo, T.M.; Razolli, D.S.; Doneda, D.L.; Paschoal, L.B.; Lellis-Santos, C.; Bertolini, G.L.; Velloso, L.A.; et al. Melatonin acts through MT1/MT2 receptors to activate hypothalamic Akt and suppress hepatic gluconeogenesis in rats. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E230–E242. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Liang, H.; Dong, X.; Zhao, G.; Jin, Z.; Zhai, M.; Yang, Y.; Chen, W.; Liu, J.; Yi, W.; et al. Reduced silent information regulator 1 signaling exacerbates myocardial ischemia-reperfusion injury in type 2 diabetic rats and the protective effect of melatonin. J. Pineal Res. 2015, 59, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Costes, S.; Boss, M.; Thomas, A.P.; Matveyenko, A.V. Activation of melatonin signaling promotes beta-cell survival and function. Mol. Endocrinol. 2015, 29, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Wongchitrat, P.; Lansubsakul, N.; Kamsrijai, U.; Sae-Ung, K.; Mukda, S.; Govitrapong, P. Melatonin attenuates the high-fat diet and streptozotocin-induced reduction in rat hippocampal neurogenesis. Neurochem. Int. 2016, 100, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Yildirimturk, S.; Batu, S.; Alatli, C.; Olgac, V.; Firat, D.; Sirin, Y. The effects of supplemental melatonin administration on the healing of bone defects in streptozotocin-induced diabetic rats. J. Appl. Oral Sci. 2016, 24, 239–249. [Google Scholar] [CrossRef] [PubMed]
- WHO. Obesity and Overweight. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 18 December 2016).
- Szewczyk-Golec, K.; Wozniak, A.; Reiter, R.J. Inter-relationships of the chronobiotic, melatonin, with leptin and adiponectin: Implications for obesity. J. Pineal Res. 2015, 59, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Aranda, A.; Fernandez-Vazquez, G.; Campos, D.; Tassi, M.; Velasco-Perez, L.; Tan, D.X.; Reiter, R.J.; Agil, A. Melatonin induces browning of inguinal white adipose tissue in Zucker diabetic fatty rats. J. Pineal Res. 2013, 55, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Winiarska, K.; Focht, D.; Sierakowski, B.; Lewandowski, K.; Orlowska, M.; Usarek, M. NADPH oxidase inhibitor, apocynin, improves renal glutathione status in Zucker diabetic fatty rats: A comparison with melatonin. Chem. Biol. Interact. 2014, 218, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Sharabiani, M.T.; Vermeulen, R.; Scoccianti, C.; Hosnijeh, F.S.; Minelli, L.; Sacerdote, C.; Palli, D.; Krogh, V.; Tumino, R.; Chiodini, P.; et al. Immunologic profile of excessive body weight. Biomarkers 2011, 16, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Favero, G.; Stacchiotti, A.; Castrezzati, S.; Bonomini, F.; Albanese, M.; Rezzani, R.; Rodella, L.F. Melatonin reduces obesity and restores adipokine patterns and metabolism in obese (ob/ob) mice. Nutr. Res. 2015, 35, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Chatterjee, S.; Li, L.; Kim, J.M.; Lee, J.; Yechoor, V.K.; Minze, L.J.; Hsueh, W.; Ma, K. The clock gene, brain and muscle Arnt-like 1, regulates adipogenesis via Wnt signaling pathway. FASEB J. 2012, 26, 3453–3463. [Google Scholar] [CrossRef] [PubMed]
- Otway, D.T.; Mantele, S.; Bretschneider, S.; Wright, J.; Trayhurn, P.; Skene, D.J.; Robertson, M.D.; Johnston, J.D. Rhythmic diurnal gene expression in human adipose tissue from individuals who are lean, overweight, and type 2 diabetic. Diabetes 2011, 60, 1577–1581. [Google Scholar] [CrossRef] [PubMed]
- McFadden, E.; Jones, M.E.; Schoemaker, M.J.; Ashworth, A.; Swerdlow, A.J. The relationship between obesity and exposure to light at night: Cross-sectional analyses of over 100,000 women in the Breakthrough Generations Study. Am. J. Epidemiol. 2014, 180, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Nduhirabandi, F.; Huisamen, B.; Strijdom, H.; Blackhurst, D.; Lochner, A. Short-term melatonin consumption protects the heart of obese rats independent of body weight change and visceral adiposity. J. Pineal Res. 2014, 57, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, A.; Favero, G.; Giugno, L.; Lavazza, A.; Reiter, R.J.; Rodella, L.F.; Rezzani, R. Mitochondrial and metabolic dysfunction in renal convoluted tubules of obese mice: Protective role of melatonin. PLoS ONE 2014, 9, e111141. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, X.; Chen, J.; Song, K.; Gusdon, A.M.; Li, L.; Bu, L.; Qu, S. Melatonin improves non-alcoholic fatty liver disease via MAPK-JNK/P38 signaling in high-fat-diet-induced obese mice. Lipids Health Dis. 2016, 15, 202. [Google Scholar] [CrossRef] [PubMed]
- Sagrillo-Fagundes, L.; Assuncao Salustiano, E.M.; Yen, P.W.; Soliman, A.; Vaillancourt, C. Melatonin in pregnancy: Effects on brain development and CNS programming disorders. Curr. Pharm. Des. 2016, 22, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Wang, H.; Xu, J.; Li, T.; Zhang, L.; Ding, Y.; Zhu, L.; He, J.; Zhou, M. Melatonin stimulates antioxidant enzymes and reduces oxidative stress in experimental traumatic brain injury: The Nrf2-ARE signaling pathway as a potential mechanism. Free Radic. Biol. Med. 2014, 73, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, S.; Dong, Y.; Fan, C.; Zhao, L.; Yang, X.; Li, J.; Di, S.; Yue, L.; Liang, G.; et al. Melatonin prevents cell death and mitochondrial dysfunction via a SIRT1-dependent mechanism during ischemic-stroke in mice. J. Pineal Res. 2015, 58, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, G.; Li, J.; Qian, C.; Mo, H.; Gu, C.; Yan, F.; Yan, W.; Wang, L. Melatonin attenuates inflammatory response-induced brain edema in early brain injury following a subarachnoid hemorrhage: A possible role for the regulation of pro-inflammatory cytokines. J. Pineal Res. 2014, 57, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Lee, M.Y.; Chen, H.Y.; Hsu, Y.S.; Wu, T.S.; Chen, S.T.; Chang, G.L. Melatonin attenuates gray and white matter damage in a mouse model of transient focal cerebral ischemia. J. Pineal Res. 2005, 38, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Pazar, A.; Kolgazi, M.; Memisoglu, A.; Bahadir, E.; Sirvanci, S.; Yaman, A.; Yegen, B.C.; Ozek, E. The neuroprotective and anti-apoptotic effects of melatonin on hemolytic hyperbilirubinemia-induced oxidative brain damage. J. Pineal Res. 2016, 60, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Keskin, I.; Kaplan, S.; Kalkan, S.; Sutcu, M.; Ulkay, M.B.; Esener, O.B. Evaluation of neuroprotection by melatonin against adverse effects of prenatal exposure to a nonsteroidal anti-inflammatory drug during peripheral nerve development. Int. J. Dev. Neurosci. 2015, 41, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Aranda, M.L.; Fleitas, M.F.G.; De Laurentiis, A.; Sarmiento, M.I.K.; Chianelli, M.; Sande, P.H.; Dorfman, D.; Rosenstein, R.E. Neuroprotective effect of melatonin in experimental optic neuritis in rats. J. Pineal Res. 2016, 60, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Willis, G.L. Parkinson’s disease as a neuroendocrine disorder of circadian function: Dopamine-melatonin imbalance and the visual system in the genesis and progression of the degenerative process. Rev. Neurosci. 2008, 19, 245–316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, P.; Ren, L.; Hu, C.; Bi, J. Protective effect of melatonin on soluble Abeta1–42-induced memory impairment, astrogliosis, and synaptic dysfunction via the Musashi1/Notch1/Hes1 signaling pathway in the rat hippocampus. Alzheimers Res. Ther. 2016, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Breen, D.P.; Nombela, C.; Vuono, R.; Jones, P.S.; Fisher, K.; Burn, D.J.; Brooks, D.J.; Reddy, A.B.; Rowe, J.B.; Barker, R.A. Hypothalamic volume loss is associated with reduced melatonin output in Parkinson’s disease. Mov. Disord. 2016, 31, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ran, D.; Zhao, X.; Huang, Y.; Long, S.; Liang, F.; Guo, W.; Nucifora, F.C., Jr.; Gu, H.; Lu, X.; et al. Melatonin attenuates hLRRK2-induced sleep disturbances and synaptic dysfunction in a Drosophila model of Parkinson’s disease. Mol. Med. Rep. 2016, 13, 3936–3944. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P.; Vigo, D.E.; Olivar, N.; Vidal, M.F.; Furio, A.M.; Brusco, L.I. Therapeutic application of melatonin in mild cognitive impairment. Am. J. Neurodegener. Dis. 2012, 1, 280–291. [Google Scholar] [PubMed]
- Mukda, S.; Panmanee, J.; Boontem, P.; Govitrapong, P. Melatonin administration reverses the alteration of amyloid precursor protein-cleaving secretases expression in aged mouse hippocampus. Neurosci. Lett. 2016, 621, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Waseem, M.; Tabassum, H.; Parvez, S. Neuroprotective effects of melatonin as evidenced by abrogation of oxaliplatin induced behavioral alterations, mitochondrial dysfunction and neurotoxicity in rat brain. Mitochondrion 2016, 30, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Li, J.G.; Zhang, G.W.; Meng, Z.Z.; Wang, L.Z.; Liu, H.Y.; Liu, Q.; Buren, B. Neuroprotective effect of acute melatonin treatment on hippocampal neurons against irradiation by inhibition of caspase-3. Exp. Ther. Med. 2016, 11, 2385–2390. [Google Scholar] [CrossRef] [PubMed]
- Koc, G.E.; Kaplan, S.; Altun, G.; Gumus, H.; Deniz, O.G.; Aydin, I.; Onger, M.E.; Altunkaynak, Z. Neuroprotective effects of melatonin and omega-3 on hippocampal cells prenatally exposed to 900 MHz electromagnetic fields. Int. J. Radiat. Biol. 2016, 92, 590–595. [Google Scholar]
- Letra-Vilela, R.; Sanchez-Sanchez, A.M.; Rocha, A.M.; Martin, V.; Branco-Santos, J.; Puente-Moncada, N.; Santa-Marta, M.; Outeiro, T.F.; Antolin, I.; Rodriguez, C.; et al. Distinct roles of N-acetyl and 5-methoxy groups in the antiproliferative and neuroprotective effects of melatonin. Mol. Cell Endocrinol. 2016, 434, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Acuna-Castroviejo, D.; Escames, G.; Venegas, C.; Diaz-Casado, M.E.; Lima-Cabello, E.; Lopez, L.C.; Rosales-Corral, S.; Tan, D.X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef] [PubMed]
- Tresguerres, I.F.; Tamimi, F.; Eimar, H.; Barralet, J.E.; Prieto, S.; Torres, J.; Calvo-Guirado, J.L.; Tresguerres, J.A. Melatonin dietary supplement as an anti-aging therapy for age-related bone loss. Rejuvenation Res. 2014, 17, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Hibaoui, Y.; Reutenauer-Patte, J.; Patthey-Vuadens, O.; Ruegg, U.T.; Dorchies, O.M. Melatonin improves muscle function of the dystrophic mdx5Cv mouse, a model for Duchenne muscular dystrophy. J. Pineal Res. 2011, 51, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Shin, I.S.; Park, J.W.; Shin, N.R.; Jeon, C.M.; Kwon, O.K.; Lee, M.Y.; Kim, H.S.; Kim, J.C.; Oh, S.R.; Ahn, K.S. Melatonin inhibits MUC5AC production via suppression of MAPK signaling in human airway epithelial cells. J. Pineal Res. 2014, 56, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Sehajpal, J.; Kaur, T.; Bhatti, R.; Singh, A.P. Role of progesterone in melatonin-mediated protection against acute kidney injury. J. Surg. Res. 2014, 191, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Elbe, H.; Vardi, N.; Esrefoglu, M.; Ates, B.; Yologlu, S.; Taskapan, C. Amelioration of streptozotocin-induced diabetic nephropathy by melatonin, quercetin, and resveratrol in rats. Hum. Exp. Toxicol. 2015, 34, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.Z.; He, T.; Gao, J.X.; Liu, Y.; Liu, J.Q.; Han, S.C.; Li, Y.; Shi, J.H.; Han, J.T.; Tao, K.; et al. Melatonin prevents acute kidney injury in severely burned rats via the activation of SIRT1. Sci. Rep. 2016, 6, 32199. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Young, Y.K.; Fradette, J.; Eliopoulos, N. Melatonin pretreatment of human adipose tissue-derived mesenchymal stromal cells enhances their prosurvival and protective effects on human kidney cells. Am. J. Physiol. Ren. Physiol. 2015, 308, F1474–F1483. [Google Scholar] [CrossRef] [PubMed]
- Wierrani, F.; Grin, W.; Hlawka, B.; Kroiss, A.; Grunberger, W. Elevated serum melatonin levels during human late pregnancy and labour. J. Obstet. Gynaecol. 1997, 17, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Takayama, H.; Nakamura, Y.; Tamura, H.; Yamagata, Y.; Harada, A.; Nakata, M.; Sugino, N.; Kato, H. Pineal gland (melatonin) affects the parturition time, but not luteal function and fetal growth, in pregnant rats. Endocr. J. 2003, 50, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Tola, E.N.; Mungan, M.T.; Uguz, A.C.; Naziroglu, M. Intracellular Ca2+ and antioxidant values induced positive effect on fertilisation ratio and oocyte quality of granulosa cells in patients undergoing in vitro fertilisation. Reprod. Fertil. Dev. 2013, 25, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Bromfield, E.G.; Aitken, R.J.; Anderson, A.L.; McLaughlin, E.A.; Nixon, B. The impact of oxidative stress on chaperone-mediated human sperm-egg interaction. Hum. Reprod. 2015, 30, 2597–2613. [Google Scholar] [CrossRef] [PubMed]
- Kose, O.; Arabaci, T.; Kara, A.; Yemenoglu, H.; Kermen, E.; Kizildag, A.; Gedikli, S.; Ozkanlar, S. Effects of melatonin on oxidative stress index and Alveolar bone loss in diabetic rats with periodontitis. J. Periodontol. 2016, 87, e82–e90. [Google Scholar] [CrossRef] [PubMed]
- Arabaci, T.; Kermen, E.; Ozkanlar, S.; Kose, O.; Kara, A.; Kizildag, A.; Duman, S.B.; Ibisoglu, E. Therapeutic effects of melatonin on alveolar bone resorption after experimental periodontitis in rats: A biochemical and immunohistochemical study. J. Periodontol. 2015, 86, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin and the theories of aging: A critical appraisal of melatonin’s role in antiaging mechanisms. J. Pineal Res. 2013, 55, 325–356. [Google Scholar] [CrossRef] [PubMed]
- Wahab, M.H.; Akoul, E.S.; Abdel-Aziz, A.A. Modulatory effects of melatonin and vitamin E on doxorubicin-induced cardiotoxicity in Ehrlich ascites carcinoma-bearing mice. Tumori 2000, 86, 157–162. [Google Scholar] [PubMed]
- Andersen, L.P.; Rosenberg, J.; Gogenur, I. Perioperative melatonin: Not ready for prime time. Br. J. Anaesth. 2014, 112, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Seabra, M.L.; Bignotto, M.; Pinto, L.R., Jr.; Tufik, S. Randomized, double-blind clinical trial, controlled with placebo, of the toxicology of chronic melatonin treatment. J. Pineal Res. 2000, 29, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Jahnke, G.; Marr, M.; Myers, C.; Wilson, R.; Travlos, G.; Price, C. Maternal and developmental toxicity evaluation of melatonin administered orally to pregnant Sprague-Dawley rats. Toxicol. Sci. 1999, 50, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Wright, B.; Sims, D.; Smart, S.; Alwazeer, A.; Alderson-Day, B.; Allgar, V.; Whitton, C.; Tomlinson, H.; Bennett, S.; Jardine, J.; et al. Melatonin versus placebo in children with autism spectrum conditions and severe sleep problems not amenable to behaviour management strategies: A randomised controlled crossover trial. J. Autism Dev. Disord. 2011, 41, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Gringras, P.; Gamble, C.; Jones, A.P.; Wiggs, L.; Williamson, P.R.; Sutcliffe, A.; Montgomery, P.; Whitehouse, W.P.; Choonara, I.; Allport, T.; et al. Melatonin for sleep problems in children with neurodevelopmental disorders: Randomised double masked placebo controlled trial. BMJ 2012, 345, e6664. [Google Scholar] [CrossRef] [PubMed]
- Holliman, B.J.; Chyka, P.A. Problems in assessment of acute melatonin overdose. South. Med. J. 1997, 90, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Gitto, E.; Aversa, S.; Salpietro, C.D.; Barberi, I.; Arrigo, T.; Trimarchi, G.; Reiter, R.J.; Pellegrino, S. Pain in neonatal intensive care: Role of melatonin as an analgesic antioxidant. J. Pineal Res. 2012, 52, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Gitto, E.; Karbownik, M.; Reiter, R.J.; Tan, D.X.; Cuzzocrea, S.; Chiurazzi, P.; Cordaro, S.; Corona, G.; Trimarchi, G.; Barberi, I. Effects of melatonin treatment in septic newborns. Pediatr. Res. 2001, 50, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Gitto, E.; Romeo, C.; Reiter, R.J.; Impellizzeri, P.; Pesce, S.; Basile, M.; Antonuccio, P.; Trimarchi, G.; Gentile, C.; Barberi, I.; et al. Melatonin reduces oxidative stress in surgical neonates. J. Pediatr. Surg. 2004, 39, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Gitto, E.; Reiter, R.J.; Amodio, A.; Romeo, C.; Cuzzocrea, E.; Sabatino, G.; Buonocore, G.; Cordaro, V.; Trimarchi, G.; Barberi, I. Early indicators of chronic lung disease in preterm infants with respiratory distress syndrome and their inhibition by melatonin. J. Pineal Res. 2004, 36, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Acil, M.; Basgul, E.; Celiker, V.; Karagoz, A.H.; Demir, B.; Aypar, U. Perioperative effects of melatonin and midazolam premedication on sedation, orientation, anxiety scores and psychomotor performance. Eur. J. Anaesthesiol. 2004, 21, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Samarkandi, A.H. The comparative dose-response effects of melatonin and midazolam for premedication of adult patients: A double-blinded, placebo-controlled study. Anesth. Analg. 2000, 91, 473–479. [Google Scholar] [PubMed]
- Edmonds, K.E.; Stetson, M.H. Pineal gland and melatonin affect testicular status in the adult marsh rice rat (Oryzomys palustris). Gen. Comp. Endocrinol. 1995, 99, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Kennaway, D.J.; Rowe, S.A. Controlled-release melatonin implants delay puberty in rats without altering melatonin rhythmicity. J. Pineal Res. 1997, 22, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Kelestimur, H.; Ozcan, M.; Kacar, E.; Alcin, E.; Yilmaz, B.; Ayar, A. Melatonin elicits protein kinase C-mediated calcium response in immortalized GT1–7 GnRH neurons. Brain Res. 2012, 1435, 24–28. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).