Evidence of Some Natural Products with Antigenotoxic Effects. Part 1: Fruits and Polysaccharides
Abstract
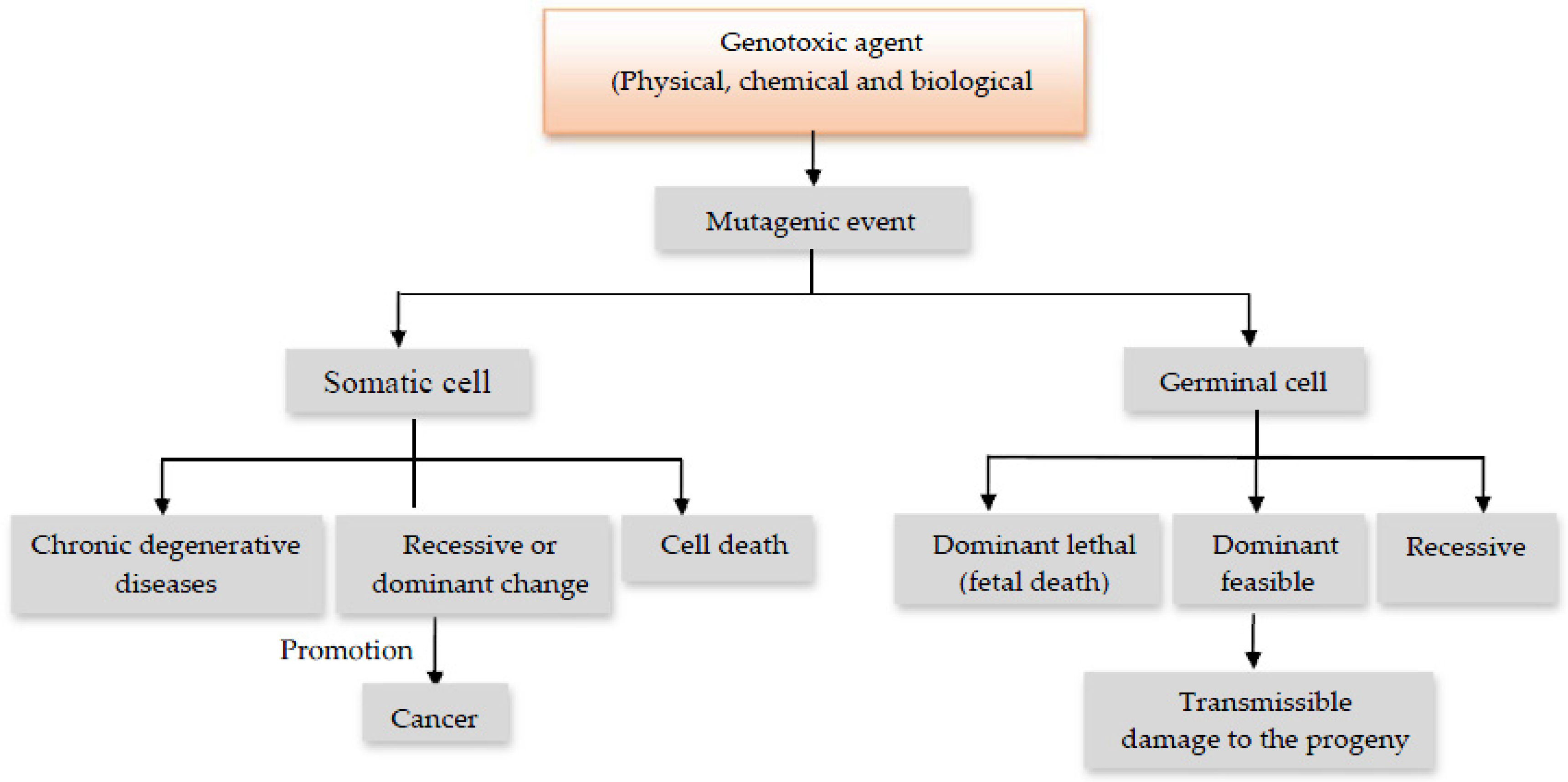
:1. Introduction
2. Antigenotoxic Fruits
2.1. Pomegranate (Punica granatum L.)
2.2. Guava (Psidium guajava L.)
2.3. Grapefruit (Citrus paradisi Macfad)
2.4. Pineapple (Ananas comosus L.)
2.5. Mango (Mangifera indica L.)
2.6. Blueberries/Cranberries (Vaccinium spp.)
3. Polysaccharides
3.1. β-Glucans, α-Mannans and Glucomannans
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Bhattacharya, S. Natural antimutagens: A review. Res. J. Med. Plants 2011, 5, 116–126. [Google Scholar] [CrossRef]
- Nagarathna, P.K.M.; Johnson-Wesley, M.; Sriram-Reddy, P.; Reena, K. Review on Genotoxicity, its Molecular Mechanisms and Prevention. Int. J. Pharm. Sci. Rev. Res. 2013, 22, 236–243. [Google Scholar]
- Słoczyńska, K.; Powroźnik, B.; Pękala, E.; Waszkielewicz, A.M. Antimutagenic compounds and their possible mechanisms of action. J. Appl. Genet. 2014, 55, 273–285. [Google Scholar]
- Ferguson, L.R.; Philpott, M. Nutrition and mutagenesis. Annu. Rev. Nutr. 2008, 28, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R. Natural and human-made mutagens and carcinogens in the human diet. Toxicology 2002, 181–182, 79–82. [Google Scholar] [CrossRef]
- Teaf, C.; Middendorf, P.J. Mutagenesis and Genetic Toxicology. In Principles of Toxicology: Environmental and Industrial Applications; Williams, P.L., James, R.C., Roberts, S.M., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2000; pp. 239–265. [Google Scholar]
- De Flora, S.; Ferguson, L.R. Overview of mechanisms of cancer chemopreventive agents. Mutat. Res. 2005, 591, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R.; Bronzetti, G.; De Flora, S. Mechanistic approaches to chemoprevention of mutation and cancer. Mutat Res. 2005, 591, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R.; Philpott, M.; Karunasinghe, N. Dietary cancer and prevention using antimutagens. Toxicology 2004, 198, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Brusick, D. Evolution of testing strategies for genetic toxicity. Mutat. Res. 1988, 205, 69–78. [Google Scholar] [CrossRef]
- Abilev, S.K.; Glaser, V.M. Genetic toxicology: Findings and challenges. Genetika 2013, 49, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Marzin, D. Theory and practice of genetic toxicology tests. Tests on eukaryotes. Ann. Biol. Clin. 1986, 44, 656–661. [Google Scholar]
- Mortelmans, K.; Zeiger, E. The Ames Salmonella/microsome mutagenicity assay. Mutat. Res. 2000, 455, 29–60. [Google Scholar] [CrossRef]
- Costa, S.; Carvalho, S.; Costa, C.; Coelho, P.; Silva, S.; Santos, L.S.; Gaspar, J.F.; Porto, B.; Laffon, B.; Teixeira, J.P. Increased levels of chromosomal aberrations and DNA damage in a group of workers exposed to formaldehyde. Mutagenesis 2015, 30, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Majer, B.J.; Laky, B.; Knasmüller, S.; Kassie, F. Use of the micronucleus assay with exfoliated epithelial cells as a biomarker for monitoring individuals at elevated risk of genetic damage and in chemoprevention trials. Mutat. Res. 2001, 489, 147–172. [Google Scholar] [CrossRef]
- Liao, W.; McNutt, M.A.; Zhu, W.G. The comet assay: A sensitive method for detecting DNA damage in individual cells. Methods 2009, 48, 46–53. [Google Scholar] [CrossRef] [PubMed]
- López-Mejia, O.A.; López-Malo, A.; Palou, E. Granada (Punica granatum L.): Una fuente de antioxidantes de interés actual. Temas Selectos de Ingeniería de Alimentos 2010, 4, 64–73. [Google Scholar]
- Lansky, E.P.; Newman, R.A. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J. Ethnopharmacol. 2007, 109, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, H.R.; Arastoo, M.; Ostad, S.N. A Comprehensive Review of Punicagranatum (Pomegranate) Properties in Toxicological, Pharmacological, Cellular and Molecular Biology Researches. Iran J. Pharm. Res. 2012, 11, 385–400. [Google Scholar] [PubMed]
- Jurenka, J.S. Therapeutic applications of pomegranate (Punicagranatum L.): A review. Altern. Med. Rev. 2008, 13, 128–144. [Google Scholar] [PubMed]
- Alekperov, U.K. Plant antimutagens and their mixtures in inhibition of genotoxic effects of xenobiotics and aging processes. Eur. J. Cancer Prev. 2002, 11, S8–S11. [Google Scholar] [PubMed]
- Sánchez-Lamar, A.; Cozzi, R.; Cundari, E.; Fiore, M.; Ricordy, R.; Gensabella, G.; Degrassi, F.; De Salvia, R. Punica granatum L. whole fruit extract as a protection against the hydrogen peroxide-induced damage. Rev. Cubana Plant. Med. 2005, 10, 1–9. [Google Scholar]
- Dassprakash, M.V.; Arun, R.; Abraham, S.K.; Premkumar, K. In vitro and in vivo evaluation of antioxidant and antigenotoxic potential of Punica granatum leaf extract. Pharm. Biol. 2012, 50, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Forouzanfar, F.; Afkhami Goli, A.; Asadpour, E.; Ghorbani, A.; Sadeghnia, H.R. Protective efecto of Punicagranatum L. against serum/glucose deprivation-induced PC12cells injury. Evid. Based Complement. Altern. Med. 2013, 2013, 716–730. [Google Scholar] [CrossRef] [PubMed]
- Zahin, M.; Ahmad, I.; Gupta, R.C.; Aqil, F. Punica laginand ellagic acid demonstrate antimutagenic activity an dinhibition of benzo[a]pyrene induced DNA adducts. Biomed. Res. Int. 2014, 2014, 467465. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gutiérrez, M.; Izquierdo-Vega, J.A.; Chimal-Cazares, F.; Betanzos-Cabrera, G.; Morales-González, J.A.; Madrigal-Santillán, E. Evaluation of Anticlastogenic Capacity of a Microencapsulated of Pomegranate against the Damage Caused by Acrylamide in Mice. I Congreso Nacional de Advances en Alimentación Biotecnología; Autonomous University of Yucatan: Mérida, México, 2016; p. 36. [Google Scholar]
- Shruthi, S.D.; Roshan, A.; Timilsina, S.S.; Sunita, S. A review on the medicinal plant psidium guajava linn. (myrtaceae). J. Drug Deliv. Ther. 2013, 3, 162–168. [Google Scholar]
- Barbalho, S.M.; Farinazzi-Machado, F.M.; De Alvares-Goulart, R.; Brunnati, A.C.; Ottoboni, A.M. Psidium guajava (Guava): A plant of multipurpose medicinal plants. Med. Aromat. Plants. 2012, 1, 104. [Google Scholar]
- Gutiérrez, R.M.; Mitchell, S.; Solis, R.V. Psidium guajava: A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2008, 117, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Grover, I.S.; Bala, S. Studies on antimutagenic effects of guava (Psidium guajava) in Salmonella typhimurium. Mutat. Res. 1993, 300, 1–3. [Google Scholar] [CrossRef]
- Matsuo, T.; Hanamure, N.; Shimoi, K.; Nakamura, Y.; Tomita, I. Identification of (+)-gallocatechin as a bio-antimutagenic compound in Psidium guava leaves. Phytochemistry 1994, 36, 1027–1029. [Google Scholar] [CrossRef]
- Roncada, T.; Vicentini, V.E.; Mantovani, M.S. Possible modulating actions of plant extracts on the chromosome breaking activity of MMC and Ara-C in human lymphocytes in vitro. Toxicol. In Vitro 2004, 18, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Bartolome, A.; Mandap, K.; David, K.J.; Sevilla, F., 3rd; Villanueva, J. SOS-red fluorescent protein (RFP) bioassay system for monitoring of antigenotoxic activity in plant extracts. Biosens. Bioelectron. 2006, 21, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Yen, M.; Chiu, C.; Wu, H.; Huang, S.; Tai, S.; Wang, B. The inhibitory effects of aqueous extract from guava twigs, Psidium guajava L., on mutation and oxidative damage. J. Chem. 2013, 2013. [Google Scholar] [CrossRef]
- Huang, C.S.; Yin, M.C.; Chiu, L.C. Antihyperglycemic and antioxidativepotential of Psidium guajava fruit in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2011, 49, 2189–2195. [Google Scholar] [CrossRef] [PubMed]
- Amith, K.; Reshma, K.; Rajalakshmi-Rai, B.S.; Satish, R. Radiomodulatory Role of Psidium guajava Leaf Extracts against X-ray Induced Genotoxicity, Oxidative stress and Apoptosis in Wistar Rat Model. J. Appl. Pharm. Sci. 2016, 6, 058–065. [Google Scholar]
- Gupta, V.; Kohli, K.; Ghaiye, P.; Bansal, P.; Lather, A. Pharmacological potentials of citrus paradise-An overview. Int. J. Phytother. Res. 2011, 1, 8–17. [Google Scholar]
- Madrigal-Santillán, E.; Madrigal-Bujaidar, E.; Álvarez-González, I.; Sumaya-Martínez, M.T.; Gutiérrez-Salinas, J.; Bautista, M.; Morales-González, A.; García-Luna, M.; González-Rubio, M.; Aguilar-Faisal, J.L.; et al. Review of natural products with hepatoprotective effects. World J. Gastroenterol. 2014, 20, 14787–14804. [Google Scholar] [CrossRef] [PubMed]
- Monroe, K.R.; Murphy, S.P.; Kolonel, L.N.; Pike, M.C. Prospective study of grapefruit intake and risk of breast cancer in postmenopausal women: The Multiethnic Cohort Study. Br. J. Cancer 2007, 97, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dogra, S.; Prakash, A. Protective effect of naringin, a citrus flavonoid, against colchicine-induced cognitive dysfunction and oxidative damage in rats. J. Med. Food. 2010, 13, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.M.; Andrades, N.E.; Paulino, N.; Sawaya, A.C.; Eberlin, M.N.; Marcucci, M.C.; Favero, G.M.; Novak, E.M.; Bydlowski, S.P. Synthesis and characterization of a metal complex containing naringin and Cu, and its antioxidant, antimicrobial, anti-inflammatory and tumor cell cytotoxicity. Molecules 2007, 12, 1352–1366. [Google Scholar] [CrossRef] [PubMed]
- Miyata, M.; Takano, H.; Takahashi, K.; Sasaki, Y.F.; Yamazoe, Y. Suppression of 2-amino-1-methyl-6-phenylimidazo(4,5-b)pyridine-induced DNA damage in rat colon after grapefruit juice intake. Cancer Lett. 2002, 183, 17–22. [Google Scholar] [CrossRef]
- Miyata, M.; Takano, H.; Guo, L.Q.; Nagata, K.; Yamazoe, Y. Grapefruit juice intake does not enhance but rather protects against aflatoxin B1-induced liver DNA damage through a reduction in hepatic CYP3A activity. Carcinogenesis 2004, 25, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-González, I.; Madrigal-Bujaidar, E.; Martino-Roaro, L.; Espinosa-Aguirre, J.J. Antigenotoxic and antioxidant effect of grapefruit juice in mice treated with daunorubicin. Toxicol. Lett. 2004, 152, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-González, I.; Madrigal-Bujaidar, E.; Sánchez-García, V.Y. Inhibitory effect of grapefruit juice on the genotoxic damage induced by ifosfamide in mouse. Plant Foods Hum. Nutr. 2010, 65, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Platt, K.L.; Edenharder, R.; Aderhold, S.; Muckel, E.; Glatt, H. Fruits and vegetables protect against the genotoxicity of heterocyclic aromatic amines activated by human xenobiotic-metabolizing enzymes expressed in immortal mammalian cells. Mutat. Res. 2010, 703, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Gonzalez, I.; Mojica, R.; Madrigal-Bujaidar, E.; Camacho-Carranza, R.; Escobar-García, D.; Espinosa-Aguirre, J.J. The antigenotoxic effects of grapefruit juice on the damage induced by benzo(a)pyrene and evaluation of its interaction with hepatic and intestinal Cytochrome P450 (Cyp) 1A1. Food Chem. Toxicol. 2011, 49, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Razo-Aguilera, G.; Baez-Reyes, R.; Alvarez-González, I.; Paniagua-Pérez, R.; Madrigal-Bujaidar, E. Inhibitory effect of grapefruit juice on the genotoxicity induced by hydrogen peroxide in human lymphocytes. Food Chem. Toxicol. 2011, 49, 2947–2953. [Google Scholar] [CrossRef] [PubMed]
- Sakr, S.A.; Zoil, M.; El-Shafey, S.S. Ameliorative effect of grapefruit juice on amiodarone-induced cytogenetic and testicular damage in albino rats. Asian Pac. J. Trop. Biomed. 2013, 3, 573–579. [Google Scholar] [CrossRef]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Caturla, N.; Castillo, J.; Benavente-García, O.; Alcaraz, M.; Micol, V. Protective effects of citrus and rosemary extracts on UV-induced damage in skin cell model and human volunteers. J. Photochem. Photobiol. B. 2014, 136, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Kanno, S.; Shouji, A.; Asou, K.; Ishikawa, M. Effects of naringin on hydrogen peroxide-induced cytotoxicity and apoptosis in P388 cells. J. Pharmacol. Sci. 2003, 92, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Kanno, S.; Shouji, A.; Hirata, R.; Asou, K.; Ishikawa, M. Effects of naringin on cytosine arabinoside (Ara-C)-induced cytotoxicity and apoptosis in P388 cells. Life Sci. 2004, 75, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-González, I.; Madrigal-Bujaidar, E.; Dorado, V.; Espinosa-Aguirre, J.J. Inhibitory effect of naringin on the micronuclei induced by ifosfamide in mouse, and evaluation of its modulatory effect on the Cyp3a subfamily. Mutat. Res. 2001, 480–481, 171–178. [Google Scholar] [CrossRef]
- Jagetia, A.; Jagetia, G.C.; Jha, S. Naringin, a grapefruit flavanone, protects V79 cells against the bleomycin-induced genotoxicity and decline in survival. J. Appl. Toxicol. 2007, 27, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, D.; Aydemir, N.C.; Vatan, O.; Tüzün, E.; Bilaloglu, R. Influence of naringin on cadmium-induced genomic damage in human lymphocytes in vitro. Toxicol. Ind. Health 2012, 28, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Gross-Steinmeyer, K.; Eaton, D.L. A Dietary modulation of the biotrasnformation and genotoxicity aflatoxin B(1). Toxicology 2012, 299, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.G.; Malcolm, J.; Arnold, O.; Spence, J.D. Grapefruit juice–drug interactions. Br. J. Clin. Pharmacol. 1998, 46, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Ushigome, F.; Koyabu, N.; Morimoto, N.; Shoyama, Y.; Uchiumi, T.; Kuwano, M.; Ohtani, H.; Sawada, Y. Effects of grapefruit juice and orange juice components on P-glycoprotein- and MRP2- mediated drug efflux. Br. J. Pharmacol. 2004, 143, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Romiti, N.; Tramonti, G.; Donati, A.; Chieli, E. Effects of grapefruit juice on the multidrug transporter P-glycoprotein in the human proximal tubular cell line HK-2. Life Sci. 2004, 76, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, J.; Ming, R. Genomic analyses of the CAM plant pineapple. J. Exp. Bot. 2014, 65, 3395–3404. [Google Scholar] [CrossRef] [PubMed]
- López-García, B.; Hernández, M.; Segundo, B.S. Bromelain, a cysteine protease from pineapple (Ananas comosus) stem, is an inhibitor of fungal plant pathogens. Lett. Appl. Microbiol. 2012, 55, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.E.; Yeap, S.K.; Lim, K.L.; Yusof, H.M.; Beh, B.K.; Tan, S.W.; Ho, W.Y.; Sharifuddin, S.A.; Jamaluddin, A.; Long, K.; et al. Antioxidant effects of pineapple vinegar in reversing of paracetamol-induced liver damage in mice. Chin. Med. 2015, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Pavan, R.; Jain, S.; Shraddha; Kumar, A. Properties and therapeutic application of bromelain: A review. Biotechnol. Res. Int. 2012, 2012, 976203. [Google Scholar] [CrossRef] [PubMed]
- Maurer, H.R. Bromelain: Biochemistry, pharmacology and medical use. Cell Mol. Life Sci. 2001, 58, 1234–1245. [Google Scholar] [CrossRef] [PubMed]
- Ikken, Y.; Morales, P.; Martínez, A.; Marín, M.L.; Haza, A.I.; Cambero, M.I. Antimutagenic effect of fruit and vegetable ethanolic extracts against N-nitrosamines evaluated by the Ames Test. J. Agric. Food Chem. 1999, 47, 3257–3264. [Google Scholar] [CrossRef] [PubMed]
- Sah, B.N.; Vasiljevic, T.; McKechnie, S.; Donkor, O.N. Effect of refrigerated storage on probiotic viability and the production and stability of antimutagenic and antioxidant peptides in yogurt supplemented with pineapple peel. J. Dairy Sci. 2015, 98, 5905–5916. [Google Scholar] [CrossRef] [PubMed]
- Stopper, H.; Schinzel, R.; Sebekova, K.; Heidland, A. Genotoxicity of advanced glycation end products in mammalian cells. Cancer Lett. 2003, 190, 151–156. [Google Scholar] [CrossRef]
- Shah, K.A.; Patel, M.B.; Patel, R.J.; Parmar, P.K. Mangifera Indica (Mango). Pharmacogn. Rev. 2010, 4, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Jahurul, M.H.; Zaidul, I.S.; Ghafoor, K.; Al-Juhaimi, F.Y.; Nyam, K.L.; Norulaini, N.A.; Sahena, F.; Mohd Omar, A.K. Mango (Mangifera indica L.) by-products and their valuable components: A review. Food Chem. 2015, 183, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Wall-Medrano, A.; Olivas-Aguirre, F.J.; Velderrain-Rodríguez, G.R.; González-Aguilar, A.; De La Rosa, L.A.; López-Díaz, J.A.; Álvarez-Parrilla, E. Mango: Agroindustrial aspects, nutritional/functional value and health effects. Nutr. Hosp. 2014, 31, 67–75. [Google Scholar] [PubMed]
- Benard, O.; Chi, Y. Medicinal properties of mangiferin, structural features, derivative synthesis, pharmacokinetics and biological activities. Mini-Rev. Med. Chem. 2015, 15, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Kumar Yadav, V.; Srivastava, S.; Shukla, Y. Protective effects of lupeol against benzo(a)pyrene induced clastogenicity in mouse bone marrow cells. Mol. Nutr. Food Res. 2008, 52, 1117–1120. [Google Scholar] [CrossRef] [PubMed]
- Morffi, J.; Rodeiro, I.; Hernández, S.L.; González, L.; Herrera, J.; Espinosa-Aguirre, J.J. Antimutagenic properties of Mangifera indica L. stembark extract and evaluation of its effects on hepatic CYP1A1. Plant. Foods Hum. Nutr. 2012, 67, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Rodeiro, I.; Delgado, R.; Garrido, G. Effects of a Mangifera indica L. stem bark extract and mangiferin on radiation-induced DNA damage in human lymphocytes and lymphoblastoid cells. Cell Prolif. 2014, 47, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Rodeiro, I.; Hernandez, S.; Morffi, J.; Herrera, J.A.; Gómez-Lechón, M.J.; Delgado, R.; Espinosa-Aguirre, J.J. Evaluation of genotoxicity and DNA protective effects of mangiferin, a glucosylxanthoneisolated from Mangifera indica L. stembarkextract. Food Chem. Toxicol. 2012, 50, 3360–3366. [Google Scholar] [CrossRef] [PubMed]
- Satish Rao, B.S.; Sreedevi, M.V.; Nageshwar Rao, B. Cytoprotective and antigenotoxic potential of Mangiferin, a glucosylxanthone against cadmium chloride induced toxicity in HepG2 cells. Food Chem. Toxicol. 2009, 47, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Nageshwar Rao, B.; Satish Rao, B.S. Mangiferin attenuates methylmercury induced cytotoxicity against IMR-32, human neuroblastoma cells by the inhibition of oxidative stress and free radical scavenging potential. Chem. Biol. Interact. 2011, 193, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Kaivalya, M.; Nageshwar Rao, B.N.; Satish Rao, B.S. Mangiferin: A xanthone attenuates mercury chloride induced cytotoxicity and genotoxicity in HepG2 cells. J. Biochem. Mol. Toxicol. 2011, 25, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhao, J.; Li, S.; Zeng, L.; Chen, Y.; Fang, J. Mangiferin activates the Nrf2-ARE pathway and reduces etoposide-induced DNA damage in human umbilical cord mononuclear blood cells. Pharm. Biol. 2015, 53, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Ekambaram, G.; Sakthisekaran, D. Protective role of mangiferin against Benzo(a)pyrene induced lung carcinogenesis in experimental animals. Biol. Pharm. Bull. 2008, 31, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Jagetia, G.C.; Venkatesha, V.A. Effect of mangiferin on radiation-induced micronucleus formation in cultured human peripheral blood lymphocytes. Environ. Mol. Mutagen. 2005, 46, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Viswanadh, E.K.; Rao, B.N.; Rao, B.S. Antigenotoxic effect of mangiferin and changes in antioxidant enzyme levels of Swiss albino mice treated with cadmium chloride. Hum. Exp. Toxicol. 2010, 29, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Madrigal-Santillán, E.; Fragoso-Antonio, S.; Valadez-Vega, C.; Solano-Solano, G.; Pérez, C.Z.; Sánchez-Gutiérrez, M.; Izquierdo-Vega, J.A.; Gutiérrez-Salinas, J.; Esquivel-Soto, J.; Esquivel-Chirino, C.; et al. Investigation on the protective effects of cranberry against the DNA damage induced by benzo[a]pyrene. Molecules 2012, 17, 4435–4451. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P. Berry fruits: Compositional elements, biochemicalactivities, and the impact of their intake on human health, performance, and disease. J. Agric. Food Chem. 2008, 56, 627–629. [Google Scholar] [CrossRef] [PubMed]
- Neto, C.C. Cranberry and its phytochemicals: A review of invitro anticancer studies. J. Nutr. 2007, 137, 186S–193S. [Google Scholar] [PubMed]
- Edenharder, R.; Sager, J.W.; Glatt, H.; Muckel, E.; Platt, K.L. Protection by beverages, fruits, vegetables, herbs, and flavonoids against genotoxicity of 2-acetylaminofluorene and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in metabolically competent V79 cells. Mutat. Res. 2002, 521, 57–72. [Google Scholar] [CrossRef]
- Pepe, G.; Grossi, M.R.; Berni, A.; Filippi, S.; Shanmugakani, R.K.; Papeschi, C.; Mosesso, P.; Natarajan, A.T.; Palitti, F. Effect of blueberries (BB) on micronuclei induced by N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) and 7,12-dimethylbenz(a)anthracene (DMBA) in mammalian cells, assessed in in vitro and in vivo assays. Mutat. Res. 2013, 758, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Calò, R.; Marabini, L. Protective effect of Vaccinium myrtillus extract against UVA- and UVB-induced damage in a humankeratinocyte cell line (HaCaT cells). J. Photochem. Photobiol. B 2014, 132, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Barros, D.; Amaral, O.B.; Izquierdo, I.; Geracitano, L.; do Carmo Bassols Raseira, M.; Henriques, A.T.; Ramirez, M.R. Behavioral and genoprotective effects of Vaccinium berries intake in mice. Pharmacol. Biochem. Behav. 2006, 84, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Del Bó, C.; Martini, D.; Vendrame, S.; Riso, P.; Ciappellano, S.; Klimis-Zacas, D.; Porrini, M. Improvement of lymphocyte resistance against H(2)O(2)-induced DNA damage in Sprague-Dawley rats after eight weeks of a wild blueberry (Vaccinium angustifolium)-enriched diet. Mutat. Res. 2010, 703, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Wilms, L.C.; De Boer, V.C.; Maas, L.M.; Pachen, D.M.; Gottschalk, R.W.; Ketelslegers, H.B.; Godschalk, R.W.; Haenen, G.R.; Van Schooten, F.J.; Kleinjans, J.C. Impact of multiple genetic polymorphisms on effects of a 4-week blueberry juice intervention on ex vivo induced lymphocytic DNA damage in human volunteers. Carcinogenesis 2007, 28, 1800–1806. [Google Scholar] [CrossRef] [PubMed]
- Del Bó, C.; Riso, P.; Campolo, J.; Møller, P.; Loft, S.; Klimis-Zacas, D.; Brambilla, A.; Rizzolo, A.; Porrini, M. A single portion of blueberry (Vaccinium corymbosum L.) improves protection against DNA damage but not vascular function in healthy male volunteers. Nutr. Res. 2013, 33, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Del Bó, C.; Fracassetti, D.; Lanti, C.; Porrini, M.; Riso, P. Comparison of DNA damage by the comet assay in fresh versus cryopreserved peripheral blood mononuclear cells obtained following dietary intervention. Mutagenesis 2015, 30, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Philpott, M.; Lim, C.C.; Ferguson, L.R. Dietary Protection Against Free Radicals: A Case for Multiple Testing to Establish Structure-activity Relationships for Antioxidant Potential of Anthocyanic Plant Species. Int. J. Mol. Sci. 2009, 10, 1081–1103. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lu, X.; He, G.; Gao, X.; Li, M.; Wu, J.; Li, Z.; Wu, J.; Wang, J.; Luo, C. Cytosolic protection against ultraviolet induced DNA damage by blueberry anthocyanins and anthocyanidins in hepatocarcinoma HepG2 cells. Biotechnol. Lett. 2013, 35, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Soccol, C.R.; Vandenberghe, L.P.S.; Spier, M.R.; Medeiros, A.B.P.; Yamaguishi, C.T.; De Dea, L.J.; Pandey, A.; Thomaz-Soccol, V. The potential of probiotics: A review. Food Technol. Biotech. 2010, 48, 413–434. [Google Scholar]
- Kumar, M.; Kumar, A.; Nagpal, R.; Mohania, D.; Behare, P.; Verma, V.; Kumar, P.; Poddar, D.; Aggarwal, P.K.; Henry, C.J.; et al. Cancer-preventing attributes of probiotics: An update. Int. J. Food Sci. Nutr. 2010, 61, 473–496. [Google Scholar] [CrossRef] [PubMed]
- Oelschlaeger, T.A. Mechanisms of probiotic actions—A review. Int. J. Med. Microbiol. 2010, 300, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Al Ibrahim, R.M.; Whelan, S.J.; Pierce, K.M.; Campion, D.P.; Gath, V.P.; Mulligan, F.J. Effect of timing of post-partum introduction to pasture and supplementation with Saccharomyces cerevisiae on milk production, metabolic status, energy balance and some reproductive parameters in early lactation dairy cows. J. Anim. Physiol. Anim. Nutr. (Berl.) 2013, 97 (Suppl. 1), 105–114. [Google Scholar] [CrossRef] [PubMed]
- González Canga, A.; Fernández Martínez, N.; Sahagún, A.M.; García Vieitez, J.J.; Díez Liébana, M.J.; Calle Pardo, A.P.; Castro Robles, L.J.; Sierra Vega, M. Glucomannan: Properties and therapeutic applications. Nutr. Hosp. 2004, 19, 45–50. [Google Scholar] [PubMed]
- Madrigal-Santillán, E.; Morales-González, J.A.; Sánchez-Gutiérrez, M.; Reyes-Arellano, A.; Madrigal-Bujaidar, E. Investigation on the protective effect of α-mannan against the DNA damage induced by aflatoxin B1 in mouse hepatocytes. Int. J. Mol. Sci. 2009, 10, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Madrigal-Bujaidar, E.; Morales-González, J.A.; Sánchez-Gutiérrez, M.; Izquierdo-Vega, J.A.; Reyes-Arellano, A.; Álvarez-González, I.; Pérez-Pasten, R.; Madrigal-Santillán, E. Prevention of Aflatoxin B1-Induced DNA Breaks by β-d-Glucan. Toxins 2015, 7, 2145–2158. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, M.S.; Bellini, M.F.; Angeli, J.P.; Oliveira, R.J.; Silva, A.F.; Ribeiro, L.R. Beta-Glucans in promoting health: Preventionagainst mutation and cancer. Mutat. Res. 2008, 658, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Kogan, G.; Pajtinka, M.; Babincova, M.; Miadoková, E.; Rauko, P.; Slamenova, D.; Korolenko, T.A. Yeast Cell Wall Polysaccharides as Antioxidants and Antimutagens: Can They Fight Cancer? Neoplasma 2008, 55, 387–393. [Google Scholar] [PubMed]
- Chorvatovicová, D.; Navarová, J. Suppressing effects of glucan on micronuclei induced by cyclophosphamide in mice. Mutat. Res. 1992, 282, 147–150. [Google Scholar] [CrossRef]
- Chorvatovicová, D.; Kováciková, Z.; Sandula, J.; Navarová, J. Protective effect of sulfoethylglucan against hexavalent chromium. Mutat. Res. 1993, 302, 207–211. [Google Scholar] [CrossRef]
- Chorvatovicová, D.; Machová, E.; Sandula, J. Ultrasonication: The way to achieve antimutagenic effect of carboxymethyl-chitin-glucan by oral administration. Mutat. Res. 1998, 412, 83–89. [Google Scholar] [CrossRef]
- Oliveira, R.J.; Ribeiro, L.R.; da Silva, A.F.; Matuo, R.; Mantovani, M.S. Evaluation of antimutagenic activity and mechanisms of action of beta-glucan from barley, in CHO-k1 and HTC cell lines using the micronucleus test. Toxicol. In Vitro 2006, 20, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.J.; Pesarini, J.R.; Sparça Salles, M.J.; Nakamura Kanno, T.Y.; Dos Santos Lourenço, A.C.; Da Silva Leite, V.; Da Silva, A.F.; Matiazi, H.J.; Ribeiro, L.R.; Mantovani, M.S. Effects of β-glucan polysaccharide revealed by the dominant lethal assay and micronucleus assays, and reproductive performance of male mice exposed to cyclophosphamide. Genet. Mol. Biol. 2014, 37, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Slamenová, D.; Lábaj, J.; Krizková, L.; Kogan, G.; Sandula, J.; Bresgen, N.; Eckl, P. Protective effects of fungal (1→3)-beta-d-glucan derivatives against oxidative DNA lesions in V79 hamster lung cells. Cancer Lett. 2003, 198, 153–160. [Google Scholar] [CrossRef]
- Lazarová, M.; Lábaj, J.; Eckl, P.; Kogan, G.; Slamenová, D. Effects of dietary intake of a fungal beta-d-glucan derivative on the level of DNA damage induced in primary rat hepatocytes by various carcinogens. Nutr. Cancer 2006, 56, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Angeli, J.P.; Ribeiro, L.R.; Gonzaga, M.L.; Soares Sde, A.; Ricardo, M.P.; Tsuboy, M.S.; Stidl, R.; Knasmueller, S.; Linhares, R.E.; Mantovani, M.S. Protective effects of beta-glucan extracted from Agaricus brasiliensis against chemically induced DNA damage in human lymphocytes. Cell. Biol. Toxicol. 2006, 22, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Slamenova, D.; Sramkova, M.; Chalupa, I.; Smigova, J.; Kogan, G. Reduction of genotoxic effects of N-nitrosomorpholine in human hepatoma cells and hamster lung cells by carboxymethyl chitin-glucan. Neoplasma 2008, 55, 280–285. [Google Scholar] [PubMed]
- Horvathova, E.; Eckl, P.M.; Bresgen, N.; Slamenova, D. Evaluation of genotoxic and cytotoxic effects of H2O2 and DMNQ on freshly isolated rat hepatocytes; protective effects of carboxymethyl chitin-glucan. Neuro Endocrinol. Lett. 2008, 29, 644–648. [Google Scholar] [PubMed]
- Angeli, J.P.; Ribeiro, L.R.; Angeli, J.L.; Mantovani, M.S. Protective effects of beta-glucan extracted from barley against benzo(a)pyrene-induced DNA damage in hepatic cell HepG2. Exp. Toxicol. Pathol. 2009, 61, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Angeli, J.P.; Ribeiro, L.R.; Bellini, M.F.; Mantovani, M.S. Beta-glucan extracted from the medicinal mushroom Agaricus blazei prevents the genotoxic effects of benzo(a)pyrene in the human hepatoma cell line HepG2. Arch. Toxicol. 2009, 83, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Slamenová, D.; Kováciková, I.; Horváthová, E.; Wsólová, L.; Navarová, J. Carboxymethyl chitin-glucan (CM-CG) protects human HepG2 and HeLa cells against oxidative DNA lesions and stimulates DNA repair of lesions induced by alkylating agents. Toxicol. In Vitro 2010, 24, 1986–1992. [Google Scholar]
- Ghavami, L.; Goliaei, B.; Taghizadeh, B.; Nikoofar, A. Effects of barley β-glucan on radiation damage in the human hepatoma cell line HepG2. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 775–776, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pillai, T.G.; Maurya, D.K.; Salvi, V.P.; Janardhanan, K.K.; Nair, C.K. Fungal beta glucan protects radiation induced DNA damage in human lymphocytes. Ann. Transl. Med. 2014, 2, 13. [Google Scholar] [PubMed]
- Lazarová, M.; Lábaj, J.; Kováciková, Z.; Slamenová, D. Diet containing fungal (1→3)-beta-d-glucan derivative exhibits protective effects against DNA lesions induced in freshly isolated rat cells. Neoplasma 2004, 51, 431–435. [Google Scholar] [PubMed]
- Magnani, M.; Castro-Gomez, R.J.; Mori, M.P.; Kuasne, H.; Gregório, E.P.; Libos, F., Jr.; De Syllos Cólus, I.M. Protective effect of carboxymethyl-glucan (CM-G) against DNA damage in patients with advanced prostate cancer. Genet. Mol. Biol. 2011, 34, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, C.E.; Cruz, I.B.; Cadoná, F.C.; Machado, A.K.; Assmann, C.; Schlemmer, K.B.; Zanette, R.A.; Leal, D.B.; Santurio, J.M. Cytoprotective and genoprotective effects of β-glucans against aflatoxin B1-induced DNA damage in broiler chicken lymphocytes. Toxicol. In Vitro 2015, 29, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Krizková, L.; Zitnanová, I.; Mislovicová, D.; Masárová, J.; Sasinková, V.; Duracková, Z.; Krajcovic, J. Antioxidant and antimutagenic activity of mannan neoglycoconjugates: Mannan-human serum albumin and mannan-penicillin G acylase. Mutat. Res. 2006, 606, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Madrigal-Santillán, E.; Alvarez-González, I.; Márquez-Márquez, R.; Velázquez-Guadarrama, N.; Madrigal-Bujaidar, E. Inhibitory effect of mannan on the toxicity produced in mice fed aflatoxin B1 contaminated corn. Arch. Environ. Contam. Toxicol. 2007, 53, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Chorvatovicová, D.; Machová, E.; Sandula, J.; Kogan, G. Protective effect of the yeast glucomannan against cyclophosphamide-induced mutagenicity. Mutat. Res. 1999, 444, 117–122. [Google Scholar] [CrossRef]
| Types of Mechanisms | Examples of Dietary Antimutagens |
|---|---|
| Extracellular | |
| 1. Inhibition of mutagen uptake | Dietary fibres, probiotics, grapefruit (naringenin). |
| 2. Inhibition of endogenous formation (a) Inhibition of nitrosation (b) Modification of the intestinal flora | Vitamins (ascorbic acid), sulphur compounds (cysteine, glutathione). Prebiotics, probiotics. |
| 3. Complexation and/or deactivation | Dietary fibres, chlorophyllin. |
| 4. Favouring absorption of protective agents | Vitamin D3. |
| Intracellular | |
| 5. Blocking or competition (a) Scavenging of reactive oxygen species (b) Protection of DNA nucleophilic sites | Mango (polyphenols), guava (gallocatechin) vitamins (β-carotene, α-tocopherol, ascorbic acid), pineapple, blueberries (anthocyanins). Ellagic acid, retinoids, polyamines. |
| 6. Stimulation of trapping and detoxification in non-target cells | N-Acetyl cysteine. |
| 7. Modification of transmembrane transport | Short chain fatty acids (caproate), dietary calcium. |
| 8. Modulation of xenobiotic metabolising enzymes (a) Inhibition of promutagen activation (b) Induction of detoxification pathways (c) Inhibition of metabolic enzymes | Isothiocyanates, monocyclic monoterpenoids (limonene, methol), flavonoids, wheat bran. Polyphenols, indoles, diterpene esters. Grapefruit (naringin, naringenin). |
| 9. Modulation of DNA metabolism and repair | Cinnamaldehyde, vanillin. |
| 10. Regulation of signaling pathways | Pomegranate (polyphenols), β-glucans. |
| 11. Enhancement of apoptosis | Retinoids, flavonoids. |
| 12. Maintenance of genomic stability | Vitamins (folic acid, B12), minerals (selenium, zinc), polyphenols. |
| Table modified from Ferguson et al. (2004). | |
| Prokaryote and Eukaryote Models | Germinal Cell | ||
|---|---|---|---|
| In vitro | In vivo | ||
| I. Gene Mutations | |||
| Bacteria (Ames assay, SOS chromotest) Yeast /Fungus (S. cerevisiae assay, A. nidulans assay) | Mouse spot test Somatic mutations and recombination test (SMART) DNA microarrays Serial analysis of gene expression (SAGE) Specific genes targeting | Recessive lethal Specific locus test Abnormalities of the sperm | |
| II. Chromosome Changes | |||
| Fibroblast culture Lymphocyte culture Mouse lymphoma assay | Micronucleus assay (MN) | Dominant lethal Heritable translocations Cytogenetic sperm Aneuploidy | |
| III. Indicators Biological Damage | |||
| Gene recombination Unscheduled DNA synthesis (UDS) assay | Comet assay Sister chromatid exchange (SCE) MN | Comet assay MN, SCE UDS assay | |
| Country | Traditional Uses |
|---|---|
| Amazonia | Diarrhea, dysentery, menstrual disorders, stomachache, vertigo. |
| Brasil | Diarrhea, anorexia, cholera, digestive problems, dysentery, gastric insufficiency, inflamed mucous membranes, skin problems, sore throat, ulcers, vaginal discharge. |
| Cuba | Dysentery, dispepsia. |
| Ghana | Diarrhea, dysentery, coughs, toothache. |
| Haiti | Diarrhea, dysentery, stomachache, epilepsy, itch, piles, scabies, skin sores, sore throat, wounds and as an antiseptic and astringent. |
| India | Anorexia, cerebral ailments, childbirth, chorea, convulsions, epilepsy, nephritis. |
| Malaya | Diarrhea, dermatosis, epilepsy, hysteria, menstrual disorders. |
| Mexico | Diarrhea, stomachache, deafnessitch, scabies, swelling, ulcer, worms, wounds. |
| Peru | Diarrhea, dysentery, conjunctivitis, cough, digestive problems, edema, gout, hemorrhages, gastroenteritis, gastritis, lung problems, shock, vaginal discharge, vertigo, worms. |
| Philippines | Sores, wounds and as an astringent. |
| Trinidad | Diarrhea, dysentery, bacterial infections, blood cleansing. |
| Year | Main Objetive | Type of Study | Assay Employed | Reference |
|---|---|---|---|---|
| Fruit, juice or extract | ||||
| 2002 | Suppression of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine-induced DNA damage in rat colon after grapefruit juice intake. | In vivo | Comet assay | [42] |
| 2004 | Influence of grapefruit juice intake on AFB1-induced liver DNA damage. | In vivo | Comet assay | [43] |
| 2004 | Capacity of the grapefruit juice to inhibit the rate of micronucleated polychromatic erythrocytes (MNPE) produced by daunorubicin. | In vivo | Micronucleus | [44] |
| 2010 | Capacity of the grapefruit juice to inhibit the rate of micronucleated polychromatic erythrocytes (MNPE) and Sister chromatid exchange (SCE) induced by ifosfamide. | In vivo | Micronucleus & SCE | [45] |
| 2010 | Capacity of 15 fruit juices to protect against the genotoxic effect produced by 2-amino-3-methylimidazo [4,5-f] quinoline and 2-amino-1-methyl-6-phenylimidazo [4,5-b] pyridine. | In vitro | Comet assay | [46] |
| 2011 | Capacity of grapefruit juice to inhibit the rate of micronucleated polychromatic erythrocytes (MNPE) in mice treated with benzo(a)pyrene. | In vivo | Micronucleus | [47] |
| 2011 | Evaluation of the genotoxic effect of H2O2 and the reduction of this damage by grapefruit juice in human lymphocytes. | In vitro | Comet assay | [48] |
| 2013 | Evaluation of the ameliorative role of grapefruit juice on the cytogenetic and testicular damage induced by the amiodarone in albino rats. | In vivo | Chromosomal aberrations | [49] |
| 2014 | Combination of rosemary and citrus bioflavonoids extracts from grape fruit against the genotoxicity induced by X-rays in human lymphocytes. | In vitro | Comet assay & Micronucleus | [50] |
| Phytochemical (naringin and naringenin) | ||||
| 2001 | Antigenotoxic effect of naringin against the damage induced by ifosfamide. | In vivo | Micronucleus | [51] |
| 2003 | Evaluation of the protective effect of naringin on H2O2-induced cytotoxicity and apoptosis in mouse leukemia P388 cells. | In vitro | Comet assay | [52] |
| 2004 | Effect of naringin on the cytotoxicity and apoptosis in mouse leukemia P388 cells treated with Ara-C. | In vitro | Comet assay | [53] |
| 2007 | Evaluation of the protective effect of naringin on the genomic damage induced by bleomycin. | In vitro | Micronucleus | [54] |
| 2012 | Influence of naringin on cadmium-induced genomic damage in human lymphocytes. | In vitro | Cromosomal aberrations & SCE | [55] |
| Year | Main Objetive | Assay Employed | Reference |
|---|---|---|---|
| In vitro studies | |||
| 2014 | Effect of MGN on gamma radiation-induced DNA damage in human lymphocytes and lymphoblastoid cells. | Comet assay | [74] |
| 2012 | Evaluation of protective effects of MGN against several mutagens (bleomycin, CP, AFB1, B[a]P, 2-AAF, H2O2, sodium azide, cisplatin, MMC, and DMNA). | Ames test, SOS Chromotest assay & Comet assay | [75] |
| 2009 | Cytoprotective and antigenotoxic potential of mangiferine against cadmium chloride CdCl2-induced toxicity in HepG2 cells. | Comet assay & Micronucleus | [76] |
| 2011 | Evaluation of the protector effect of MGN against methylmercury (MeHg) induced neurotoxicity by employing IMR-32 (human neuroblastoma) cell line. | Comet assay & Micronucleus | [77] |
| 2011 | Antigenotoxic potential of MGN against mercuric chloride (HgCl2)-induced genotoxicity in HepG2 cell line. | Comet assay & Micronucleus | [78] |
| 2015 | Evaluation of the Mangiferin to reduce etoposide-induced DNA damage in human umbilical cord mononuclear blood cells. | Comet assay & Micronucleus | [79] |
| In vivo studies | |||
| 2008 | Protective role of mangiferin against B[a]P induced lung carcinogenesis in experimental animals. | Comet assay | [80] |
| 2005 | Ability of the mangiferin to reduce the frequency of radiation-induced micronucleated binucleate cells (MNBNCs) in cultured human peripheral blood lymphocytes. | Micronucleus | [81] |
| 2010 | The protective role of mangiferin (MGN) against cadmium chloride CdCl2-induced genotoxicity studied in Swiss albino mice. | Micronucleus | [82] |
| Year | Aim of the Study | Technique Used | Conclusion | Reference |
|---|---|---|---|---|
| In vitro studies | ||||
| 2002 | Protective effect of blueberries and blackberries against genotoxicity of 2-acetylaminofluorene (AAF) & 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in V79 cells. | CA | Genotoxic activity of AAF and PhIP was strongly reduced in a dose-related manner by berry fruits. Demonstrating that protection of fruits against genotoxicity of heterocyclic aromatic amines may take place within metabolically competent mammalian cells. | [86] |
| 2010 | Protective effect of the juices of blueberry, and blackberry against genotoxicity of 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) &PhIP in V79 cells. | CA | These berry fruits showed a inhibitory effect on genotoxicity of both mutagens. The best protection was for the blueberry juice. As one possible mechanism of antigenotoxicity, is the inhibition of activating enzymes of xenobiotics, such as CYP1A2. | [46] |
| 2013 | Protective effect of blueberries against genotoxicity of N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) & 7,12-dimethylbenz(a)anthracene (DMBA) assessed in HepG2. | MN | Blueberries were not toxic in vitro. The pre-treatment with blueberries reduced the micronucleus frequency induced by MNNG but the same effect is not present with DMBA. | [87] |
| 2014 | Protective effect of Vaccinium myrtillus extract against UVA- and UVB-induced damage in HaCaT cells. | MN & CA | The extract showed its free radical scavenging properties reducing oxidative stress and apoptotic markers, especially in UVA-irradiated cells. | [88] |
| In vivo studies | ||||
| 2006 | Protective effect of extract of Vaccinium ashei berries on DNA damage in the hippocampus and cerebral cortex, as well as on cognitive performance in mice. | CA | The extract reduced oxidative DNA damage in brain tissue. Suggesting that supplementation with V. ashei berries to mice improves the memory and has a protective effect on DNA damage, possibly due to the antioxidant activity. | [89] |
| 2010 | Protective effect of 8 weeks administration of a wild blueberry (Vaccinium angustifolium) against H2O2-induced DNA damage in lymphocytes of rats. | CA | The level of DNA damage was significantly lower in rats fed with the wild blueberry at the end of the eight weeks. | [90] |
| 2012 | Protective effect of cranberry ethanolic extract (CEE), (Vaccinium macrocarpon) against the DNA damage induced by B[a]P. | MN | The CEE was not genotoxic. On the contrary, reduces the frequency of micronucleus induced by B[a]P. Suggesting that the anticlastogenic effect of the CEE is related to the antioxidant capacity of the combination of phytochemicals present in its chemical composition. | [83] |
| 2013 | Protective effect of blueberries against genotoxicity of MNNG and DMBA assessed in polychromatic erythrocytes (PCEs) from the bone marrow of mice. | MN | Blueberries fruits reduced the frequency of micronucleus induced by MNNG and DMBA. However, in the case of DMBA, a dramatic reduction in the percentage of PCEs was observed, suggesting increased cytotoxicity. | [87] |
| Clinical Studies | ||||
| 2007 | Impact of multiple genetic polymorphisms on effects of a 4-week blueberry/apple juice intervention on ex vivo induced lymphocytic DNA damage in 168 healthy human volunteers. | CA | The analysis of 34 genetic polymorphisms showed that DNA damage is reduced faster in some individuals per share of micronutrients in the blueberry/apple juice. | [91] |
| 2012 | Protective effect blueberry (Vaccinium corymbosum L) on markers of oxidative stress and DNA damage induced by H2O2 in healthy volunteers. | CA | Blueberries significantly reduced H2O2-induced DNA damage in blood mononuclear cells. No significant differences were observed in peripheral arterial function and nitric oxide levels after blueberry intake. In conclusion, one portion of blueberries seems sufficient to improve cell antioxidant defense against DNA damage. | [92] |
| 2015 | Compare DNA damage in fresh versus cryopreserved peripheral blood mononuclear cells (PBMCs) obtained from subjects following a 6-week intervention with wild blueberry drink. | CA | The decrease in H2O2-induced DNA damage observed in the cryopreserved cells masked the protective effect of the wild blueberry drink documented in the fresh samples. Suggesting that H2O2-induced DNA damage could be significantly modified following the long-term storage of samples obtained from individuals participating in adietary intervention. | [93] |
| Year | Type of β-glucan | Type Cell | Mutagen or Carcinog | Reference |
|---|---|---|---|---|
| In vitro studies | ||||
| 2003 | β-glucan from S. cerevisiae β-glucan-chitin from A. niger | V79 hamster lung cells | H2O2 | [110] |
| 2006 | Carboxymethyl chitin-glucan (CM-CG) from A. niger | Primary rat hepatocytes | BaP, N-nitrosomorpholine (NMOR) & dimethyldibenzocarbazole | [111] |
| 2006 | β-glucan from A. brasiliensis | Human peripheral lymphocytes | 3-amino-1-methyl-5H-pyrido[4,3-b]indole, (+/−)-anti-B[a]P-7,8-dihydrodiol-9,10-epoxide & H2O2 | [112] |
| 2008 | CM-CG from A. niger | Human hepatoma cells (HepG2) & V79 hamster lung cells | NMOR | [113] |
| 2008 | CM-CGfrom A. niger | Primary rat hepatocytes | H2O2 & 2,3-dimethoxy-1,4-naphthoquinone | [114] |
| 2009 | β-glucan from barley | HepG2 | BaP | [115] |
| 2009 | β-glucan from Agaricus blazei | HepG2 | BaP | [116] |
| 2010 | CM-CG from A. niger | HepG2 & HeLa cells | H2O2, Methylmethane sulfonate (MMS) & N-methyl-N′-nitro-N-nitrosoquanidine (MNNG) | [117] |
| 2014 | β-glucan from barley | HepG2 | Radiation (6 Gy) | [118] |
| 2014 | β-glucan from Ganoderma lucidum | Human peripheral lymphocytes | Radiation (1, 2 & 4 Gy) | [119] |
| In vivo studies | ||||
| 2004 | CM-CG from A. niger | Lymphocytes, testicular cells & epithelial II cells from Sprague Dawley rats | H2O2 | [120] |
| 2011 | CM-G from S. cerevisiae | Cells from patients with advanced prostate cancer (PCa) | Prostate cancer | [121] |
| 2015 | β-glucan from S. cerevisiae | Broiler chicken lymphocytes | AFB1 | [122] |
| 2015 | β-glucan from S. cerevisiae | Mouse hepatocytes | AFB1 | [102] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izquierdo‐Vega, J.A.; Morales‐González, J.A.; SánchezGutiérrez, M.; Betanzos‐Cabrera, G.; Sosa‐Delgado, S.M.; Sumaya‐Martínez, M.T.; Morales‐González, Á.; Paniagua‐Pérez, R.; Madrigal‐Bujaidar, E.; Madrigal‐Santillán, E. Evidence of Some Natural Products with Antigenotoxic Effects. Part 1: Fruits and Polysaccharides. Nutrients 2017, 9, 102. https://doi.org/10.3390/nu9020102
Izquierdo‐Vega JA, Morales‐González JA, SánchezGutiérrez M, Betanzos‐Cabrera G, Sosa‐Delgado SM, Sumaya‐Martínez MT, Morales‐González Á, Paniagua‐Pérez R, Madrigal‐Bujaidar E, Madrigal‐Santillán E. Evidence of Some Natural Products with Antigenotoxic Effects. Part 1: Fruits and Polysaccharides. Nutrients. 2017; 9(2):102. https://doi.org/10.3390/nu9020102
Chicago/Turabian StyleIzquierdo‐Vega, Jeannett Alejandra, José Antonio Morales‐González, Manuel SánchezGutiérrez, Gabriel Betanzos‐Cabrera, Sara M. Sosa‐Delgado, María Teresa Sumaya‐Martínez, Ángel Morales‐González, Rogelio Paniagua‐Pérez, Eduardo Madrigal‐Bujaidar, and Eduardo Madrigal‐Santillán. 2017. "Evidence of Some Natural Products with Antigenotoxic Effects. Part 1: Fruits and Polysaccharides" Nutrients 9, no. 2: 102. https://doi.org/10.3390/nu9020102
APA StyleIzquierdo‐Vega, J. A., Morales‐González, J. A., SánchezGutiérrez, M., Betanzos‐Cabrera, G., Sosa‐Delgado, S. M., Sumaya‐Martínez, M. T., Morales‐González, Á., Paniagua‐Pérez, R., Madrigal‐Bujaidar, E., & Madrigal‐Santillán, E. (2017). Evidence of Some Natural Products with Antigenotoxic Effects. Part 1: Fruits and Polysaccharides. Nutrients, 9(2), 102. https://doi.org/10.3390/nu9020102





