Acute Effects of Nitrate-Rich Beetroot Juice on Blood Pressure, Hemostasis and Vascular Inflammation Markers in Healthy Older Adults: A Randomized, Placebo-Controlled Crossover Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Study Design
2.3. Blood Pressure Measurements and Power Density Spectral Analysis
2.4. Blood Sample Collections
2.5. Plasma Nitrite and Nitrate
2.6. Rotational Thrombelastometry
2.7. Plasma Hemostasis Analysis
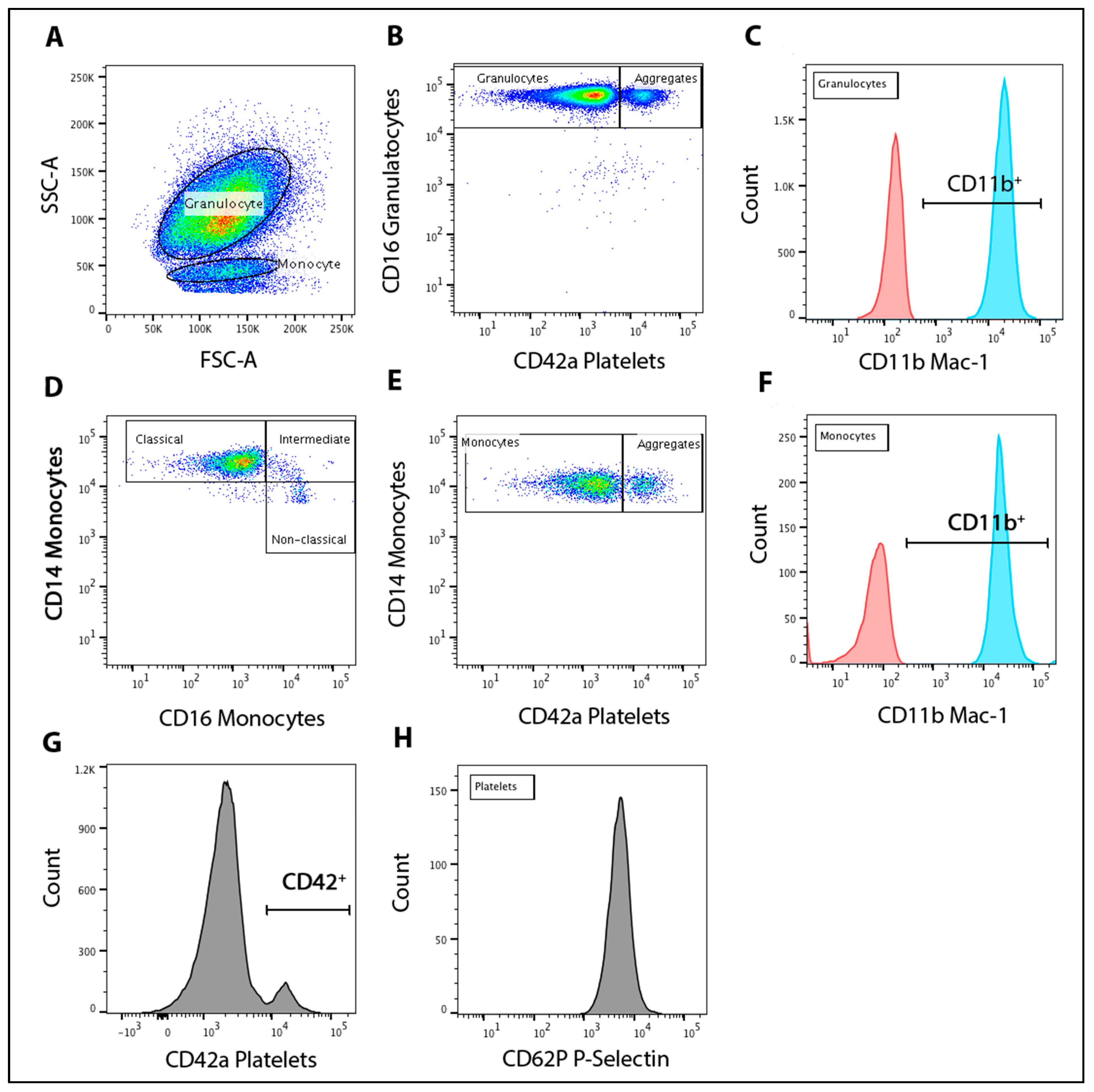
2.8. Flow Cytometry
2.9. Leukocyte-Platelet Aggregation and Activation
2.10. Monocyte Population Subsets
2.11. Platelet P-Selectin
2.12. Statistical Analysis
3. Results
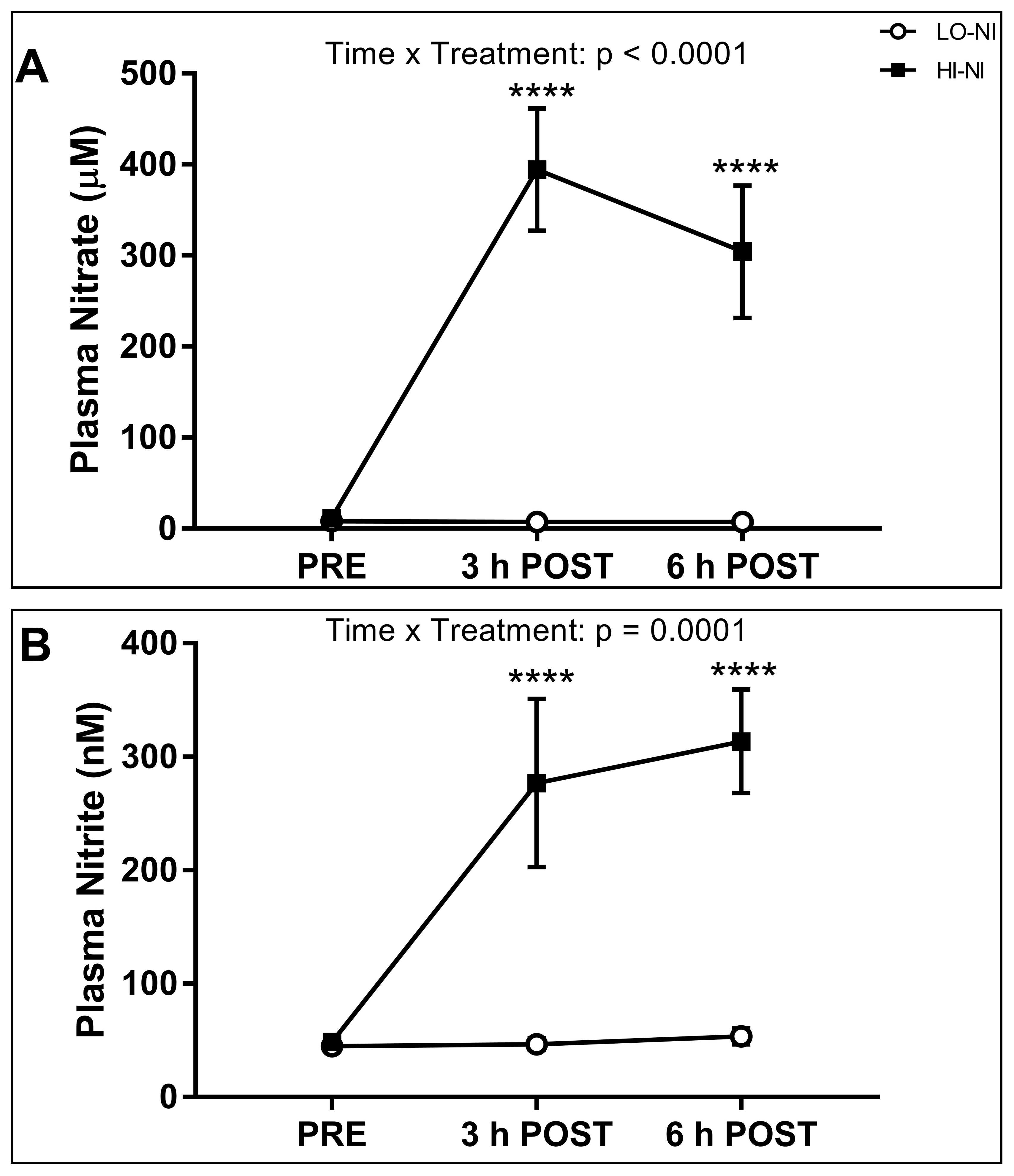
3.1. Plasma Nitrate and Nitrite Levels
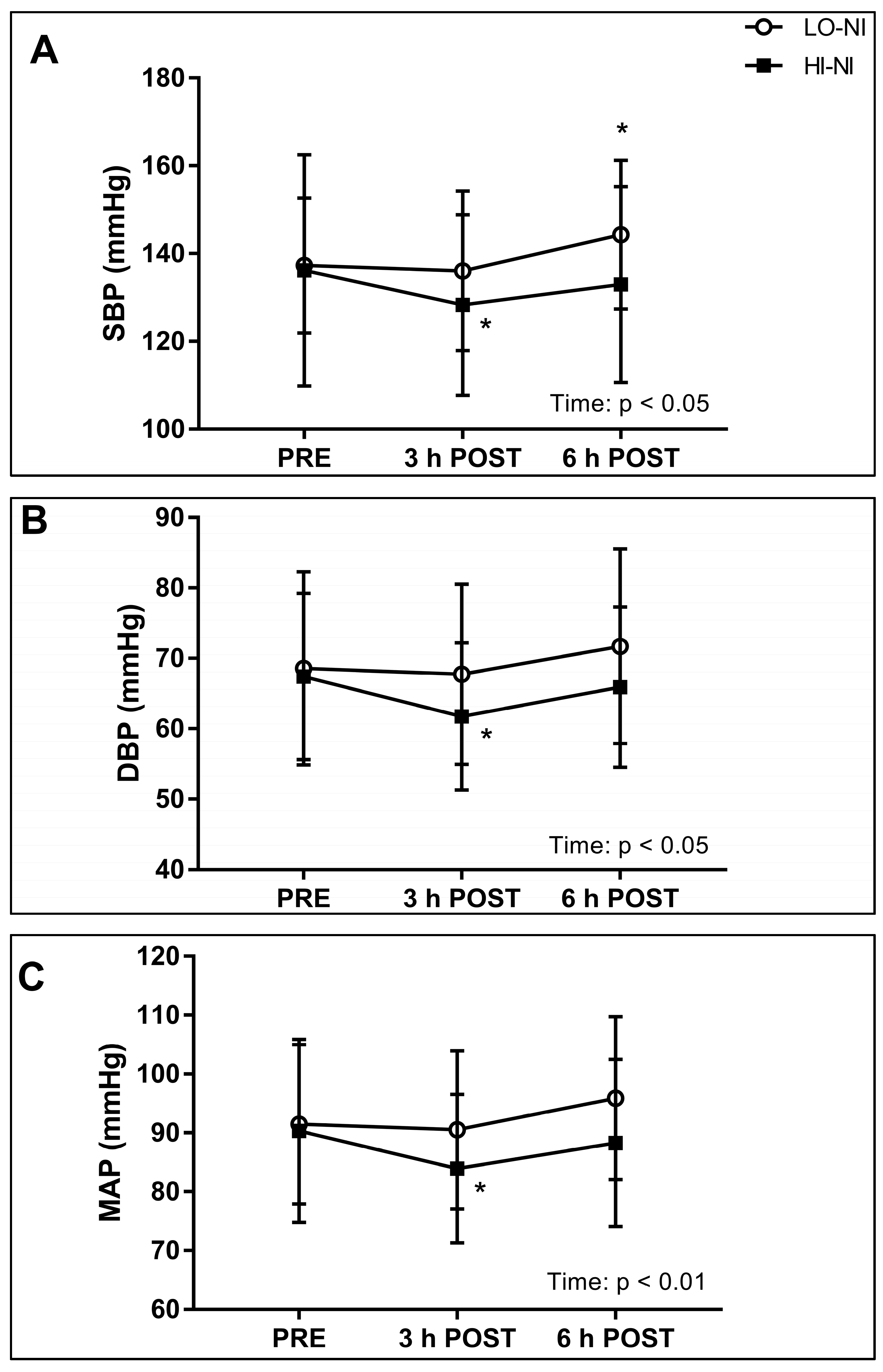
3.2. Systolic, Diastolic and Mean Arterial Blood Pressure, and Power Spectral Density of Systolic Blood Pressure
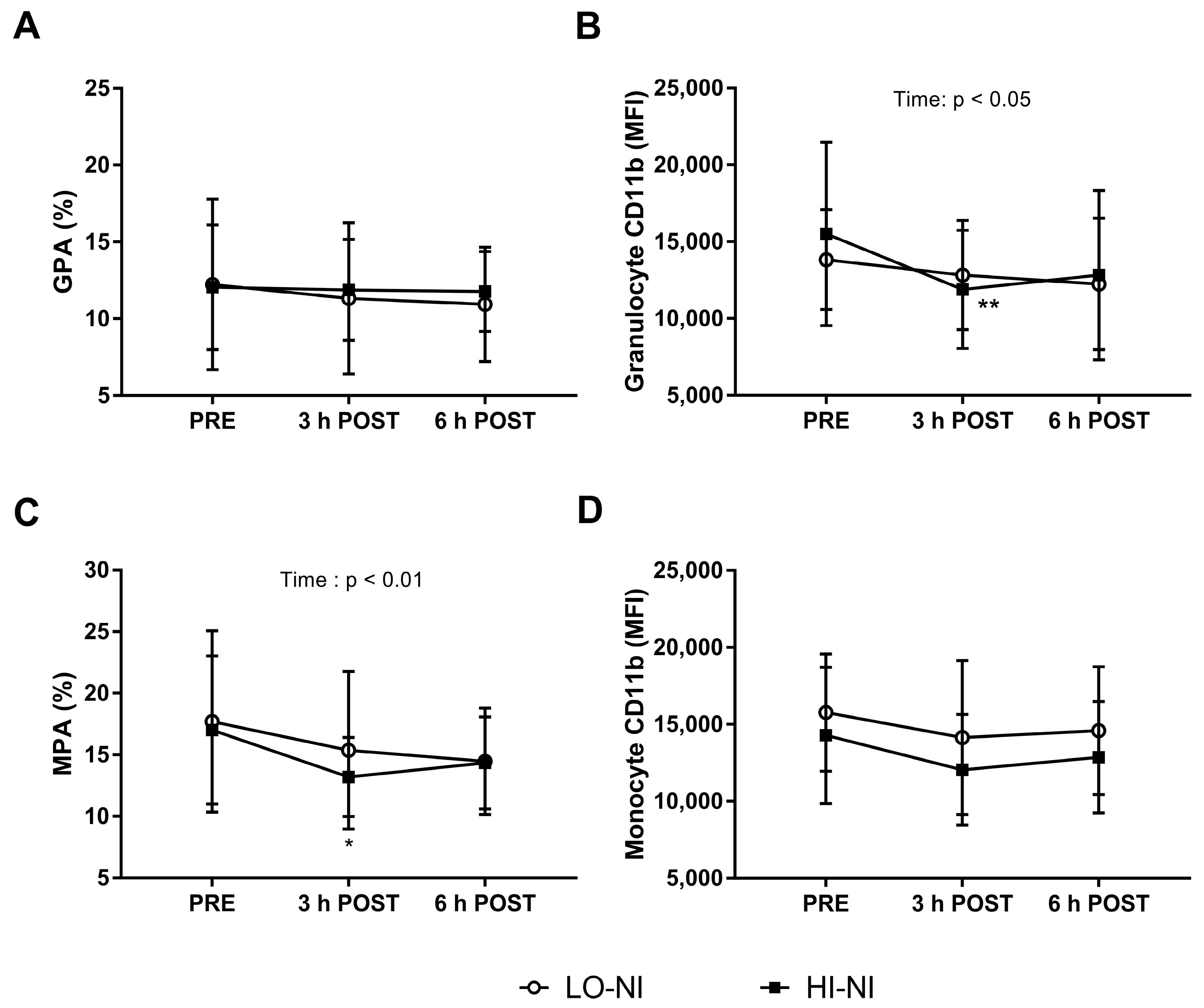
3.3. Granulocyte-Platelet and Monocyte-Platelet Aggregation
3.4. Expression of CD11b in Granulocytes with HI-NO Beetroot Juice
3.5. Monocyte Subset Populations and Platelet P-Selectin Expression
3.6. Whole Blood Coagulation
3.7. Effects on Plasma Hemostasis Biomarkers
4. Discussion
4.1. Effects of Nitrate-Rich Beetroot Juice on Plasma Nitrate and Nitrite
4.2. Effects of Nitrate-Rich Beetroot Juice on Blood Pressure and Frequency Components of Blood Pressure Variability
4.3. Effects of Beetroot Juice on Markers of Vascular Inflammation and Platelet Activation
4.4. Effects of Nitrate-Rich Beetroot Juice on Hemostasis
4.5. Theoretical and Practical Implications
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Seals, D.R.; Kaplon, R.E.; Gioscia-Ryan, R.A.; LaRocca, T.J. You’re only as old as your arteries: Translational strategies for preserving vascular endothelial function with aging. Physiology 2014, 29, 250–264. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, C.P.; Croft, K.D.; Ward, N.; Considine, M.J.; Hodgson, J.M. Dietary flavonoids and nitrate: Effects on nitric oxide and vascular function. Nutr. Rev. 2015, 73, 216–235. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, C.; Palomo, I.; Fuentes, E. Primary and secondary haemostasis changes related to aging. Mech. Ageing Dev. 2015, 150, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, K.; Altmann, H.M.; Straub, A.C.; Isenberg, J.S. Nitric oxide: What’s new to NO? Am. J. Physiol. Cell Physiol. 2017, 312, C254–C262. [Google Scholar] [CrossRef] [PubMed]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Weitzberg, E.; Lundberg, J.O. Novel aspects of dietary nitrate and human health. Annu. Rev. Nutr. 2013, 33, 129–159. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Gladwin, M.T.; Weitzberg, E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat. Rev. Drug Discov. 2015, 14, 623–641. [Google Scholar] [CrossRef] [PubMed]
- Siervo, M.; Lara, J.; Ogbonmwan, I.; Mathers, J.C. Inorganic nitrate and beetroot juice supplementation reduces blood pressure in adults: A systematic review and meta-analysis. J. Nutr. 2013, 143, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Jajja, A.; Sutyarjoko, A.; Lara, J.; Rennie, K.; Brandt, K.; Qadir, O.; Siervo, M. Beetroot supplementation lowers daily systolic blood pressure in older, overweight subjects. Nutr. Res. 2014, 34, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Rammos, C.; Hendgen-Cotta, U.B.; Sobierajski, J.; Bernard, A.; Kelm, M.; Rassaf, T. Dietary nitrate reverses vascular dysfunction in older adults with moderately increased cardiovascular risk. J. Am. Coll. Cardiol. 2014, 63, 1584–1585. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.J.; Patel, N.; Loukogeorgakis, S.; Okorie, M.; Aboud, Z.; Misra, S.; Rashid, R.; Miall, P.; Deanfield, J.; Benjamin, N.; et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 2008, 51, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, S.; Gan, J.M.; Rathod, K.S.; Khambata, R.S.; Ghosh, S.M.; Hartley, A.; Van Eijl, S.; Sagi-Kiss, V.; Chowdhury, T.A.; Curtis, M.; et al. Dietary nitrate improves vascular function in patients with hypercholesterolemia: A randomized, double-blind, placebo-controlled study. Am. J. Clin. Nutr. 2016, 103, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.; Fulford, J.; Vanhatalo, A.; Blackwell, J.R.; French, O.; Bailey, S.J.; Gilchrist, M.; Winyard, P.G.; Jones, A.M. Effects of short-term dietary nitrate supplementation on blood pressure, O2 uptake kinetics, and muscle and cognitive function in older adults. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R73–R83. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, M.; Winyard, P.G.; Aizawa, K.; Anning, C.; Shore, A.; Benjamin, N. Effect of dietary nitrate on blood pressure, endothelial function, and insulin sensitivity in type 2 diabetes. Free Radic. Biol. Med. 2013, 60, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Larbi, A.; Franceschi, C.; Mazzatti, D.; Solana, R.; Wikby, A.; Pawelec, G. Aging of the immune system as a prognostic factor for human longevity. Physiology 2008, 23, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Woessner, M.; Smoliga, J.M.; Tarzia, B.; Stabler, T.; Van Bruggen, M.; Allen, J.D. A stepwise reduction in plasma and salivary nitrite with increasing strengths of mouthwash following a dietary nitrate load. Nitric Oxide 2016, 54, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Stauss, H.M. Identification of blood pressure control mechanisms by power spectral analysis. Clin. Exp. Pharmacol. Physiol. 2007, 34, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Sathishkumar, M.; Wilson, T.E.; Shibasaki, M.; Davis, S.L.; Crandall, C.G. Spectral characteristics of skin sympathetic nerve activity in heat-stressed humans. Am. J. Physiol. Heart Circ. Physiol. 2006, 290. [Google Scholar] [CrossRef] [PubMed]
- Bangash, M.F.; Xie, A.; Skatrud, J.B.; Reichmuth, K.J.; Barczi, S.R.; Morgan, B.J. Cerebrovascular response to arousal from NREM and REM sleep. Sleep 2008, 31, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Luddington, R.J. Thrombelastography/thromboelastometry. Clin. Lab. Haematol. 2005, 27, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Solomon, C.; Ranucci, M.; Hochleitner, G.; Schochl, H.; Schlimp, C.J. Assessing the Methodology for Calculating Platelet Contribution to Clot Strength (Platelet Component) in Thromboelastometry and Thrombelastography. Anesth. Analg. 2015, 121, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.J.; Liu, Y.J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.H.; Witkiewitz, K.; Andre, J.S.; Reilly, S. Methods for Handling Missing Data in the Behavioral Neurosciences: Don’t Throw the Baby Rat out with the Bath Water. J. Undergrad. Neurosci. Educ. 2007, 5, A71–A77. [Google Scholar] [PubMed]
- Wylie, L.J.; Kelly, J.; Bailey, S.J.; Blackwell, J.R.; Skiba, P.F.; Winyard, P.G.; Jeukendrup, A.E.; Vanhatalo, A.; Jones, A.M. Beetroot juice and exercise: Pharmacodynamic and dose-response relationships. J. Appl. Physiol. (1985) 2013, 115, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Presley, T.D.; Morgan, A.R.; Bechtold, E.; Clodfelter, W.; Dove, R.W.; Jennings, J.M.; Kraft, R.A.; King, S.B.; Laurienti, P.J.; Rejeski, W.J.; et al. Acute effect of a high nitrate diet on brain perfusion in older adults. Nitric Oxide 2011, 24, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Rossier, B.C.; Bochud, M.; Devuyst, O. The Hypertension Pandemic: An Evolutionary Perspective. Physiology 2017, 32, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Notay, K.; Incognito, A.V.; Millar, P.J. Acute beetroot juice supplementation on sympathetic nerve activity: A randomized, double-blind, placebo-controlled proof-of-concept study. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H59–H65. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhai, K.; Chen, Y.; Zhang, X.; Miao, L.; Wei, B.; Ji, G. Nitric oxide mediates stretch-induced Ca2+ oscillation in smooth muscle. J. Cell Sci. 2016, 129, 2430–2437. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, J.; Gamu, D.; Heigenhauser, G.J.F.; van Loon, L.J.C.; Spriet, L.L.; Tupling, A.R.; Holloway, G.P. Beetroot Juice Increases Human Muscle Force Without Changing Ca2+-handling Proteins. Med. Sci Sports Exerc. 2017, 49, 2016–2024. [Google Scholar] [CrossRef] [PubMed]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Jadert, C.; Petersson, J.; Massena, S.; Ahl, D.; Grapensparr, L.; Holm, L.; Lundberg, J.O.; Phillipson, M. Decreased leukocyte recruitment by inorganic nitrate and nitrite in microvascular inflammation and NSAID-induced intestinal injury. Free Radic. Biol. Med. 2012, 52, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Khambata, R.S.; Ghosh, S.M.; Rathod, K.S.; Thevathasan, T.; Filomena, F.; Xiao, Q.; Ahluwalia, A. Antiinflammatory actions of inorganic nitrate stabilize the atherosclerotic plaque. Proc. Natl. Acad. Sci. USA 2017, 114, E550–E559. [Google Scholar] [CrossRef] [PubMed]
- Rondina, M.T.; Weyrich, A.S.; Zimmerman, G.A. Platelets as cellular effectors of inflammation in vascular diseases. Circ. Res. 2013, 112, 1506–1519. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, C.P.; Yang, X.; Croft, K.D.; Considine, M.J.; Ward, N.C.; Rich, L.; Puddey, I.B.; Swinny, E.; Mubarak, A.; Hodgson, J.M. Flavonoid-rich apples and nitrate-rich spinach augment nitric oxide status and improve endothelial function in healthy men and women: A randomized controlled trial. Free Radic. Biol. Med. 2012, 52, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. The potential benefits of red beetroot supplementation in health and disease. Nutrients 2015, 7, 2801–2822. [Google Scholar] [CrossRef] [PubMed]
- Rogacev, K.S.; Cremers, B.; Zawada, A.M.; Seiler, S.; Binder, N.; Ege, P.; Grosse-Dunker, G.; Heisel, I.; Hornof, F.; Jeken, J.; et al. CD14++CD16+ monocytes independently predict cardiovascular events: A cohort study of 951 patients referred for elective coronary angiography. J. Am. Coll. Cardiol. 2012, 60, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, S.; Kapil, V.; Ghosh, S.M.; Davies, S.; McKnight, A.; Aboud, Z.; Khambata, R.S.; Webb, A.J.; Poole, A.; Ahluwalia, A. Antiplatelet effects of dietary nitrate in healthy volunteers: Involvement of cGMP and influence of sex. Free Radic. Biol. Med. 2013, 65, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Piknova, B.; Nghiem, K.; Lozier, J.N.; Schechter, A.N. Inhibitory effect of nitrite on coagulation processes demonstrated by thrombelastography. Nitric Oxide 2014, 40, 45–51. [Google Scholar] [CrossRef] [PubMed]
| Anthropometric Characterististics and Medications | Baseline Values |
|---|---|
| Age (Years; Indicated as Mean Age and Range) | 64 (57–71) |
| Sex (male:female) | 5:7 |
| Body mass index (BMI) (kg/m2) | 25.7 ± 4.2 |
| Baseline systolic blood pressure (mmHg) | 133.0 ± 16.6 |
| Baseline diastolic blood pressure (mmHg) | 88.6 ± 8.8 |
| Waist circumference (cm) | 96.0 ± 12.2 |
| Medications (n) | 4 |
| ACE inhibitors | 1 |
| PPI | 1 |
| SSNRI | 1 |
| XOI | 1 |
| Breakfast | ||||||
| Food | Brand | Amount | Energy (kJ) | Fat (g) | Carbo-Hydrates (g) | Protein (g) |
| High-fiber low-sugar cereal biscuit | Weet-bix, Sanitarium | 4 biscuits | 983 | 0.9 | 44.2 | 8.2 |
| Milk † | Devondale | 200 mL | 538 | 6.8 | 10.2 | 6.6 |
| Apple Juice | Just Juice | 200 mL | 374 | <1 | 21 | <1 |
| Total | 1895 | 7.7 | 75.4 | 14.8 | ||
| Snack | ||||||
| Food | Brand | Amount | Energy (kJ) | Fat (g) | Carbo-Hydrates (g) | Protein (g) |
| Oat Slice ʎ | Uncle Toby’s | 1 bar | 600 | 6.1 | 19.1 | 2.1 |
| Apple * | Gala | 1 whole fruit | 441 | 0 | 22.9 | 0.6 |
| Total | 1041 | 6.1 | 42 | 2.7 | ||
| Variable | LO-NI | HI-NI | 2-Way ANOVA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PRE | 3 h POST | 6 h POST | PRE | 3 h POST | 6 h POST | Time | Treatment | Time × Treatment | |
| SBP variability, VLF, mmHg2 | 29.1 ± 4.7 | 26.6 ± 7.5 | 28.6 ± 8.1 | 29.9 ± 5.5 | 33.0 ± 10.5 | 27.6 ± 5.1 | 0.530 | 0.368 | 0.065 |
| SBP variability, LF, mmHg2 | 24.7 ± 9.4 | 25.5 ± 7.1 | 26.7 ± 8.4 | 28.3 ± 9.9 | 31.5 ± 11.3 | 27.3 ± 8.1 | 0.578 | 0.258 | 0.389 |
| SBP variability, HF, mmHg2 | 24.9 ± 9.2 | 24.6 ± 5.9 | 26.4 ± 9.2 | 26.2 ± 8.2 | 28.3 ± 11.7 | 24.5 ± 7.9 | 0.820 | 0.740 | 0.270 |
| Heart rate, beats/min | 68.9 ± 4.5 | 71.3 ± 8.4 | 69.8 ± 7.8 | 70.0 ± 6.8 | 73.7 ± 7.7 | 71.7 ± 7.4 | 0.059 | 0.499 | 0.857 |
| Variable | LO-NI | HI-NI | 2-Way ANOVA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PRE | 3 h POST | 6 h POST | PRE | 3 h POST | 6 h POST | Time | Treatment | Time × Treatment | |
| Classical CD14++ CD16− monocytes | 90.0 ± 4.1 | 91.6 ± 2.8 | 91.2 ± 3.7 | 91.1 ± 3.6 | 92.1 ± 3.2 | 89.7 ± 2.2 | 0.047 | 0.991 | 0.112 |
| Intermediate CD14++ CD16+ monocytes | 4.3 ± 1.5 | 3.9 ± 1.5 | 3.3 ± 1.6 * | 4.0 ± 1.5 | 3.5 ± 1.3 | 4.9 ± 1.0 * | 0.183 | 0.532 | 0.001 |
| Non-classical CD14+ CD16++ monocytes | 5.4 ± 2.7 | 4.4 ± 1.4 | 5.0 ± 2.4 | 4.2 ± 2.0 | 4.4 ± 2.1 | 5.4 ± 1.8 | 0.235 | 0.722 | 0.239 |
| P-selectin (CD42a) | 3083 ± 1657 | 3731 ± 1682 | 3104 ± 1202 | 3908 ± 1404 | 3810 ± 2073 | 3575 ± 1937 | 0.385 | 0.442 | 0.495 |
| Variable | Reference Range | LO-NI | HI-NI | 2-Way ANOVA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PRE | 3 h POST | 6 h POST | PRE | 3 h POST | 6 h POST | Time | Treatment | Time × Treatment | ||
| Whole Blood Thromobelastometry | ||||||||||
| EXTEM CT, s | 42–78 | 68.9 ± 4.4 | 71.5 ± 4.4 | 71.6 ± 5.0 | 70.3 ± 6.0 | 68.7 ± 4.7 | 68.3 ± 7.9 | 0.904 | 0.400 | 0.102 |
| EXTEM CFT, s | 53–144 | 91.8 ± 18.0 | 93.4 ± 17.2 | 91.8 ± 14.7 | 89.8 ± 16.5 | 94.3 ± 17.7 | 93.8 ± 21.3 | 0.271 | 0.969 | 0.533 |
| EXTEM MCF, mm | 48–70 | 65.8 ± 4.6 | 65.8 ± 4.1 | 66.2 ± 3.6 | 66.2 ± 3.5 | 65.3 ± 2.6 | 65.7 ± 3.7 | 0.429 | 0.867 | 0.495 |
| EXTEM α angle, ° | 63–83 | 72.6 ± 3.7 | 72.4 ± 3.3 | 72.8 ± 3.2 | 72.8 ± 3.0 | 72.1 ± 2.8 | 72.3 ± 4.0 | 0.389 | 0.781 | 0.304 |
| EXTEM LI30, % | 94–100 | 99.3 ± 0.9 | 98.8 ± 1.4 | 99.3 ± 0.8 | 98.8 ± 2.3 | 98.3 ± 2.1 | 99.1 ± 0.8 | 0.151 | 0.407 | 0.904 |
| INTEM CT, s | 134–218 | 208.0 ± 17.9 | 200.3 ± 15.0 | 199.0 ± 12.7 | 205.3 ± 18.5 | 193.1 ± 14.2 * | 197.1 ± 20.8 | 0.010 | 0.490 | 0.698 |
| INTEM CFT, s | 52–116 | 72.6 ± 17.5 | 66.0 ± 13.5 | 67.7 ± 11.9 | 66.8 ± 14.7 | 66.8 ± 11.9 | 64.9 ± 12.3 | 0.180 | 0.614 | 0.279 |
| INTEM MCF, mm | 47–69 | 65.4 ± 4.6 | 65.7 ± 4.2 | 65.1 ± 3.7 | 66.3 ± 3.7 | 65.4 ± 3.3 | 66.3 ± 3.6 | 0.814 | 0.704 | 0.280 |
| INTEM α angle, ° | 70–83 | 75.3 ± 3.5 | 76.6 ± 2.7 | 76.1 ± 2.5 | 76.5 ± 2.8 | 76.5 ± 2.5 | 76.8 ± 2.5 | 0.247 | 0.562 | 0.287 |
| INTEM LI30, % | 94–100 | 99.7 ± 0.5 | 99.3 ± 1.2 | 99.3 ± 0.8 | 99.5 ± 0.7 | 98.4 ± 2.5 | 99.3 ± 0.8 | 0.066 | 0.367 | 0.378 |
| APTEM CT, s | 42–78 | 66.1 ± 5.4 | 67.6 ± 4.7 | 67.7 ± 3.6 | 68.8 ± 6.9 | 68.6 ± 5.2 | 64.8 ± 5.4 * | 0.254 | 0.879 | 0.043 |
| APTEM MCF, mm | 48–70 | 66.3 ± 4.1 | 65.9 ± 4.2 | 65.8 ± 3.3 | 66.3 ± 4.3 | 65.3 ± 2.5 | 66.8 ± 3.7 | 0.234 | 0.954 | 0.232 |
| FIBTEM MCF, mm | 7–21 | 14.9 ± 4.3 | 14.8 ± 4.6 | 14.4 ± 4.0 | 14.5 ± 3.8 | 14.8 ± 3.8 | 14.9 ± 3.8 | 0.943 | 0.973 | 0.405 |
| MCE | 180.0 ± 37.5 | 178.7 ± 35.2 | 178.2 ± 25.8 | 181.6 ± 28.9 | 171.8 ± 21.2 | 176.7 ± 28.7 | 0.432 | 0.842 | 0.614 | |
| Plasma Hemostasis Analysis | ||||||||||
| Prothrombin time, s | 11–15 | 11.9 ± 0.2 | 11.8 ± 0.2 | 11.8 ± 0.2 | 11.9 ± 0.2 | 12.0 ± 0.2 | 12.0 ± 0.2 | 0.851 | 0.605 | 0.508 |
| Activated partial thromboplastin time, s | 26–37 | 33.0 ± 1.0 | 32.5 ± 1.0 | 32.5 ± 1.0 | 32.8 ± 1.2 | 32.5 ± 1.2 | 32.5 ± 1.1 | 0.297 | 0.956 | 0.912 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raubenheimer, K.; Hickey, D.; Leveritt, M.; Fassett, R.; Ortiz de Zevallos Munoz, J.; Allen, J.D.; Briskey, D.; Parker, T.J.; Kerr, G.; Peake, J.M.; et al. Acute Effects of Nitrate-Rich Beetroot Juice on Blood Pressure, Hemostasis and Vascular Inflammation Markers in Healthy Older Adults: A Randomized, Placebo-Controlled Crossover Study. Nutrients 2017, 9, 1270. https://doi.org/10.3390/nu9111270
Raubenheimer K, Hickey D, Leveritt M, Fassett R, Ortiz de Zevallos Munoz J, Allen JD, Briskey D, Parker TJ, Kerr G, Peake JM, et al. Acute Effects of Nitrate-Rich Beetroot Juice on Blood Pressure, Hemostasis and Vascular Inflammation Markers in Healthy Older Adults: A Randomized, Placebo-Controlled Crossover Study. Nutrients. 2017; 9(11):1270. https://doi.org/10.3390/nu9111270
Chicago/Turabian StyleRaubenheimer, Kyle, Danica Hickey, Michael Leveritt, Robert Fassett, Joaquin Ortiz de Zevallos Munoz, Jason D. Allen, David Briskey, Tony J. Parker, Graham Kerr, Jonathan M. Peake, and et al. 2017. "Acute Effects of Nitrate-Rich Beetroot Juice on Blood Pressure, Hemostasis and Vascular Inflammation Markers in Healthy Older Adults: A Randomized, Placebo-Controlled Crossover Study" Nutrients 9, no. 11: 1270. https://doi.org/10.3390/nu9111270
APA StyleRaubenheimer, K., Hickey, D., Leveritt, M., Fassett, R., Ortiz de Zevallos Munoz, J., Allen, J. D., Briskey, D., Parker, T. J., Kerr, G., Peake, J. M., Pecheniuk, N. M., & Neubauer, O. (2017). Acute Effects of Nitrate-Rich Beetroot Juice on Blood Pressure, Hemostasis and Vascular Inflammation Markers in Healthy Older Adults: A Randomized, Placebo-Controlled Crossover Study. Nutrients, 9(11), 1270. https://doi.org/10.3390/nu9111270







