Isoflavones: Anti-Inflammatory Benefit and Possible Caveats
Abstract
:1. Introduction
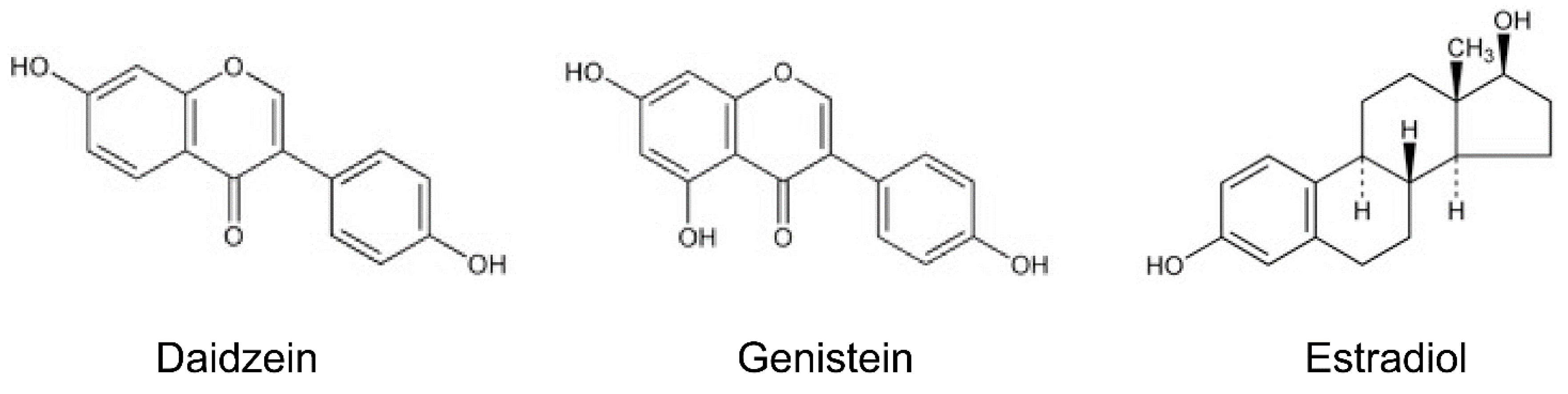
2. Isoflavones and Anti-Inflammatory Effects
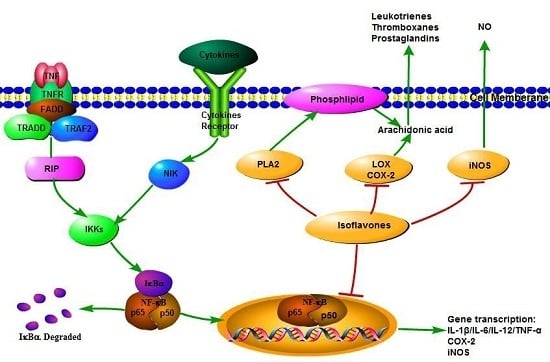
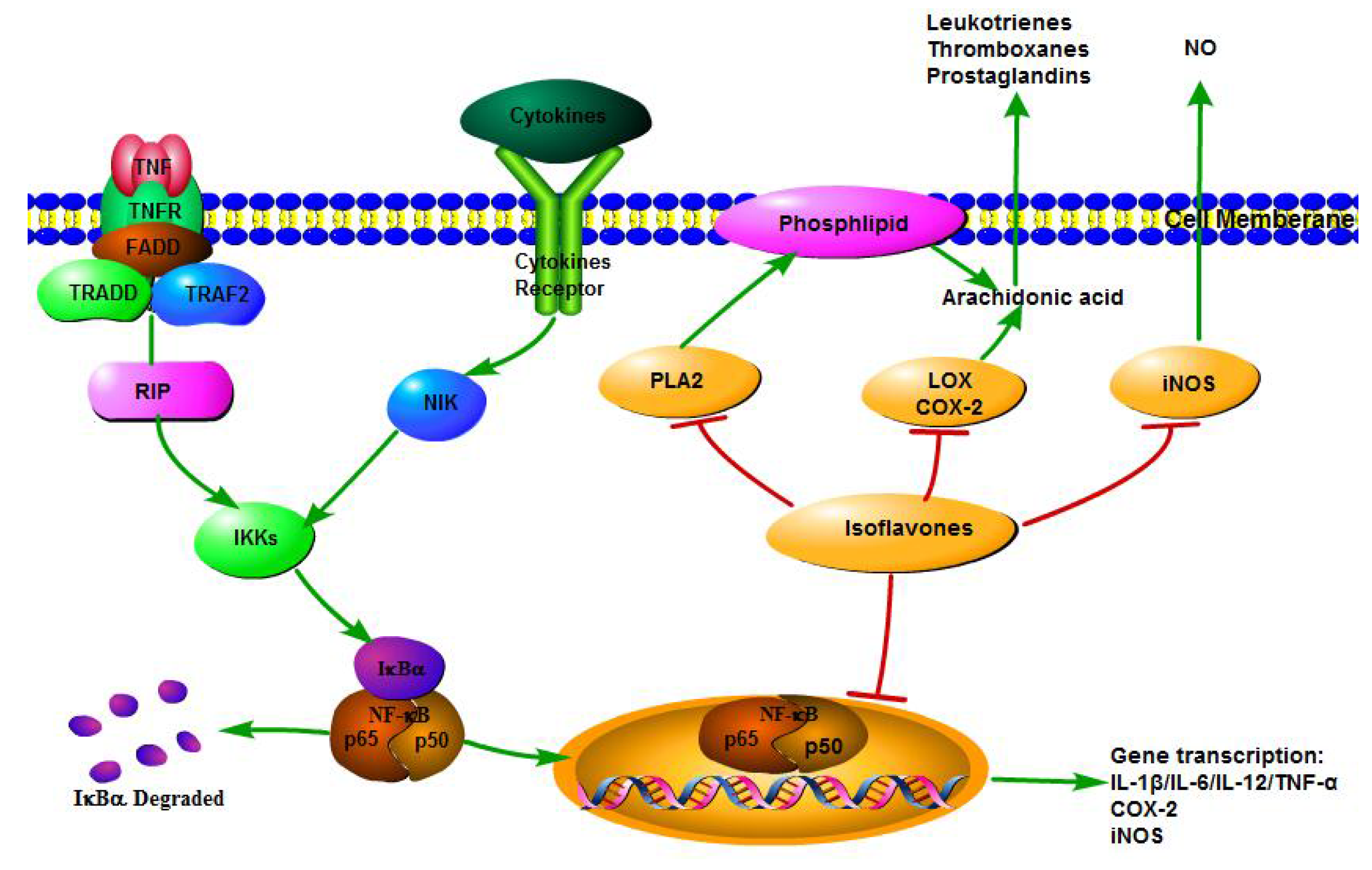
3. Anti-Inflammatory Mechanisms of Isoflavones
3.1. Antioxidative Activities
3.2. Pro-Inflammatory Cytokines and Chemokines Production
3.3. Pro-Inflammatory Enzyme Activities
3.4. NF-κB Transcriptional System
4. Potential Health Risks of Isoflavones Intake
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barton, G.M. A calculated response: Control of inflammation by the innate immune system. J. Clin. Investig. 2008, 118, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lafuente, A.; Guillamon, E.; Villares, A.; Rostagno, M.A.; Martinez, J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Samad, F.; Ruf, W. Inflammation, obesity, and thrombosis. Blood 2013, 122, 3415–3422. [Google Scholar] [CrossRef] [PubMed]
- Khandpur, R.; Carmona-Rivera, C.; Vivekanandan-Giri, A.; Gizinski, A.; Yalavarthi, S.; Knight, J.S.; Friday, S.; Li, S.; Patel, R.M.; Subramanian, V.; et al. Nets are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 2013, 5, 178ra140. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.; Elbaz, H.A.; Lee, I.; Zielske, S.P.; Malek, M.H.; Huttemann, M. Molecular mechanisms and therapeutic effects of (-)-epicatechin and other polyphenols in cancer, inflammation, diabetes, and neurodegeneration. Oxidative Med. Cell. Longev. 2015, 2015, 181260. [Google Scholar] [CrossRef] [PubMed]
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.; Flavell, R.A. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 2013, 13, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Coxib and traditional NSAID Trialists’ (CNT) Collaboration; Bhala, N.; Emberson, J.; Merhi, A.; Abramson, S.; Arber, N.; Baron, J.A.; Bombardier, C.; Cannon, C.; Farkouh, M.E.; et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: Meta-analyses of individual participant data from randomised trials. Lancet 2013, 382, 769–779. [Google Scholar]
- Trichopoulou, A.; Bamia, C.; Trichopoulos, D. Anatomy of health effects of mediterranean diet: Greek epic prospective cohort study. BMJ 2009, 338, b2337. [Google Scholar] [CrossRef] [PubMed]
- Ros, E.; Hu, F.B. Consumption of plant seeds and cardiovascular health: Epidemiological and clinical trial evidence. Circulation 2013, 128, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.S.; Kwok, T.C.; Celermajer, D.S. Vegan diet, subnormal vitamin b-12 status and cardiovascular health. Nutrients 2014, 6, 3259–3273. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, B. Traditional Chinese medicine network pharmacology: Theory, methodology and application. Chin. J. Nat. Med. 2013, 11, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Yang, G.; Ma, Y.; Xu, B.; Hu, M.; You, M.; Gao, S. Developing an activity and absorption-based quality control platform for chinese traditional medicine: Application to zeng-sheng-ping (antitumor b). J. Ethnopharmacol. 2015, 172, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Bo, Y.; Yuan, L.P.; Zhang, J.J.; Meng, D.D.; Jing, H.; Dai, H.J. Total flavonoids of bidens bipinnata l. A traditional Chinese medicine inhibits the production of inflammatory cytokines of vessel endothelial cells stimulated by sera from henoch-schonlein purpura patients. J. Pharm. Pharmacol. 2012, 64, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Dower, J.I.; Geleijnse, J.M.; Gijsbers, L.; Schalkwijk, C.; Kromhout, D.; Hollman, P.C. Supplementation of the pure flavonoids epicatechin and quercetin affects some biomarkers of endothelial dysfunction and inflammation in (pre)hypertensive adults: A randomized double-blind, placebo-controlled, crossover trial. J. Nutr. 2015, 145, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- Dower, J.I.; Geleijnse, J.M.; Gijsbers, L.; Zock, P.L.; Kromhout, D.; Hollman, P.C. Effects of the pure flavonoids epicatechin and quercetin on vascular function and cardiometabolic health: A randomized, double-blind, placebo-controlled, crossover trial. Am. J. Clin. Nutr. 2015, 101, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.; Chen, A.; Grace, M.H.; Komarnytsky, S.; Lila, M.A. Inhibitory effects of wild blueberry anthocyanins and other flavonoids on biomarkers of acute and chronic inflammation in vitro. J. Agric. Food Chem. 2014, 62, 7022–7028. [Google Scholar] [CrossRef] [PubMed]
- Terashima, M.; Kakuno, Y.; Kitano, N.; Matsuoka, C.; Murase, M.; Togo, N.; Watanabe, R.; Matsumura, S. Antioxidant activity of flavonoids evaluated with myoglobin method. Plant Cell Rep. 2012, 31, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Kogiso, M. Soy isoflavones and immunity. J. Med. Investig. 2008, 55, 167–173. [Google Scholar] [CrossRef]
- Marzocchella, L.; Fantini, M.; Benvenuto, M.; Masuelli, L.; Tresoldi, I.; Modesti, A.; Bei, R. Dietary flavonoids: Molecular mechanisms of action as anti-inflammatory agents. Recent Pat. Inflamm. Allergy Drug Discov. 2011, 5, 200–220. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Hanley, B.; Lamuela-Raventos, R.M. Isoflavones, lignans and stilbenes—Origins, metabolism and potential importance to human health. J. Sci. Food Agric. 2000, 80, 1044–1062. [Google Scholar] [CrossRef]
- Perez-Jimenez, J.; Neveu, V.; Vos, F.; Scalbert, A. Systematic analysis of the content of 502 polyphenols in 452 foods and beverages: An application of the phenol-explorer database. J. Agric. Food Chem. 2010, 58, 4959–4969. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Nardi, E.; Battezzati, P.-M.; Asciutti, S.; Castellani, D.; Perriello, G.; Clerici, C. Novel soy germ pasta enriched in isoflavones ameliorates gastroparesis in type 2 diabetes a pilot study. Diabetes Care 2013, 36, 3495–3497. [Google Scholar] [CrossRef] [PubMed]
- Barros, R.P.; Gustafsson, J.Å. Estrogen receptors and the metabolic network. Cell Metab. 2011, 14, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic activity, biological effect and bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Conklin, C.M.; Bechberger, J.F.; MacFabe, D.; Guthrie, N.; Kurowska, E.M.; Naus, C.C. Genistein and quercetin increase connexin43 and suppress growth of breast cancer cells. Carcinogenesis 2007, 28, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Dhayakaran, R.P.A.; Neethirajan, S.; Xue, J.; Shi, J. Characterization of antimicrobial efficacy of soy isoflavones against pathogenic biofilms. LWT Food Sci. Technol. 2015, 63, 859–865. [Google Scholar] [CrossRef]
- Rodríguez-Roque, M.J.; Rojas-Graü, M.A.; Elez-Martínez, P.; Martín-Belloso, O. Soymilk phenolic compounds, isoflavones and antioxidant activity as affected by in vitro gastrointestinal digestion. Food Chem. 2013, 136, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Chacko, B.K.; Chandler, R.T.; D’Alessandro, T.L.; Mundhekar, A.; Khoo, N.K.; Botting, N.; Barnes, S.; Patel, R.P. Anti-inflammatory effects of isoflavones are dependent on flow and human endothelial cell ppargamma. J. Nutr. 2007, 137, 351–356. [Google Scholar] [PubMed]
- Kuiper, G.G.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.-A.K. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [PubMed]
- Phetnoo, N.; Werawatganon, D.; Siriviriyakul, P. Genistein could have a therapeutic potential for gastrointestinal diseases. Thai J. Gastroenterol. 2013 2013, 14, 120–125. [Google Scholar]
- Marugame, T.; Katanoda, K. International comparisons of cumulative risk of breast and prostate cancer, from cancer incidence in five continents vol. Viii. Jpn. J. Clin. Oncol. 2006, 36, 399–400. [Google Scholar] [CrossRef] [PubMed]
- Medjakovic, S.; Mueller, M.; Jungbauer, A. Potential health-modulating effects of isoflavones and metabolites via activation of ppar and ahr. Nutrients 2010, 2, 241–279. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.H.; Ziegler, R.G.; Nomura, A.; West, D.W.; Kolonel, L.N.; Horn-Ross, P.L.; Hoover, R.N.; Pike, M.C. Soy intake and risk of breast cancer in asians and asian americans. Am. J. Clin. Nutr. 1998, 68, 1437S–1443S. [Google Scholar] [PubMed]
- Nielsen, I.L.; Williamson, G. Review of the factors affecting bioavailability of soy isoflavones in humans. Nutr. Cancer 2007, 57, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vergne, S.; Titier, K.; Bernard, V.; Asselineau, J.; Durand, M.; Lamothe, V.; Potier, M.; Perez, P.; Demotes-Mainard, J.; Chantre, P.; et al. Bioavailability and urinary excretion of isoflavones in humans: Effects of soy-based supplements formulation and equol production. J. Pharm. Biomed. Anal. 2007, 43, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; DuPont, M.S.; Ridley, S.; Rhodes, M.; Rhodes, M.J.; Morgan, M.R.; Williamson, G. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver β-glucosidase activity. FEBS Lett. 1998, 436, 71–75. [Google Scholar] [CrossRef]
- Setchell, K.D.; Brown, N.M.; Desai, P.; Zimmer-Nechemias, L.; Wolfe, B.E.; Brashear, W.T.; Kirschner, A.S.; Cassidy, A.; Heubi, J.E. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J. Nutr. 2001, 131, 1362S–1375S. [Google Scholar] [PubMed]
- Barnes, S. The biochemistry, chemistry and physiology of the isoflavones in soybeans and their food products. Lymphat. Res. Biol. 2010, 8, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [PubMed]
- Barnes, S. Evolution of the health benefits of soy isoflavones. Proc. Soc. Exp. Biol. Med. 1998, 217, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Messina, M. Soy foods, isoflavones, and the health of postmenopausal women. Am. J. Clin. Nutr. 2014, 100, 423S–430S. [Google Scholar] [CrossRef] [PubMed]
- Verdrengh, M.; Jonsson, I.M.; Holmdahl, R.; Tarkowski, A. Genistein as an anti-inflammatory agent. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2003, 52, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Paradkar, P.N.; Blum, P.S.; Berhow, M.A.; Baumann, H.; Kuo, S.M. Dietary isoflavones suppress endotoxin-induced inflammatory reaction in liver and intestine. Cancer Lett. 2004, 215, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Kao, T.H.; Wu, W.M.; Hung, C.F.; Wu, W.B.; Chen, B.H. Anti-inflammatory effects of isoflavone powder produced from soybean cake. J. Agric. Food Chem. 2007, 55, 11068–11079. [Google Scholar] [CrossRef] [PubMed]
- Carrara, V.S.; Melo, J.O.; Filho, B.; Bersani-Amado, C.A.; Nakamura, C.V.; Mandarino, J.; Cortez, L.; Cortez, D. Anti-inflammatory activity of the soybean methanolic fraction containing isoflavones. Planta Medica 2008, 74, 1179. [Google Scholar] [CrossRef]
- Lim, D.W.; Lee, C.; Kim, I.H.; Kim, Y.T. Anti-inflammatory effects of total isoflavones from pueraria lobata on cerebral ischemia in rats. Molecules 2013, 18, 10404–10412. [Google Scholar] [CrossRef] [PubMed]
- Ganai, A.A.; Khan, A.A.; Malik, Z.A.; Farooqi, H. Genistein modulates the expression of nf-kappab and mapk (p-38 and erk1/2), thereby attenuating d-galactosamine induced fulminant hepatic failure in wistar rats. Toxicol. Appl. Pharmacol. 2015, 283, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Kuo, I.C.; Selvarajan, S.; Chua, K.Y.; Bay, B.H.; Wong, W.S. Antiinflammatory effects of genistein, a tyrosine kinase inhibitor, on a guinea pig model of asthma. Am. J. Respir. Crit. Care Med. 2003, 167, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, S.; Soung do, Y.; Lucas, E.A.; Madihally, S.V.; Levenson, C.W.; Arjmandi, B.H. Genistein reduces the production of proinflammatory molecules in human chondrocytes. J. Nutr. Biochem. 2007, 18, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Pan, L.; Ke, Y.S.; Batnasan, E.; Jin, X.Q.; Liu, Z.Y.; Ba, X.Q. Daidzein suppresses pro-inflammatory chemokine cxcl2 transcription in tnf-alpha-stimulated murine lung epithelial cells via depressing parp-1 activity. Acta Pharmacol. Sin. 2014, 35, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Ohgo, Y.; Katayanagi, Y.; Yasui, K.; Hiramoto, S.; Ikemoto, H.; Nakata, Y.; Miyoshi, N.; Isemura, M.; Ohashi, N.; et al. Anti-inflammatory effects of green soybean extract irradiated with visible light. Sci. Rep. 2014, 4, 4732. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Wu, H.; Li, W.; Gao, P. Protective effects of genistein in homocysteine-induced endothelial cell inflammatory injury. Mol. Cell. Biochem. 2015, 403, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Azadbakht, L.; Kimiagar, M.; Mehrabi, Y.; Esmaillzadeh, A.; Hu, F.B.; Willett, W.C. Soy consumption, markers of inflammation, and endothelial function a cross-over study in postmenopausal women with the metabolic syndrome. Diabetes Care 2007, 30, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Nasca, M.M.; Zhou, J.-R.; Welty, F.K. Effect of soy nuts on adhesion molecules and markers of inflammation in hypertensive and normotensive postmenopausal women. Am. J. Cardiol. 2008, 102, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Fanti, P.; Asmis, R.; Stephenson, T.J.; Sawaya, B.P.; Franke, A.A. Positive effect of dietary soy in esrd patients with systemic inflammation—Correlation between blood levels of the soy isoflavones and the acute-phase reactants. Nephrol. Dial. Transplant. 2006, 21, 2239–2246. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.F.; Ryan, M.F.; Gibney, E.R.; Brennan, L.; Roche, H.M.; Reilly, M.P. Dietary isoflavone intake is associated with evoked responses to inflammatory cardiometabolic stimuli and improved glucose homeostasis in healthy volunteers. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Shu, X.O.; Chow, W.-H.; Xiang, Y.-B.; Zhang, X.; Li, H.-L.; Cai, Q.; Ji, B.-T.; Cai, H.; Rothman, N. Soy food intake and circulating levels of inflammatory markers in Chinese women. J. Acad. Nutr. Diet. 2012, 112, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Mangano, K.M.; Hutchins-Wiese, H.L.; Kenny, A.M.; Walsh, S.J.; Abourizk, R.H.; Bruno, R.S.; Lipcius, R.; Fall, P.; Kleppinger, A.; Kenyon-Pesce, L. Soy proteins and isoflavones reduce interleukin-6 but not serum lipids in older women: A randomized controlled trial. Nutr. Res. 2013, 33, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Acharjee, S.; Zhou, J.-R.; Elajami, T.K.; Welty, F.K. Effect of soy nuts and equol status on blood pressure, lipids and inflammation in postmenopausal women stratified by metabolic syndrome status. Metabolism 2015, 64, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Kang, S.W. Reactive oxygen species and tumor metastasis. Mol. Cells 2013, 35, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C.; Neher, J.J. Microglial phagocytosis of live neurons. Nat. Rev. Neurosci. 2014, 15, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Niki, E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: In vitro and in vivo evidence. Free Radic. Biol. Med. 2014, 66, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-T.; Yang, C.-M. Role of nadph oxidase/ros in pro-inflammatory mediators-induced airway and pulmonary diseases. Biochem. Pharmacol. 2012, 84, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, J.; Rimarčík, J.; Vagánek, A.; Klein, E. On the radical scavenging activity of isoflavones: Thermodynamics of O-H bond cleavage. Phys. Chem. Chem. Phys. 2013, 15, 10895–10903. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, T.; Prasain, J.; Benton, M.; Botting, N.; Moore, R.; Darley-Usmar, V.; Patel, R.; Barnes, S. Polyphenols, inflammatory response, and cancer prevention: Chlorination of isoflavones by human neutrophils. J. Nutr. 2003, 133, 3773S–3777S. [Google Scholar] [PubMed]
- Patel, R.P.; Boersma, B.J.; Crawford, J.H.; Hogg, N.; Kirk, M.; Kalyanaraman, B.; Parks, D.A.; Barnes, S.; Darley-Usmar, V. Antioxidant mechanisms of isoflavones in lipid systems: Paradoxical effects of peroxyl radical scavenging. Free Radic. Biol. Med. 2001, 31, 1570–1581. [Google Scholar] [CrossRef]
- Boersma, B.J.; Patel, R.P.; Kirk, M.; Jackson, P.L.; Muccio, D.; Darley-Usmar, V.M.; Barnes, S. Chlorination and nitration of soy isoflavones. Arch. Biochem. Biophys. 1999, 368, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.H.; Yen, G.C. Inhibitory effect of isoflavones on peroxynitrite-mediated low-density lipoprotein oxidation. Biosci. Biotechnol. Biochem. 2002, 66, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Tie, L.; An, Y.; Han, J.; Xiao, Y.; Xiaokaiti, Y.; Fan, S.; Liu, S.; Chen, A.F.; Li, X. Genistein accelerates refractory wound healing by suppressing superoxide and foxo1/inos pathway in type 1 diabetes. J. Nutr. Biochem. 2013, 24, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejczyk-Czepas, J.; Wachowicz, B.; Moniuszko-Szajwaj, B.; Kowalska, I.; Oleszek, W.; Stochmal, A. Antioxidative effects of extracts from trifolium species on blood platelets exposed to oxidative stress. J. Physiol. Biochem. 2013, 69, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Lai, H.H. Inhibition of reactive nitrogen species effects in vitro and in vivo by isoflavones and soy-based food extracts. J. Agric. Food Chem. 2003, 51, 7892–7900. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Jiang, Z.; Zhang, J.; Hu, Y.; Gao, K.; Wang, L.; Yang, X. Isoflavone ameliorates H2O2 induced injury by activating the antioxidant system of sow mammary gland cell. Nat. Sci. 2015, 7, 571. [Google Scholar]
- Jin, L.; Zhao, X.; Qin, Y.; Zhu, W.; Zhang, W.; Liu, A.; Luo, Z. Soy isoflavones protect against H2O2-induced injury in human umbilical vein endothelial cells. Mol. Med. Rep. 2015, 12, 4476–4482. [Google Scholar] [CrossRef] [PubMed]
- Mann, G.E.; Bonacasa, B.; Ishii, T.; Siow, R.C. Targeting the redox sensitive nrf2–keap1 defense pathway in cardiovascular disease: Protection afforded by dietary isoflavones. Curr. Opin. Pharmacol. 2009, 9, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Lin, M.; Zhang, F.; Hu, Y.; Xu, X.; Li, Y.; Liu, K.; Ma, X.; Tian, X.; Yao, J. Dietary flavonoid genistein induces nrf2 and phase ii detoxification gene expression via erks and pkc pathways and protects against oxidative stress in caco-2 cells. Mol. Nutr. Food Res. 2013, 57, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Blay, M.; Espinel, A.; Delgado, M.; Baiges, I.; Blade, C.; Arola, L.; Salvado, J. Isoflavone effect on gene expression profile and biomarkers of inflammation. J. Pharm. Biomed. Anal. 2010, 51, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Zhang, Y.; Yang, Q.; Cheng, S.; Hao, J.; Zhao, X.; Jiang, Z. Genistein suppresses lps-induced inflammatory response through inhibiting nf-κb following amp kinase activation in raw 264.7 macrophages. PLoS ONE 2012, 7, e53101. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Fang, L.; Liu, F.; Zong, C.; Cai, R.; Chen, X.; Qi, Y. Suppressive effects of irisflorentin on lps-induced inflammatory responses in raw 264.7 macrophages. Exp. Biol. Med. 2014, 239, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Satsu, H.; Hyun, J.S.; Shin, H.S.; Shimizu, M. Suppressive effect of an isoflavone fraction on tumor necrosis factor-α-induced interleukin-8 production in human intestinal epithelial caco-2 cells. J. Nutr. Sci. Vitaminol. 2009, 55, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Watanabe, T.; Yamori, M.; Takebe, M.; Wakatsuki, Y. Isoflavones regulate innate immunity and inhibit experimental colitis. J. Gastroenterol. Hepatol. 2009, 24, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Lesinski, G.B.; Reville, P.K.; Mace, T.A.; Young, G.S.; Ahn-Jarvis, J.; Thomas-Ahner, J.; Vodovotz, Y.; Ameen, Z.; Grainger, E.; Riedl, K.; et al. Consumption of soy isoflavone enriched bread in men with prostate cancer is associated with reduced proinflammatory cytokines and immunosuppressive cells. Cancer Prev. Res. 2015, 8, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- De Paula, M.L.; Rodrigues, D.H.; Teixeira, H.C.; Barsante, M.M.; Souza, M.A.; Ferreira, A.P. Genistein down-modulates pro-inflammatory cytokines and reverses clinical signs of experimental autoimmune encephalomyelitis. Int. Immunopharmacol. 2008, 8, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Yoo, N.Y.; Jeon, S.; Nam, Y.; Park, Y.J.; Won, S.B.; Kwon, Y.H. Dietary supplementation of genistein alleviates liver inflammation and fibrosis mediated by a methionine-choline-deficient diet in db/db mice. J. Agric. Food Chem. 2015, 63, 4305–4311. [Google Scholar] [CrossRef] [PubMed]
- Langenbach, R.; Morham, S.G.; Tiano, H.F.; Loftin, C.D.; Ghanayem, B.I.; Chulada, P.C.; Mahler, J.F.; Lee, C.A.; Goulding, E.H.; Kluckman, K.D. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell 1995, 83, 483–492. [Google Scholar] [CrossRef]
- Kuehl, F.A.; Egan, R.W. Prostaglandins, arachidonic acid, and inflammation. Science 1980, 210, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.R.; Mitchell, J.A.; Appleton, I.; Tomlinson, A.; Bishop-Bailey, D.; Croxtall, J.; Willoughby, D.A. Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Proc. Natl. Acad. Sci. USA 1994, 91, 2046–2050. [Google Scholar] [CrossRef] [PubMed]
- Xiang, B.; Zhang, G.; Guo, L.; Li, X.-A.; Morris, A.J.; Daugherty, A.; Whiteheart, S.W.; Smyth, S.S.; Li, Z. Platelets protect from septic shock by inhibiting macrophage-dependent inflammation via the cyclooxygenase 1 signalling pathway. Nat. Commun. 2013, 4, 2657. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Dharmappa, K.K.; Mohamed, R.; Shivaprasad, H.V.; Vishwanath, B.S. Genistein, a potent inhibitor of secretory phospholipase a2: A new insight in down regulation of inflammation. Inflammopharmacology 2010, 18, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, R.M.; Rabello, M.M.; Araújo, R.M.; Silveira, E.R.; Fagundes, F.H.; Diz-Filho, E.; Buzzo, S.C.; Soares, V.C.; Toyama, D.D.O.; Gaeta, H.H. Inhibition of neurotoxic secretory phospholipases a2 enzymatic, edematogenic, and myotoxic activities by harpalycin 2, an isoflavone isolated from harpalyce brasiliana benth. Evid. Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Suleyman, H.; Demircan, B.; Karagoz, Y. Anti-inflammatory and side effects of cyclooxygenase inhibitors. Pharmacol. Rep. 2007, 59, 247–258. [Google Scholar] [PubMed]
- Vera, R.; Galisteo, M.; Villar, I.C.; Sánchez, M.; Zarzuelo, A.; Pérez-Vizcaíno, F.; Duarte, J. Soy isoflavones improve endothelial function in spontaneously hypertensive rats in an estrogen-independent manner: Role of nitric-oxide synthase, superoxide, and cyclooxygenase metabolites. J. Pharmacol. Exp. Ther. 2005, 314, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Ramasamy, K.; Jaafar, S.M.; Majeed, A.B.; Mani, V. Total isoflavones from soybean and tempeh reversed scopolamine-induced amnesia, improved cholinergic activities and reduced neuroinflammation in brain. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2014, 65, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Lau, T.Y.; Leung, L.K. Soya isoflavones suppress phorbol 12-myristate 13-acetate-induced cox-2 expression in mcf-7 cells. Br. J. Nutr. 2006, 96, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Wu, L.P.; Li, K.H.; Liu, Y.P.; Xiang, R.; Zhang, S.B.; Zhu, L.Y.; Zhang, L.Y. Involvement of nuclear factor kappab (nf-kappab) in the downregulation of cyclooxygenase-2 (cox-2) by genistein in gastric cancer cells. J. Int. Med. Res. 2011, 39, 2141–2150. [Google Scholar] [CrossRef] [PubMed]
- Mahesha, H.; Singh, S.A.; Rao, A.A. Inhibition of lipoxygenase by soy isoflavones: Evidence of isoflavones as redox inhibitors. Arch. Biochem. Biophys. 2007, 461, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Mascayano, C.; Espinosa, V.; Sepúlveda-Boza, S.; Hoobler, E.K.; Perry, S.; Diaz, G.; Holman, T.R. Enzymatic studies of isoflavonoids as selective and potent inhibitors of human leukocyte 5-lipo-oxygenase. Chem. Biol. Drug Des. 2015, 86, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Mascayano, C.; Espinosa, V.; Sepúlveda-Boza, S.; Hoobler, E.K.; Perry, S. In vitro study of isoflavones and isoflavans as potent inhibitors of human 12-and 15-lipoxygenases. Chem. Biol. Drug Des. 2013, 82, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Palmer, R.; Higgs, E. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar] [PubMed]
- Salvemini, D.; Kim, S.F.; Mollace, V. Reciprocal regulation of the nitric oxide and cyclooxygenase pathway in pathophysiology: Relevance and clinical implications. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R473–R487. [Google Scholar] [CrossRef] [PubMed]
- Sheu, F.; Lai, H.-H.; Yen, G.-C. Suppression effect of soy isoflavones on nitric oxide production in raw 264.7 macrophages. J. Agric. Food Chem. 2001, 49, 1767–1772. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Woo, M.-S.; Kim, D.-H.; Hyun, J.-W.; Kim, W.-K.; Lee, J.-C.; Kim, H.-S. Anti-inflammatory mechanisms of isoflavone metabolites in lipopolysaccharide-stimulated microglial cells. J. Pharmacol. Exp. Ther. 2007, 320, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Chinta, S.J.; Ganesan, A.; Reis-Rodrigues, P.; Lithgow, G.J.; Andersen, J.K. Anti-inflammatory role of the isoflavone diadzein in lipopolysaccharide-stimulated microglia: Implications for Parkinson’s disease. Neurotox. Res. 2013, 23, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, M.; Tachibana, H.; Yamada, K. Isoflavone genistein and daidzein up-regulate lps-induced inducible nitric oxide synthase activity through estrogen receptor pathway in raw264. 7 cells. Biochem. Pharmacol. 2005, 71, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Valachovicova, T.; Slivova, V.; Bergman, H.; Shuherk, J.; Sliva, D. Soy isoflavones suppress invasiveness of breast cancer cells by the inhibition of nf-κb/ap-1-dependent and-independent pathways. Int. J. Oncol. 2004, 25, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.G.; Oakley, F. Nuclear factor-kappab1: Regulation and function. Int. J. Biochem. Cell Biol. 2008, 40, 1425–1430. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Karin, M. Nuclear factor-kappab: A pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997, 336, 1066–1071. [Google Scholar] [PubMed]
- Yang, F.; Tang, E.; Guan, K.; Wang, C.Y. Ikk beta plays an essential role in the phosphorylation of rela/p65 on serine 536 induced by lipopolysaccharide. J. Immunol. 2003, 170, 5630–5635. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Vallee, S.; Wu, J.; Vu, D.; Sondek, J.; Ghosh, G. Inhibition of nf-kappab activity by ikappabbeta in association with kappab-ras. Mol. Cell. Biol. 2004, 24, 3048–3056. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.N.; Kucuk, O.; Djuric, Z.; Sarkar, F.H. Soy isoflavone supplementation in healthy men prevents nf-κb activation by tnf-α in blood lymphocytes. Free Radic. Biol. Med. 2001, 30, 1293–1302. [Google Scholar] [CrossRef]
- Kim, J.W.; Jin, Y.C.; Kim, Y.M.; Rhie, S.; Kim, H.J.; Seo, H.G.; Lee, J.H.; Ha, Y.L.; Chang, K.C. Daidzein administration in vivo reduces myocardial injury in a rat ischemia/reperfusion model by inhibiting nf-kb activation. Life Sci. 2009, 84, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Shi, J.-X.; Zhang, D.-M.; Chen, H.-L.; Qi, M.; Yin, H.-X. Genistein, a soybean isoflavone, reduces the production of pro-inflammatory and adhesion molecules induced by hemolysate in brain microvascular endothelial cells. Acta Neurol. Belg. 2009, 109, 32. [Google Scholar] [PubMed]
- Eo, H.; Ann, J.-Y.; Lim, Y. Dietary supplementation of genistein attenuates inflammatory responses and oxidative stress during cutaneous wound healing in diabetic mice. J. Agric. Sci. 2015, 7, 80. [Google Scholar] [CrossRef]
- Hilakivi-Clarke, L.; Andrade, J.E.; Helferich, W. Is soy consumption good or bad for the breast? J. Nutr. 2010, 140, 2326S–2334S. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, D.; Rajora, A.K.; Greco, F.; Osborn, H.M. Flavonoids as prospective compounds for anti-cancer therapy. Int. J. Biochem. Cell Biol. 2013, 45, 2821–2831. [Google Scholar] [CrossRef] [PubMed]
- Boucher, B.A.; Cotterchio, M.; Anderson, L.N.; Kreiger, N.; Kirsh, V.A.; Thompson, L.U. Use of isoflavone supplements is associated with reduced postmenopausal breast cancer risk. Int. J. Cancer 2013, 132, 1439–1450. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Sobue, T.; Kobayashi, M.; Sasaki, S.; Tsugane, S. Soy, isoflavones, and breast cancer risk in japan. J. Natl. Cancer Inst. 2003, 95, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Mizoue, T.; Tanaka, K.; Tsuji, I.; Tamakoshi, A.; Matsuo, K.; Wakai, K.; Inoue, M.; Tsugane, S.; Sasazuki, S. Soy intake and breast cancer risk: An evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn. J. Clin. Oncol. 2014, 44, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Rao, Y.; Zheng, Y.; Wei, S.; Li, Y.; Guo, T.; Yin, P. Association between soy isoflavone intake and breast cancer risk for pre-and post-menopausal women: A meta-analysis of epidemiological studies. PLoS ONE 2014, 9, e89288. [Google Scholar] [CrossRef] [PubMed]
- Mense, S.M.; Hei, T.K.; Ganju, R.K.; Bhat, H.K. Phytoestrogens and breast cancer prevention: Possible mechanisms of action. Environ. Health Perspect. 2008, 116, 426. [Google Scholar] [CrossRef] [PubMed]
- Allred, C.D.; Allred, K.F.; Ju, Y.H.; Clausen, L.M.; Doerge, D.R.; Schantz, S.L.; Korol, D.L.; Wallig, M.A.; Helferich, W.G. Dietary genistein results in larger mnu-induced, estrogen-dependent mammary tumors following ovariectomy of sprague-dawley rats. Carcinogenesis 2004, 25, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Kijkuokool, P.; Parhar, I.S.; Malaivijitnond, S. Genistein enhances N-nitrosomethylurea-induced rat mammary tumorigenesis. Cancer Lett. 2006, 242, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Mas, A.; Elam, L.; Trevino, L.; Berry, T.; Walker, C.; Diamond, M.; Al-Hendy, A. Early life exposure to genistein permanently decreases myometrial DNA repair capacity, which may contribute to increased risk of uterine fibroid development. Fertil. Steril. 2015, 104, e30. [Google Scholar] [CrossRef]
- Greathouse, K.L.; Bredfeldt, T.; Everitt, J.I.; Lin, K.; Berry, T.; Kannan, K.; Mittelstadt, M.L.; Ho, S.-M.; Walker, C.L. Environmental estrogens differentially engage the histone methyltransferase ezh2 to increase risk of uterine tumorigenesis. Mol. Cancer Res. 2012, 10, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.; Bray, T.M.; Helferich, W.G.; Doerge, D.R.; Ho, E. Differential effects of whole soy extract and soy isoflavones on apoptosis in prostate cancer cells. Exp. Biol. Med. 2010, 235, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Rietjens, I.M.; Sotoca, A.M.; Vervoort, J.; Louisse, J. Mechanisms underlying the dualistic mode of action of major soy isoflavones in relation to cell proliferation and cancer risks. Mol. Nutr. Food Res. 2013, 57, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Yellayi, S.; Naaz, A.; Szewczykowski, M.A.; Sato, T.; Woods, J.A.; Chang, J.; Segre, M.; Allred, C.D.; Helferich, W.G.; Cooke, P.S. The phytoestrogen genistein induces thymic and immune changes: A human health concern? Proc. Natl. Acad. Sci. USA 2002, 99, 7616–7621. [Google Scholar] [CrossRef] [PubMed]
- Yellayi, S.; Zakroczymski, M.A.; Selvaraj, V.; Valli, V.E.; Ghanta, V.; Helferich, W.G.; Cooke, P.S. The phytoestrogen genistein suppresses cell-mediated immunity in mice. J. Endocrinol. 2003, 176, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Ebaid, H.M.; Elgawish, R.A.R.; Abdelrazek, H.M.; Gaffer, G.; Tag, H.M. Prenatal exposure to soy isoflavones altered the immunological parameters in female rats. Int. J. Toxicol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Portman, M.A. Kawasaki disease and soy: Potential role for isoflavone interaction with fcγ receptors. Pediatr. Res. 2012, 73, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Rogan, W.J. Isoflavones in soy infant formula: A review of evidence for endocrine and other activity in infants. Annu. Rev. Nutr. 2004, 24, 33–54. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.E.; Ju, Y.H.; Baker, C.; Doerge, D.R.; Helferich, W.G. Long-term exposure to dietary sources of genistein induces estrogen-independence in the human breast cancer (mcf-7) xenograft model. Mol. Nutr. Food Res. 2015, 59, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.D.; Park, K.-S.; Ro, J.; Kim, J. Differential influence of dietary soy intake on the risk of breast cancer recurrence related to her2 status. Nutr. Cancer 2012, 64, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Lofamia, E.A.A.; Ramos, G.B.; Mamon, M.A.C.; Salido, F.M.; Su, G.S.; de Vera, M.P. Isoflavone maternal-supplementation during periconception period: Influence on the reproductive organs of the first generation (f1) murine weanling-stage offspring. Asian Pac. J. Reprod. 2014, 3, 268–274. [Google Scholar] [CrossRef]
- Delclos, K.B.; Bucci, T.J.; Lomax, L.G.; Latendresse, J.R.; Warbritton, A.; Weis, C.C.; Newbold, R.R. Effects of dietary genistein exposure during development on male and female cd (sprague-dawley) rats. Reprod. Toxicol. 2001, 15, 647–663. [Google Scholar] [CrossRef]
- Xiao, Y.; Mao, X.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Huang, Z.; Chen, D. Potential risk of isoflavones: Toxicological study of daidzein supplementation in piglets. J. Agric. Food Chem. 2015, 63, 4228–4235. [Google Scholar] [CrossRef] [PubMed]
- Ziberna, L.; Lunder, M.; Moze, S.; Vanzo, A.; Tramer, F.; Passamonti, S.; Drevensek, G. Acute cardioprotective and cardiotoxic effects of bilberry anthocyanins in ischemia–reperfusion injury: Beyond concentration-dependent antioxidant activity. Cardiovasc. Toxicol. 2010, 10, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lin, Y.C.; Ma, X.Y.; Jiang, Z.Y.; Lan, S.P. High concentrations of genistein exhibit pro-oxidant effects in primary muscle cells through mechanisms involving 5-lipoxygenase-mediated production of reactive oxygen species. Food Chem. Toxicol. 2014, 67, 72–79. [Google Scholar] [CrossRef] [PubMed]
| Sources | Approximate Contents (mg/100 g) | Reference |
|---|---|---|
| Soy bean | 26–381 | [20,22] |
| Roasted soy bean | 246 | [23] |
| Soy tempe | 148 | [23] |
| Soy flour | 83–466 | [22,23] |
| Tofu | 8–67 | [20,22] |
| Miso | 25–89 | [22] |
| Tempeh | 86.5 | [20] |
| Treatments | Dosage | Effects | Models | Reference | |
|---|---|---|---|---|---|
| Genistein | 30 mg/kg every 2nd day | Granulocytes, monocytes, and lymphocytes | ↓ | Mice | [44] |
| Soybean cake | 0.3 mL aqueous solution | leukocyte number, IL-1β, IL-6, NO, and PGE2 | ↓ | Mice | [46] |
| Soybean methanolic fraction | 2.5 mg/kg | Acute toxicity Inflammation | ↓ | Mice | [47] |
| Puerarin | 12.5 mg/kg | COX-2, astrocyte and microglia | ↓ | Mice | [48] |
| Genestein | 5 mg/kg·BW/day | iNOS, COX-2, NF-ĸB, IKK α/β, MAPK | ↓ | Rats | [49] |
| Genestein | 15 mg/kg | Bronchoconstriction, peroxidase | ↓ | Guinea pigs | [50] |
| Daidzein | 400 mg/kg | Cxcl2 Poly-adenosine Diphosphate-ribosylation | ↓ | Murine lung epithelial cells | [52] |
| Genistein | 10, 50, 100 μM | Cell morphology, ROS | ↓ | Vascular endothelial cells | [54] |
| Genistein | 894 mg/kg | Metallothionein, IL-6, STAT3 Pro-inflammatory cytokine | ↓ | Mice, Caco-2 cells | [45] |
| Mn-SOD | ↑ | ||||
| Green soybean extract | 50 mg/mL | IL-6, IL-12 and TNF-α expression levels | ↓ | Human THP-1 | [53] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Bi, X.; Yu, B.; Chen, D. Isoflavones: Anti-Inflammatory Benefit and Possible Caveats. Nutrients 2016, 8, 361. https://doi.org/10.3390/nu8060361
Yu J, Bi X, Yu B, Chen D. Isoflavones: Anti-Inflammatory Benefit and Possible Caveats. Nutrients. 2016; 8(6):361. https://doi.org/10.3390/nu8060361
Chicago/Turabian StyleYu, Jie, Xiaojuan Bi, Bing Yu, and Daiwen Chen. 2016. "Isoflavones: Anti-Inflammatory Benefit and Possible Caveats" Nutrients 8, no. 6: 361. https://doi.org/10.3390/nu8060361
APA StyleYu, J., Bi, X., Yu, B., & Chen, D. (2016). Isoflavones: Anti-Inflammatory Benefit and Possible Caveats. Nutrients, 8(6), 361. https://doi.org/10.3390/nu8060361