Antibacterial Effects of Cinnamon: From Farm to Food, Cosmetic and Pharmaceutical Industries
Abstract
:1. Introduction
2. History
3. Cultivation of Cinnamon
4. Chemical Composition of Cinnamon
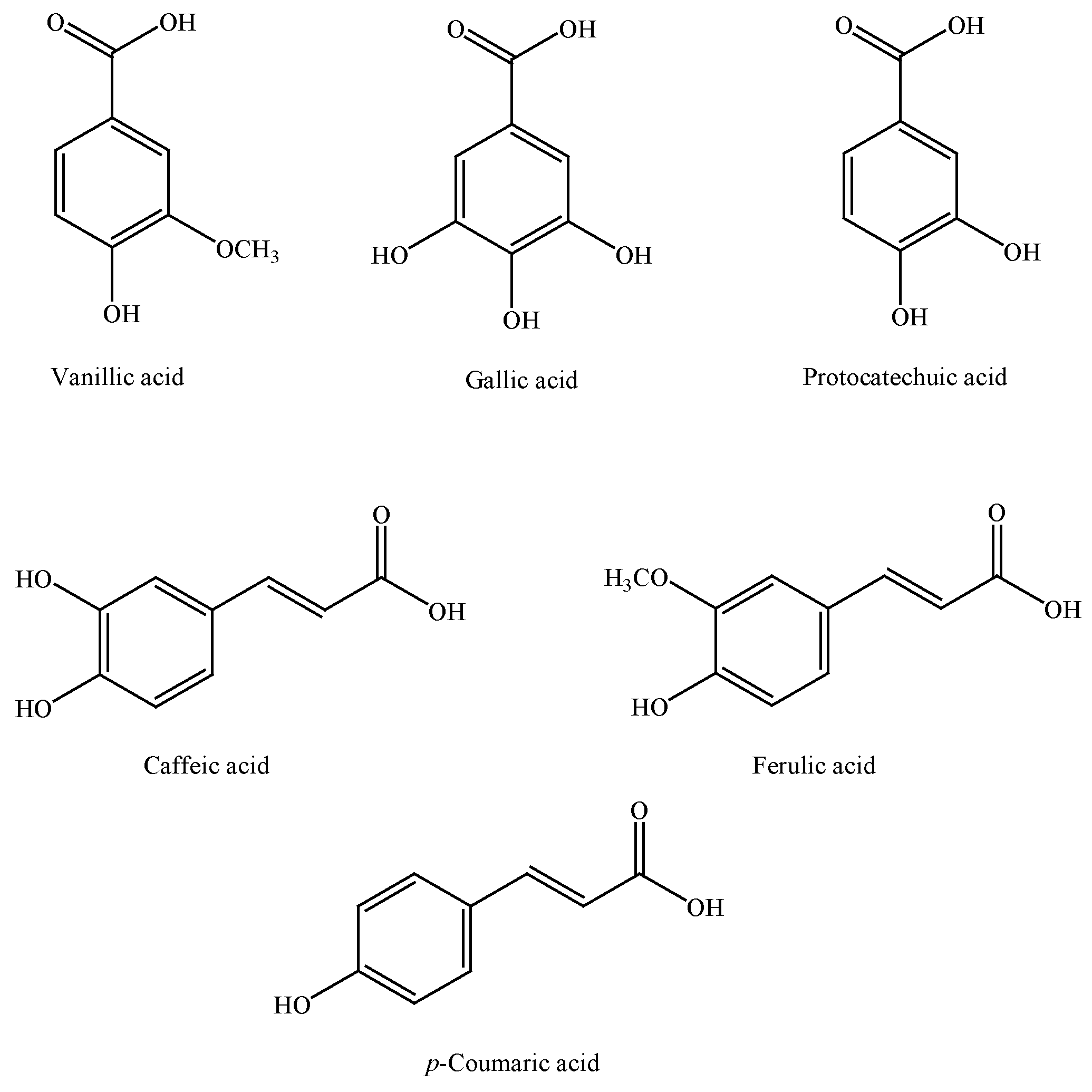
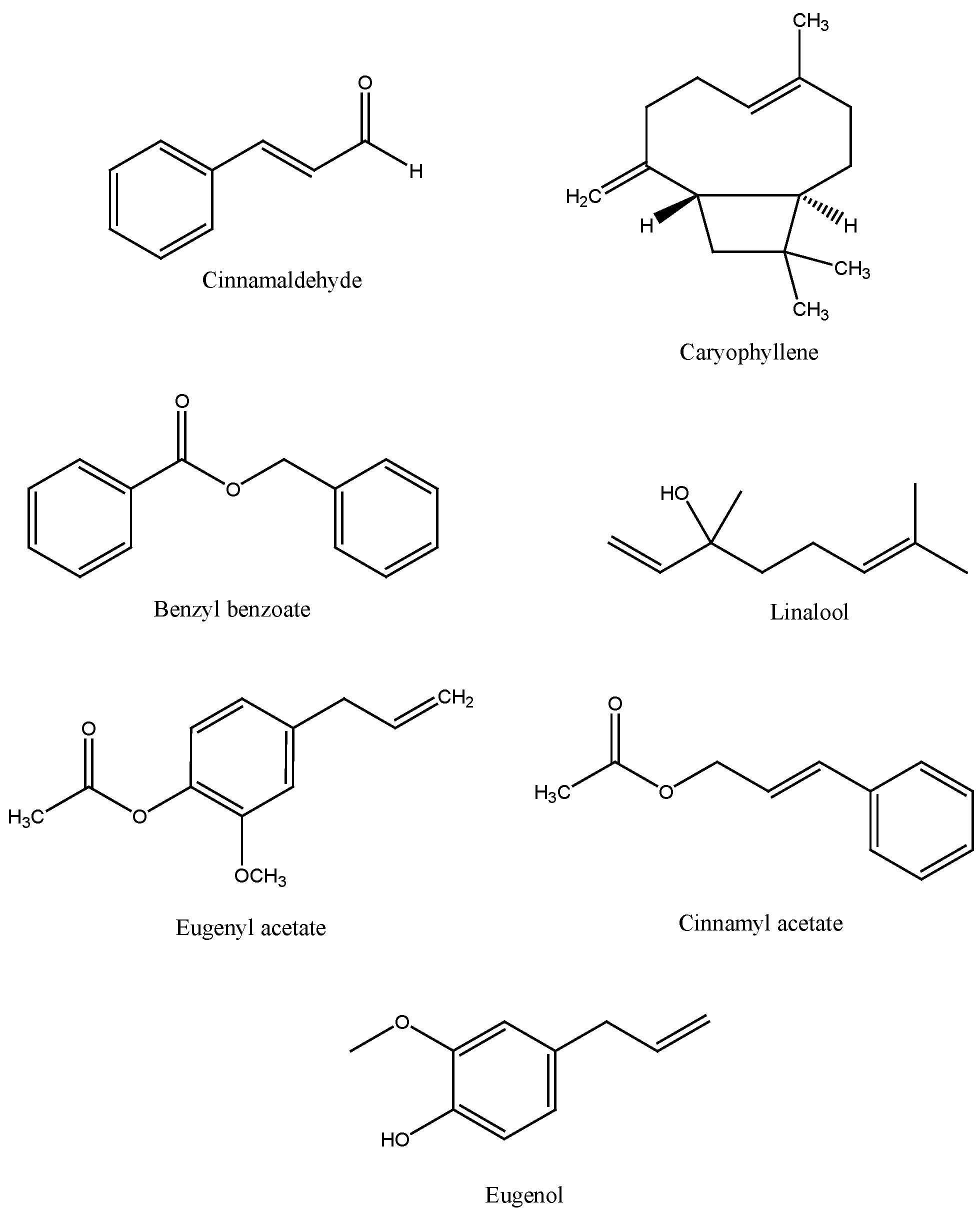
5. Traditional Uses
6. Clinical Impacts
| Clinical Trials | Title | Primary Outcome Measures and Treatments | Results |
|---|---|---|---|
| NCT02074423 | A Human Clinical Trial Evaluating the Effect of MealShape™ on Blood Glucose Level Following Consumption of Standard Meal | measurements of blood glucose incremental area under the curve between 0 and 120 min, after consumption of a standard meal, compared the consumption of MealShape cinnamon extract (acute administration of 1 g corresponding to 2 capsules of 500 mg) | Cinnamon hydro-alcoholic extract may provide a natural and safe solution for the reduction of postprandial hyperglycemia and therefore help to reduce the risks of developing metabolic disorders. |
| NCT00846898 | Is There a Metabolic Effect of Cinnamon on glycosylated hemoglobin A1c (HbA1c), Blood Pressure and Serum Lipids in Type 2 Diabetes Mellitus? (cinnamon) | measurements of blood profiles of HbA1c levels, after administration of cinnamon capsules (2 g per day for 12 weeks) | No study results posted on ClinicalTrials.gov* [44] |
| NCT00331279 | The Effect of Cinnamon Extract on Insulin Resistance Parameters in Polycystic Ovary Syndrome: A Pilot Study | measurements of fasting glucose, fasting insulin, Homeostasis Model Assessment – Insulin Resistance (HOMA-IR), Quantitative Insulin Sensitivity Check Index (QUICKI), insulin sensitivity index (Matsuda), after administration of 2 cinnamon tablets (500 mg of purified aqueous extract of cinnamon for 8 weeks). | No study results posted on ClinicalTrials.gov* [44] |
| NCT00951639 | Cassia Cinnamon for Glucose Uptake In Young Women | measurements of blood glucose, after the treatment with a cinnamon food supplement (5 g encapsulated ground bark administered once in experimental session) | No study results posted on ClinicalTrials.gov* [44] |
| NCT00237640 | Effect of Cinnamon on Glucose and Lipid Levels in Non-Insulin Dependent Type 2 Diabetes Mellitus | measurements of HbA1c, glucose, total cholesterol, low-density lipoprotein (LDL cholesterol), high-density lipoprotein (HDL cholesterol), and triglycerides levels, after the treatment with cinnamon (500 mg capsule twice daily for 3 months) | Cinnamon taken at a dose of 1 g daily for 3 months produced no significant change in fasting glucose, lipid, A1C, or insulin levels. |
| NCT00371800 | The Effect of Cinnamon on HbA1c Among Adolescents With Type I Diabetes | measurements of blood profiles of HbA1c levels, after the treatment with cinnamon (1 gram/day for 90 days). | No study results posted on ClinicalTrials.gov* [44] |
| NCT01350284 | The Effect of Natural Food Flavourings on Gastrointestinal and Cardiovascular Physiological Responses. (CinnGastEmpt) | measurements of the effect of 3 g cinnamon on gastric emptying half time | An aliquot of 3 g cinnamon did not alter the postprandial response to a high-fat test meal. No evidence was found to support the use of 3 g cinnamon supplementation for the prevention or treatment of metabolic disease |
| NCT01027585 | The Effects of Cinnamon on Postprandial Blood Glucose, and Insulin in Subjects With Impaired Glucose Tolerance | measurements of postprandial blood glucose, and plasma concentrations of insulin in subjects with impaired glucose tolerance, after the treatment with cinnamon capsules (doses not provided, for 5 months) | No study results posted on ClinicalTrials.gov* [44] |
| NCT01085019 | Impact of Spices and Herbs on Endothelial Function | measurements of circulating level of plasma lipoproteins-lipids, oxidative stress, endothelial activation and inflammatory markers, after daily consumption of spices and herbs, among which cinnamon in capsules (2.8 g/day for 4 weeks) | No study results posted on ClinicalTrials.gov* [44] |
| NCT00718796 | Naturopathic Treatment for the Prevention of Cardiovascular Disease (CVD) | evaluation of metabolic syndrome and general cardiovascular risk profile (Framingham Heart Study), after naturopathic approach with some spices (among which cinnamon) to CVD prevention over the course of 1 year | Naturopathic approach to CVD primary prevention significantly reduced CVD risk over usual care plus biometric screening and reduced costs to society and employers in this multi-worksite-based study. |
| NCT02193438 | Physiologic Effect of Spices Ingestion | determination of resting energy expenditure, calculation of the resting energy expenditure from continuous measurement of oxygen consumption and carbon dioxid production (indirect calorimetry), heart rate variability, power spectral analysis of heart rate variability from continuous measurement of very low, low and high frequency range electrocardiographic signals, after the ingestion of a single dose of cinnamon extract (dose not provided). | No study results posted on ClinicalTrials.gov* [44] |
| NCT02234206 | A Clinical Trial to Study the Safety and Efficacy of Chandrakanthi Choornam in Patients With Low Sperm Count | measurements of sperm concentration, proportion of sperm motility changes in the percentage of total and progressive motility of sperm proportion of sperm morphology changes in the percentage of sperm cells with normal forms, after the treatment with Chandrakanthi Choornam (dose non provided), which is a formulation consisting of 25 ingredients, among which Cinnamomum verum (bark) and Cinnamomum tamala (leaf) for 3 months | No study results posted on ClinicalTrials.gov* [44] |
| NCT00954902 | Effects of Antioxidants on Cardiovascular Risk Measures (Spice Study) | measurements of Interleukin 6 (IL-6) response to psychological stress at time points equal to and greater than 90 min post task, after treatment with a high antioxidant spice blend (14.5 g blend of spice, among which cinnamon, incorporated into a delivery meal | Inclusion of spices may attenuate postprandial lipemia via inhibition of Phospholipase (PL) and Phospholipase A2 (PLA2). |
| NCT01752868 | Can Fish Oil and Phytochemical Supplements Mimic Anti-Aging Effects of Calorie Restriction? | measurements of carotid-femoral pulse wave velocity, after the treatment with a combination of 10 nutritional supplements, among which cinnamon bark, for 6 months | No study results posted on ClinicalTrials.gov* [44] |
| NCT01667523 | The Effect of Capsaicin and Cinnamaldehyde on Intestinal Permeability | evaluation of the effect of capsaicin and cinnamaldehyde infusion on intestinal permeability, after the administration of cinnamaldehyde (70 mg per intervention administered intraduodenally) | No study results posted on ClinicalTrials.gov* [44] |
| NCT01895816 | Herbal Tonic Fertile Supplement(ZO2C5) | measurements of sperm count variation and semen analysis according World Health Organization methods, after the treatment with mixed herbals drug, in which cinnamon is one of the bioactive components for 6 months (dose not provided) | No study results posted on ClinicalTrials.gov* [44] |
7. Antibacterial Effects of Cinnamon Essential Oil and Cinnamon Extracts
7.1. Antibacterial Activity of Cinnamon against Bacteria Responsible for Human Infectious Diseases
7.2. Examples of Cinnamon Applications in Food and Cosmetic Industries
| Type of Sample | Bacteria | References |
|---|---|---|
| BARK extracts, obtained with different organic solvents (ethyl acetate, acetone and methanol) | Klebsiella pneumonia 13883 | [45] |
| Bacillus megaterium NRS | ||
| Pseudomonas aeroginosa ATCC 27859 | ||
| Staphylococcus aureus 6538 P | ||
| Escherichia coli ATCC 8739 | ||
| Enterobacter cloaca ATCC 13047 | ||
| Corynebacterium xerosis UC 9165 | ||
| Streptococcus faecalis DC 74 | ||
| STEM BARK Ethanolic extract | Staphylococcus aureus (MRSA) | [46] |
| BARK AND CLOVE POWDER Hydroethanolic extract | Moraxella catarrhalis | [47] |
| Combination of piperacillin and cinnamon BARK essential oil | E. coli (β-lactamase-producing) | [49] |
| Essential oil obtained by hydro-distillation of cinnamon BARK | Salmonella typhi | [50] |
| Salmonella paratyphi A | ||
| Escherichia coli | ||
| Staphylococcus aureus | ||
| Pseudomonas fluorescens | ||
| Bacillus licheniformis | ||
| Essential oil obtained by hydro-steam distillation of cinnamon BARK | Escherichia coli O157:H7 | [51] [52] [53] |
| Yersinia enterocolitica O9 | ||
| Proteus spp. | ||
| Klebsiella pneumonia | ||
| Essential oil (BARK and fresh LEAVES) | Escherichia coli O157:H7 | [52] [53] [54] [56] [58] [59] [60] [61] [63] |
| Yersinia enterocolitica O9 | ||
| Proteus spp. | ||
| Klebsiella pneumonia | ||
| Streptococcus mutans | ||
| Lactobacillus acidophilus (fresh leaves) | ||
| Mycoplasma hominis (bark) | ||
| Haemophilus ducreyi (E. O from C. verum) | ||
| L. monocytogenes (bark) (E.O from C. cassia) | ||
| Salmonella typhimurium | ||
| Methanolic extract of cinnamon BARK | Escherichia coli | [55] |
| Enterobacter aerogenes | ||
| Providencia stuartii | ||
| Pseudomonas aeruginosa | ||
| Klebsiella pneumoniae | ||
| Enterobacter cloacae | ||
| Fresh LEAF extract | Escherichia coli O157:H7 | [52] [53] [57] |
| Yersinia enterocolitica O9 | ||
| Proteus spp. | ||
| Klebsiella pneumonia | ||
| Enterococcus faecalis | ||
| Aqueous, hydroalcoholic and alcoholic dried inner BARK extracts (Soxhlet) | Propionibacterium acnes (hydroethanolic extracts inactive) | [30] |
| Staphylococcus epidermidis | ||
| Cinnamon BARK extracts | Salmonella typhimurium | [62] |
| S. aureus | ||
| E. coli | ||
| Extract obtained from cinnamon STICK | L. monocytogenes | [65] |
| S. aureus | ||
| Salmonella enterica | ||
| BARK essential oil (tested in liquid and vapor phases) | Cronobacter sakazakii | [66] |
| C. malonaticus | ||
| Commercial essential oils from C. cassia (LEAF-BRANCH) and C. verum (BARK) | L. monocytogenes NCTC 11994 | [67] [5] [64] |
| L. monocytogenes S0580 | ||
| S. typhimurium ATCC 14028 | ||
| S. typhimurium | ||
| E. coli O157:H7 ATCC 35150 | ||
| E. coli O157:H7 S0575 | ||
| Brochothrix thermosphacta ATCC 11509 | ||
| P. fluorescens ATCC 13525 | ||
| P. aeruginosa ATCC 27853 | ||
| E. coli ATCC 25922 | ||
| S. aureus ATCC 29213 | ||
| Arcobacter butzeiri | ||
| Arcobacter skirrowii | ||
| Nanoparticles loaded with cinnamon BARK extract | L. monocytogenes | [68] |
| S. typhimurium |
8. Toxicological Aspects
9. Conclusions and Recommendations
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Alinezhad, H.; Azimi, R.; Zare, M.; Ebrahimzadeh, M.A.; Eslami, S.; Nabavi, S.F.; Nabavi, S.M. Antioxidant and Antihemolytic Activities of Ethanolic Extract of Flowers, Leaves, and Stems of Hyssopus officinalis L. Var. angustifolius. Int. J. Food Prop. 2013, 16, 1169–1178. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Daglia, M.; Moghaddam, A.H.; Habtemariam, S.; Nabavi, S.M. Curcumin and Liver Disease: From Chemistry to Medicine. Compr. Rev. Food Sci. Saf. 2014, 13, 62–77. [Google Scholar] [CrossRef]
- Curti, V.; Capelli, E.; Boschi, F.; Nabavi, S.F.; Bongiorno, A.I.; Habtemariam, S.; Nabavi, S.M.; Daglia, M. Modulation of human miR-17–3p expression by methyl 3-O-methyl gallate as explanation of its in vivo protective activities. Mol. Nutr. Food Res. 2014, 58, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Nabavi, S.M.; Habtemariam, S.; Moghaddam, A.H.; Sureda, A.; Jafari, M.; Latifi, A.M. Hepatoprotective effect of gallic acid isolated from Peltiphyllum peltatum against sodium fluoride-induced oxidative stress. Ind. Crop. Prod. 2013, 44, 50–55. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P.; Domagalska, B.W.; Młynarczyk, A. Essential oils and herbal extracts as antimicrobial agents in cosmetic emulsion. Indian J. Microbiol. 2013, 53, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.M.; Marchese, A.; Izadi, M.; Curti, V.; Daglia, M.; Nabavi, S.F. Plants belonging to the genus Thymus as antibacterial agents: From farm to pharmacy. Food Chem. 2015, 173, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Simoes, M.; Bennett, R.N.; Rosa, E.A. Understanding antimicrobial activities of phytochemicals against multidrug resistant bacteria and biofilms. Nat. Prod. Rep. 2009, 26, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Coppo, E.; Sobolev, A.P.; Rossi, D.; Mannina, L.; Daglia, M. Influence of in vitro simulated gastroduodenal digestion on the antibacterial activity, metabolic profiling and polyphenols content of green tea (Camellia sinensis). Food Res. Int. 2014, 63, 182–191. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Jayawardana, R.; Galappaththy, P.; Constantine, G.; de Vas Gunawardana, N.; Katulanda, P. Efficacy and safety of ‘true’cinnamon (Cinnamomum zeylanicum) as a pharmaceutical agent in diabetes: A systematic review and meta-analysis. Diabetic Med. 2012, 29, 1480–1492. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, P.; Shylaja, M.; Nirmal Babu, K.; Krishnamoorthy, B. Botany and crop improvement of cinnamon and cassia. In Cinnamon and Cassia—The Genus Cinnamomum; Ravindran, P.N., Babu, K.N., Eds.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Jakhetia, V.; Patel, R.; Khatri, P.; Pahuja, N.; Garg, S.; Pandey, A.; Sharma, S. Cinnamon: A pharmacological review. JASR 2010, 1, 19–23. [Google Scholar]
- Krishnamoorthy, B.; Rema, J. End uses of cinnamon and cassia. In Cinnamon and Cassia: The Genus Cinnamomum; Ravindran, P.N., Babu, K.N., Eds.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Khasnavis, S.; Pahan, K. Sodium benzoate, a metabolite of cinnamon and a food additive, upregulates neuroprotective parkinson disease protein DJ-1 in astrocytes and neurons. J. Neuroimmune Pharmacol. 2012, 7, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Hyun, S.H.; Choung, S.Y. Anti-diabetic effect of cinnamon extract on blood glucose in db/db mice. J. Ethnopharmacol. 2006, 104, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Brierley, S.M.; Kelber, O. Use of natural products in gastrointestinal therapies. Curr. Opin. Pharmacol. 2011, 11, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Al-Jiffri, O.; El-Sayed, Z.; Al-Sharif, F. Urinary tract infection with Esherichia coli and antibacterial activity of some plants extracts. Int. J. Microbiol. Res. 2011, 2, 1–7. [Google Scholar]
- Leung, E.; Weil, D.E.; Raviglione, M.; Nakatani, H. The WHO policy package to combat antimicrobial resistance. Bull. World Health Organ. 2011, 89, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Högberg, L.D.; Heddini, A.; Cars, O. The global need for effective antibiotics: Challenges and recent advances. Trends Pharmacol. Sci. 2010, 31, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Wijesekera, R. Historical overview of the cinnamon industry. Crit. Rev. Food Sci. Nutr. 1997, 10, 1–30. [Google Scholar] [CrossRef]
- Sulaiman, S.A.B. Extraction of Essential Oil from Cinnamomum Zeylanicum by Various Methods as a Perfume Oil. Bachelor Thesis, University of Malaysia Pahang, Gambang, Pahang, Malaysia, 2013. [Google Scholar]
- Barceloux, D.G. Cinnamon (Cinnamomum Species). Dis.-a-Month 2009, 55, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Madan, M.; Kannan, S. Economics and Marketing of Cinnamon and Cassia–A Global View. In Cinnamon and Cassia: The Genus Cinnamomum; Ravindran, P.N., Babu, K.N., Eds.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Ranatunga, J.; Senanayake, U.; Wijesekera, R. Cultivation and management of cinnamon. In Cinnamon and Cassia: The Genus Cinnamomum; Ravindran, P.N., Babu, K.N., Eds.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Thankamani, C.; Sivaraman, K.; Kandiannan, K.; Peter, K. Agronomy of tree spices (clove, nutmeg, cinnamon and allspice)—A review. J. Spices Aromat. Crops 1994, 3, 105–123. [Google Scholar]
- Muchuweti, M.; Kativu, E.; Mupure, C.H.; Chidewe, C.; Ndhlala, A.R.; Benhura, M.A.N. Phenolic composition and antioxidant properties of some spices. Am. J. Food Technol. 2007, 2, 414–420. [Google Scholar]
- Wong, Y.C.; Ahmad-Mudzaqqirand, M.Y.; Wan-Nurdiyana, W.A. Extraction of Essential Oil from Cinnamon (Cinnamomum zeylanicum). Orient. J. Chem. 2014, 30, 37–47. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Singh, R.P.; Pereira, J.; Sakariah, K.K. Limonoids from Citrus reticulata and their moult inhibiting activity in mosquito Culex quinquefasciatus larvae. Phytochemistry 1997, 44, 843–846. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Jagan Mohan Rao, L.; Sakariah, K.K. Chemical composition of the flower oil of Cinnamomum zeylanicum blume. J. Agric. Food Chem. 2000, 48, 4294–4295. [Google Scholar] [CrossRef] [PubMed]
- Filoche, S.K.; Soma, K.; Sissons, C.H. Antimicrobial effects of essential oils in combination with chlorhexidine digluconate. Oral Microbiol. Immunol. 2005, 20, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.S.; Tariq, M.; Zaman, R.; Imtiyaz, S. The In vitro anti-acne activity of two unani drugs. Anc. Sci. Life 2013, 33, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, K.; Thampuran, R.A. Pharmacology and Toxicology of Cinnamon and Cassia. In Cinnamon and Cassia: The Genus Cinnamomum; Ravindran, P.N., Babu, K.N., Eds.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- European Medicines Agency. Assessment Report on Cinnamomum verum J.S. Presl, cortex and corticis aetheroleum. EMA/HMPC/246773/2009. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_HMPC_assessment_report/2011/08/WC500110090.pdf (accessed on 10 May 2011).
- Hossein, N.; Zahra, Z.; Abolfazl, M.; Mahdi, S.; Ali, K. Effect of Cinnamon zeylanicum essence and distillate on the clotting time. J. Med. Plants Res. 2013, 7, 1339–1343. [Google Scholar]
- Rao, P.V.; Gan, S.H. Cinnamon: A Multifaceted Medicinal Plant. Evid. Based Complement. Alternat. Med. 2014, 2014, 642942. [Google Scholar] [CrossRef] [PubMed]
- Reichling, J.; Schnitzler, P.; Suschke, U.; Saller, R. Essential oils of aromatic plants with antibacterial, antifungal, antiviral, and cytotoxic properties–an overview. Forsch. Komplementärmedizin/Res. Complement. Med. 2009, 16, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-T.; Cheng, S.-S. Antitermitic activity of leaf essential oils and components from Cinnamomum osmophleum. J. Agric. Food Chem. 2002, 50, 1389–1392. [Google Scholar] [CrossRef] [PubMed]
- Mancini-Filho, J.; Van-Koiij, A.; Mancini, D.; Cozzolino, F.; Torres, R. Antioxidant activity of cinnamon (Cinnamomum Zeylanicum, Breyne) extracts. Boll. Chim. Farm. 1998, 137, 443–447. [Google Scholar] [PubMed]
- Tung, Y.-T.; Chua, M.-T.; Wang, S.-Y.; Chang, S.-T. Anti-inflammation activities of essential oil and its constituents from indigenous cinnamon (Cinnamomum osmophloeum) twigs. Bioresour. Technol. 2008, 99, 3908–3913. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-S.; Liu, J.-Y.; Tsai, K.-H.; Chen, W.-J.; Chang, S.-T. Chemical composition and mosquito larvicidal activity of essential oils from leaves of different Cinnamomum osmophloeum provenances. J. Agric. Food Chem. 2004, 52, 4395–4400. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.-O.; Lee, S.-M.; Moon, Y.-S.; Lee, S.-G.; Ahn, Y.-J. Nematicidal activity of cassia and cinnamon oil compounds and related compounds toward Bursaphelenchus xylophilus (Nematoda: Parasitaphelenchidae). J. Nematol. 2007, 39, 31. [Google Scholar] [PubMed]
- Moselhy, S.S.; Ali, H.K. Hepatoprotective effect of cinnamon extracts against carbon tetrachloride induced oxidative stress and liver injury in rats. Biol. Res. 2009, 42, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Alqasoumi, S.; Al-Dosary, M.; Al-Yahya, M.; Al-Mofleh, I. Gastroprotective effect of a popular spice cinnamon “Cinnamomum zeylanicum” in rats. Eur. J. Pharmacol. 2011, 668, e42. [Google Scholar]
- Hur, M.H.; Lee, M.S.; Seong, K.Y.; Lee, M.K. Aromatherapy massage on the abdomen for alleviating menstrual pain in high school girls: A preliminary controlled clinical study. Evid.-Based Complement. Altern. Med. 2012, 2012, 187163. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Institutes of Health, ClinicalTrials.gov. Available online: http://clinicaltrial.gov/ (accessed on 1 November 2014).
- U.S. National Library of Medicine, PubMed database. Available online: http://www.ncbi.nlm.nih.gov/pubmed (accessed on 1 November 2014).
- Keskin, D.; Toroglu, S. Studies on antimicrobial activities of solvent extracts of different spices. J. Environ. Biol. 2011, 32, 251–256. [Google Scholar] [PubMed]
- Mandal, S.; DebMandal, M.; Saha, K.; Pal, N.K. In vitro Antibacterial Activity of three Indian Spices against Methicillin-Resistant Staphylococcus aureus. Oman Med. J. 2011, 26, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, M.U.; Thajuddin, N. Effect of medicinal plants on Moraxella cattarhalis. Asian Pac J. Trop. Med. 2011, 4, 133–136. [Google Scholar] [PubMed]
- Guerra, F.Q.; Mendes, J.M.; Sousa, J.P.; Morais-Braga, M.F.; Santos, B.H.; Melo Coutinho, H.D.; Lima Ede, O. Increasing antibiotic activity against a multidrug-resistant Acinetobacter spp by essential oils of Citrus limon and Cinnamomum zeylanicum. Nat. Prod. Res. 2012, 26, 2235–2258. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.S.; Lim, S.H.; Hu, C.P.; Yiap, B.C. Combination of essential oils and antibiotics reduce antibiotic resistance in plasmid-conferred multidrug resistant bacteria. Phytomedicine 2013, 20, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Naveed, R.; Hussain, I.; Tawab, A.; Tariq, M.; Rahman, M.; Hameed, S.; Mahmood, M.S.; Siddique, A.B.; Iqbal, M. Antimicrobial activity of the bioactive components of essential oils from Pakistani spices against Salmonella and other multi-drug resistant bacteria. BMC Complement. Altern. Med. 2013, 13, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Al-Mariri, A.; Safi, M. In vitro Antibacterial Activity of Several Plant Extracts and Oils against Some Gram-Negative Bacteria. Iran J. Med. Sci. 2014, 39, 36–43. [Google Scholar] [PubMed]
- Bardají, D.K.; Reis, E.B.; Medeiros, T.C.; Lucarini, R.; Crotti, A.E.; Martins, C.H. Antibacterial activity of commercially available plant-derived essential oils against oral pathogenic bacteria. Nat. Prod. Res. 2015, in press. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Bhatti, H.N.; Jilani, M.I.; Hanif, M.A. Bioanalytical evaluation of Cinnamomum zeylanicum essential oil. Nat. Prod. Res. 2015, in press. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Lee, J.H.; Kim, S.I.; Baek, K.H.; Lee, J. Cinnamon bark oil and its components inhibit biofilm formation and toxin production. Int. J. Food Microbiol. 2015, 195, 30–39. [Google Scholar] [CrossRef]
- Seukep, J.A.; Fankam, A.G.; Djeussi, D.E.; Voukeng, I.K.; Tankeo, S.B.; Noumdem, J.A.K.; Kuete, A.H.L.N.; Kuete, V. Antibacterial activities of the methanol extracts of seven Cameroonian dietary plants against bacteria expressing MDR phenotypes. SpringerPlus 2013, 2, 363. [Google Scholar] [CrossRef]
- Chaudhari, L.K.; Jawale, B.A.; Sharma, S.; Sharma, H.; Kumar, C.D.; Kulkarni, P.A. Antimicrobial activity of commercially available essential oils against Streptococcus mutans. J. Contemp. Dent. Pract. 2012, 13, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Duhan, J.; Tewari, S.; Sangwan, P.; Yadav, A.; Singh, G.; Juneja, R.; Saini, H. Comparative evaluation of antimicrobial efficacy of Syzygium aromaticum, Ocimum sanctum and Cinnamomum zeylanicum plant extracts against Enterococcus faecalis: A preliminary study. Int. Endod. J. 2013, 46, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.B.; Cates, R.G.; Lawrence, M.; Soria, J.A.; Espinoza, L.V.; Martinez, J.V.; Arbizú, D.A. The antibacterial and antifungal activity of essential oils. Pharm. Biol. 2015, 53, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Sleha, R.; Mosio, P.; Vydrzalova, M.; Jantovska, A.; Bostikova, V.; Mazurova, J. In vitro antimicrobial activities of cinnamon bark oil, anethole, carvacrol, eugenol and guaiazulene against Mycoplasma hominis clinical isolates. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2014, 158, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Lindeman, Z.; Waggoner, M.; Batdorff, A.; Humphreys, T.L. Assessing the antibiotic potential of essential oils against Haemophilus ducreyi. BMC Complement. Altern. Med. 2014, 14, 172. [Google Scholar] [CrossRef] [PubMed]
- Dussault, D.; Vu, K.D.; Lacroix, M. In vitro evaluation of antimicrobial activities of various commercial essential oils, oleoresin and pure compounds against food pathogens and application in ham. Meat Sci. 2014, 96, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Tayel, A.A.; El-Tras, W.F.; Moussa, S.H.; El-Sabbagh, S.M. Surface decontamination and quality enhancement in meat steaks using plant extracts as natural biopreservatives. Foodborne Pathog. Dis. 2012, 9, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Ravishankar, S.; Marchello, J.; Friedman, M. Antimicrobial activity of plant compounds against Salmonella Typhimurium DT104 in ground pork and the influence of heat and storage on the antimicrobial activity. J. Food Prot. 2013, 6, 1264–1269. [Google Scholar] [CrossRef] [PubMed]
- Irkin, R.; Abay, S.; Aydin, F. Inhibitory effects of some plant essential oils against Arcobacter butzleri and potential for rosemary oil as a natural food preservative. J. Med. Food 2011, 14, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; Cai, Y.Z.; Brooks, J.D.; Corke, H. Potential application of spice and herb extracts as natural preservatives in cheese. J. Med. Food 2011, 14, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Frankova, A.; Marounek, M.; Mozrova, V.; Weber, J.; Kloucek, P.; Lukesova, D. Antibacterial Activities of Plant-Derived Compounds and Essential Oils toward Cronobacter sakazakii and Cronobacter malonaticus. Foodborne Pathog. Dis. 2014, 11, 795–797. [Google Scholar] [CrossRef] [PubMed]
- Mith, H.; Dure´, R.; Delcenserie, V.; Zhiri, A.; Daube, G.; Clinquart, A. Antimicrobial activities of commercial essential oils and their components against food-borne pathogens and food spoilage bacteria. Food Sci. Nutr. 2014, 2, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Hill, L.E.; Taylor, T.M.; Gomes, C. Antimicrobial efficacy of poly (dl-lactide-co-glycolide) (PLGA) nanoparticles with entrapped cinnamon bark extract against Listeria monocytogenes and Salmonella typhimurium. J. Food Sci. 2013, 78, 626–632. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health; U.S. Department of Health and Human Services. Herbs at a glance. Cinnamon. Available online: http://cinnamonvogue.com/DOWNLOADS/Cinnamon%20Side%20Effects.pdf (accessed on 7 September 2015).
- Price, S.; Price, L. Aromatherapy for Health Professionals, 3rd ed.; Churchill Livingstone Elsevier: London, UK, 2007. [Google Scholar]
- Keller, K.; Hänsel, R.; Chandler, R.F. Adverse Effects of Herbal Drugs: Cinnamomum Species; De Smet, P.A.G.M., Ed.; Springer Verlag: Heidelberg, Germany, 1992; Volume 1. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nabavi, S.F.; Di Lorenzo, A.; Izadi, M.; Sobarzo-Sánchez, E.; Daglia, M.; Nabavi, S.M. Antibacterial Effects of Cinnamon: From Farm to Food, Cosmetic and Pharmaceutical Industries. Nutrients 2015, 7, 7729-7748. https://doi.org/10.3390/nu7095359
Nabavi SF, Di Lorenzo A, Izadi M, Sobarzo-Sánchez E, Daglia M, Nabavi SM. Antibacterial Effects of Cinnamon: From Farm to Food, Cosmetic and Pharmaceutical Industries. Nutrients. 2015; 7(9):7729-7748. https://doi.org/10.3390/nu7095359
Chicago/Turabian StyleNabavi, Seyed Fazel, Arianna Di Lorenzo, Morteza Izadi, Eduardo Sobarzo-Sánchez, Maria Daglia, and Seyed Mohammad Nabavi. 2015. "Antibacterial Effects of Cinnamon: From Farm to Food, Cosmetic and Pharmaceutical Industries" Nutrients 7, no. 9: 7729-7748. https://doi.org/10.3390/nu7095359
APA StyleNabavi, S. F., Di Lorenzo, A., Izadi, M., Sobarzo-Sánchez, E., Daglia, M., & Nabavi, S. M. (2015). Antibacterial Effects of Cinnamon: From Farm to Food, Cosmetic and Pharmaceutical Industries. Nutrients, 7(9), 7729-7748. https://doi.org/10.3390/nu7095359






