Epigallocatechin Gallate: A Review of Its Beneficial Properties to Prevent Metabolic Syndrome
Abstract
:1. Introduction
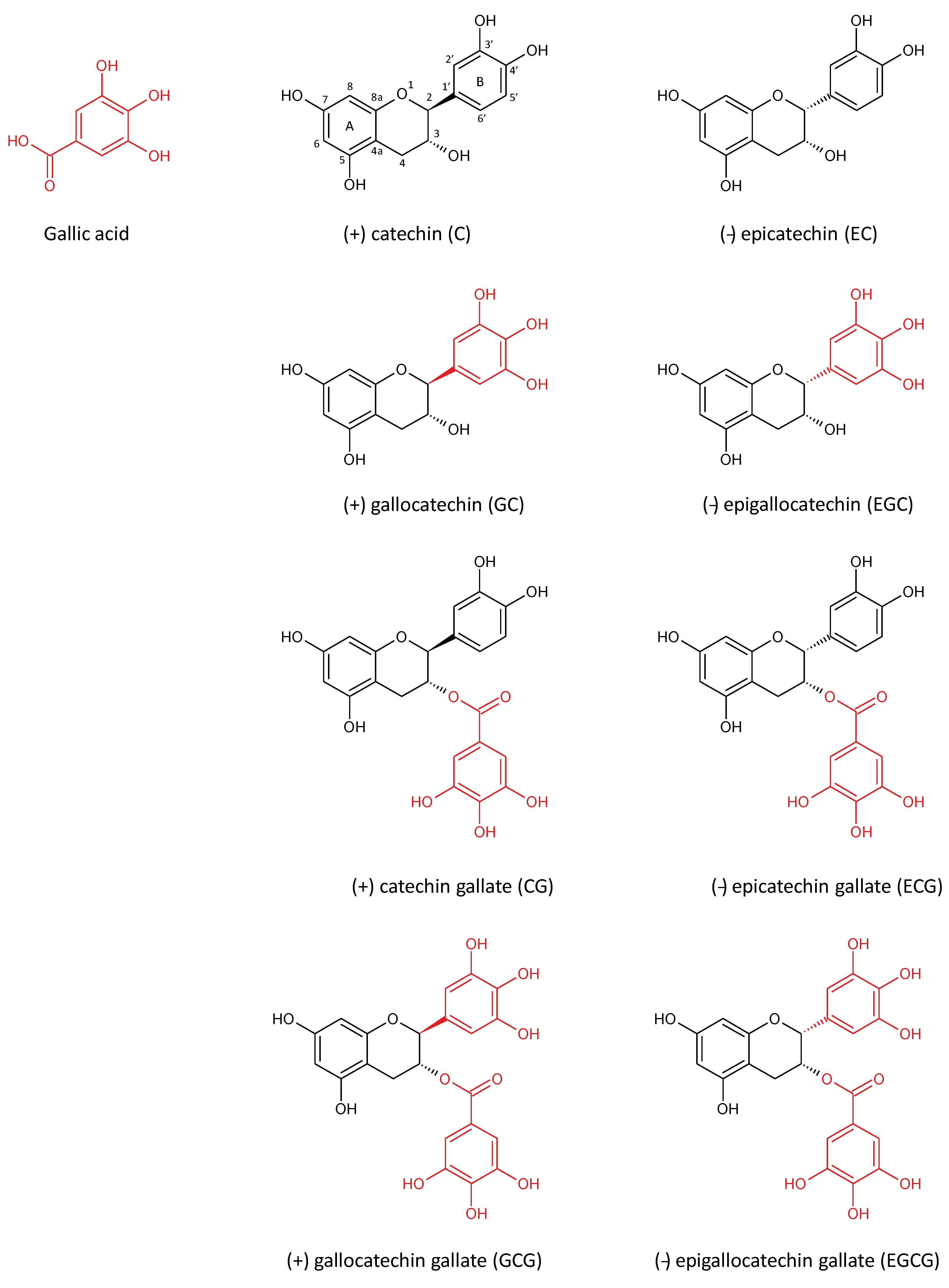
2. Green Tea Polyphenols
| Compound | % Weight of Solid Extracts |
|---|---|
| Flavonoids | 37–56 |
| Carbohydrates | 10–15 |
| Amino acids | 8–12 |
| Organic acids | 7.5–9.5 |
| Methylxanthines | 7–9 |
| Minerals | 6–8 |
| Polymers and tannins | 3–4 |
| Volatiles | Traces |
| Catchins | ConntraIion (mg/mL, Mean ± SD) |
|---|---|
| (+) catechin (C) | 19.70 ± 0.10 |
| (−) epicatechin (EC) | 123.43 ± 0.13 |
| (+) gallocatechin (GC) | 51.10 ± 1.13 |
| (−) epigallocatechin (EGC) | 279.87 ± 1.87 |
| (+) catechin gallate (CG) | nd |
| (−) epicatechin gallate (ECG) | 108.55 ± 0.11 |
| (+) gallocatechin gallate (GCG) | 3.90 ± 0.06 |
| (−) epigallocatechin gallate (EGCG) | 324.54 ± 0.17 |
| TOTAL | 911.09 |
3. Properties of EGCG in the Control of Oxidative Stress
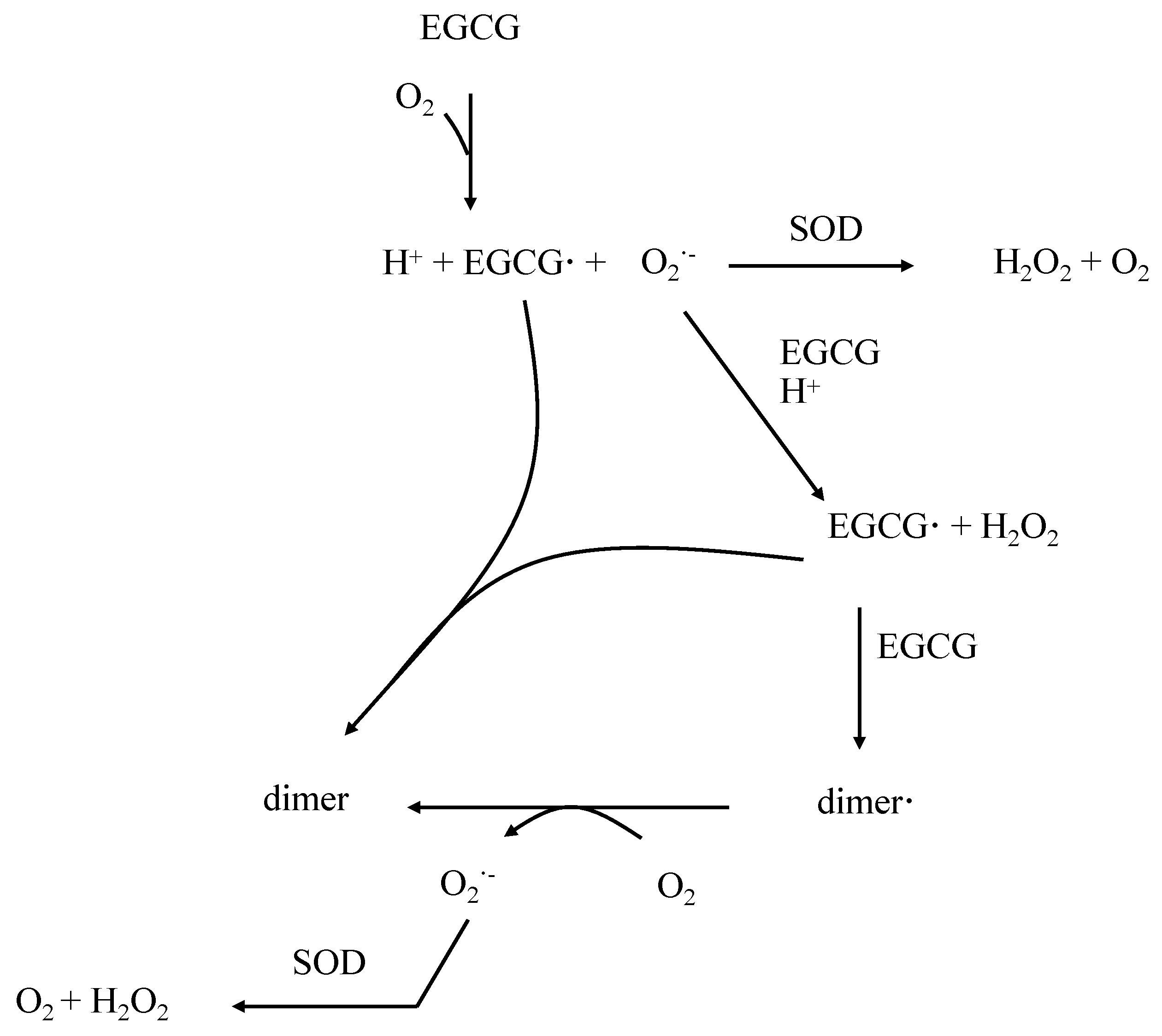
4. Pharmacokinetical Properties of EGCG in Humans
5. Roles of EGCG in Obesity
6. Involvement of EGCG in Insulin Resistance
7. Influence of EGCG in Dyslipidemia
8. Roles of EGCG in Hypertension
9. Conclusions and Perspectives
| Subjects | Dose | Duration | Results | Ref |
|---|---|---|---|---|
| 115 obese women | 12 weeks | ↓ body weight ↓ BMI ↓ total cholesterol ↓ LDL cholesterol | [146] | |
| 56 obese, hypertensive patients | 379 mg/day | 12 weeks | ↓ SBP, ↓ DBP ↓ serum glucose ↓ insulin resistance ↓ LDL cholesterol ↓ TG | [147] |
| 46 obese patients | 379 mg/day | 12 weeks | ↓ BMI ↓ body weight ↓ serum glucose ↓ total cholesterol ↓ LDL cholesterol ↓ TG | [148] |
| 35 obese patients with MS | 870 mg/day | 8 weeks | ↓ body weight ↓ BMI ↓ LDL cholesterol ↓ LDL/HDL ratio | [149] |
| 88 obese patients | 800 mg/day | 8 weeks | ↓ DBP | [150] |
| 40 obese children | 576 mg/day | 24 weeks | ↓ body weight ↓ SBP ↓ LDL cholesterol | [151] |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G. Metabolic syndrome: Definitions and controversies. BMC Med. 2011, 9, 48. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; american heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Van Rooy, M.J.; Pretorius, E. Metabolic syndrome, platelet activation and the development of transient ischemic attack or thromboembolic stroke. Thromb. Res. 2015, 135, 434–442. [Google Scholar] [CrossRef]
- Keske, M.A.; Ng, H.L.; Premilovac, D.; Rattigan, S.; Kim, J.A.; Munir, K.; Yang, P.; Quon, M.J. Vascular and metabolic actions of the green tea polyphenol epigallocatechin gallate. Curr. Med. Chem. 2015, 22, 59–69. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.N.; Ziaullah; Rupasinghe, H.P. Long chain fatty acid acylated derivatives of quercetin-3-O-glucoside as antioxidants to prevent lipid oxidation. Biomolecules 2014, 4, 980–993. [Google Scholar] [CrossRef]
- Wu, C.M.; Lin, K.W.; Teng, C.H.; Huang, A.M.; Chen, Y.C.; Yen, M.H.; Wu, W.B.; Pu, Y.S.; Lin, C.N. Chalcone derivatives inhibit human platelet aggregation and inhibit growth in human bladder cancer cells. Biol. Pharm. Bull. 2014, 37, 1191–1198. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, R.M.; Alkharfy, K.M. Effects of selected bioactive natural products on the vascular endothelium. J. Cardiovasc. Pharmacol. 2013, 62, 111–121. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G. Flavonoid intake and risk of CVD: A systematic review and meta-analysis of prospective cohort studies. Br. J. Nutr. 2014, 111, 1–11. [Google Scholar] [CrossRef]
- Graham, H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef]
- McKay, D.L.; Blumberg, J.B. The role of tea in human health: An update. J. Am. Coll. Nutr. 2002, 21, 1–13. [Google Scholar] [CrossRef]
- Cabrera, C.; Giménez, R.; López, M.C. Determination of tea components with antioxidant activity. J. Agric. Food Chem. 2003, 51, 4427–4435. [Google Scholar] [CrossRef]
- Cabrera, C.; Artacho, R.; Giménez, R. Beneficial effects of green tea—A review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef]
- Komes, D.; Belscak-Cvitanovic, A.; Horzic, D.; Rusak, G.; Likic, S.; Berendika, M. Phenolic composition and antioxidant properties of some traditionally used medicinal plants affected by the extraction time and hydrolysis. Phytochem. Anal. 2011, 22, 172–180. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Mullen, W.; Burns, J.; Lean, M.E.; Brighenti, F.; Crozier, A. HPLC-MSn analysis of phenolic compounds and purine alkaloids in green and black tea. J. Agric. Food Chem. 2004, 52, 2807–2815. [Google Scholar] [CrossRef]
- Higdon, J.V.; Frei, B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef]
- Rice-Evans, C. Implications of the mechanisms of action of tea polyphenols as antioxidants in vitro for chemoprevention in humans. Proc. Soc. Exp. Biol. Med. 1999, 220, 262–266. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Leake, D.; Bruckdorfer, K.R.; Diplock, A.T. Practical approaches to low density lipoprotein oxidation: Whys, wherefores and pitfalls. Free Radic. Res. 1996, 25, 285–311. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef]
- Mandel, S.A.; Amit, T.; Kalfon, L.; Reznichenko, L.; Weinreb, O.; Youdim, M.B. Cell signaling pathways and iron chelation in the neurorestorative activity of green tea polyphenols: Special reference to epigallocatechin gallate (EGCG). J. Alzheimers Dis. 2008, 15, 211–222. [Google Scholar]
- Hyung, S.J.; DeToma, A.S.; Brender, J.R.; Lee, S.; Vivekanandan, S.; Kochi, A.; Choi, J.S.; Ramamoorthy, A.; Ruotolo, B.T.; Lim, M.H. Insights into antiamyloidogenic properties of the green tea extract (−)-epigallocatechin-3-gallate toward metal-associated amyloid-β species. Proc. Natl. Acad. Sci. USA 2013, 110, 3743–3748. [Google Scholar] [CrossRef]
- Pirker, K.F.; Baratto, M.C.; Basosi, R.; Goodman, B.A. Influence of pH on the speciation of copper(II) in reactions with the green tea polyphenols, epigallocatechin gallate and gallic acid. J. Inorg. Biochem. 2012, 112, 10–16. [Google Scholar] [CrossRef]
- Wu, F.; Sun, H.; Kluz, T.; Clancy, H.A.; Kiok, K.; Costa, M. Epigallocatechin-3-gallate (EGCG) protects against chromate-induced toxicity in vitro. Toxicol. Appl. Pharmacol. 2012, 258, 166–175. [Google Scholar] [CrossRef]
- Abib, R.T.; Peres, K.C.; Barbosa, A.M.; Peres, T.V.; Bernardes, A.; Zimmermann, L.M.; Quincozes-Santos, A.; Fiedler, H.D.; Leal, R.B.; Farina, M.; et al. Epigallocatechin-3-gallate protects rat brain mitochondria against cadmium-induced damage. Food Chem. Toxicol. 2011, 49, 2618–2623. [Google Scholar] [CrossRef]
- An, Z.; Qi, Y.; Huang, D.; Gu, X.; Tian, Y.; Li, P.; Li, H.; Zhang, Y. EGCG inhibits Cd2+-induced apoptosis through scavenging ros rather than chelating Cd2+ in HL-7702 cells. Toxicol. Mech. Methods 2014, 24, 259–267. [Google Scholar] [CrossRef]
- Morel, I.; Lescoat, G.; Cillard, P.; Cillard, J. Role of flavonoids and iron chelation in antioxidant action. Methods Enzymol. 1994, 234, 437–443. [Google Scholar]
- Nakagawa, H.; Hasumi, K.; Woo, J.T.; Nagai, K.; Wachi, M. Generation of hydrogen peroxide primarily contributes to the induction of Fe(II)-dependent apoptosis in jurkat cells by (−)-epigallocatechin gallate. Carcinogenesis 2004, 25, 1567–1574. [Google Scholar] [CrossRef]
- Azam, S.; Hadi, N.; Khan, N.U.; Hadi, S.M. Prooxidant property of green tea polyphenols epicatechin and epigallocatechin-3-gallate: Implications for anticancer properties. Toxicol. In Vitro 2004, 18, 555–561. [Google Scholar] [CrossRef]
- Miura, Y.H.; Tomita, I.; Watanabe, T.; Hirayama, T.; Fukui, S. Active oxygens generation by flavonoids. Biol. Pharm. Bull. 1998, 21, 93–96. [Google Scholar] [CrossRef]
- Hou, Z.; Sang, S.; You, H.; Lee, M.J.; Hong, J.; Chin, K.V.; Yang, C.S. Mechanism of action of (−)-epigallocatechin-3-gallate: Auto-oxidation-dependent inactivation of epidermal growth factor receptor and direct effects on growth inhibition in human esophageal cancer KYSE 150 cells. Cancer Res. 2005, 65, 8049–8056. [Google Scholar]
- Sang, S.; Lee, M.J.; Hou, Z.; Ho, C.T.; Yang, C.S. Stability of tea polyphenol (−)-epigallocatechin-3-gallate and formation of dimers and epimers under common experimental conditions. J. Agric. Food Chem. 2005, 53, 9478–9484. [Google Scholar] [CrossRef]
- Ran, Z.H.; Chen, C.; Xiao, S.D. Epigallocatechin-3-gallate ameliorates rats colitis induced by acetic acid. Biomed. Pharmacother. 2008, 3, 189–196. [Google Scholar] [CrossRef]
- Meng, Q.; Velalar, C.N.; Ruan, R. Effects of epigallocatechin-3-gallate on mitochondrial integrity and antioxidative enzyme activity in the aging process of human fibroblast. Free Radic. Biol. Med. 2008, 44, 1032–1041. [Google Scholar] [CrossRef]
- Brückner, M.; Westphal, S.; Domschke, W.; Kucharzik, T.; Lügering, A. Green tea polyphenol epigallocatechin-3-gallate shows therapeutic antioxidative effects in a murine model of colitis. J. Crohns Colitis 2008, 2, 226–235. [Google Scholar] [CrossRef]
- Min, N.Y.; Kim, J.H.; Choi, J.H.; Liang, W.; Ko, Y.J.; Rhee, S.; Bang, H.; Ham, S.W.; Park, A.J.; Lee, K.H. Selective death of cancer cells by preferential induction of reactive oxygen species in response to (−)-epigallocatechin-3-gallate. Biochem. Biophys. Res. Commun. 2012, 421, 91–97. [Google Scholar] [CrossRef]
- Mazzanti, G.; Menniti-Ippolito, F.; Moro, P.A.; Cassetti, F.; Raschetti, R.; Santuccio, C.; Mastrangelo, S. Hepatotoxicity from green tea: A review of the literature and two unpublished cases. Eur. J. Clin. Pharmacol. 2009, 65, 331–341. [Google Scholar] [CrossRef]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef]
- Li, G.X.; Chen, Y.K.; Hou, Z.; Xiao, H.; Jin, H.; Lu, G.; Lee, M.J.; Liu, B.; Guan, F.; Yang, Z.; et al. Pro-oxidative activities and dose-response relationship of (−)-epigallocatechin-3-gallate in the inhibition of lung cancer cell growth: A comparative study in vivo and in vitro. Carcinogenesis 2010, 31, 902–910. [Google Scholar] [CrossRef]
- Lee, M.J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomarkers Prev. 2002, 11, 1025–1032. [Google Scholar]
- Williamson, G.; Dionisi, F.; Renouf, M. Flavanols from green tea and phenolic acids from coffee: Critical quantitative evaluation of the pharmacokinetic data in humans after consumption of single doses of beverages. Mol. Nutr. Food Res. 2011, 55, 864–873. [Google Scholar] [CrossRef]
- Miller, R.J.; Jackson, K.G.; Dadd, T.; Mayes, A.E.; Brown, A.L.; Lovegrove, J.A.; Minihane, A.M. The impact of the catechol-O-methyltransferase genotype on vascular function and blood pressure after acute green tea ingestion. Mol. Nutr. Food Res. 2012, 56, 966–975. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar]
- Moore, R.J.; Jackson, K.G.; Minihane, A.M. Green tea (Camellia sinensis) catechins and vascular function. Br. J. Nutr. 2009, 102, 1790–1802. [Google Scholar] [CrossRef]
- Ullmann, U.; Haller, J.; Decourt, J.P.; Girault, N.; Girault, J.; Richard-Caudron, A.S.; Pineau, B.; Weber, P. A single ascending dose study of epigallocatechin gallate in healthy volunteers. J. Int. Med. Res. 2003, 31, 88–101. [Google Scholar] [CrossRef]
- Chen, L.; Lee, M.J.; Li, H.; Yang, C.S. Absorption, distribution, elimination of tea polyphenols in rats. Drug Metab. Dispos. 1997, 25, 1045–1050. [Google Scholar]
- Chu, K.O.; Wang, C.C.; Chu, C.Y.; Chan, K.P.; Rogers, M.S.; Choy, K.W.; Pang, C.P. Pharmacokinetic studies of green tea catechins in maternal plasma and fetuses in rats. J. Pharm. Sci. 2006, 95, 1372–1381. [Google Scholar] [CrossRef]
- Lin, L.C.; Wang, M.N.; Tseng, T.Y.; Sung, J.S.; Tsai, T.H. Pharmacokinetics of (−)-epigallocatechin-3-gallate in conscious and freely moving rats and its brain regional distribution. J. Agric. Food Chem. 2007, 55, 1517–1524. [Google Scholar] [CrossRef]
- Scholey, A.; Downey, L.A.; Ciorciari, J.; Pipingas, A.; Nolidin, K.; Finn, M.; Wines, M.; Catchlove, S.; Terrens, A.; Barlow, E.; et al. Acute neurocognitive effects of epigallocatechin gallate (EGCG). Appetite 2012, 58, 767–770. [Google Scholar] [CrossRef]
- Meng, X.; Sang, S.; Zhu, N.; Lu, H.; Sheng, S.; Lee, M.J.; Ho, C.T.; Yang, C.S. Identification and characterization of methylated and ring-fission metabolites of tea catechins formed in humans, mice, and rats. Chem. Res. Toxicol. 2002, 15, 1042–1050. [Google Scholar] [CrossRef]
- Schantz, M.; Erk, T.; Richling, E. Metabolism of green tea catechins by the human small intestine. Biotechnol. J. 2010, 5, 1050–1059. [Google Scholar] [CrossRef]
- Van’t Slot, G.; Humpf, H.U. Degradation and metabolism of catechin, epigallocatechin-3-gallate (EGCG), and related compounds by the intestinal microbiota in the pig cecum model. J. Agric. Food Chem. 2009, 57, 8041–8048. [Google Scholar] [CrossRef]
- Lee, M.J.; Wang, Z.Y.; Li, H.; Chen, L.; Sun, Y.; Gobbo, S.; Balentine, D.A.; Yang, C.S. Analysis of plasma and urinary tea polyphenols in human subjects. Cancer Epidemiol. Biomarkers Prev. 1995, 4, 393–399. [Google Scholar]
- Natsume, M.; Osakabe, N.; Yasuda, A.; Osawa, T.; Terao, J. Inhibitory effects of conjugated epicatechin metabolites on peroxynitrite-mediated nitrotyrosine formation. J. Clin. Biochem. Nutr. 2008, 42, 50–53. [Google Scholar] [CrossRef]
- Basu-Modak, S.; Gordon, M.J.; Dobson, L.H.; Spencer, J.P.; Rice-Evans, C.; Tyrrell, R.M. Epicatechin and its methylated metabolite attenuate UVA-induced oxidative damage to human skin fibroblasts. Free Radic. Biol. Med. 2003, 35, 910–921. [Google Scholar] [CrossRef]
- Steffen, Y.; Gruber, C.; Schewe, T.; Sies, H. Mono-O-methylated flavanols and other flavonoids as inhibitors of endothelial NADPH oxidase. Arch. Biochem. Biophys. 2008, 469, 209–219. [Google Scholar] [CrossRef]
- Yiannakopoulou, E.Ch. Effect of green tea catechins on breast carcinogenesis: A systematic review of in vitro and in vivo experimental studies. Eur. J. Cancer Prev. 2014, 23, 84–89. [Google Scholar] [CrossRef]
- Mereles, D.; Hunstein, W. Epigallocatechin-3-gallate (EGCG) for clinical trials: More pitfalls than promises? Int. J. Mol. Sci. 2011, 12, 5592–5603. [Google Scholar] [CrossRef]
- Visioli, F.; Davalos, A. Polyphenols and cardiovascular disease: A critical summary of the evidence. Mini Rev. Med. Chem. 2011, 11, 1186–1190. [Google Scholar]
- Babu, S.; Uppu, S.; Claville, M.O.; Uppu, R.M. Prooxidant actions of bisphenol A (BPA) phenoxyl radicals: Implications to BPA-related oxidative stress and toxicity. Toxicol. Mech. Methods 2013, 23, 273–280. [Google Scholar] [CrossRef]
- Halberg, N.; Wernstedt-Asterholm, I.; Scherer, P.E. The adipocyte as an endocrine cell. Endocrinol. Metab. Clin. N. Am. 2008, 37, 753–768. [Google Scholar] [CrossRef]
- Lau, D.C.; Dhillon, B.; Yan, H.; Szmitko, P.E.; Verma, S. Adipokines: Molecular links between obesity and atheroslcerosis. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, 2031–2041. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Mendonca, F.M.; de Sousa, F.R.; Barbosa, A.L.; Martins, S.C.; Araujo, R.L.; Soares, R.; Abreu, C. Metabolic syndrome and risk of cancer: Which link? Metabolism 2015, 64, 182–189. [Google Scholar] [CrossRef]
- Cohen, D.H.; LeRoith, D. Obesity, type 2 diabetes, and cancer: The insulin and IGF connection. Endocr. Relat. Cancer 2012, 19, 27–45. [Google Scholar] [CrossRef]
- Maury, E.; Brichard, S.M. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol. Cell. Endocrinol. 2010, 314, 1–16. [Google Scholar] [CrossRef]
- Maffei, M.; Halaas, J.; Ravussin, E.; Pratley, R.E.; Lee, G.H.; Zhang, Y.; Fei, H.; Kim, S.; Lallone, R.; Ranganathan, S.; et al. Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat. Med. 1995, 1, 1155–1161. [Google Scholar] [CrossRef]
- Hutley, L.; Prins, J.B. Fat as an endocrine organ: Relationship to the metabolic syndrome. Am. J. Med. Sci. 2005, 330, 280–289. [Google Scholar] [CrossRef]
- DePaoli, A.M. 20 years of leptin: Leptin in common obesity and associated disorders of metabolism. J. Endocrinol. 2014, 223, 71–81. [Google Scholar] [CrossRef]
- Shirasaka, T.; Takasaki, M.; Kannan, H. Cardiovascular effects of leptin and orexins. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, 639–651. [Google Scholar] [CrossRef]
- Momin, A.U.; Melikian, N.; Shah, A.M.; Grieve, D.J.; Wheatcroft, S.B.; John, L.; El Gamel, A.; Desai, J.B.; Nelson, T.; Driver, C.; et al. Leptin is an endothelial-independent vasodilator in humans with coronary artery disease: Evidence for tissue specificity of leptin resistance. Eur. Heart J. 2006, 27, 2294–2299. [Google Scholar] [CrossRef]
- Adya, R.; Tan, B.K.; Randeva, H.S. Differential effects of leptin and adiponectin in endothelial angiogenesis. J. Diabetes Res. 2015, 2015, 648239. [Google Scholar] [CrossRef]
- Naseem, K.M. The role of nitric oxide in cardiovascular diseases. Mol. Aspects Med. 2005, 26, 33–65. [Google Scholar] [CrossRef]
- Yamagishi, S.I.; Edelstein, D.; Du, X.L.; Kaneda, Y.; Guzman, M.; Brownlee, M. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J. Biol. Chem. 2001, 276, 25096–25100. [Google Scholar] [CrossRef]
- Cooper, D.; Stokes, K.Y.; Tailor, A.; Granger, D.N. Oxidative stress promotes blood cell-endothelial cell interactions in the microcirculation. Cardiovasc. Toxicol. 2002, 2, 165–180. [Google Scholar] [CrossRef]
- Snoussi, C.; Ducroc, R.; Hamdaoui, M.H.; Dhaouadi, K.; Abaidi, H.; Cluzeaud, F.; Nazaret, C.; Le Gall, M.; Bado, A. Green tea decoction improves glucose tolerance and reduces weight gain of rats fed normal and high-fat diet. J. Nutr. Biochem. 2014, 25, 557–564. [Google Scholar] [CrossRef]
- Fiorini, R.N.; Donovan, J.L.; Rodwell, D.; Evans, Z.; Cheng, G.; May, H.D.; Milliken, C.E.; Markowitz, J.S.; Campbell, C.; Haines, J.K.; et al. Short-term administration of (−)-epigallocatechin gallate reduces hepatic steatosis and protects against warm hepatic ischemia/reperfusion injury in steatotic mice. Liver Transpl. 2005, 11, 298–308. [Google Scholar] [CrossRef]
- Friedrich, M.; Petzke, K.J.; Raederstorff, D.; Wolfram, S.; Klaus, S. Acute effects of epigallocatechin gallate from green tea on oxidation and tissue incorporation of dietary lipids in mice fed a high-fat diet. Int. J. Obes. 2012, 36, 735–743. [Google Scholar] [CrossRef]
- Grove, K.A.; Sae-tan, S.; Kennett, M.J.; Lambert, J.D. (−)-Epigallocatechin-3-gallate inhibits pancreatic lipase and reduces body weight gain in high fat-fed obese mice. Obesity 2012, 20, 2311–2313. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Z.; Dong, S.; Liu, Y. Molecular interactions between (−)-epigallocatechin gallate analogs and pancreatic lipase. PLoS ONE 2014, 9, e111143. [Google Scholar] [CrossRef]
- Ikeda, I.; Tsuda, K.; Suzuki, Y.; Kobayashi, M.; Unno, T.; Tomoyori, H.; Goto, H.; Kawata, Y.; Imaizumi, K.; Nozawa, A.; et al. Tea catechins with a galloyl moiety suppress postprandial hypertriacylglycerolemia by delaying lymphatic transport of dietary fat in rats. J. Nutr. 2005, 135, 155–159. [Google Scholar]
- Shishikura, Y.; Khokhar, S.; Murray, B.S. Effects of tea polyphenols on emulsification of olive oil in a small intestine model system. J. Agric. Food Chem. 2006, 54, 1906–1913. [Google Scholar] [CrossRef]
- Huang, J.B.; Zhang, Y.; Zhou, Y.B.; Wan, X.C.; Zhang, J.S. Effects of epigallocatechin gallate on lipid metabolism and its underlying molecular mechanism in broiler chickens. J. Anim. Physiol. Anim. Nutr. 2014. [Google Scholar] [CrossRef]
- Sae-Tan, S.; Grove, K.A.; Kennett, M.J.; Lambert, J.D. (−)-Epigallocatechin-3-gallate increases the expression of genes related to fat oxidation in the skeletal muscle of high fat-fed mice. Food Funct. 2011, 2, 111–116. [Google Scholar] [CrossRef]
- Nakagawa, S.; Kojima, Y.; Sekino, K.; Yamato, S. Effect of polyphenols on 3-hydroxy-3-methylglutaryl-coenzyme a lyase activity in human hepatoma Hep G2 cell extracts. Biol. Pharm. Bull. 2013, 36, 1902–1906. [Google Scholar] [CrossRef]
- Kaur, J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014. [Google Scholar] [CrossRef]
- Petersen, K.F.; Shulman, G.I. Etiology of insulin resistance. Am. J. Med. 2006, 119, 10–16. [Google Scholar] [CrossRef]
- Muniyappa, R.; Montagnani, M.; Koh, K.K.; Quon, M.J. Cardiovascular actions of insulin. Endocr. Rev. 2007, 28, 463–491. [Google Scholar] [CrossRef]
- Muniyappa, R.; Yavuz, S. Metabolic actions of angiotensin II and insulin: A microvascular endothelial balancing act. Mol. Cell. Endocrinol. 2013, 378, 59–69. [Google Scholar] [CrossRef]
- Ortsater, H.; Grankvist, N.; Wolfram, S.; Kuehn, N.; Sjoholm, A. Diet supplementation with green tea extract epigallocatechin gallate prevents progression to glucose intolerance in db/db mice. Nutr. Metab. 2012, 9, 11. [Google Scholar] [CrossRef]
- Fu, Z.; Zhen, W.; Yuskavage, J.; Liu, D. Epigallocatechin gallate delays the onset of type 1 diabetes in spontaneous non-obese diabetic mice. Br. J. Nutr. 2011, 105, 1218–1225. [Google Scholar] [CrossRef]
- Zhang, Z.; Ding, Y.; Dai, X.; Wang, J.; Li, Y. Epigallocatechin-3-gallate protects pro-inflammatory cytokine induced injuries in insulin-producing cells through the mitochondrial pathway. Eur. J. Pharmacol. 2011, 670, 311–316. [Google Scholar] [CrossRef]
- Silva, K.C.; Rosales, M.A.; Hamassaki, D.E.; Saito, K.C.; Faria, A.M.; Ribeiro, P.A.; Faria, J.B.; Faria, J.M. Green tea is neuroprotective in diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1325–1336. [Google Scholar] [CrossRef]
- Yamabe, N.; Yokozawa, T.; Oya, T.; Kim, M. Therapeutic potential of (−)-epigallocatechin 3-O-gallate on renal damage in diabetic nephropathy model rats. J. Pharmacol. Exp. Ther. 2006, 319, 228–236. [Google Scholar] [CrossRef]
- Raposo, D.; Morgado, C.; Pereira-Terra, P.; Tavares, I. Nociceptive spinal cord neurons of laminae I-III exhibit oxidative stress damage during diabetic neuropathy which is prevented by early antioxidant treatment with epigallocatechin-gallate (EGCG). Brain Res. Bull. 2015, 110, 68–75. [Google Scholar] [CrossRef]
- Nicholas, S.B.; Liu, J.; Kim, J.; Ren, Y.; Collins, A.R.; Nguyen, L.; Hsueh, W.A. Critical role for osteopontin in diabetic nephropathy. Kidney Int. 2010, 77, 588–600. [Google Scholar] [CrossRef]
- Junaid, A.; Amara, F.M. Osteopontin: Correlation with interstitial fibrosis in human diabetic kidney and PI3-kinase-mediated enhancement of expression by glucose in human proximal tubular epithelial cells. Histopathology 2004, 44, 136–146. [Google Scholar] [CrossRef]
- Yoon, S.P.; Maeng, Y.H.; Hong, R.; Lee, B.R.; Kim, C.G.; Kim, H.L.; Chung, J.H.; Shin, B.C. Protective effects of epigallocatechin gallate (EGCG) on streptozotocin-induced diabetic nephropathy in mice. Acta Histochem. 2014, 116, 1210–1215. [Google Scholar] [CrossRef]
- Yan, J.; Feng, Z.; Liu, J.; Shen, W.; Wang, Y.; Wertz, K.; Weber, P.; Long, J. Enhanced autophagy plays a cardinal role in mitochondrial dysfunction in type 2 diabetic Goto-Kakizaki (GK) rats: Ameliorating effects of (−)-epigallocatechin-3-gallate. J. Nutr. Biochem. 2012, 23, 716–724. [Google Scholar] [CrossRef]
- Kim, J.J.; Tan, Y.; Xiao, L.; Sun, Y.L.; Qu, X. Green tea polyphenol epigallocatechin-3-gallate enhance glycogen synthesis and inhibit lipogenesis in hepatocytes. Biomed. Res. Int. 2013. [Google Scholar] [CrossRef]
- Cordero-Herrera, I.; Martin, M.A.; Goya, L.; Ramos, S. Cocoa flavonoids attenuate high glucose-induced insulin signalling blockade and modulate glucose uptake and production in human Hep G2 cells. Food Chem. Toxicol. 2014, 64, 10–19. [Google Scholar] [CrossRef]
- Reynolds, C.M.; McGillicuddy, F.C.; Harford, K.A.; Finucane, O.M.; Mills, K.H.; Roche, H.M. Dietary saturated fatty acids prime the NLRP3 inflammasome via TLR4 in dendritic cells-implications for diet-induced insulin resistance. Mol. Nutr. Food Res. 2012, 56, 1212–1222. [Google Scholar] [CrossRef]
- Pal, D.; Dasgupta, S.; Kundu, R.; Maitra, S.; Das, G.; Mukhopadhyay, S.; Ray, S.; Majumdar, S.S.; Bhattacharya, S. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat. Med. 2012, 18, 1279–1285. [Google Scholar] [CrossRef]
- Bao, S.; Cao, Y.; Fan, C.; Fan, Y.; Bai, S.; Teng, W.; Shan, Z. Epigallocatechin gallate improves insulin signaling by decreasing toll-like receptor 4 (TLR4) activity in adipose tissues of high-fat diet rats. Mol. Nutr. Food Res. 2014, 58, 677–686. [Google Scholar] [CrossRef]
- Eckel, R.H.; Alberti, K.G.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2010, 375, 181–183. [Google Scholar] [CrossRef]
- Taghibiglou, C.; Rashid-Kolvear, F.; van Iderstine, S.C.; Le-Tien, H.; Fantus, I.G.; Lewis, G.F.; Adeli, K. Hepatic very low density lipoprotein-ApoB overproduction is associated with attenuated hepatic insulin signaling and overexpression of protein-tyrosine phosphatase 1B in a fructose-fed hamster model of insulin resistance. J. Biol. Chem. 2002, 277, 793–803. [Google Scholar] [CrossRef]
- Leonard, A.; Tun, T.K.; Gaffney, R.; Sharma, J.; Gibney, J.; Boran, G. Factors influencing elevated serum apolipoprotein B48 in diabetic and control participants. Br. J. Biomed. Sci. 2014, 71, 145–150. [Google Scholar]
- Lee, C.C.; Lorenzo, C.; Haffner, S.M.; Wagenknecht, L.E.; Goodarzi, M.O.; Stefanovski, D.; Norris, J.M.; Rewers, M.J.; Hanley, A.J. Components of metabolic syndrome and 5-year change in insulin clearance—The insulin resistance atherosclerosis study. Diabetes Obes. Metab. 2013, 15, 441–447. [Google Scholar] [CrossRef]
- Katsiki, N.; Nikolic, D.; Montalto, G.; Banach, M.; Mikhailidis, D.P.; Rizzo, M. The role of fibrate treatment in dyslipidemia: An overview. Curr. Pharm. Des. 2013, 19, 3124–3131. [Google Scholar] [CrossRef]
- Goto, T.; Saito, Y.; Morikawa, K.; Kanamaru, Y.; Nagaoka, S. Epigallocatechin gallate changes mRNA expression level of genes involved in cholesterol metabolism in hepatocytes. Br. J. Nutr. 2012, 107, 769–773. [Google Scholar] [CrossRef]
- Moreno, M.F.; de Laquila, R.; Okuda, M.H.; Lira, F.S.; de Souza, G.I.; de Souza, C.T.; Telles, M.M.; Ribeiro, E.B.; do Nascimento, C.M.; Oyama, L.M. Metabolic profile response to administration of epigallocatechin-3-gallate in high-fat-fed mice. Diabetol. Metab. Syndr. 2014, 6, 84. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, R.S.; Butt, M.S.; Sultan, M.T.; Mushtaq, Z.; Ahmad, S.; Dewanjee, S.; de Feo, V.; Zia-Ul-Haq, M. Preventive role of green tea catechins from obesity and related disorders especially hypercholesterolemia and hyperglycemia. J. Transl. Med. 2015, 13, 79. [Google Scholar] [CrossRef]
- Luo, M.; Kannar, K.; Wahlqvist, M.L.; O’Brien, R.C. Inhibition of LDL oxidation by green tea extract. Lancet 1997, 349, 360–361. [Google Scholar] [CrossRef]
- Yang, T.T.; Koo, M.W. Inhibitory effect of chinese green tea on endothelial cell-induced LDL oxidation. Atherosclerosis 2000, 148, 67–73. [Google Scholar] [CrossRef]
- Ferrannini, E.; Natali, A. Essential hypertension, metabolic disorders, and insulin resistance. Am. Heart J. 1991, 121, 1274–1282. [Google Scholar] [CrossRef]
- Fogari, R.; Zoppi, A.; Ferrari, I.; Mugellini, A.; Preti, P.; Derosa, G. Time to achieve blood pressure goal with a combination versus a conventional monotherapy approach in hypertensive patients with metabolic syndrome. Clin. Exp. Hypertens. 2010, 32, 245–250. [Google Scholar] [CrossRef]
- Malhotra, A.; Kang, B.P.; Cheung, S.; Opawumi, D.; Meggs, L.G. Angiotensin II promotes glucose-induced activation of cardiac protein kinase C isozymes and phosphorylation of troponin I. Diabetes 2001, 50, 1918–1926. [Google Scholar] [CrossRef]
- Mahmood, I.H.; Abed, M.N.; Merkhan, M.M. Effects of blocking of angiotensin system on the prevalence of metabolic syndrome in type 2 diabetic patients. Pak. J. Med. Sci. 2013, 29, 140–143. [Google Scholar]
- Goodfriend, T.L.; Calhoun, D.A. Resistant hypertension, obesity, sleep apnea, and aldosterone: Theory and therapy. Hypertension 2004, 43, 518–524. [Google Scholar] [CrossRef]
- Endemann, D.H.; Schiffrin, E.L. Endothelial dysfunction. J. Am. Soc. Nephrol. 2004, 15, 1983–1992. [Google Scholar] [CrossRef]
- Kraemer-Aguiar, L.G.; Laflor, C.M.; Bouskela, E. Skin microcirculatory dysfunction is already present in normoglycemic subjects with metabolic syndrome. Metabolism 2008, 57, 1740–1746. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Moreno, J.J.; Pryor, W.A.; Ischiropoulos, H.; Beckman, J.S. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem. Res. Toxicol. 1992, 5, 834–842. [Google Scholar] [CrossRef]
- Madamanchi, N.R.; Moon, S.K.; Hakim, Z.S.; Clark, S.; Mehrizi, A.; Patterson, C.; Runge, M.S. Differential activation of mitogenic signaling pathways in aortic smooth muscle cells deficient in superoxide dismutase isoforms. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 950–956. [Google Scholar] [CrossRef]
- Shrivastava, A.K.; Singh, H.V.; Raizada, A.; Singh, S.K.; Pandey, A.; Singh, N.; Yadav, D.S.; Sharma, H. Inflammatory markers in patients with rheumatoid arthritis. Allergol. Immunopathol. 2015, 43, 81–87. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, D.; Fan, Y.; Xie, P.; Li, H. Epigallocatechin-3-gallate enhances ischemia/reperfusion-induced apoptosis in human umbilical vein endothelial cells via AKT and MAPK pathways. Apoptosis 2009, 14, 1245–1254. [Google Scholar] [CrossRef]
- Prigent-Tessier, A.; Quirie, A.; Maguin-Gate, K.; Szostak, J.; Mossiat, C.; Nappey, M.; Devaux, S.; Marie, C.; Demougeot, C. Physical training and hypertension have opposite effects on endothelial brain-derived neurotrophic factor expression. Cardiovasc. Res. 2013, 100, 374–382. [Google Scholar] [CrossRef]
- Avogaro, A.; de Kreutzenberg, S.V.; Fadini, G. Endothelial dysfunction: Causes and consequences in patients with diabetes mellitus. Diabetes Res. Clin. Pract. 2008, 82, S94–S101. [Google Scholar] [CrossRef]
- Mudau, M.; Genis, A.; Lochner, A.; Strijdom, H. Endothelial dysfunction: The early predictor of atherosclerosis. Cardiovasc. J. Afr. 2012, 23, 222–231. [Google Scholar] [CrossRef]
- Tang, E.H.; Vanhoutte, P.M. Endothelial dysfunction: A strategic target in the treatment of hypertension? Pflugers Arch. 2010, 459, 995–1004. [Google Scholar] [CrossRef] [Green Version]
- Jiang, D.J.; Jiang, J.L.; Tan, G.S.; Huang, Z.Z.; Deng, H.W.; Li, Y.J. Demethylbellidifolin inhibits adhesion of monocytes to endothelial cells via reduction of tumor necrosis factor alpha and endogenous nitric oxide synthase inhibitor level. Planta Med. 2003, 69, 1150–1152. [Google Scholar]
- Böger, R.H.; Sydow, K.; Borlak, J.; Thum, T.; Lenzen, H.; Schubert, B.; Tsikas, D.; Bode-Böger, S.M. LDL cholesterol upregulates synthesis of asymmetrical dimethylarginine in human endothelial cells: Involvement of S-adenosylmethionine-dependent methyltransferases. Circ. Res. 2000, 87, 99–105. [Google Scholar] [CrossRef]
- Kimoto, M.; Whitley, G.S.; Tsuji, H.; Ogawa, T. Detection of NG, NG-dimethylarginine dimethylaminohydrolase in human tissues using a monoclonal antibody. J. Biochem. 1995, 117, 237–238. [Google Scholar] [CrossRef]
- Xuan, C.; Tian, Q.W.; Li, H.; Zhang, B.B.; He, G.W.; Lun, L.M. Levels of asymmetric dimethylarginine (ADMA), an endogenous nitric oxide synthase inhibitor, and risk of coronary artery disease: A meta-analysis based on 4713 participants. Eur. J. Prev. Cardiol. 2015. [Google Scholar] [CrossRef]
- Tang, W.J.; Hu, C.P.; Chen, M.F.; Deng, P.Y.; Li, Y.J. Epigallocatechin gallate preserves endothelial function by reducing the endogenous nitric oxide synthase inhibitor level. Can. J. Physiol. Pharmacol. 2006, 84, 163–171. [Google Scholar] [CrossRef]
- Kurita, I.; Kim, J.H.; Auger, C.; Kinoshita, Y.; Miyase, T.; Ito, T.; Schini-Kerth, V.B. Hydroxylation of (−)-epigallocatechin-3-O-gallate at 3′′, but not 4′′, is essential for the PI3-kinase/Akt-dependent phosphorylation of endothelial NO synthase in endothelial cells and relaxation of coronary artery rings. Food Funct. 2013, 4, 249–257. [Google Scholar] [CrossRef]
- Hall, J.E.; Henegar, J.R.; Dwyer, T.M.; Liu, J.; Da Silva, A.A.; Kuo, J.J.; Tallam, L. Is obesity a major cause of chronic kidney disease? Adv. Ren. Replace. Ther. 2004, 11, 41–54. [Google Scholar] [CrossRef]
- Briones, A.M.; Nguyen Dinh Cat, A.; Callera, G.E.; Yogi, A.; Burger, D.; He, Y.; Correa, J.W.; Gagnon, A.M.; Gomez-Sanchez, C.E.; Gomez-Sanchez, E.P.; et al. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: Implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension 2012, 59, 1069–1078. [Google Scholar] [CrossRef]
- Ondetti, M.A.; Cushman, D.W. Inhibition of the renin-angiotensin system. A new approach to the therapy of hypertension. J. Med. Chem. 1981, 24, 355–361. [Google Scholar] [CrossRef]
- Li, F.; Takahashi, Y.; Yamaki, K. Inhibitory effect of catechin-related compounds on renin activity. Biomed. Res. 2013, 34, 167–171. [Google Scholar] [CrossRef]
- Potenza, M.A.; Marasciulo, F.L.; Tarquinio, M.; Tiravanti, E.; Colantuono, G.; Federici, A.; Kim, J.A.; Quon, M.J.; Montagnani, M. EGCG, a green tea polyphenol, improves endothelial function and insulin sensitivity, reduces blood pressure, and protects against myocardial I/R injury in SHR. Am. J. Physiol. Endocrinol. Metab. 2007, 292, 1378–1387. [Google Scholar] [CrossRef]
- Kiskinis, E.; Hallberg, M.; Christian, M.; Olofsson, M.; Dilworth, S.M.; White, R.; Parker, M.G. RIP140 directs histone and DNA methylation to silence Ucp1 expression in white adipocytes. EMBO J. 2007, 26, 4831–4840. [Google Scholar] [CrossRef]
- Montague, C.T.; Farooqi, I.S.; Whitehead, J.P.; Soos, M.A.; Rau, H.; Wareham, N.J.; Sewter, C.P.; Digby, J.E.; Mohammed, S.N.; Hurst, J.A.; et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 1997, 387, 903–908. [Google Scholar]
- Lee, H.A.; Lee, D.Y.; Lee, H.J.; Han, H.S.; Kim, I. Enrichment of (pro)renin receptor promoter with activating histone codes in the kidneys of spontaneously hypertensive rats. J. Renin Angiotensin Aldosterone Syst. 2012, 13, 11–18. [Google Scholar] [CrossRef]
- Jiang, Q.; Yuan, H.; Xing, X.; Liu, J.; Huang, Z.; Du, X. Methylation of adrenergic β1 receptor is a potential epigenetic mechanism controlling antihypertensive response to metoprolol. Indian J. Biochem. Biophys. 2011, 48, 301–307. [Google Scholar]
- Boqué, N.; de la Iglesia, R.; de la Garza, A.L.; Milagro, F.I.; Olivares, M.; Banuelos, O.; Soria, A.C.; Rodriguez-Sanchez, S.; Martinez, J.A.; Campion, J. Prevention of diet-induced obesity by apple polyphenols in wistar rats through regulation of adipocyte gene expression and DNA methylation patterns. Mol. Nutr. Food Res. 2013, 57, 1473–1478. [Google Scholar] [CrossRef]
- Gerhauser, C. Epigenetic impact of dietary isothiocyanates in cancer chemoprevention. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 405–410. [Google Scholar] [CrossRef]
- Chen, I.J.; Liu, C.Y.; Chiu, J.P.; Hsu, C.H. Therapeutic effect of high-dose green tea extract on weight reduction: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2015. [Google Scholar] [CrossRef]
- Bogdanski, P.; Suliburska, J.; Szulinska, M.; Stepien, M.; Pupek-Musialik, D.; Jablecka, A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr. Res. 2012, 32, 421–427. [Google Scholar] [CrossRef]
- Suliburska, J.; Bogdanski, P.; Szulinska, M.; Stepien, M.; Pupek-Musialik, D.; Jablecka, A. Effects of green tea supplementation on elements, total antioxidants, lipids, and glucose values in the serum of obese patients. Biol. Trace Elem. Res. 2012, 149, 315–322. [Google Scholar] [CrossRef]
- Basu, A.; Sanchez, K.; Leyva, M.J.; Wu, M.; Betts, N.M.; Aston, C.E.; Lyons, T.J. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J. Am. Coll. Nutr. 2010, 29, 31–40. [Google Scholar] [CrossRef]
- Brown, A.L.; Lane, J.; Coverly, J.; Stocks, J.; Jackson, S.; Stephen, A.; Bluck, L.; Coward, A.; Hendrickx, H. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: Randomized controlled trial. Br. J. Nutr. 2009, 101, 886–894. [Google Scholar] [CrossRef]
- Matsuyama, T.; Tanaka, Y.; Kamimaki, I.; Nagao, T.; Tokimitsu, I. Catechin safely improved higher levels of fatness, blood pressure, and cholesterol in children. Obesity 2008, 16, 1338–1348. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Legeay, S.; Rodier, M.; Fillon, L.; Faure, S.; Clere, N. Epigallocatechin Gallate: A Review of Its Beneficial Properties to Prevent Metabolic Syndrome. Nutrients 2015, 7, 5443-5468. https://doi.org/10.3390/nu7075230
Legeay S, Rodier M, Fillon L, Faure S, Clere N. Epigallocatechin Gallate: A Review of Its Beneficial Properties to Prevent Metabolic Syndrome. Nutrients. 2015; 7(7):5443-5468. https://doi.org/10.3390/nu7075230
Chicago/Turabian StyleLegeay, Samuel, Marion Rodier, Laetitia Fillon, Sébastien Faure, and Nicolas Clere. 2015. "Epigallocatechin Gallate: A Review of Its Beneficial Properties to Prevent Metabolic Syndrome" Nutrients 7, no. 7: 5443-5468. https://doi.org/10.3390/nu7075230
APA StyleLegeay, S., Rodier, M., Fillon, L., Faure, S., & Clere, N. (2015). Epigallocatechin Gallate: A Review of Its Beneficial Properties to Prevent Metabolic Syndrome. Nutrients, 7(7), 5443-5468. https://doi.org/10.3390/nu7075230





