Postprandial Differences in the Amino Acid and Biogenic Amines Profiles of Impaired Fasting Glucose Individuals after Intake of Highland Barley
Abstract
:1. Introduction
2. Experimental Section
2.1. Study 1: Amino Acid and Biogenic Amine Profiles Change in IFG Individuals during 120 min OGTT
| Parameter | Control (n = 50) | IFG (n = 50) | p value |
|---|---|---|---|
| Sex (femeal/male) | 16/14 | 15/15 | 0.82 |
| Age (years) | 44.86 ± 9.48 | 45.86 ± 10.55 | 0.16 |
| Smoker/non-smoker | 12/18 | 13/17 | 0.67 |
| Protein (g/day) | 80.34 ± 12.23 | 81.21 ± 11.13 | 0.56 |
| Fat (g/day) | 71.45 ± 21.27 | 72.10 ± 22.09 | 0.39 |
| Carbohydrate (g/day) | 324.01 ± 108.17 | 325.07 ± 109.21 | 0.68 |
| BMI (kg/m2) | 22.24 ± 1.66 | 24.61 ± 5.30 | <0.001 |
| TC (mmmoL/L) | 4.16 ± 0.51 | 4.59 ± 1.24 | <0.001 |
| TG (mmmoL/L) | 0.90 ± 0.32 | 1.59 ± 0.53 | <0.001 |
| Fasting glucose (mmmoL/L) | 4.03 ± 0.47 | 5.69 ± 0.42 | <0.001 |
| 2 h-glucose (mmmoL/L) | 4.67 ± 0.99 | 6.13 ± 1.14 | <0.001 |
| SBP (mmHg) | 112.70 ± 6.43 | 136.62 ± 15.40 | <0.001 |
| DBP (mmHg) | 75.07 ± 6.02 | 80.16 ± 9.44 | <0.001 |
| HAc1 (%) | 5.2 ± 0.2 | 6.2 ± 0.3 | 0.008 |
| Fasting insulin (mU/L) | 6.07 ± 2.12 | 7.19 ± 2.42 | 0.02 |
| 2 h-insulin (mU/L) | 8.55 ± 2.08 | 23.45 ± 2.5 | <0.001 |
| HOMR-IR | 1.09 ± 0.46 | 1.90 ± 0.46 | <0.001 |
2.2. Study 2: Postprandial Amino Acid and Biogenic Amine Profiles after Three Test Loads in IFG Individuals
2.3. Biochemical Measurements
2.4. Serum Preparation
2.5. UPLC-TQ-MS Analysis
2.6. Statistical Analysis
3. Results
3.1. Study 1: The Amino Acid and Biogenic Amine Profiles Change in the Control and IFG Participants during 120 min OGTT
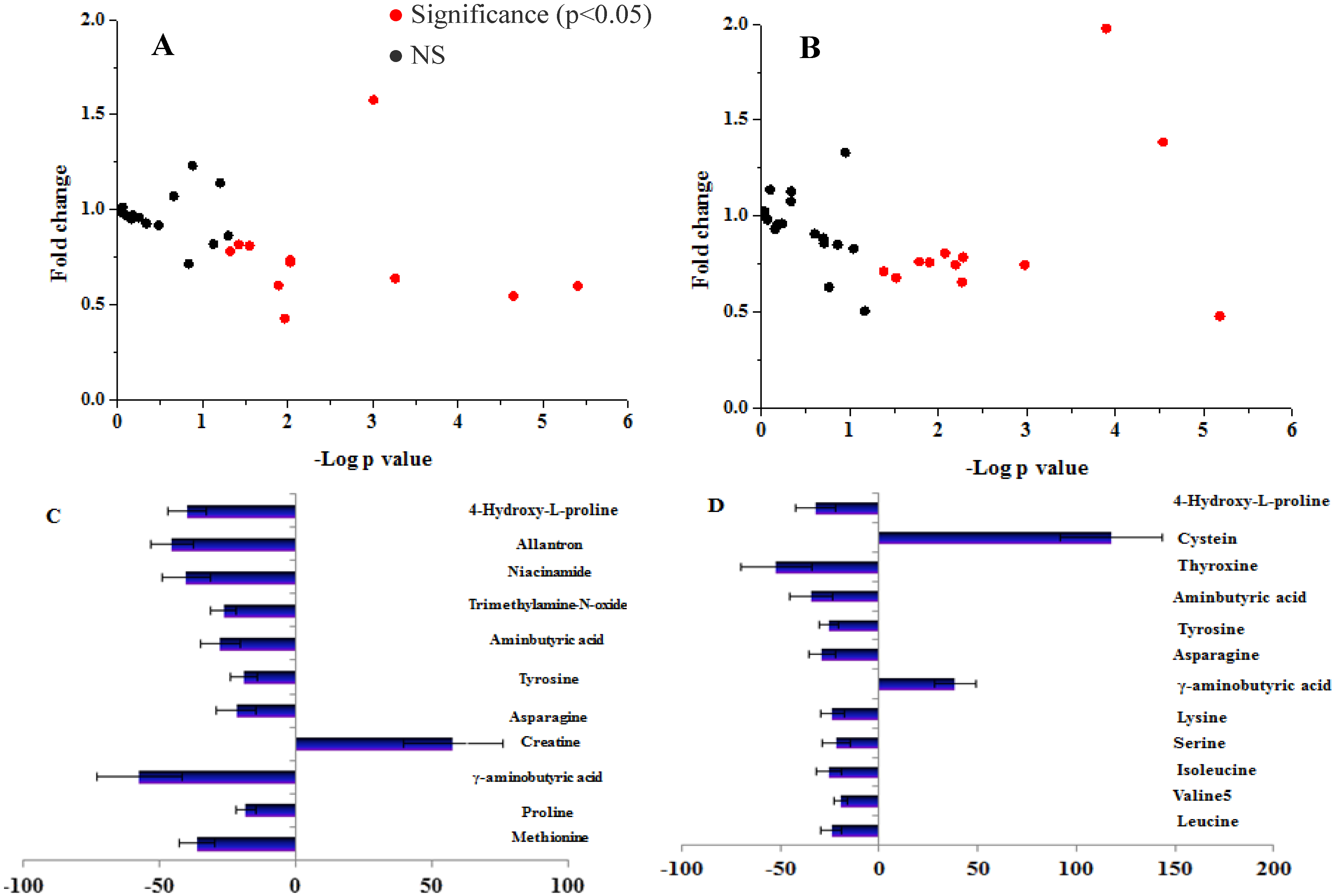
3.2. Study 2: The Postprandial Amino Acid and Biogenic Amine Profiles after Three Test Loads in the IFG Group
3.2.1. Glucose and Insulin Profiles after Three Test Loads
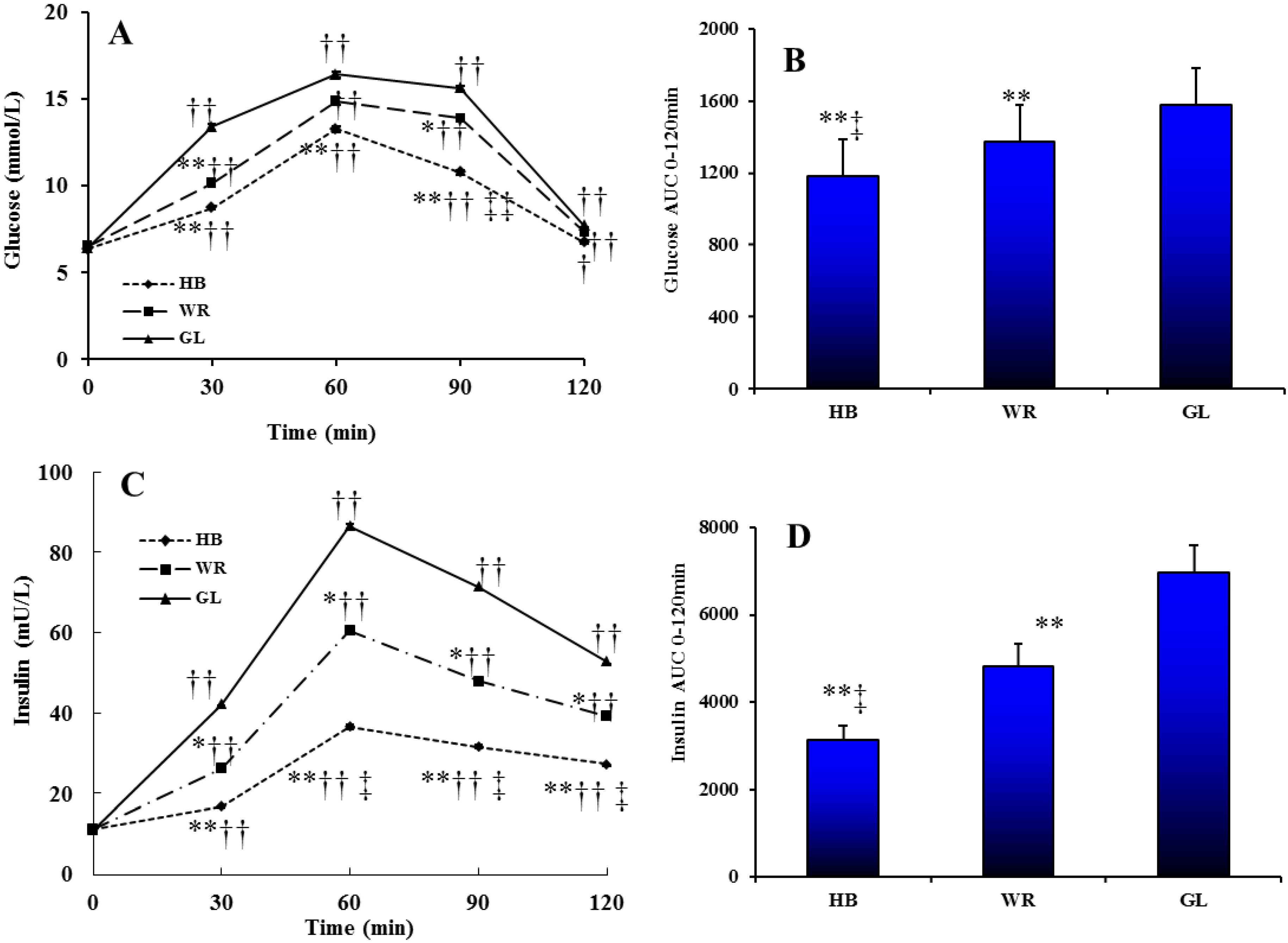
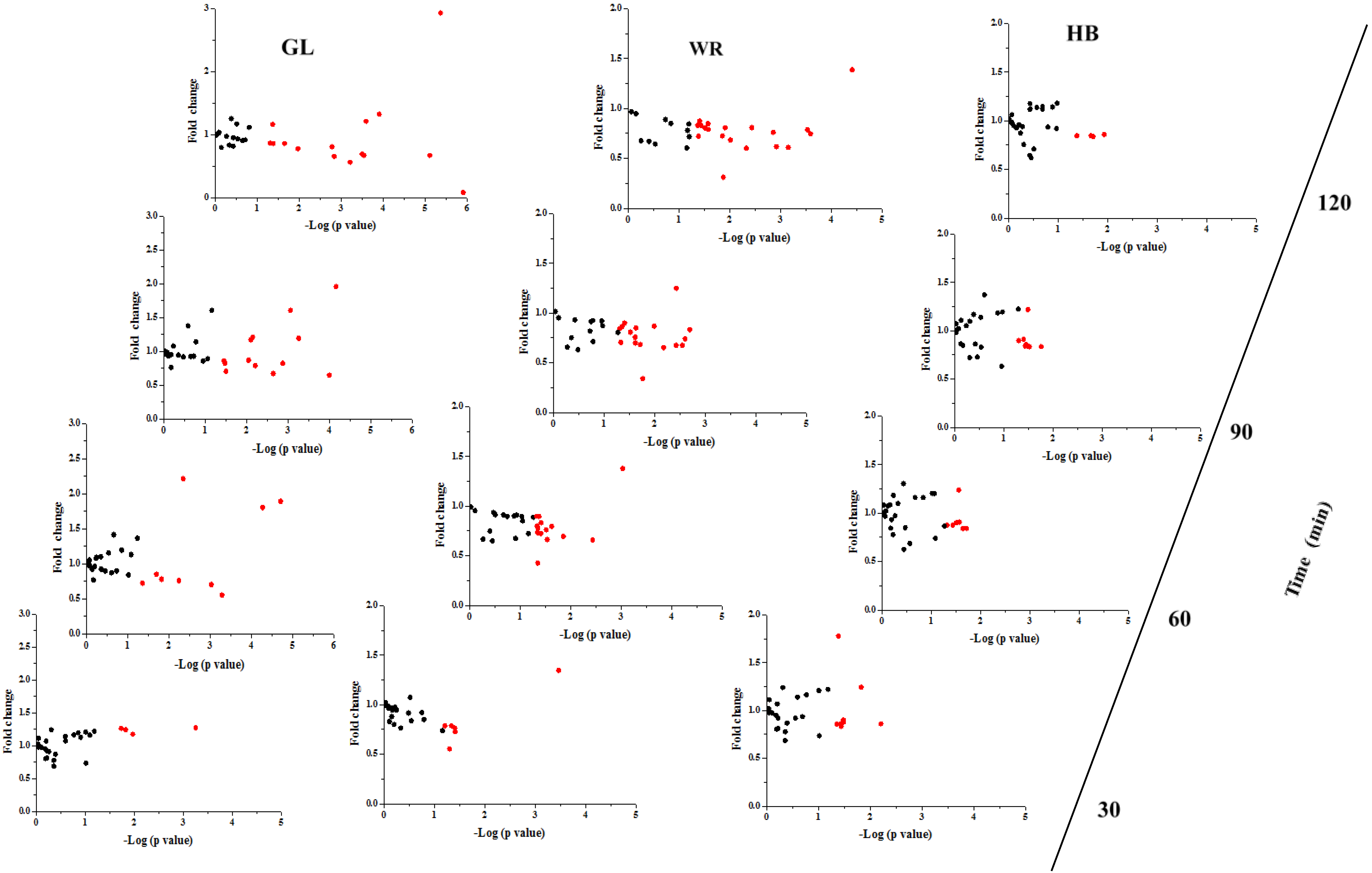
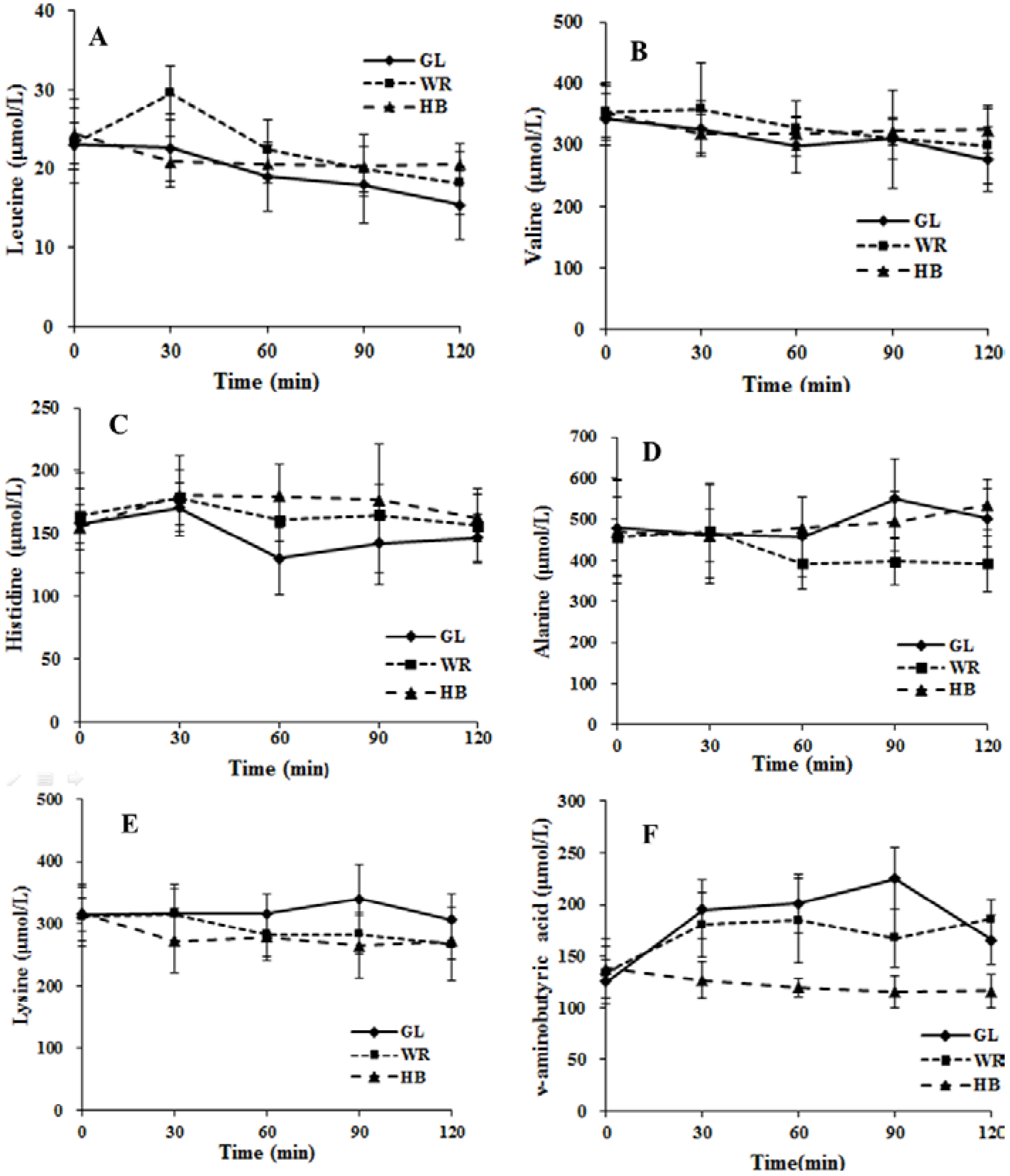
3.2.2. The Postprandial Amino Acid and Biogenic Amine Profiles after Three Test Loads
3.2.3. Correlation between Postprandial Metabolites, Glucose and Insulin Following Different Loads
| Metabolites | GL | |||
|---|---|---|---|---|
| Fasting Glucose | 2 h-glucose | Fasting Insulin | 2 h-Insulin | |
| Leucine | −0.647 (0.024) | |||
| Valine | ||||
| Histidine | −0.604 (0.029) | |||
| Alanine | −0.600 (0.048) | |||
| Lysine | −0.587 (0.034) | |||
| γ-aminobutyric acid | 0.621 (0.023) | 0.551 (0.040) | ||
| HB | ||||
| Fasting glucose | 2 h-glucose | Fasting insulin | 2 h-insulin | |
| Leucine | −0.774 (0.023) | |||
| Valine | ||||
| Histidine | −0.637 (0.045) | |||
| Alanine | −0.607 (0.041) | |||
| Lysine | −0.548 (0.042) | |||
| γ-aminobutyric acid | 0.614 (0.038) | 0.514 (0.044) | ||
| WR | ||||
| Fasting glucose | 2 h-glucose | Fasting insulin | 2 h-insulin | |
| Leucine | −0.627 (0.038) | |||
| Valine | ||||
| Histidine | 0.691 (0.018) | −0.621 (0.029) | ||
| Alanine | −0.761 (0.006) | |||
| Lysine | −0.568 (0.042) | |||
| γ-aminobutyric acid | −0.572 (0.041) | |||
4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgment
Author Contributions
Conflicts of Interest
References
- Lim, S.C.; Tai, E.S.; Tan, B.Y.; Chew, S.K.; Tan, C.E. Cardiovascular risk profile in individuals with borderline glycemia: The effect of the 1997 american diabetes association diagnostic criteria and the 1998 world health organization provisional report. Diabetes Care 2000, 23, 278–282. [Google Scholar]
- Cowie, C.C.; Rust, K.F.; Byrd-Holt, D.D.; Eberhardt, M.S.; Flegal, K.M.; Engelgau, M.M.; Saydah, S.H.; Williams, D.E.; Geiss, L.S.; Gregg, E.W. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. Population: National health and nutrition examination survey 1999–2002. Diabetes Care 2006, 29, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ford, E.S.; Zhao, G.; Mokdad, A.H. Prevalence of pre-diabetes and its association with clustering of cardiometabolic risk factors and hyperinsulinemia among U.S. Adolescents: National health and nutrition examination survey 2005–2006. Diabetes Care 2009, 32, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Q.; Hu, G.; Tuomilehto, J.; Nakagami, T.; Balkau, B.; Borch-Johnsen, K.; Ramachandran, A.; Mohan, V.; Iyer, S.R.; Tominaga, M.; et al. Age- and sex-specific prevalence of diabetes and impaired glucose regulation in 11 asian cohorts. Diabetes Care 2003, 26, 1770–1780. [Google Scholar]
- Abdul-Ghani, M.A.; Tripathy, D.; DeFronzo, R.A. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 2006, 29, 1130–1139. [Google Scholar]
- Kim, S.H.; Reaven, G.M. Isolated impaired fasting glucose and peripheral insulin sensitivity: Not a simple relationship. Diabetes Care 2008, 31, 347–352. [Google Scholar]
- Felig, P.; Marliss, E.; Cahill, G.F., Jr. Plasma amino acid levels and insulin secretion in obesity. N. Engl. J. Med. 1969, 281, 811–816. [Google Scholar]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar]
- Shah, S.H.; Crosslin, D.R.; Haynes, C.S.; Nelson, S.; Turer, C.B.; Stevens, R.D.; Muehlbauer, M.J.; Wenner, B.R.; Bain, J.R.; Laferrere, B.; et al. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia 2012, 55, 321–330. [Google Scholar]
- Kamaura, M.; Nishijima, K.; Takahashi, M.; Ando, T.; Mizushima, S.; Tochikubo, O. Lifestyle modification in metabolic syndrome and associated changes in plasma amino acid profiles. Circ. J. 2010, 74, 2434–2440. [Google Scholar]
- Perseghin, G.; Ghosh, S.; Gerow, K.; Shulman, G.I. Metabolic defects in lean nondiabetic offspring of niddm parents: A cross-sectional study. Diabetes 1997, 46, 1001–1009. [Google Scholar]
- Shaham, O.; Wei, R.; Wang, T.J.; Ricciardi, C.; Lewis, G.D.; Vasan, R.S.; Carr, S.A.; Thadhani, R.; Gerszten, R.E.; Mootha, V.K. Metabolic profiling of the human response to a glucose challenge reveals distinct axes of insulin sensitivity. Mol. Syst. Biol. 2008, 4, 214. [Google Scholar]
- Bentley-Lewis, R.; Xiong, G.; Lee, H.; Yang, A.; Huynh, J.; Kim, C. Metabolomic analysis reveals amino acid responses to an oral glucose tolerance test in women with prior history of gestational diabetes mellitus. J. Clin. Transl. Endocrinol. 2014, 1, 38–43. [Google Scholar]
- Bondia-Pons, I.; Nordlund, E.; Mattila, I.; Katina, K.; Aura, A.M.; Kolehmainen, M.; Oresic, M.; Mykkanen, H.; Poutanen, K. Postprandial differences in the plasma metabolome of healthy finnish subjects after intake of a sourdough fermented endosperm rye bread versus white wheat bread. Nutr. J. 2011, 10, 116. [Google Scholar]
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997, 20, 1183–1197. [Google Scholar]
- Liu, L.; Feng, R.; Guo, F.; Li, Y.; Jiao, J.; Sun, C. Targeted metabolomic analysis reveals the association between the postprandial change in palmitic acid, branched-chain amino acids and insulin resistance in young obese subjects. Diabetes Res. Clin. Pract. 2015, 108, 84–93. [Google Scholar]
- Tai, E.S.; Tan, M.L.; Stevens, R.D.; Low, Y.L.; Muehlbauer, M.J.; Goh, D.L.; Ilkayeva, O.R.; Wenner, B.R.; Bain, J.R.; Lee, J.J.; et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in chinese and asian-indian men. Diabetologia 2010, 53, 757–767. [Google Scholar]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar]
- Makelainen, H.; Anttila, H.; Sihvonen, J.; Hietanen, R.M.; Tahvonen, R.; Salminen, E.; Mikola, M.; Sontag-Strohm, T. The effect of beta-glucan on the glycemic and insulin index. Eur. J. Clin. Nutr. 2007, 61, 779–785. [Google Scholar]
- Orskov, C.; Wettergren, A.; Holst, J.J. Secretion of the incretin hormones glucagon-like peptide-1 and gastric inhibitory polypeptide correlates with insulin secretion in normal man throughout the day. Scand. J. Gastroenterol. 1996, 31, 665–670. [Google Scholar]
- Salehi, M.; Gastaldelli, A.; D’Alessio, D.A. Evidence from a single individual that increased plasma glp-1 and glp-1-stimulated insulin secretion after gastric bypass are independent of foregut exclusion. Diabetologia 2014, 57, 1495–1499. [Google Scholar]
- Li, J.; Zhang, N.; Hu, L.; Li, Z.; Li, R.; Li, C.; Wang, S. Improvement in chewing activity reduces energy intake in one meal and modulates plasma gut hormone concentrations in obese and lean young chinese men. Am. J. Clin. Nutr. 2011, 94, 709–716. [Google Scholar]
- Geidenstam, N.; Spegel, P.; Mulder, H.; Filipsson, K.; Ridderstrale, M.; Danielsson, A.P. Metabolite profile deviations in an oral glucose tolerance test-a comparison between lean and obese individuals. Obesity (Silver Spring) 2014, 22, 2388–2395. [Google Scholar]
- Christensen, K.L.; Hedemann, M.S.; Laerke, H.N.; Jorgensen, H.; Mutt, S.J.; Herzig, K.H.; Bach Knudsen, K.E. Concentrated arabinoxylan but not concentrated beta-glucan in wheat bread has similar effects on postprandial insulin as whole-grain rye in porto-arterial catheterized pigs. J. Agric. Food Chem. 2013, 61, 7760–7768. [Google Scholar]
- Holecek, M. The bcaa-bcka cycle: Its relation to alanine and glutamine synthesis and protein balance. Nutrition 2001, 17, 70. [Google Scholar]
- Doi, M.; Yamaoka, I.; Nakayama, M.; Sugahara, K.; Yoshizawa, F. Hypoglycemic effect of isoleucine involves increased muscle glucose uptake and whole body glucose oxidation and decreased hepatic gluconeogenesis. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1683–1693. [Google Scholar]
- Lynch, C.J.; Gern, B.; Lloyd, C.; Hutson, S.M.; Eicher, R.; Vary, T.C. Leucine in food mediates some of the postprandial rise in plasma leptin concentrations. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E621–630. [Google Scholar]
- DeFronzo, R.A.; Ferrannini, E.; Simonson, D.C. Fasting hyperglycemia in non-insulin-dependent diabetes mellitus: Contributions of excessive hepatic glucose production and impaired tissue glucose uptake. Metabolism 1989, 38, 387–395. [Google Scholar]
- Thomas-Reetz, A.; Hell, J.W.; During, M.J.; Walch-Solimena, C.; Jahn, R.; de Camilli, P. A gamma-aminobutyric acid transporter driven by a proton pump is present in synaptic-like microvesicles of pancreatic beta cells. Proc. Natl. Acad. Sci. USA 1993, 90, 5317–5321. [Google Scholar]
- Dong, H.; Kumar, M.; Zhang, Y.; Gyulkhandanyan, A.; Xiang, Y.Y.; Ye, B.; Perrella, J.; Hyder, A.; Zhang, N.; Wheeler, M.; et al. Gamma-aminobutyric acid up- and downregulates insulin secretion from beta cells in concert with changes in glucose concentration. Diabetologia 2006, 49, 697–705. [Google Scholar]
- Nakagawa, T.; Yokozawa, T.; Kim, H.J.; Shibahara, N. Protective effects of gamma-aminobutyric acid in rats with streptozotocin-induced diabetes. J. Nutr. Sci. Vitaminol. (Tokyo) 2005, 51, 278–282. [Google Scholar]
- Cosentino, F.; Hishikawa, K.; Katusic, Z.S.; Luscher, T.F. High glucose increases nitric oxide synthase expression and superoxide anion generation in human aortic endothelial cells. Circulation 1997, 96, 25–28. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Wang, X.; Li, Y.; Sun, C. Postprandial Differences in the Amino Acid and Biogenic Amines Profiles of Impaired Fasting Glucose Individuals after Intake of Highland Barley. Nutrients 2015, 7, 5556-5571. https://doi.org/10.3390/nu7075238
Liu L, Wang X, Li Y, Sun C. Postprandial Differences in the Amino Acid and Biogenic Amines Profiles of Impaired Fasting Glucose Individuals after Intake of Highland Barley. Nutrients. 2015; 7(7):5556-5571. https://doi.org/10.3390/nu7075238
Chicago/Turabian StyleLiu, Liyan, Xinyang Wang, Ying Li, and Changhao Sun. 2015. "Postprandial Differences in the Amino Acid and Biogenic Amines Profiles of Impaired Fasting Glucose Individuals after Intake of Highland Barley" Nutrients 7, no. 7: 5556-5571. https://doi.org/10.3390/nu7075238
APA StyleLiu, L., Wang, X., Li, Y., & Sun, C. (2015). Postprandial Differences in the Amino Acid and Biogenic Amines Profiles of Impaired Fasting Glucose Individuals after Intake of Highland Barley. Nutrients, 7(7), 5556-5571. https://doi.org/10.3390/nu7075238




