Micronutrient Status in Patients with Short Bowel Syndrome Weaned off Parenteral Support
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Follow-Up
2.2. Data Collection
2.3. Measurements
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Nutritional Characteristics at Weaning
3.3. Nutritional Characteristics
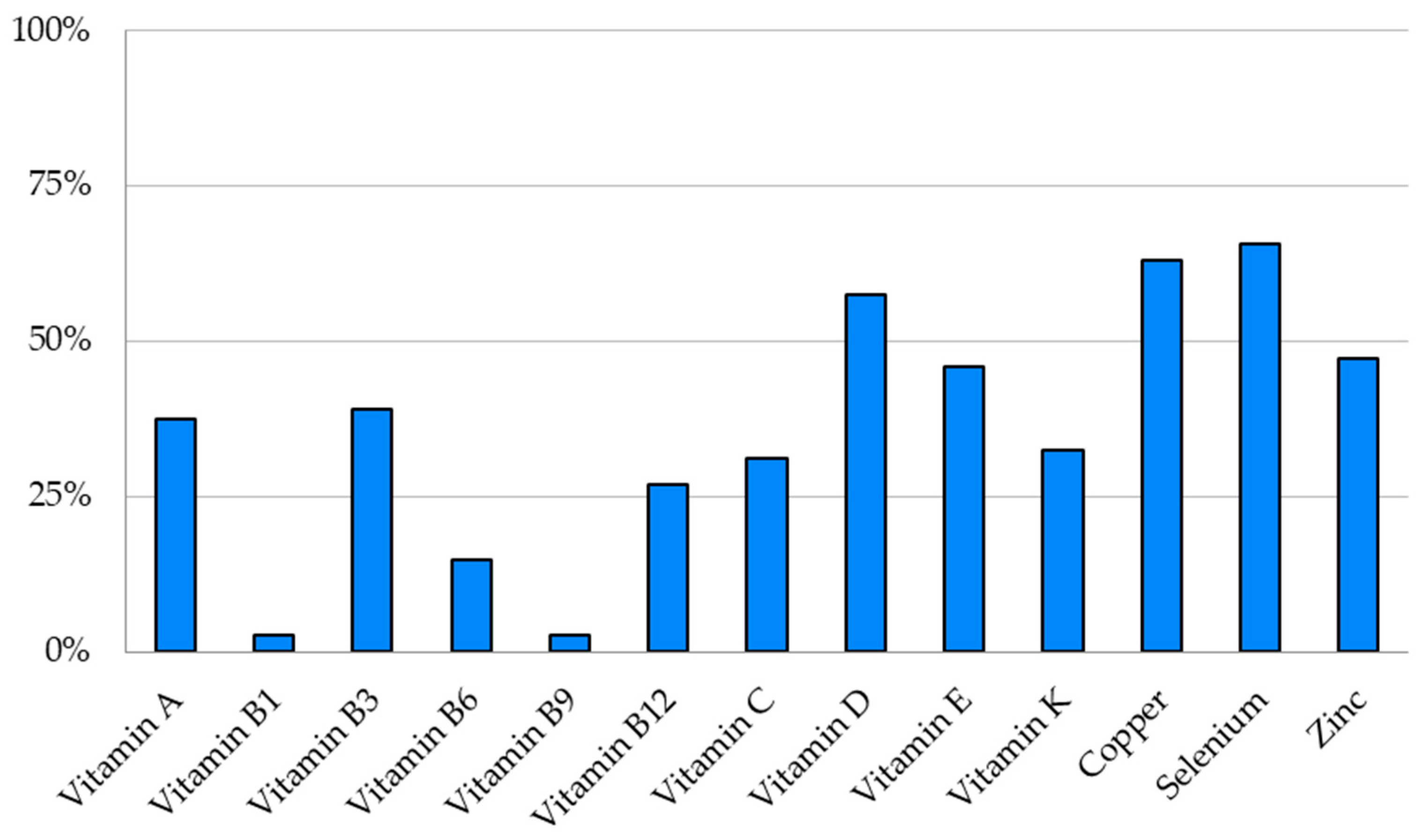
3.4. Micronutrient Depletions (Vitamins and Trace Elements)
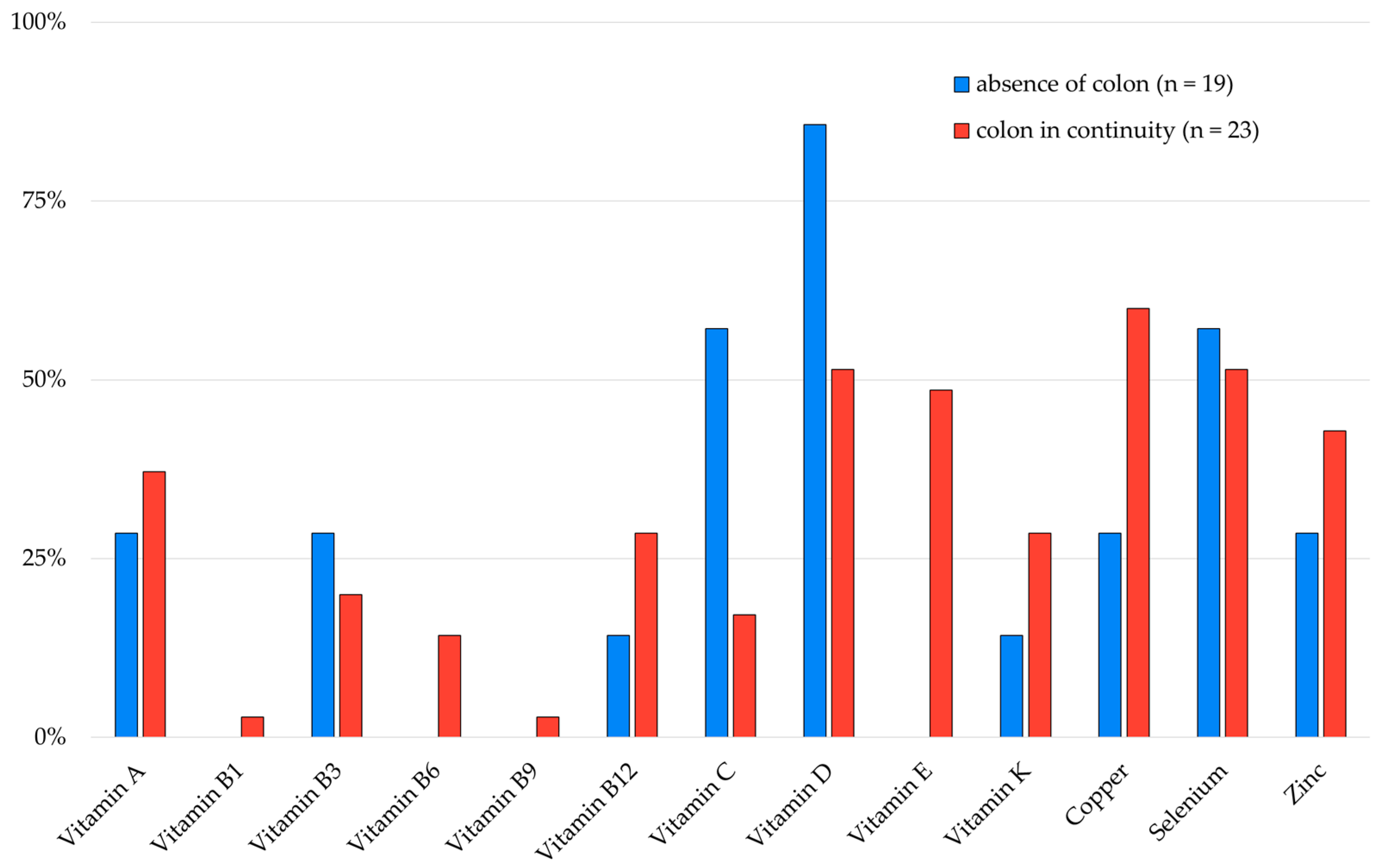
3.5. Micronutrient Depletions According to SBS Characteristics
3.6. Depletions’ Risk Factors (Table 7)
| SBS Characteristics | Number of Depletions (mean +/− SD) |
|---|---|
| SBS type | |
| Type 1 | 3.1 ± 1.5 |
| Type 2 | 4.0 ± 2.0 |
| Type 3 | 4.4 ± 1.8 |
| SBS etiology | |
| IBD | 3.5 ± 1.9 |
| Mesenteric ischemia | 3.7 ± 2.1 |
| Surgical complications | 4.1 ± 2.5 |
| Other | 4.2 ± 0.9 |
| Severity of intestinal failure at weaning based on the daily mean of the total volume infused per week * | |
| <1 L/day | 3.7 ± 1.8 |
| 1–2 L/day | 4.5 ± 2.1 |
| 2–3 L/day | 4.3 ± 2.3 |
| >3 L/day | 6.5 ± 0.7 |
| Severity of intestinal failure at weaning based on the daily mean of the total kcal infused per week, adjusted on weight ** | |
| <1 kcal/kg/day | 4.0 ± 0 |
| 1–10 kcal/kg/day | 3.8 ± 1.8 |
| 11–20 kcal/kg/day | 3.5 ± 1.6 |
| >20 kcal/kg/day | 5.8 ± 1.6 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SBS | Short bowel syndrome |
| IBD | Inflammatory bowel disease |
| PN | Parenteral nutrition |
| PS | Parenteral support |
| ICN | Intensive clinical nutrition |
| BMI | Body mass index |
| CRP | C-reactive protein |
| IM | Intramuscular |
| PPIs | Proton pump inhibitors |
| RBP | Retinol binding protein |
| EN | Enteral nutrition |
References
- Pironi, L. Definition, classification, and causes of short bowel syndrome. Nutr. Clin. Prac. 2023, 38, S9–S16. [Google Scholar] [CrossRef] [PubMed]
- Brandt, C.F.; Hvistendahl, M.; Naimi, R.M.; Tribler, S.; Staun, M.; Brøbech, P.; Jeppesen, P.B. Home Parenteral Nutrition in Adult Patients With Chronic Intestinal Failure: The Evolution Over 4 Decades in a Tertiary Referral Center. J. Parenter. Enter. Nutr. 2017, 41, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Pironi, L.; Goulet, O.; Buchman, A.; Messing, B.; Gabe, S.; Candusso, M.; Bond, G.; Gupte, G.; Pertkiewicz, M.; Steiger, E.; et al. Outcome on home parenteral nutrition for benign intestinal failure: A review of the literature and benchmarking with the European prospective survey of ESPEN. Clin. Nutr. 2012, 31, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Crenn, P.; Coudray–Lucas, C.; Thuillier, F.; Cynober, L.; Messing, B. Postabsorptive plasma citrulline concentration is a marker of absorptive enterocyte mass and intestinal failure in humans. Gastroenterology 2000, 119, 1496–1505. [Google Scholar] [CrossRef]
- Amiot, A.; Messing, B.; Corcos, O.; Panis, Y.; Joly, F. Determinants of home parenteral nutrition dependence and survival of 268 patients with non-malignant short bowel syndrome. Clin. Nutr. 2013, 32, 368–374. [Google Scholar] [CrossRef]
- Reboul, E. Absorption intestinale des vitamines liposolubles. OCL 2011, 18, 53–58. [Google Scholar] [CrossRef]
- Bonnefond-Ortega, M.; Goudable, J.; Chambrier, C.; Bétry, C. L’absorption intestinale des vitamines hydrosolubles et liposolubles en pratique clinique. Nutr. Clin. Métabolisme 2018, 32, 57–66. [Google Scholar] [CrossRef]
- Reboul, E. Vitamin E Bioavailability: Mechanisms of Intestinal Absorption in the Spotlight. Antioxidants 2017, 6, 95. [Google Scholar] [CrossRef]
- Berger, M.M.; Shenkin, A.; Schweinlin, A.; Amrein, K.; Augsburger, M.; Biesalski, H.K.; Bischoff, S.C.; Casaer, M.P.; Gundogan, K.; Lepp, H.-L.; et al. ESPEN micronutrient guideline. Clin. Nutr. 2022, 41, 1357–1424. [Google Scholar] [CrossRef]
- Berger, M.M.; Shenkin, A.; Schweinlin, A.; Amrein, K.; Augsburger, M.; Biesalski, H.K.; Bischoff, S.C.; Casaer, M.P.; Gundogan, K.; Lepp, H.L.; et al. Corrigendum to “ESPEN micronutrient guideline” [Clin Nutr 41 (2022) 1357–1424/YCLNU5151]. Clin. Nutr. 2024, 43, 1024. [Google Scholar] [CrossRef]
- Chatzidaki, V.; Wood, R.; Alegakis, A.; Lawson, M.; Fagbemi, A. Parenteral support and micronutrient deficiencies in children with short bowel syndrome: A comprehensive retrospective study. Clin. Nutr. ESPEN 2023, 58, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Ubesie, A.C.; Kocoshis, S.A.; Mezoff, A.G.; Henderson, C.J.; Helmrath, M.A.; Cole, C.R. Multiple Micronutrient Deficiencies among Patients with Intestinal Failure during and after Transition to Enteral Nutrition. J. Pediatr. 2013, 163, 1692–1696. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.F.; Duro, D.; Zurakowski, D.; Lee, M.; Jaksic, T.; Duggan, C. High Prevalence of Multiple Micronutrient Deficiencies in Children with Intestinal Failure: A Longitudinal Study. J. Pediatr. 2011, 159, 39–44.e1. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Zhang, T.; Yan, W.; Lu, L.; Tao, Y.; Cai, W.; Wang, Y. Micronutrient deficiencies in pediatric short bowel syndrome: A 10-year review from an intestinal rehabilitation center in China. Pediatr. Surg. Int. 2020, 36, 1481–1487. [Google Scholar] [CrossRef]
- Fan, S.; Ni, X.; Wang, J.; Zhang, Y.; Tao, S.; Kong, W.; Li, Y.; Li, J. High Prevalence of Suboptimal Vitamin D Status and Bone Loss in Adult Short Bowel Syndrome Even After Weaning Off Parenteral Nutrition. Nutr. Clin. Prac. 2017, 32, 258–265. [Google Scholar] [CrossRef]
- Mercer-Smith, G.W.; Kirk, C.; Gemmell, L.; Mountford, C.; Nightingale, J.; Thompson, N. British Intestinal Failure Alliance (BIFA) guidance—Haematological and biochemical monitoring of adult patients receiving home parenteral nutrition. Frontline Gastroenterol. 2021, 12, 656–663. [Google Scholar] [CrossRef]
- Pironi, L.; Cuerda, C.; Jeppesen, P.B.; Joly, F.; Jonkers, C.; Krznarić, Ž.; Lal, S.; Lamprecht, G.; Lichota, M.; Mundi, M.S.; et al. ESPEN guideline on chronic intestinal failure in adults—Update 2023. Clin. Nutr. 2023, 42, 1940–2021. [Google Scholar]
- Osland, E.J.; Ali, A.; Nguyen, T.; Davis, M.; Gillanders, L. Australasian society for parenteral and enteral nutrition (AuSPEN) adult vitamin guidelines for parenteral nutrition. Asia Pac. J. Clin. Nutr. 2016, 25, 636–650. [Google Scholar]
- Siepler, J. Principles and Strategies for Monitoring Home Parenteral Nutrition. Nutr. Clin. Prac. 2007, 22, 340–350. [Google Scholar] [CrossRef]
- HAS: Diagnostic de la dénutrition chez l’enfant, l’adulte, et la personne de 70 ans et plus. Available online: https://www.has-sante.fr/upload/docs/application/pdf/2021-11/reco368_fiche_outil_denutrition_pa_cd_20211110_v1.pdf (accessed on 30 March 2025).
- Pironi, L.; Konrad, D.; Brandt, C.; Joly, F.; Wanten, G.; Agostini, F.; Chambrier, C.; Aimasso, U.; Zeraschi, S.; Kelly, D.; et al. Clinical classification of adult patients with chronic intestinal failure due to benign disease: An international multicenter cross-sectional survey. Clin. Nutr. 2018, 37, 728–738. [Google Scholar] [CrossRef]
- Van Der Werf, G.M.; Van Rijssen, N.M.; Jonkers, C.F.; Boermeester, M.A.; Serlie, M.J. Trace-Element Status In Patients With Intestinal Failure Type II And III. Clin. Nutr. ESPEN 2023, 54, 621–622. [Google Scholar] [CrossRef]
- Wu, J.; Tang, Q.; Feng, Y.; Huang, J.; Tao, Y.; Wang, Y.; Cai, W.; Shi, C. Nutrition assessment in children with short bowel syndrome weaned off parenteral nutrition: A long-term follow-up study. J. Pediatr. Surg. 2007, 42, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- Cole, C.R.; Ziegler, T.R. Small bowel bacterial overgrowth: A negative factor in gut adaptation in pediatric SBS. Curr. Gastroenterol. Rep. 2007, 9, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Thurnham, D.I.; Davies, J.A.; Crump, B.J.; Situnayake, R.D.; Davis, M. The Use of Different Lipids to Express Serum Tocopherol: Lipid Ratios for the Measurement of Vitamin E Status. Ann. Clin. Biochem. 1986, 23, 514–520. [Google Scholar] [CrossRef]
- Jeejeebhoy, K.N. Short bowel syndrome: A nutritional and medical approach. CMAJ 2002, 166, 1297–1302. [Google Scholar]
- Kumar, A.; Sharma, E.; Marley, A.; Samaan, M.A.; Brookes, M.J. Iron deficiency anaemia: Pathophysiology, assessment, practical management. BMJ Open Gastroenterol. 2022, 9, e000759. [Google Scholar] [CrossRef]
- Tafaro, L.; Nati, G.; Leoni, E.; Baldini, R.; Cattaruzza, M.S.; Mei, M.; Falaschi, P. Adherence to anti-osteoporotic therapies: Role and determinants of “spot therapy”. Osteoporos. Int. 2013, 24, 2319–2323. [Google Scholar] [CrossRef]
- Haastrup, P.F.; Thompson, W.; Søndergaard, J.; Jarbøl, D.E. Side Effects of Long-Term Proton Pump Inhibitor Use: A Review. Basic Clin. Pharma Tox. 2018, 123, 114–121. [Google Scholar] [CrossRef]
- Joly, F.; Mayeur, C.; Messing, B.; Lavergne-Slove, A.; Cazals-Hatem, D.; Noordine, M.L.; Cherbuy, C.; Duée, P.-H.; Thomas, M. Morphological adaptation with preserved proliferation/transporter content in the colon of patients with short bowel syndrome. Am. J. Physiol.-Gastrointest. Liver Physiol. 2009, 297, G116–G123. [Google Scholar] [CrossRef]
- Lam, J.R.; Schneider, J.L.; Zhao, W.; Corley, D.A. Proton Pump Inhibitor and Histamine 2 Receptor Antagonist Use and Vitamin B12 Deficiency. JAMA 2013, 310, 2435. [Google Scholar] [CrossRef]
- Braga, C.B.M.; Vannucchi, H.; Freire, C.M.M.; Marchini, J.S.; Júnior, A.A.J.; De Carvalho Da Cunha, S.F. Serum Vitamins in Adult Patients With Short Bowel Syndrome Receiving Intermittent Parenteral Nutrition. J. Parenter. Enter. Nutr. 2011, 35, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Amrein, K.; Barazzoni, R.; Bindels, L.; Bretón, I.; Calder, P.C.; Cappa, S.; Cuerda, C.; D’Amelio, P.; de Man, A.; et al. The science of micronutrients in clinical practice—Report on the ESPEN symposium. Clin. Nutr. 2024, 43, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Pironi, L.; Corcos, O.; Forbes, A.; Holst, M.; Joly, F.; Jonkers, C.; Klek, S.; Lal, S.; Blaser, A.R.; Rollins, K.E.; et al. Intestinal failure in adults: Recommendations from the ESPEN expert groups. Clin. Nutr. 2018, 37, 1798–1809. [Google Scholar] [CrossRef] [PubMed]

| Micronutrients | Absorption Sites |
|---|---|
| Vitamin A | Proximal jejunum |
| Vitamin E | Jejunum |
| Vitamin D | Distal jejunum and ileum |
| Vitamin K | Ileum |
| Vitamin B1 | Proximal jejunum |
| Vitamin B3 | Proximal jejunum |
| Vitamin B6 | Proximal jejunum |
| Vitamin B9 | Jejunum |
| Vitamin B12 | Ileum |
| Vitamin C | Distal jejunum and ileum |
| Zinc | Jejunum |
| Copper | Stomach and duodenum |
| Selenium | Duodenum and proximal jejunum |
| Micronutrient | Laboratory Technique | Reference Range |
|---|---|---|
| Vitamin A | LC-UV on heparinized plasma | 1.4–3.2 µmol/L |
| Vitamin B1 | LC-FLD on whole blood | 66–200 nmol/L |
| Vitamin B3 | UPLC on whole blood | 38–56 µmol/L |
| Vitamin B6 | LC-FLD on whole blood | 15–73 µmol/L |
| Vitamin B9 | ECLIA on serum | 7–39.6 µg/L |
| Vitamin B12 | ECLIA on serum | 187–974 ng/L |
| Vitamin C | LC-ECD on heparinized plasma | 25–85 µmol/L |
| Vitamin D | ECLIA on serum | 75–150 nmol/L |
| Vitamin E | LC-UV on heparinized plasma | 20–35 µmol/L |
| Vitamin K | HPLC-MS on serum | 100–1000 ng/L |
| Copper | ICP-OES on serum | 14–19 µmol/L |
| Selenium | ICP-MS on serum | 0.9–1.5 µmol/L |
| Zinc | ICP-OES on serum | 9.4–15.2 µmol/L |
| Characteristics | |
|---|---|
| Age (years) (mean +/− SD) | 62 +/− 18 |
| Gender, male, n (%) | 17 (40.5%) |
| SBS sub-type, n (%) | |
| I | 7 (17%) |
| II | 27 (64%) |
| III | 8 (19%) |
| SBS etiology, n (%) | |
| Mesenteric ischemia | 17 |
| Surgical complications | 11 |
| Inflammatory bowel diseases (IBD) | 6 |
| Oncology | 3 |
| Trauma | 3 |
| Radiation enteritis | 0 |
| Chronic intestinal pseudo-obstruction (CIPO) | 0 |
| Other (small bowel volvulus) | 2 |
| Comorbidities | |
| Hypertension | 15 (36%) |
| Oncological history | 9 (28%) |
| Obesity or history of obesity | 9 (28%) |
| Chronic kidney disease | 13 (31%) |
| Dyslipidemia | 6 (19%) |
| Diabetes | 4 (10%) |
| Heart failure | 5 (12%) |
| Small bowel length in situ (cm) (mean +/− SD) | 109 +/− 42 |
| Percentage of colon in situ (%) (mean +/− SD) | 75 +/− 31 |
| History of enterocutaneous fistula, n (%) | 6 (14%) |
| History of total colectomy, n (%) | 4 (10%) |
| History of bowel continuity restoration, n (%) | 23 (55%) |
| Citrullinemia (n = 16) | |
| Citrullinemia (μmol/L) (mean +/− SD) | 29 +/− 123 |
| Patients with citrullinemia < 20 μmol/L, n (%) | 3 (19%) |
| Number of patients with kidney failure (GFR < 60 mL/min/1.73 m2), n (%) | 13 (81%) |
| Characteristics | |
|---|---|
| BMI with usual weight before SBS (kg/m2) (mean +/− SD) | 27.0 +/− 7.6 |
| BMI with weight at initiation of PS (kg/m2) (mean +/− SD) | 23.4 +/− 6.6 |
| BMI with weight at reassessment (kg/m2) (mean +/− SD) | 24.6 +/− 6.4 |
| Duration on PS before weaning off (months) [Med (IQR)] | 45.5 (40.5) |
| Severity of intestinal failure at weaning based on the daily mean of the total volume infused per week * | |
| <1 L/day | 35 (83%) |
| 1–2 L/day | 2 (5%) |
| 2–3 L/day | 3 (7%) |
| >3 L/day | 2 (5%) |
| Severity of intestinal failure at weaning based on the daily mean of the total kcal infused per week, adjusted on weight ** | |
| <1 kcal/kg/day | 1 (2%) |
| 1–10 kcal/kg/day | 28 (67%) |
| 11–20 kcal/kg/day | 8 (19%) |
| >20 kcal/kg/day | 5 (12%) |
| Serum albumin at reassessment (g/L) (mean +/− SD) | 38.0 +/− 7.7 |
| C-Reactive Protein (CRP) at reassessment (mg/L) (mean +/− SD) | 6.6 +/− 1.1 |
| Ongoing Supplementation | Number of Patients |
|---|---|
| Vitamin D | 35 |
| Vitamin B12 | 34 |
| Vitamin E | 8 |
| Vitamin K | 8 |
| Micronutrient mixture | 6 |
| Vitamin A | 5 |
| Vitamin C | 4 |
| Vitamin B9 | 3 |
| Vitamin B6 | 2 |
| Vitamin B1 | 1 |
| Selenium | 1 |
| Micronutrient | Patients with Depletion | Plasma Concentration of Micronutrient in Patients with Depletions Med (IQR) |
|---|---|---|
| Fat-soluble vitamins | ||
| Vitamin A (µmol/L) | 15/40 | 1.2 (0.4) |
| Vitamin E (µmol/L) | 17/37 | 15.2 (5.1) |
| Vitamin K1 (ng/L) | 11/34 | 83.0 (25.5) |
| Vitamin D (nmol/L) | 23/40 | 48.0 (28. 3) |
| Water-soluble vitamins | ||
| Vitamin B1 (nmol/L) | 1/36 | 47.1 (0) |
| Vitamin B3 (µmol/L) | 9/23 | 34.0 (8.0) |
| Vitamin B6 (nmol/L) | 5/34 | 7 (0.9) |
| Vitamin B9 (µg/L) | 1/36 | 1.5 (0) |
| Vitamin B12 (ng/L) | 11/41 | 131.0 (48.0) |
| Vitamin C (µmol/L) | 10/32 | 14.0 (5.8) |
| Trace elements | ||
| Copper (µmol/L) | 22/35 | 10.7 (3.6) |
| Selenium (µmol/L) | 23/35 | 0.7 (0.2) |
| Zinc (µmol/L) | 17/36 | 8.3 (1.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattio, N.; Juin, C.; Lauverjat, M.; Chambrier, C.; Bergoin, C.; Couronne, T. Micronutrient Status in Patients with Short Bowel Syndrome Weaned off Parenteral Support. Nutrients 2025, 17, 1598. https://doi.org/10.3390/nu17091598
Mattio N, Juin C, Lauverjat M, Chambrier C, Bergoin C, Couronne T. Micronutrient Status in Patients with Short Bowel Syndrome Weaned off Parenteral Support. Nutrients. 2025; 17(9):1598. https://doi.org/10.3390/nu17091598
Chicago/Turabian StyleMattio, Nastasia, Charlotte Juin, Madeleine Lauverjat, Cécile Chambrier, Charlotte Bergoin, and Thomas Couronne. 2025. "Micronutrient Status in Patients with Short Bowel Syndrome Weaned off Parenteral Support" Nutrients 17, no. 9: 1598. https://doi.org/10.3390/nu17091598
APA StyleMattio, N., Juin, C., Lauverjat, M., Chambrier, C., Bergoin, C., & Couronne, T. (2025). Micronutrient Status in Patients with Short Bowel Syndrome Weaned off Parenteral Support. Nutrients, 17(9), 1598. https://doi.org/10.3390/nu17091598





