Enteral Nutrition in Neonatal Cholestasis: An Up-to-Date Overview
Abstract
1. Introduction
- The role of EN on the prevention or reversal of neonatal cholestasis in IFALD cholestatic patients;
- The role of EN on the prevention of neonatal cholestasis (whenever possible) and management of NOT IFALD cholestatic patients;
- The effect of nutritional management and specific nutritional interventions on growth of both IFALD and NOT IFALD cholestatic patients.
2. Materials and Methods
3. The IFALD Cholestatic Patient
3.1. Definition
3.2. Incidence
3.3. Etiology
3.4. The Role of Enteral Nutrition in the Prevention or Reversal of IFALD
3.4.1. Human Milk Versus Infant Milk Formulas
3.4.2. Mode of Delivery: Oral Versus Tube Feeding
3.4.3. Mode of Delivery: Bolus Versus Continuous Feeding
3.4.4. Initiation of Feeding
3.4.5. Feeding Advancement
3.4.6. Role of Specific Nutrients
4. The NOT IFALD Cholestatic Patient
4.1. Definition
4.2. Incidence
4.3. Etiology
4.4. The Role of Enteral Nutrition in the Prevention and Management of NOT IFALD Diseases
4.5. The Role of Enteral Nutrition in the Prevention of PNAC/PNALD
4.6. Enteral Nutrition Management of Chronic Cholestatic Liver Diseases
4.6.1. Energy Requirements
4.6.2. Fluids and Electrolytes
4.6.3. Carbohydrates
4.6.4. Proteins
4.6.5. Lipids
4.6.6. Fat Soluble Vitamins
4.6.7. Water Soluble Vitamins and Trace Elements
4.6.8. Human Milk Versus Infant Milk Formulas
4.6.9. Mode of Delivery
5. The Effect of Nutritional Management on the Growth of the Cholestatic Newborn
5.1. IFALD Cholestatic Patients
5.2. NOT IFALD Cholestatic Patients
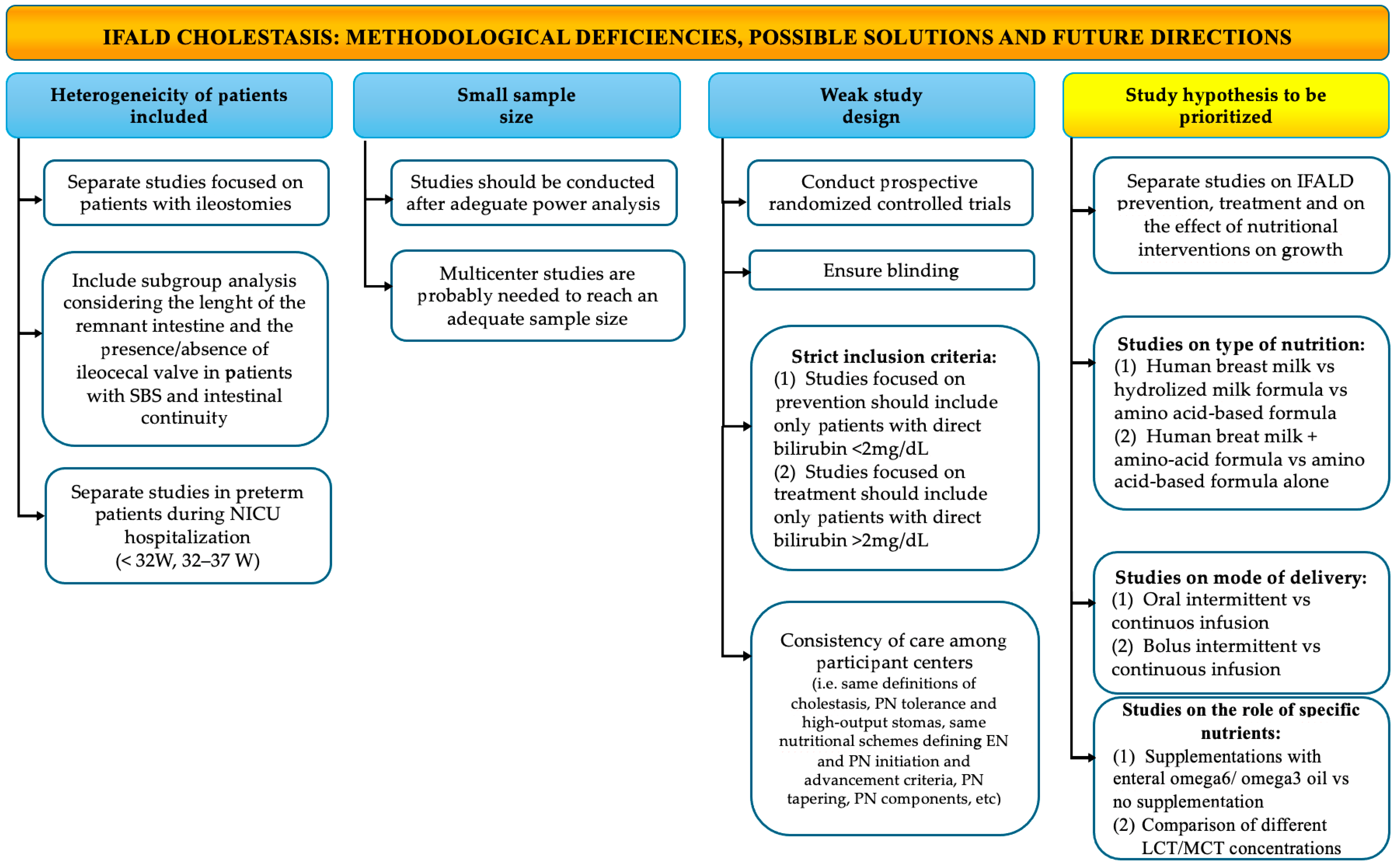
6. Strengths and Limitations of This Study
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NICU | Neonatal intensive care unit |
| AAP | American Academy of Pediatrics |
| IF | Intestinal failure |
| NEC | Necrotizing enterocolitis |
| SBS | Short bowel syndrome |
| IFALD | Intestinal failure associated liver disease |
| PN | Parenteral nutrition |
| EN | Enteral nutrition |
| PNAC | Parenteral nutrition-associated cholestasis |
| PNALD | Parenteral nutrition-associated liver disease |
| ESLD | End-stage liver disease |
| RCT | Randomized clinical trial |
| VEGF | Vascular endothelial growth factor |
| EGF | Epidermal growth factor |
| SIBO | Small intestinal bacterial overgrowth |
| LCPUFA | Long-chain polyunsaturated fatty acids |
| PFIC | Progressive familial intrahepatic cholestasis |
| TORCH | Toxoplasmosis, Others, Rubeola, Cytomegalovirus, Herpes |
| AAAs | Aromatic amino acids |
| BCAAs | Branched-chain amino acids |
| MCT | Medium-chain triglycerides |
| LCT | Long-chain triglycerides |
| EFA | Essential fatty acid |
| RDA | Recommended Dietary Allowances |
| REE | Resting energy expenditure |
References
- Feldman, A.G.; Sokol, R.J. Neonatal Cholestasis: Updates on Diagnostics, Therapeutics, and Prevention. Neoreviews 2021, 22, e819–e836. [Google Scholar] [CrossRef] [PubMed]
- Tufano, M.; Nicastro, E.; Giliberti, P.; Vegnente, A.; Raimondi, F.; Iorio, R. Cholestasis in Neonatal Intensive Care Unit: Incidence, Aetiology and Management. Acta Paediatr. 2009, 98, 1756–1761. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, W.; Yang, Q.; Lin, R.; Tang, L.; Bai, R.; Yang, D.; Zhang, J.; Zhang, Y.; Yu, W.; et al. Analysis of Risk Factors for Parenteral Nutrition-Associated Cholestasis in Preterm Infants: A Multicenter Observational Study. BMC Pediatr. 2023, 23, 250. [Google Scholar] [CrossRef]
- Fawaz, R.; Baumann, U.; Ekong, U.; Fischler, B.; Hadzic, N.; Mack, C.L.; McLin, V.A.; Molleston, J.P.; Neimark, E.; Ng, V.L.; et al. Guideline for the Evaluation of Cholestatic Jaundice in Infants. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 154–168. [Google Scholar] [CrossRef]
- Kemper, A.R.; Newman, T.B.; Slaughter, J.L.; Maisels, M.J.; Watchko, J.F.; Downs, S.M.; Grout, R.W.; Bundy, D.G.; Stark, A.R.; Bogen, D.L.; et al. Clinical Practice Guideline Revision: Management of Hyperbilirubinemia in the Newborn Infant 35 or More Weeks of Gestation. Pediatrics 2022, 150, e2022058859. [Google Scholar] [CrossRef]
- Choi, H.J.; Kim, I.; Lee, H.-J.; Oh, H.J.; Ahn, M.K.; Baek, W.I.; Kim, Y.E.; Oh, S.H.; Lee, B.S.; Namgoong, J.-M.; et al. Clinical Characteristics of Neonatal Cholestasis in a Tertiary Hospital and the Development of a Novel Prediction Model for Mortality. EBioMedicine 2022, 77, 103890. [Google Scholar] [CrossRef]
- Harpavat, S.; Garcia-Prats, J.A.; Shneider, B.L. Newborn Bilirubin Screening for Biliary Atresia. N. Engl. J. Med. 2016, 375, 605–606. [Google Scholar] [CrossRef]
- Quelhas, P.; Jacinto, J.; Cerski, C.; Oliveira, R.; Oliveira, J.; Carvalho, E.; dos Santos, J. Protocols of Investigation of Neonatal Cholestasis—A Critical Appraisal. Healthcare 2022, 10, 2012. [Google Scholar] [CrossRef]
- Costa, S.; Maggio, L.; Sindico, P.; Cota, F.; De Carolis, M.P.; Romagnoli, C. Preterm Small for Gestational Age Infants are not at Higher Risk for Parenteral Nutrition–Associated Cholestasis. J. Pediatr. 2010, 156, 575–579. [Google Scholar] [CrossRef]
- Lauriti, G.; Zani, A.; Aufieri, R.; Cananzi, M.; Chiesa, P.L.; Eaton, S.; Pierro, A. Incidence, Prevention, and Treatment of Parenteral Nutrition–Associated Cholestasis and Intestinal Failure–Associated Liver Disease in Infants and Children. J. Parenter. Enter. Nutr. 2014, 38, 70–85. [Google Scholar] [CrossRef]
- Lee, H.H.; Jung, J.M.; Nam, S.; Lim, G.; Chung, M.L. Risk Factor Analysis of Parenteral Nutrition-associated Cholestasis in Extremely Low Birth Weight Infants. Acta Paediatr. 2016, 105, e313–e319. [Google Scholar] [CrossRef] [PubMed]
- Degrassi, I.; Leonardi, I.; Di Profio, E.; Montanari, C.; Zuccotti, G.; Verduci, E. Fat-Soluble Vitamins Deficiency in Pediatric Cholestasis: A Scoping Review. Nutrients 2023, 15, 2491. [Google Scholar] [CrossRef] [PubMed]
- Norsa, L.; Nicastro, E.; Di Giorgio, A.; Lacaille, F.; D’Antiga, L. Prevention and Treatment of Intestinal Failure-Associated Liver Disease in Children. Nutrients 2018, 10, 664. [Google Scholar] [CrossRef]
- Goulet, O.; Ruemmele, F. Causes and Management of Intestinal Failure in Children. Gastroenterology 2006, 130, S16–S28. [Google Scholar] [CrossRef]
- Lacaille, F.; Gupte, G.; Colomb, V.; D’Antiga, L.; Hartman, C.; Hojsak, I.; Kolacek, S.; Puntis, J.; Shamir, R. Intestinal Failure–Associated Liver Disease: A Position Paper of the ESPGHAN Working Group of Intestinal Failure and Intestinal Transplantation. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 272–283. [Google Scholar] [CrossRef]
- Zafirovska, M.; Zafirovski, A.; Rotovnik Kozjek, N. Current Insights Regarding Intestinal Failure-Associated Liver Disease (IFALD): A Narrative Review. Nutrients 2023, 15, 3169. [Google Scholar] [CrossRef]
- Thanhaeuser, M.; Steyrl, D.; Fuiko, R.; Brandstaetter, S.; Binder, C.; Thajer, A.; Huber-Dangl, M.; Haiden, N.; Berger, A.; Repa, A. Neurodevelopmental Outcome of Extremely Low Birth Weight Infants with Cholestasis at 12 and 24 Months. Neonatology 2022, 119, 501–509. [Google Scholar] [CrossRef]
- Ukarapong, S.; Venkatarayappa, S.K.B.; Navarrete, C.; Berkovitz, G. Risk Factors of Metabolic Bone Disease of Prematurity. Early Hum. Dev. 2017, 112, 29–34. [Google Scholar] [CrossRef]
- Shores, D.R.; Alaish, S.M.; Aucott, S.W.; Bullard, J.E.; Haney, C.; Tymann, H.; Nonyane, B.A.S.; Schwarz, K.B. Postoperative Enteral Nutrition Guidelines Reduce the Risk of Intestinal Failure–Associated Liver Disease in Surgical Infants. J. Pediatr. 2018, 195, 140–147.e1. [Google Scholar] [CrossRef]
- Nader, E.A.; Lambe, C.; Talbotec, C.; Pigneur, B.; Lacaille, F.; Garnier-Lengliné, H.; Petit, L.-M.; Poisson, C.; Rocha, A.; Corriol, O.; et al. Outcome of Home Parenteral Nutrition in 251 Children over a 14-y Period: Report of a Single Center. Am. J. Clin. Nutr. 2016, 103, 1327–1336. [Google Scholar] [CrossRef]
- Ryckman, F.C.; Alonso, M.H.; Nathan, J.D.; Tiao, G. Solid Organ and Intestinal Transplantation. In Ashcraft’s Pediatric Surgery, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 578–604. ISBN 9781416061274. [Google Scholar]
- Wales, P.W.; de Silva, N.; Kim, J.H.; Lecce, L.; Sandhu, A.; Moore, A.M. Neonatal Short Bowel Syndrome. J. Pediatr. Surg. 2005, 40, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Tappenden, K.A. Intestinal Adaptation Following Resection. J. Parenter. Enter. Nutr. 2014, 38, 23S–31S. [Google Scholar] [CrossRef]
- Ksiazyk, J.; Piena, M.; Kierkus, J.; Lyszkowska, M. Hydrolyzed Versus Nonhydrolyzed Protein Diet in Short Bowel Syndrome in Children. J. Pediatr. Gastroenterol. Nutr. 2002, 35, 615–618. [Google Scholar] [CrossRef]
- Kulkarni, S.; Mercado, V.; Rios, M.; Arboleda, R.; Gomara, R.; Muinos, W.; Reeves-Garcia, J.; Hernandez, E. Breast Milk is Better Than Formula Milk in Preventing Parenteral Nutrition–Associated Liver Disease in Infants Receiving Prolonged Parenteral Nutrition. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 383–388. [Google Scholar] [CrossRef]
- Andorsky, D.J.; Lund, D.P.; Lillehei, C.W.; Jaksic, T.; DiCanzio, J.; Richardson, D.S.; Collier, S.B.; Lo, C.; Duggan, C. Nutritional and Other Postoperative Management of Neonates with Short Bowel Syndrome Correlates with Clinical Outcomes. J. Pediatr. 2001, 139, 27–33. [Google Scholar] [CrossRef]
- Ekingen, G.; Ceran, C.; Guvenc, B.H.; Tuzlaci, A.; Kahraman, H. Early Enteral Feeding in Newborn Surgical Patients. Nutrition 2005, 21, 142–146. [Google Scholar] [CrossRef]
- Shakeel, F.; Newkirk, M.; Sellers, A.; Shores, D.R. Postoperative Feeding Guidelines Improve Outcomes in Surgical Infants. J. Parenter. Enter. Nutr. 2020, 44, 1047–1056. [Google Scholar] [CrossRef]
- Wang, J.; Xu, H.; Wang, J.; Xiao, D. Evaluation of Postoperative Feeding Strategies in Children with Intestinal Atresia: A Single-Center Retrospective Study. Front. Pediatr. 2022, 10, 953852. [Google Scholar] [CrossRef]
- Savoie, K.B.; Bachier-Rodriguez, M.; Jones, T.L.; Jeffreys, K.; Papraniku, D.; Sevilla, W.M.A.; Tillman, E.; Huang, E.Y. Standardization of Feeding Advancement After Neonatal Gastrointestinal Surgery. Nutr. Clin. Pract. 2016, 31, 810–818. [Google Scholar] [CrossRef]
- Tillman, E.M.; Norman, J.L.; Huang, E.Y.; Lazar, L.F.; Crill, C.M. Evaluation of Parenteral Nutrition–Associated Liver Disease in Infants with Necrotizing Enterocolitis Before and After the Implementation of Feeding Guidelines. Nutr. Clin. Pract. 2014, 29, 234–237. [Google Scholar] [CrossRef]
- Garg, P.M.; Pittman, I.; Yi, J.; Weis, V.G.; Rodriguez, R.J.; Ladd, M.R.; Rauh, J.L.; McDonald, A.G.; Welch, C.; Premkumar, M.H.; et al. Clinical Correlates of Cholestasis in Preterm Infants with Surgical Necrotizing Enterocolitis. Newborn 2023, 2, 191–197. [Google Scholar] [CrossRef]
- Yang, Q.; Ayers, K.; Welch, C.D.; O’Shea, T.M. Randomized Controlled Trial of Early Enteral Fat Supplement and Fish Oil to Promote Intestinal Adaptation in Premature Infants with an Enterostomy. J. Pediatr. 2014, 165, 274–279.e1. [Google Scholar] [CrossRef] [PubMed]
- Tillman, E.M.; Crill, C.M.; Black, D.D.; Hak, E.B.; Lazar, L.F.; Christensen, M.L.; Huang, E.Y.; Helms, R.A. Enteral Fish Oil for Treatment of Parenteral Nutrition-Associated Liver Disease in Six Infants with Short-Bowel Syndrome. Pharmacotherapy 2011, 31, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Olieman, J.; Kastelijn, W. Nutritional Feeding Strategies in Pediatric Intestinal Failure. Nutrients 2020, 12, 177. [Google Scholar] [CrossRef]
- Ou, J.; Courtney, C.M.; Steinberger, A.E.; Tecos, M.E.; Warner, B.W. Nutrition in Necrotizing Enterocolitis and Following Intestinal Resection. Nutrients 2020, 12, 520. [Google Scholar] [CrossRef]
- Cummins, A.G. Effect of Breast Milk and Weaning on Epithelial Growth of the Small Intestine in Humans. Gut 2002, 51, 748–754. [Google Scholar] [CrossRef]
- Playford, R.J.; Macdonald, C.E.; Johnson, W.S. Colostrum and Milk-Derived Peptide Growth Factors for the Treatment of Gastrointestinal Disorders. Am. J. Clin. Nutr. 2000, 72, 5–14. [Google Scholar] [CrossRef]
- Adibi, S.A.; Morse, E.L.; Masilamani, S.S.; Amin, P.M. Evidence for Two Different Modes of Tripeptide Disappearance in Human Intestine. Uptake by Peptide Carrier Systems and Hydrolysis by Peptide Hydrolases. J. Clin. Investig. 1975, 56, 1355–1363. [Google Scholar] [CrossRef]
- De Greef, E.; Mahler, T.; Janssen, A.; Cuypers, H.; Veereman-Wauters, G. The Influence of Neocate in Paediatric Short Bowel Syndrome on PN Weaning. J. Nutr. Metab. 2010, 2010, 297575. [Google Scholar] [CrossRef][Green Version]
- Bines, J.; Francis, D.; Hill, D. Reducing Parenteral Requirement in Children with Short Bowel Syndrome: Impact of an Amino Acid-Based Complete Infant Formula. J. Pediatr. Gastroenterol. Nutr. 1998, 26, 123–128. [Google Scholar] [CrossRef]
- Schaart, M.W.; de Bruijn, A.C.J.M.; Tibboel, D.; Renes, I.B.; van Goudoever, J.B. Dietary Protein Absorption of the Small Intestine in Human Neonates. J. Parenter. Enter. Nutr. 2007, 31, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Capriati, T.; Nobili, V.; Stronati, L.; Cucchiara, S.; Laureti, F.; Liguori, A.; Tyndall, E.; Diamanti, A. Enteral Nutrition in Pediatric Intestinal Failure: Does Initial Feeding Impact on Intestinal Adaptation? Expert. Rev. Gastroenterol. Hepatol. 2017, 11, 741–748. [Google Scholar] [CrossRef]
- Diamanti, A.; Conforti, A.; Panetta, F.; Torre, G.; Candusso, M.; Bagolan, P.; Papa, R.E.; Grimaldi, C.; Fusaro, F.; Capriati, T.; et al. Long-Term Outcome of Home Parenteral Nutrition in Patients with Ultra-Short Bowel Syndrome. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 438–442. [Google Scholar] [CrossRef]
- Lapillonne, A.; Matar, M.; Adleff, A.; Chbihi, M.; Kermorvant-Duchemin, E.; Campeotto, F. Use of Extensively Hydrolysed Formula for Refeeding Neonates Postnecrotising Enterocolitis: A Nationwide Survey-Based, Cross-Sectional Study. BMJ Open 2016, 6, e008613. [Google Scholar] [CrossRef][Green Version]
- Verlato, G.; Hill, S.; Jonkers-Schuitema, C.; Macdonald, S.; Guimber, D.; Echochard-Dugelay, E.; Pulvirenti, R.; Lambe, C.; Tabbers, M. Results of an International Survey on Feeding Management in Infants with Short Bowel Syndrome-Associated Intestinal Failure. J. Pediatr. Gastroenterol. Nutr. 2021, 73, 647–653. [Google Scholar] [CrossRef]
- Boctor, D.L.; Jutteau, W.H.; Fenton, T.R.; Shourounis, J.; Galante, G.J.; Eicher, I.; Goulet, O.; Lambe, C. The Prevalence of Feeding Difficulties and Potential Risk Factors in Pediatric Intestinal Failure: Time to Consider Promoting Oral Feeds? Clin. Nutr. 2021, 40, 5399–5406. [Google Scholar] [CrossRef]
- Norsa, L.; Goulet, O.; Alberti, D.; DeKooning, B.; Domellöf, M.; Haiden, N.; Hill, S.; Indrio, F.; Köglmeier, J.; Lapillonne, A.; et al. Nutrition and Intestinal Rehabilitation of Children with Short Bowel Syndrome: A Position Paper of the ESPGHAN Committee on Nutrition. Part 1: From Intestinal Resection to Home Discharge. J. Pediatr. Gastroenterol. Nutr. 2023, 77, 281–297. [Google Scholar] [CrossRef]
- Helmrath, M.A.; Shin, C.E.; Fox, J.W.; Erwin, C.R.; Warner, B.W. Adaptation after Small Bowel Resection is Attenuated by Sialoadenectomy: The Role for Endogenous Epidermal Growth Factor. Surgery 1998, 124, 848–854. [Google Scholar] [CrossRef]
- Parvadia, J.K.; Keswani, S.G.; Vaikunth, S.; Maldonado, A.R.; Marwan, A.; Stehr, W.; Erwin, C.; Uzvolgyi, E.; Warner, B.W.; Yamano, S.; et al. Role of VEGF in Small Bowel Adaptation after Resection: The Adaptive Response is Angiogenesis Dependent. Am. J. Physiol.-Gastrointest. Liver Physiol. 2007, 293, G591–G598. [Google Scholar] [CrossRef]
- Channabasappa, N.; Girouard, S.; Nguyen, V.; Piper, H. Enteral Nutrition in Pediatric Short-Bowel Syndrome. Nutr. Clin. Pract. 2020, 35, 848–854. [Google Scholar] [CrossRef]
- Heyland, D.K.; Drover, J.W.; MacDonald, S.; Novak, F.; Lam, M. Effect of Postpyloric Feeding on Gastroesophageal Regurgitation and Pulmonary Microaspiration: Results of a Randomized Controlled Trial. Crit. Care Med. 2001, 29, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Michaud, L.; Coopman, S.; Guimber, D.; Sfeir, R.; Turck, D.; Gottrand, F. Percutaneous Gastrojejunostomy in Children: Efficacy and Safety. Arch. Dis. Child. 2012, 97, 733–734. [Google Scholar] [CrossRef] [PubMed]
- Puoti, M.G.; Köglmeier, J. Nutritional Management of Intestinal Failure Due to Short Bowel Syndrome in Children. Nutrients 2022, 15, 62. [Google Scholar] [CrossRef]
- Cole, C.R.; Kocoshis, S.A. Nutrition Management of Infants with Surgical Short Bowel Syndrome and Intestinal Failure. Nutr. Clin. Pract. 2013, 28, 421–428. [Google Scholar] [CrossRef]
- Joosten, K.F.M.; Kerklaan, D.; Verbruggen, S.C.A.T. Nutritional Support and the Role of the Stress Response in Critically Ill Children. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 226–233. [Google Scholar] [CrossRef]
- Parker, P.; Stroop, S.; Greene, H. A Controlled Comparison of Continuous versus Intermittent Feeding in the Treatment of Infants with Intestinal Disease. J. Pediatr. 1981, 99, 360–364. [Google Scholar] [CrossRef]
- Goulet, O.; Olieman, J.; Ksiazyk, J.; Spolidoro, J.; Tibboe, D.; Köhler, H.; Yagci, R.V.; Falconer, J.; Grimble, G.; Beattie, R.M. Neonatal Short Bowel Syndrome as a Model of Intestinal Failure: Physiological Background for Enteral Feeding. Clin. Nutr. 2013, 32, 162–171. [Google Scholar] [CrossRef]
- Aynsley-Green, A.; Adrian, T.E.; Bloom, S.R. Feeding and the Development of Enteroinsular Hormone Secretion in the Preterm Infant: Effects of Continuous Gastric Infusions of Human Milk Compared with Intermittent Boluses. Acta Paediatr. 1982, 71, 379–383. [Google Scholar] [CrossRef]
- Jawaheer, G.; Shaw, N.J.; Pierro, A. Continuous Enteral Feeding Impairs Gallbladder Emptying in Infants. J. Pediatr. 2001, 138, 822–825. [Google Scholar] [CrossRef]
- Avitzur, Y.; Courtney-Martin, G. Enteral Approaches in Malabsorption. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 295–307. [Google Scholar] [CrossRef]
- Fivez, T.; Kerklaan, D.; Mesotten, D.; Verbruggen, S.; Wouters, P.J.; Vanhorebeek, I.; Debaveye, Y.; Vlasselaers, D.; Desmet, L.; Casaer, M.P.; et al. Early versus Late Parenteral Nutrition in Critically Ill Children. N. Engl. J. Med. 2016, 374, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, K.B.; Duggan, C. Enteral Nutrition in the Management of Pediatric Intestinal Failure. J. Pediatr. 2014, 165, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.R.; Thunberg, B.J.; Golub, L.; Maniscalco, W.M.; Cox, C.; Shapiro, D.L. Decreased Cholestasis with Enteral Instead of Intravenous Protein in the Very Low-Birth-Weight Infant. J. Pediatr. Gastroenterol. Nutr. 1989, 9, 21–27. [Google Scholar]
- Slagle, T.A.; Gross, S.J. Effect of Early Low-Volume Enteral Substrate on Subsequent Feeding Tolerance in Very Low Birth Weight Infants. J. Pediatr. 1988, 113, 526–531. [Google Scholar] [CrossRef]
- Zamir, O.; Nussbaum, M.S.; Bhadra, S.; Subbiah, M.T.; Rafferty, J.F.; Fischer, J.E. Effect of Enteral Feeding on Hepatic Steatosis Induced by Total Parenteral Nutrition. JPEN J. Parenter. Enter. Nutr. 1994, 18, 20–25. [Google Scholar]
- Brindle, M.E.; McDiarmid, C.; Short, K.; Miller, K.; MacRobie, A.; Lam, J.Y.K.; Brockel, M.; Raval, M.V.; Howlett, A.; Lee, K.; et al. Consensus Guidelines for Perioperative Care in Neonatal Intestinal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations. World J. Surg. 2020, 44, 2482–2492. [Google Scholar] [CrossRef]
- Koseesirikul, P.; Chotinaruemol, S.; Ukarapol, N. Incidence and Risk Factors of Parenteral Nutrition-associated Liver Disease in Newborn Infants. Pediatr. Int. 2012, 54, 434–436. [Google Scholar] [CrossRef]
- Lee, S.H.; Jang, J.Y.; Kim, H.W.; Jung, M.J.; Lee, J.G. Effects of Early Enteral Nutrition on Patients After Emergency Gastrointestinal Surgery. Medicine 2014, 93, e323. [Google Scholar] [CrossRef]
- De Rose, D.U.; Lapillonne, A.; Iacobelli, S.; Capolupo, I.; Dotta, A.; Salvatori, G. Nutritional Strategies for Preterm Neonates and Preterm Neonates Undergoing Surgery: New Insights for Practice and Wrong Beliefs to Uproot. Nutrients 2024, 16, 1719. [Google Scholar] [CrossRef]
- Shores, D.R.; Bullard, J.E.; Aucott, S.W.; Stewart, F.D.; Haney, C.; Tymann, H.; Miller, M.R.; Nonyane, B.A.S.; Schwarz, K.B. Implementation of Feeding Guidelines in Infants at Risk of Intestinal Failure. J. Perinatol. 2015, 35, 941–948. [Google Scholar] [CrossRef]
- Embleton, N.D.; Jennifer Moltu, S.; Lapillonne, A.; van den Akker, C.H.P.; Carnielli, V.; Fusch, C.; Gerasimidis, K.; van Goudoever, J.B.; Haiden, N.; Iacobelli, S.; et al. Enteral Nutrition in Preterm Infants (2022): A Position Paper from the ESPGHAN Committee on Nutrition and Invited Experts. J. Pediatr. Gastroenterol. Nutr. 2023, 76, 248–268. [Google Scholar] [CrossRef] [PubMed]
- Cowles, R.A.; Ventura, K.A.; Martinez, M.; Lobritto, S.J.; Harren, P.A.; Brodlie, S.; Carroll, J.; Jan, D.M. Reversal of Intestinal Failure–Associated Liver Disease in Infants and Children on Parenteral Nutrition: Experience with 93 Patients at a Referral Center for Intestinal Rehabilitation. J. Pediatr. Surg. 2010, 45, 84–88. [Google Scholar] [CrossRef]
- Sigalet, D.; Boctor, D.; Brindle, M.; Lam, V.; Robertson, M. Elements of Successful Intestinal Rehabilitation. J. Pediatr. Surg. 2011, 46, 150–156. [Google Scholar] [CrossRef]
- Hair, A.B.; Good, M. Dilemmas in Feeding Infants with Intestinal Failure: A Neonatologist’s Perspective. J. Perinatol. 2023, 43, 114–119. [Google Scholar] [CrossRef]
- Frazer, L.C.; Gura, K.M.; Bines, J.E.; Puder, M.; Martin, C.R. Prevention and Management of Parenteral Nutrition-Associated Cholestasis and Intestinal Failure-Associated Liver Disease in the Critically Ill Infant. In Nutritional Care of Preterm Infants: Scientific Basis and Practical Guidelines; Karger Publishers: Berlin, Germany, 2021; pp. 379–399. [Google Scholar]
- Hyman, P.E.; Everett, S.L.; Harada, T. Gastric Acid Hypersecretion in Short Bowel Syndrome in Infants: Association with Extent of Resection and Enteral Feeding. J. Pediatr. Gastroenterol. Nutr. 1986, 5, 191–197. [Google Scholar]
- Sukhotnik, I.; Shiloni, E.; Krausz, M.M.; Yakirevich, E.; Sabo, E.; Mogilner, J.; Coran, A.G.; Harmon, C.M. Low-Fat Diet Impairs Postresection Intestinal Adaptation in a Rat Model of Short Bowel Syndrome. J. Pediatr. Surg. 2003, 38, 1182–1187. [Google Scholar] [CrossRef]
- Kollman, K.A.; Lien, E.L.; Vanderhoof, J.A. Dietary Lipids Influence Intestinal Adaptation After Massive Bowel Resection. J. Pediatr. Gastroenterol. Nutr. 1999, 28, 41–45. [Google Scholar] [CrossRef]
- Vanderhoof, J.A.; Park, J.H.Y.; Herrington, M.K.; Adrian, T.E. Effects of Dietary Menhaden Oil on Mucosal Adaptation after Small Bowel Resection in Rats. Gastroenterology 1994, 106, 94–99. [Google Scholar] [CrossRef]
- Yang, Q.; Lan, T.; Chen, Y.; Dawson, P.A. Dietary Fish Oil Increases Fat Absorption and Fecal Bile Acid Content without Altering Bile Acid Synthesis in 20-d-Old Weanling Rats Following Massive Ileocecal Resection. Pediatr. Res. 2012, 72, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Van Citters, G.W.; Lin, H.C. Ileal Brake: Neuropeptidergic Control of Intestinal Transit. Curr. Gastroenterol. Rep. 2006, 8, 367–373. [Google Scholar] [CrossRef]
- Gura, K.M.; Lee, S.; Valim, C.; Zhou, J.; Kim, S.; Modi, B.P.; Arsenault, D.A.; Strijbosch, R.A.M.; Lopes, S.; Duggan, C.; et al. Safety and Efficacy of a Fish-Oil–Based Fat Emulsion in the Treatment of Parenteral Nutrition–Associated Liver Disease. Pediatrics 2008, 121, e678–e686. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Welch, C.D.; Ayers, K.; Turner, C.; Pranikoff, T. Early Enteral Fat Supplementation with Microlipid® and Fish Oil in the Treatment of Two Premature Infants with Short Bowel. Neonatology 2010, 98, 348–353. [Google Scholar] [CrossRef]
- Pupillo, D.; Correani, A.; Biagetti, C.; D’Ascenzo, R.; Simonato, M.; Verlato, G.; Cogo, P.; Rocchi, M.B.L.; Carnielli, V.P. Half-Life of Plasma Phytosterols in Very Low Birth Weight Preterm Infants on Routine Parenteral Nutrition with Vegetable Oil-Based Lipid Emulsions. Clin. Nutr. 2018, 37, 262–269. [Google Scholar] [CrossRef]
- Rollins, M.D.; Scaife, E.R.; Jackson, W.D.; Meyers, R.L.; Mulroy, C.W.; Book, L.S. Elimination of Soybean Lipid Emulsion in Parenteral Nutrition and Supplementation with Enteral Fish Oil Improve Cholestasis in Infants with Short Bowel Syndrome. Nutr. Clin. Pract. 2010, 25, 199–204. [Google Scholar] [CrossRef]
- Miller, M.; Burjonrappa, S. A Review of Enteral Strategies in Infant Short Bowel Syndrome: Evidence-Based or NICU Culture? J. Pediatr. Surg. 2013, 48, 1099–1112. [Google Scholar] [CrossRef]
- Jeppesen, P.B.; Mortensen, P.B. The Influence of a Preserved Colon on the Absorption of Medium Chain Fat in Patients with Small Bowel Resection. Gut 1998, 43, 478–483. [Google Scholar] [CrossRef]
- Balistreri, W.F. Immaturity of Hepatic Excretory Function and the Ontogeny of Bile Acid Metabolism. J. Pediatr. Gastroenterol. Nutr. 1983, 2, 207–214. [Google Scholar] [CrossRef]
- Hsieh, M.-H.; Pai, W.; Tseng, H.-I.; Yang, S.-N.; Lu, C.-C.; Chen, H.-L. Parenteral Nutrition-Associated Cholestasis in Premature Babies: Risk Factors and Predictors. Pediatr. Neonatol. 2009, 50, 202–207. [Google Scholar] [CrossRef]
- Christensen, R.D.; Henry, E.; Wiedmeier, S.E.; Burnett, J.; Lambert, D.K. Identifying Patients, on the First Day of Life, at High-Risk of Developing Parenteral Nutrition-Associated Liver Disease. J. Perinatol. 2007, 27, 284–290. [Google Scholar] [CrossRef]
- Balistreri, W.F.; Bezerra, J.A.; Jansen, P.; Karpen, S.J.; Shneider, B.L.; Suchy, F.J. Intrahepatic Cholestasis: Summary of an American Association for the Study of Liver Diseases Single-Topic Conference. Hepatology 2005, 42, 222–235. [Google Scholar] [CrossRef]
- Feldman, A.G.; Sokol, R.J. Recent Developments in Diagnostics and Treatment of Neonatal Cholestasis. Semin. Pediatr. Surg. 2020, 29, 150945. [Google Scholar] [CrossRef] [PubMed]
- Dani, C.; Pratesi, S.; Raimondi, F.; Romagnoli, C. Italian Guidelines for the Management and Treatment of Neonatal Cholestasis. Ital. J. Pediatr. 2015, 41, 69. [Google Scholar] [CrossRef]
- Young, S.; Kwarta, E.; Azzam, R.; Sentongo, T. Nutrition Assessment and Support in Children with End-Stage Liver Disease. Nutr. Clin. Pract. 2013, 28, 317–329. [Google Scholar] [CrossRef]
- Leaf, A.; Dorling, J.; Kempley, S.; McCormick, K.; Mannix, P.; Linsell, L.; Juszczak, E.; Brocklehurst, P. Early or Delayed Enteral Feeding for Preterm Growth-Restricted Infants: A Randomized Trial. Pediatrics 2012, 129, e1260–e1268. [Google Scholar] [CrossRef]
- Siggers, R.H.; Siggers, J.; Thymann, T.; Boye, M.; Sangild, P.T. Nutritional Modulation of the Gut Microbiota and Immune System in Preterm Neonates Susceptible to Necrotizing Enterocolitis. J. Nutr. Biochem. 2011, 22, 511–521. [Google Scholar] [CrossRef]
- Thavamani, A.; Mhanna, M.J.; Groh-Wargo, S.; Gulati, R.; Shekhawat, P.S. Enteral Fish Oil Supplementation in the Resolution of Parenteral Nutrition Associated Cholestasis. J. Neonatal Perinat. Med. 2019, 12, 13–20. [Google Scholar] [CrossRef]
- Mouzaki, M.; Bronsky, J.; Gupte, G.; Hojsak, I.; Jahnel, J.; Pai, N.; Quiros-Tejeira, R.E.; Wieman, R.; Sundaram, S. Nutrition Support of Children with Chronic Liver Diseases. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 498–511. [Google Scholar] [CrossRef]
- Pierro, A.; Koletzko, B.; Carnielli, V.; Superina, R.A.; Roberts, E.A.; Filler, R.M.; Smith, J.; Heim, T. Resting Energy Expenditure is Increased in Infants and Children with Extrahepatic Biliary Atresia. J. Pediatr. Surg. 1989, 24, 534–538. [Google Scholar] [CrossRef]
- Tessitore, M.; Sorrentino, E.; Schiano Di Cola, G.; Colucci, A.; Vajro, P.; Mandato, C. Malnutrition in Pediatric Chronic Cholestatic Disease: An Up-to-Date Overview. Nutrients 2021, 13, 2785. [Google Scholar] [CrossRef]
- Watanabe, M.; Houten, S.M.; Mataki, C.; Christoffolete, M.A.; Kim, B.W.; Sato, H.; Messaddeq, N.; Harney, J.W.; Ezaki, O.; Kodama, T.; et al. Bile Acids Induce Energy Expenditure by Promoting Intracellular Thyroid Hormone Activation. Nature 2006, 439, 484–489. [Google Scholar] [CrossRef]
- Carpenter, A.; Ng, V.L.; Chapman, K.; Ling, S.C.; Mouzaki, M. Predictive Equations are Inaccurate in the Estimation of the Resting Energy Expenditure of Children with End-Stage Liver Disease. J. Parenter. Enter. Nutr. 2017, 41, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Weisdorf, S.A.; Freese, D.K.; Fath, J.J.; Tsai, M.Y.; Cerra, F.B. Amino Acid Abnormalities in Infants with Extrahepatic Biliary Atresia and Cirrhosis. J. Pediatr. Gastroenterol. Nutr. 1987, 6, 860–864. [Google Scholar] [CrossRef]
- Chin, S.; Shepherd, R.; Thomas, B.; Cleghorn, G.; Patrick, M.; Wilcox, J.; Ong, T.; Lynch, S.; Strong, R. Nutritional Support in Children with End-Stage Liver Disease: A Randomized Crossover Trial of a Branched-Chain Amino Acid Supplement. Am. J. Clin. Nutr. 1992, 56, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Sokal, E.M.; Baudoux, M.C.; Collette, E.; Hausleithner, V.; Lambotte, L.; Buts, J.P. Branched Chain Amino Acids Improve Body Composition and Nitrogen Balance in a Rat Model of Extra Hepatic Biliary Atresia. Pediatr. Res. 1996, 40, 66–71. [Google Scholar] [CrossRef]
- Kalivianakis, M.; Minich, D.M.; Havinga, R.; Kuipers, F.; Stellaard, F.; Vonk, R.J.; Verkade, H.J. Detection of Impaired Intestinal Absorption of Long-Chain Fatty Acids: Validation Studies of a Novel Test in a Rat Model of Fat Malabsorption. Am. J. Clin. Nutr. 2000, 72, 174–180. [Google Scholar] [CrossRef][Green Version]
- Mancell, S.; Manwani, K.; Dhawan, A.; Whelan, K. Medium-Chain Triglycerides and the Impact on Fat Absorption, Growth, Nutritional Status and Clinical Outcomes in Children with Cholestatic Liver Disease: A Scoping Review. Clin. Nutr. 2023, 42, 2159–2172. [Google Scholar] [CrossRef]
- Lapillonne, A.; Moltu, S.J. Long-Chain Polyunsaturated Fatty Acids and Clinical Outcomes of Preterm Infants. Ann. Nutr. Metab. 2016, 69, 35–44. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, J.; Hsu, H.; Ni, Y.; Chang, M.; Liu, Y.; Lai, H.; Hsu, W.; Weng, H.; Chen, H. Oral Absorbable Fat-soluble Vitamin Formulation in Pediatric Patients With Cholestasis. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 587–591. [Google Scholar] [CrossRef]
- Thébaut, A.; Nemeth, A.; Le Mouhaër, J.; Scheenstra, R.; Baumann, U.; Koot, B.; Gottrand, F.; Houwen, R.; Monard, L.; de Micheaux, S.L.; et al. Oral Tocofersolan Corrects or Prevents Vitamin E Deficiency in Children With Chronic Cholestasis. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 610–615. [Google Scholar] [CrossRef]
- Shneider, B.L.; Magee, J.C.; Bezerra, J.A.; Haber, B.; Karpen, S.J.; Raghunathan, T.; Rosenthal, P.; Schwarz, K.; Suchy, F.J.; Kerkar, N.; et al. Efficacy of Fat-Soluble Vitamin Supplementation in Infants with Biliary Atresia. Pediatrics 2012, 130, e607–e614. [Google Scholar] [CrossRef]
- Ramaccioni, V.; Soriano, H.E.; Arumugam, R.; Klish, W.J. Nutritional Aspects of Chronic Liver Disease and Liver Transplantation in Children. J. Pediatr. Gastroenterol. Nutr. 2000, 30, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Macías-Rosales, R.; Larrosa-Haro, A.; Ortíz-Gabriel, G.; Trujillo-Hernández, B. Effectiveness of Enteral Versus Oral Nutrition with a Medium-Chain Triglyceride Formula to Prevent Malnutrition and Growth Impairment in Infants with Biliary Atresia. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Binder, C.; Longford, N.; Gale, C.; Modi, N.; Uthaya, S. Body Composition Following Necrotising Enterocolitis in Preterm Infants. Neonatology 2018, 113, 242–248. [Google Scholar] [CrossRef]
- Chong, C.; van Druten, J.; Briars, G.; Eaton, S.; Clarke, P.; Tsang, T.; Yardley, I. Neonates Living with Enterostomy Following Necrotising Enterocolitis are at High Risk of Becoming Severely Underweight. Eur. J. Pediatr. 2019, 178, 1875–1881. [Google Scholar] [CrossRef] [PubMed]
- Cole, C.R.; Hansen, N.I.; Higgins, R.D.; Ziegler, T.R.; Stoll, B.J. Very Low Birth Weight Preterm Infants with Surgical Short Bowel Syndrome: Incidence, Morbidity and Mortality, and Growth Outcomes at 18 to 22 Months. Pediatrics 2008, 122, e573–e582. [Google Scholar] [CrossRef] [PubMed]
- Hintz, S.R.; Kendrick, D.E.; Stoll, B.J.; Vohr, B.R.; Fanaroff, A.A.; Donovan, E.F.; Poole, W.K.; Blakely, M.L.; Wright, L.; Higgins, R. Neurodevelopmental and Growth Outcomes of Extremely Low Birth Weight Infants After Necrotizing Enterocolitis. Pediatrics 2005, 115, 696–703. [Google Scholar] [CrossRef]
- Olieman, J.F.; Tibboel, D.; Penning, C. Growth and Nutritional Aspects of Infantile Short Bowel Syndrome for the Past 2 Decades. J. Pediatr. Surg. 2008, 43, 2061–2069. [Google Scholar] [CrossRef]
- Pichler, J.; Chomtho, S.; Fewtrell, M.; Macdonald, S.; Hill, S. Body Composition in Paediatric Intestinal Failure Patients Receiving Long-Term Parenteral Nutrition. Arch. Dis. Child. 2014, 99, 147–153. [Google Scholar] [CrossRef]
- Pichler, J.; Chomtho, S.; Fewtrell, M.; Macdonald, S.; Hill, S.M. Growth and Bone Health in Pediatric Intestinal Failure Patients Receiving Long-Term Parenteral Nutrition. Am. J. Clin. Nutr. 2013, 97, 1260–1269. [Google Scholar] [CrossRef]
- McLaughlin, C.M.; Channabasappa, N.; Pace, J.; Nguyen, H.; Piper, H.G. Growth Trajectory in Children with Short Bowel Syndrome During the First 2 Years of Life. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 484–488. [Google Scholar] [CrossRef]
- Niccum, M.; Khan, M.N.; Middleton, J.P.; Vergales, B.D.; Syed, S. Cholestasis Affects Enteral Tolerance and Prospective Weight Gain in the NICU. Clin. Nutr. ESPEN 2019, 30, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, W.F.; Lenfestey, R.W.; Rice, H.E.; Rach, E.; Goldberg, R.N.; Cotten, C.M. Dietary Fat for Infants with Enterostomies. J. Pediatr. Surg. 2007, 42, 1811–1815. [Google Scholar] [CrossRef] [PubMed]
- Choi, P.M.; Sun, R.C.; Guo, J.; Erwin, C.R.; Warner, B.W. High-Fat Diet Enhances Villus Growth During the Adaptation Response to Massive Proximal Small Bowel Resection. J. Gastrointest. Surg. 2014, 18, 286–294. [Google Scholar] [CrossRef]
- Barr, P.A.; Mally, P.V.; Caprio, M.C. Standardized Nutrition Protocol for Very Low-Birth-Weight Infants Resulted in Less Use of Parenteral Nutrition and Associated Complications, Better Growth, and Lower Rates of Necrotizing Enterocolitis. J. Parenter. Enter. Nutr. 2019, 43, 540–549. [Google Scholar] [CrossRef]
- Loomis, T.; Byham-Gray, L.; Ziegler, J.; Parrott, J.S. Impact of Standardized Feeding Guidelines on Enteral Nutrition Administration, Growth Outcomes, Metabolic Bone Disease, and Cholestasis in the NICU. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 93–98. [Google Scholar] [CrossRef]
- Giretti, I.; D’Ascenzo, R.; Correani, A.; Antognoli, L.; Monachesi, C.; Biagetti, C.; Pompilio, A.; Marinelli, L.; Burattini, I.; Cogo, P.; et al. Hypertriglyceridemia and Lipid Tolerance in Preterm Infants with a Birth Weight of Less than 1250 g on Routine Parenteral Nutrition. Clin. Nutr. 2021, 40, 4444–4448. [Google Scholar] [CrossRef]


| Category | Main Diseases | |
|---|---|---|
| IFALD | Intestinal failure | Necrotizing enterocolitis, intestinal obstruction, congenital intestinal malformations, volvulus, short bowel syndrome, intestinal neuromuscular motility disorders, congenital enteropathies |
| NOT IFALD | Obstructive anomalies of biliary system | Biliary atresia, choledochal cysts, cholelithiasis, thick bile syndrome, spontaneous perforation of common bile duct |
| Infections | Viral: CMV, rubella, HSV 1,2,6, parvovirus B19, hepatitis A, B, C, chickenpox, adenovirus, enterovirus, coxsackievirus Bacterial: Syphilis, Listeria, congenital TBC, sepsis, urinary tract infections Parasitic: Toxoplasma | |
| Toxic | Parenteral nutrition-associated cholestasis (PNALD), Drugs (Ceftriaxone, Erythromycin, Rifampicin) | |
| Endocrine | Hypothyroidism, panhypopituitarism, adrenal insufficiency | |
| Immune | Neonatal hemochromatosis (gestational alloimmune liver disease), hemophagocytic lymphohistiocytosis, congenital systemic lupus erythematosus | |
| Cardiovascular (hypoxia, ischemia, hepatic congestion) | Perinatal asphyxia, in utero growth restriction, cardiovascular diseases | |
| Genetic and metabolic disorders | Cystic fibrosis, alpha 1 antitrypsin deficiency, Alagille syndrome, galactosemia, tyrosinemia type I, hereditary fructose intolerance, bile acid synthesis defects, congenital hepatic fibrosis, citrin deficiency, bile acid conjugation defects, fatty acid oxidation defects, glycogen storage disease type IV, mitochondrial respiratory chain disorders, Niemann–Pick type C disease, peroxisomal disorders, progressive familial intrahepatic cholestasis, bile transport defects, cytoskeleton defects, Smith–Lemli–Opitz syndrome, Down syndrome | |
| Other | Idiopathic neonatal hepatitis (transient neonatal cholestasis), malignancy |
| Reference | Study Design | Study Period | Sample | Main Inquiry | Effects on Cholestasis | |
|---|---|---|---|---|---|---|
| Type of nutrition | Ksiazyk (2002) [24] | Randomized, cross-over, double blind | NS | 10 patients with SBS (aged 6 weeks–8 months) 3 out of 10 with cholestasis | Hydrolyzed protein vs. standard formula on growth and development of children with SBS. | Not mentioned. Notes: no absorptive advantage, difference in energy expenditure, or weight gain in administering hydrolyzed vs. non-hydrolyzed proteins. |
| Kulkarni (2013) [25] | Retrospective cohort | 2010–2011 | 67 newborns receiving PN for >4 weeks | Human breast milk vs. milk formula in preventing PNALD | Lower maximum DB plasma concentration and lower prevalence of PNALD in human breast milk-fed infants compared with formula milk group (35 vs. 73%; p = 0.008). | |
| Andorsky (2001) [26] | Retrospective cohort | 1986–1998 | 30 neonates with SBS and dependence on PN > 90 days after surgery | Risk factors for duration of PN and peak serum DB concentration | EN with human breast milk or an amino acid-based formula was associated with a shorter duration of PN; EN with protein hydrolysate formula was associated with a lower peak DB concentration. Not confirmed in multivariate analysis. | |
| Mode of delivery | Not found | |||||
| EN initiation and advancement | Ekingen (2005) [27] | RCT | 2000–2003 | 56 newborns who underwent upper abdominal surgery | Compare early enteral feeding (12 h post-surgery) vs. feeding initiation after ileus resolution | Not significant. Notes: Early EN (3 to 5 mL of human breast milk every hour through NGT) initiated at a mean of 12 h post-surgery promoted earlier stool passage, shorter nasogastric feeding duration, and faster full oral feeding. |
| Shakeel (2020) [28] | Multicenter, Prospective with historical controls | 2007–2018 | 409 infants < 6 months of age at risk of IF after surgery requiring >7 days of PN | Incidence of IFALD and time to reach 50% of target EN calories before and after implementation of feeding strategies | Higher initial volumes of minimal EN up to 20 mL/kg/day, and faster daily feeding advancements by 20 mL/kg/day reduced the incidence of moderate IFALD from 32% to 20%. | |
| Shores (2018) [19] | Prospective with historical controls | 2007–2016 | 164 infants < 6 months of age at risk of IF after surgery requiring >7 days of PN | Incidence of IFALD and time to reach 50% of target EN calories before and after implementation of EN strategies | Incidence of IFALD decreased from 71 to 51% (p = 0.031), and median peak DB decreased from 5.7 to 2.4 mg/dL (p = 0.001). | |
| Wang (2022) [29] | Retrospective cohort | 2019–2021 | 32 neonates requiring PN after surgery for intestinal atresia | High-dose vs. low-dose feeding strategy | Higher initial volumes (>15 mL/kg/day) and faster daily advancements (>10 mL/kg/day) were associated with significantly lower incidence of IFALD. | |
| Savoie (2016) [30] | Retrospective cohort | 2007–2011 | 163 infants who underwent intestinal surgery | Time to reach full EN in neonates fed without standardized feeding regimen vs. standardized strategy based on body weight and percentage of remaining small bowel | Cholestasis was less severe in the post-implementation group of infants and human breast milk use increased. | |
| Tillman (2014) [31] | Retrospective cohort | 2007–2011 | 64 newborns with surgical NEC | Incidence of IFALD before and after implementation of feeding strategies | Incidence of IFALD improved from 73% before to 42% after guideline implementation (p = 0.01) and degree of hyperbilirubinemia was less severe. | |
| Garg (2023) [32] | Retrospective cohort | 2013–2018 | 91 preterm infants with surgical NEC, 62/91 developed cholestasis | Clinical factors and outcomes of cholestasis in preterm infants with surgical NEC | Time from surgery to EN initiation and the duration of postoperative ileus were independently associated with mild to moderate cholestasis at two months of age. | |
| Role of specific nutrients | Yang (2014) [33] | RCT | NS | 37 preterm infants < 2 months of age with jejunostomy or ileostomy (7/37 with SBS) | Effects of early enteral fish oil supplementation on duration of PN before bowel re-anastomosis | Neonates receiving EN supplemented with safflower (omega-6) and fish oil (omega-3) had lower DB levels, required less intravenous lipids, and achieved higher enteral intake compared to those on standard EN. |
| Tillman (2011) [34] | Retrospective case series | NS | 6 PN-dependent infants with SBS and IFALD | Enteral fish oil for treatment of IFALD | IFALD reversed in 4 of 6 infants within 5 ± 2.6 weeks (range 2–8 weeks) after starting enteral fish oil supplementation. |
| Energy Nutrient | Requirement | Comments | Reference | |
|---|---|---|---|---|
| Energy | 125–140% of the recommended caloric requirement based on ideal body weight; smallest infants may require 150–160 kcal/kg/day | The Schofield equations for REE are often inaccurate; complications like sepsis, cholangitis, or variceal bleeding can further raise demands. | [100,101,102,103] | |
| Fluids and Electrolytes | Normal fluid intake Sodium: 1–2 mmol/kg/day Potassium: ~2 mmol/kg/day | In case of ascites, fluid restriction may be required. Calcium and phosphorus needs may be higher due to fat malabsorption (minimum intakes in preterms are Ca: 3.0–5.0 mmol/kg/day; P: 2.2–3.7 mmol/kg/day). | [99] | |
| Carbohydrates | 40–60% of total calories | Maltodextrins preferred due to low osmotic load. Hypoglycemia and hyperglycemia may both occur. | [99] | |
| Proteins | 2–3 g/kg/day (higher needs in preterm infants); restriction to 0.5–1.0 g/kg/day only in case of encephalopathy | Higher needs due to increased oxidation and protein loss (up to 130–150% of requirements for age); BCAA-enriched formulas may be beneficial but evidence is limited. | [100,101,104,105,106] | |
| Lipids | 30–50% of total caloric intake | Start: MCTs/LCTs = 30/70% of total fat calories; MCTs from 30% up to 70% are recommended due to better absorption, in case of poor growth; MCTs intake >80% should be avoided; LCTs required to prevent EFA deficiency (minimum 3% of total fat calories, up to 10% in cholestasis). | [95,99,101,107,108,109] | |
| Fat-Soluble Vitamins | Vitamin A | Oral <10 kg–5000 IU/day >10 kg–10,000 IU/day IM: 5000–10,000 UI/die OR up to 50,000 UI/month OS 2000–5000 IU/day | Start supplementation early, monitor regularly. Separate supplementation of the different vitamins is the best strategy to individualize therapies. Supplementation with all fat-soluble vitamins together may improve their absorption. Higher supplementation of vitamins may be required in cholestatic preterm infants (<32 weeks). | [12,72,110,111,112] |
| Vitamin D (Cholecalciferol) | OS 15–25 IU/kg/day | |||
| Vitamin E (TPGS) | OS 2–5 mg/day | |||
| Vitamin K | IM <5 kg: 1 mg/kg every 2 weeks; >5 kg: 10 mg every 2 weeks | |||
| Water-Soluble Vitamins and Trace Elements | Twice the RDA is the recommended dose (regardless of the patient’s vitamin status that is difficult to evaluate) | Multivitamin formulations can be used; zinc, selenium, and iron should be supplemented according to plasma levels. | [101] | |
| Human milk and infant milk formulas | MCT-enriched formulas if breastfeeding is not possible; consider increasing caloric density of formula to 0.8–1 kcal/mL using supplements or choosing concentrated formulas containing MCTs and maltodextrins | BCAA-enriched formulas available, but evidence for benefits is limited. | [101,113] | |
| Reference | Study Design | Study Period | Sample | Main Inquiry | Effects on Growth | |
|---|---|---|---|---|---|---|
| IFALD cholestasis | Ksiazyk (2002) [24] | Randomized, cross-over, double blind | NS | 10 patients with SBS (aged 6 weeks–8 months) 3 out of 10 with cholestasis | Hydrolyzed protein vs. standard formula on growth and development of children with SBS | No absorptive advantage, difference in energy expenditure or weight gain in administering hydrolyzed vs. non-hydrolyzed proteins |
| Niccum (2019) [123] | Retrospective cohort | 2014–2017 | 163 newborns receiving PN for >5 days | Association between cholestasis (DB > 2 mg/dL) and weight percentiles at hospital discharge and 6 months of age | Weight percentiles in cholestatic infants were lower both at hospital discharge (14 ± 19 vs. 24 ± 22, p-value < 0.005) and at 6 months of age (24 ± 28 vs. 36 ± 31, p-value = 0.05). Peak conjugated bilirubin was not associated in multivariate analysis with 6-month weight percentile | |
| Yang (2014) [33] | RCT | NS | 37 preterm infants < 2 months of age with jejunostomy or ileostomy (7/37 with SBS) | Effects of early enteral fish oil supplementation on weight gain before bowel re-anastomosis (secondary outcome) | Neonates receiving EN supplemented with safflower (omega-6) and fish oil (omega-3) had greater weight and length gain only after re-anastomosis (weight: 20 ± 9 vs. 27 ± 11, p < 0.05 and length: 0.9 ± 1.3 vs. 2.1 ± 1.5, p < 0.05 in controls and treatment group, respectively) | |
| NOT IFALD cholestasis | Thavamani (2018) [98] | Retrospective case–control study | 2011–2017 | 48 preterm infants born less than 32 weeks who developed PNALD | Effect of enteral omega-3 fatty acids supplementation (1 g/Kg/d) on postnatal growth | Infants who received enteral omega-3 fatty acids supplementation had higher average daily weight gain than their controls (22 ± 3 vs. 19 ± 4 g/day, p = 0.011) |
| Macías-Rosales (2016) [114] | RCT | 2009–2011 | 15 infants with a diagnosis of biliary atresia waiting for liver transplantation | Effect of oral vs. 18 h enteral administration via NGT of an MCT-fortified formula for 12 weeks on growth | Length/age and head circumference dropped in the per os group while it remained stable in the enteral nutrition group | |
| Chin (1992) [105] | Randomized cross-over study | 1989–1990 | 19 infants and children with ESLD waiting for liver transplantation | Effect of two matched isocaloric and isonitrogenous nutritional formulations differing only in their BCAAs content fed by nasogastric infusion over a period of 8 weeks on growth | During BCAAs supplementation, improved weight (difference: 0.41 ± 0.16, p < 0.05) and height (difference: 0.50 ± 0.21, p < 0.05), and significant increase in mid-upper arm circumference (p < 0.05) and subscapular skinfold thickness (p < 0.02) were detected |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cimadamore, E.; Palazzo, M.; Fioroni, M.C.; Cerverizzo, M.; Correani, A.; Burattini, I.; Biagetti, C. Enteral Nutrition in Neonatal Cholestasis: An Up-to-Date Overview. Nutrients 2025, 17, 1794. https://doi.org/10.3390/nu17111794
Cimadamore E, Palazzo M, Fioroni MC, Cerverizzo M, Correani A, Burattini I, Biagetti C. Enteral Nutrition in Neonatal Cholestasis: An Up-to-Date Overview. Nutrients. 2025; 17(11):1794. https://doi.org/10.3390/nu17111794
Chicago/Turabian StyleCimadamore, Elisa, Martina Palazzo, Maria Chiara Fioroni, Martina Cerverizzo, Alessio Correani, Ilaria Burattini, and Chiara Biagetti. 2025. "Enteral Nutrition in Neonatal Cholestasis: An Up-to-Date Overview" Nutrients 17, no. 11: 1794. https://doi.org/10.3390/nu17111794
APA StyleCimadamore, E., Palazzo, M., Fioroni, M. C., Cerverizzo, M., Correani, A., Burattini, I., & Biagetti, C. (2025). Enteral Nutrition in Neonatal Cholestasis: An Up-to-Date Overview. Nutrients, 17(11), 1794. https://doi.org/10.3390/nu17111794





