Investigating the Impact of Maternal Obesity on Disease Severity in a Mouse Model of Preeclampsia
Abstract
1. Introduction
2. Methods
2.1. Animal Studies
2.2. Blood Pressure Measurements
2.3. Tissue Collection
2.4. Dam Kidney and Liver Histology
2.5. Vascular Reactivity Studies
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Quantitative Polymerase Chain Reaction (qPCR)
2.8. Statistical Analysis
3. Results
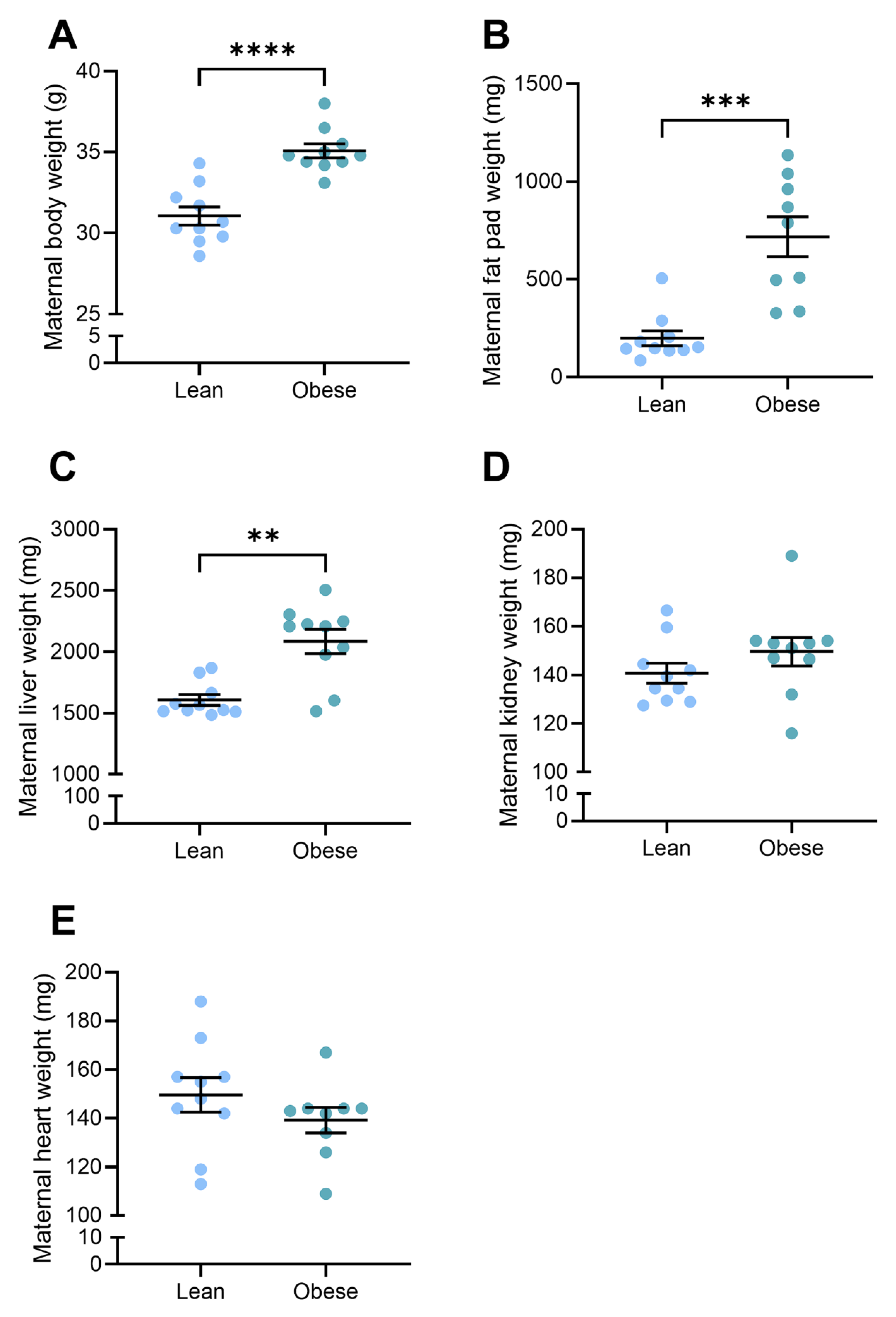
3.1. Body and Organ Weights Were Elevated in Obese Dams
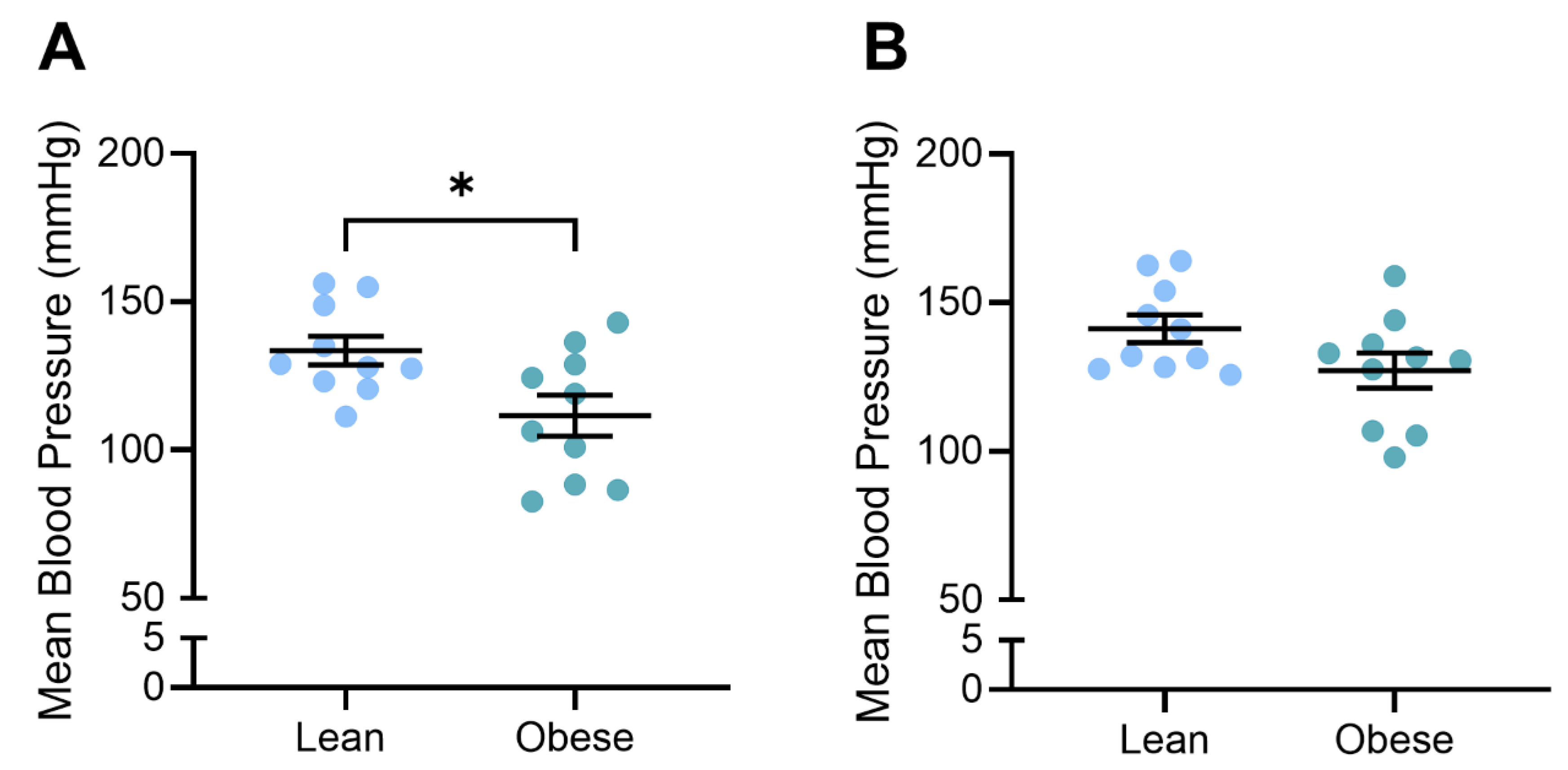
3.2. Blood Pressure Was Reduced in Obese, L-NAME-Treated Dams
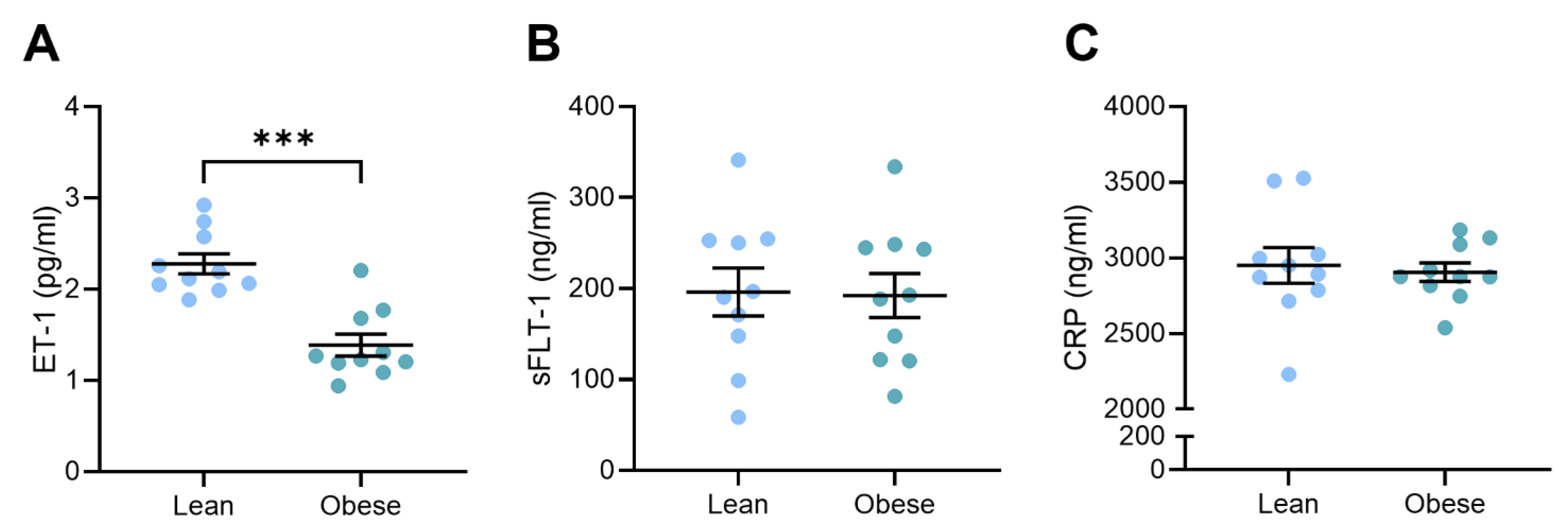
3.3. Circulating Vasoconstrictor Levels Were Reduced in Obese Dams
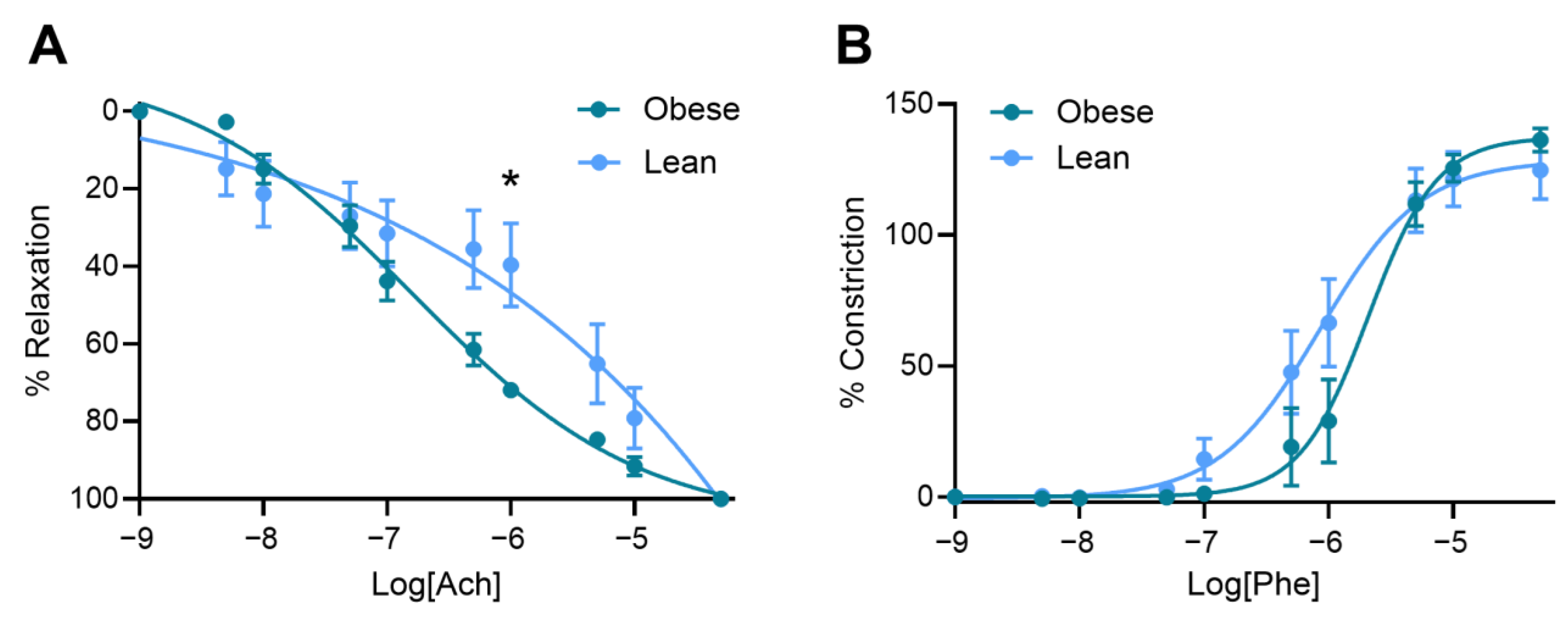
3.4. Obese Mice Had Increased Vasorelaxation Compared to Lean Mice
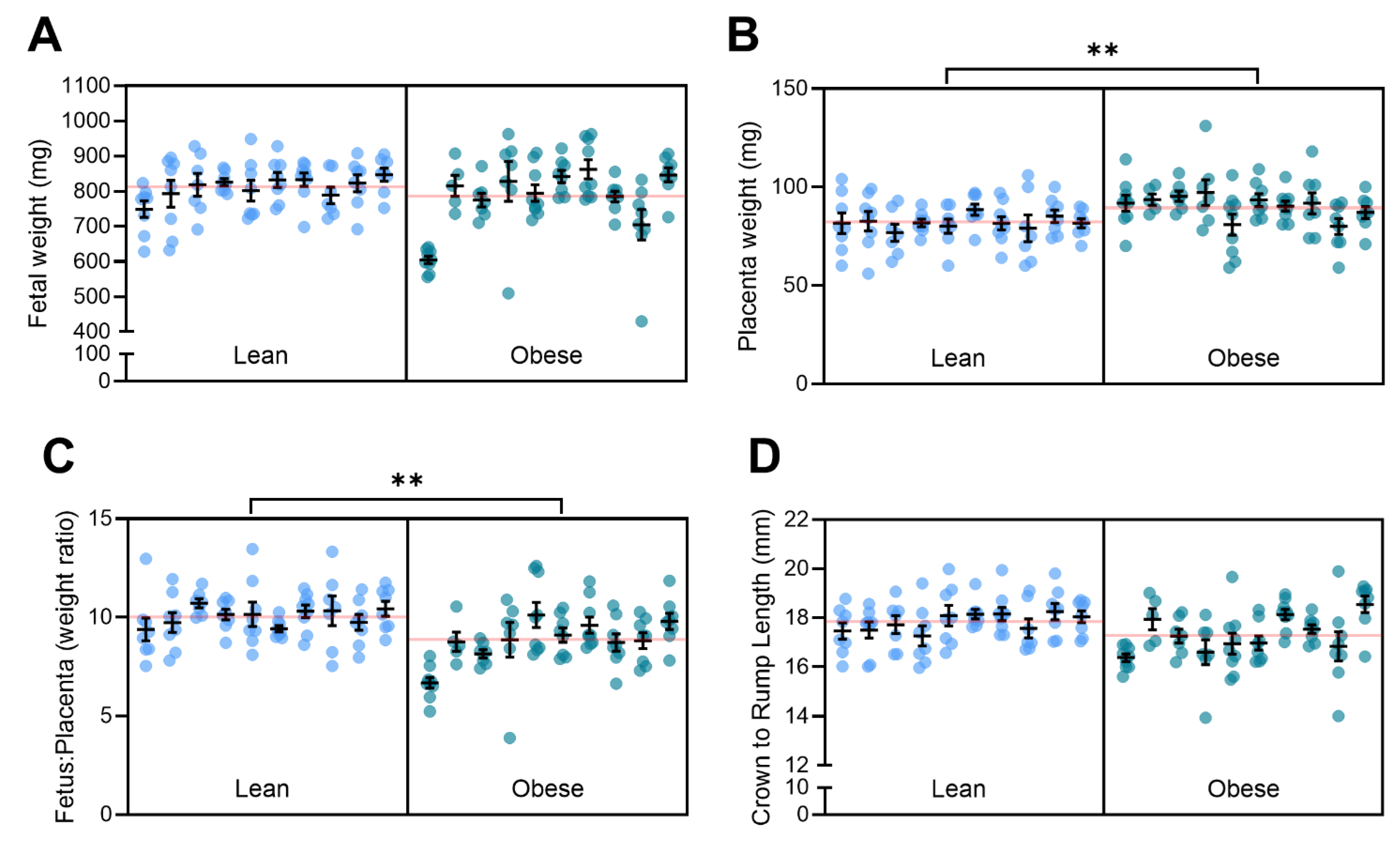
3.5. Obesity with Superimposed Preeclampsia Alters Placental Size
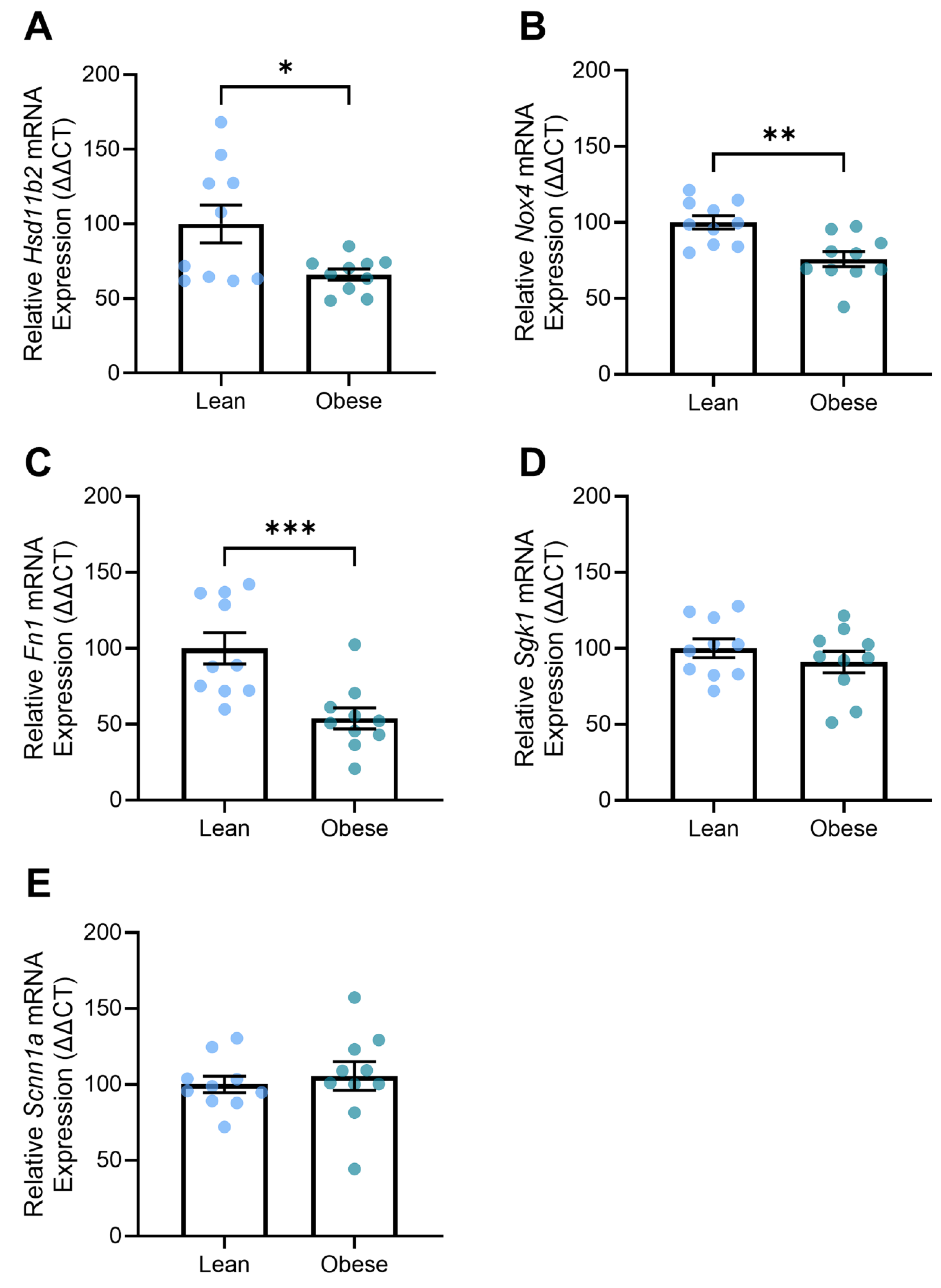
3.6. Obese Mice Demonstrated Altered Expression of Genes Associated with Kidney Function
3.7. Kidney and Liver Histology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duley, L. The Global Impact of Pre-eclampsia and Eclampsia. Semin. Perinatol. 2009, 33, 130–137. [Google Scholar] [CrossRef]
- Kuklina, E.V.; Ayala, C.; Callaghan, W.M. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet. Gynecol. 2009, 113, 1299–1306. [Google Scholar] [CrossRef]
- Paruk, F.; Moodley, J. Maternal and neonatal outcome in early- and late-onset pre-eclampsia. Semin. Neonatol. 2000, 5, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Opichka, M.A.; Rappelt, M.W.; Gutterman, D.D.; Grobe, J.L.; McIntosh, J.J. Vascular Dysfunction in Preeclampsia. Cells 2021, 10, 3055. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Woods, A.W.; Jauniaux, E.; Kingdom, J.C. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 2009, 30, 473–482. [Google Scholar] [CrossRef]
- Wang, A.; Rana, S.; Karumanchi, S.A. Preeclampsia: The role of angiogenic factors in its pathogenesis. Physiology 2009, 24, 147–158. [Google Scholar] [CrossRef]
- Venkatesha, S.; Toporsian, M.; Lam, C.; Hanai, J.; Mammoto, T.; Kim, Y.M.; Bdolah, Y.; Lim, K.H.; Yuan, H.T.; Libermann, T.A.; et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 2006, 12, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Robillard, P.Y.; Dekker, G.; Scioscia, M.; Bonsante, F.; Iacobelli, S.; Boukerrou, M.; Hulsey, T.C. Increased BMI has a linear association with late-onset preeclampsia: A population-based study. PLoS ONE 2019, 14, e0223888. [Google Scholar] [CrossRef]
- O’Brien, T.E.; Ray, J.G.; Chan, W.S. Maternal body mass index and the risk of preeclampsia: A systematic overview. Epidemiology 2003, 14, 368–374. [Google Scholar] [CrossRef]
- Jones, S.I.; Rosenthal, E.A.; Pruszynski, J.E.; Cunningham, F.G. The Dose-Dependent Effect of Obesity on Adverse Maternal and Neonatal Outcomes in a Hispanic Population. Am. J. Perinatol. 2025; ahead of print. [Google Scholar] [CrossRef]
- Sohlberg, S.; Stephansson, O.; Cnattingius, S.; Wikström, A.K. Maternal body mass index, height, and risks of preeclampsia. Am. J. Hypertens. 2012, 25, 120–125. [Google Scholar] [CrossRef]
- Chen, C.; Xu, X.; Yan, Y. Estimated global overweight and obesity burden in pregnant women based on panel data model. PLoS ONE 2018, 13, e0202183. [Google Scholar] [CrossRef] [PubMed]
- Autret, K.; Bekelman, T.A. Socioeconomic Status and Obesity. J. Endocr. Soc. 2024, 8, bvae176. [Google Scholar] [CrossRef]
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Callaway, L.K.; Prins, J.B.; Chang, A.M.; McIntyre, H.D. The prevalence and impact of overweight and obesity in an Australian obstetric population. Med. J. Aust. 2006, 184, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ. Tech. Rep. Ser. 2000, 894, 1–253.
- Poston, L.; Caleyachetty, R.; Cnattingius, S.; Corvalán, C.; Uauy, R.; Herring, S.; Gillman, M.W. Preconceptional and maternal obesity: Epidemiology and health consequences. Lancet Diabetes Endocrinol. 2016, 4, 1025–1036. [Google Scholar] [CrossRef]
- Battineni, G.; Sagaro, G.G.; Chintalapudi, N.; Amenta, F.; Tomassoni, D.; Tayebati, S.K. Impact of Obesity-Induced Inflammation on Cardiovascular Diseases (CVD). Int. J. Mol. Sci. 2021, 22, 4798. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Baig, S.; Parvaresh Rizi, E.; Chia, C.; Shabeer, M.; Aung, N.; Loh, T.P.; Magkos, F.; Vidal-Puig, A.; Seet, R.C.S.; Khoo, C.M.; et al. Genes Involved in Oxidative Stress Pathways Are Differentially Expressed in Circulating Mononuclear Cells Derived From Obese Insulin-Resistant and Lean Insulin-Sensitive Individuals Following a Single Mixed-Meal Challenge. Front. Endocrinol. 2019, 10, 256. [Google Scholar] [CrossRef]
- Malti, N.; Merzouk, H.; Merzouk, S.A.; Loukidi, B.; Karaouzene, N.; Malti, A.; Narce, M. Oxidative stress and maternal obesity: Feto-placental unit interaction. Placenta 2014, 35, 411–416. [Google Scholar] [CrossRef]
- Hunyenyiwa, T.; Hendee, K.; Matus, K.; Kyi, P.; Mammoto, T.; Mammoto, A. Obesity Inhibits Angiogenesis Through TWIST1-SLIT2 Signaling. Front. Cell Dev. Biol. 2021, 9, 693410. [Google Scholar] [CrossRef]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef]
- Chappell, L.C.; Cluver, C.A.; Kingdom, J.; Tong, S. Pre-eclampsia. Lancet 2021, 398, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.N.; Redman, L.M.; Sones, J.L. Obesity “complements” preeclampsia. Physiol. Genomics 2019, 51, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Virdis, A. Endothelial Dysfunction in Obesity: Role of Inflammation. High Blood Press. Cardiovasc. Prev. 2016, 23, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, J.E.; Ferrell, W.R.; Crawford, L.; Wallace, A.M.; Greer, I.A.; Sattar, N. Maternal Obesity Is Associated with Dysregulation of Metabolic, Vascular, and Inflammatory Pathways. J. Clin. Endocrinol. Metab. 2002, 87, 4231–4237. [Google Scholar] [CrossRef]
- Barber, E.; Ram, M.; Mor, L.; Ganor Paz, Y.; Shmueli, A.; Bornstein, S.; Barda, G.; Schreiber, L.; Weiner, E.; Levy, M. Pregnancy and placental outcomes according to maternal BMI in women with preeclampsia: A retrospective cohort study. Arch. Gynecol. Obstet. 2024, 309, 2521–2528. [Google Scholar] [CrossRef]
- Onda, K.; Tong, S.; Beard, S.; Binder, N.; Muto, M.; Senadheera, S.N.; Parry, L.; Dilworth, M.; Renshall, L.; Brownfoot, F.; et al. Proton Pump Inhibitors Decrease Soluble fms-Like Tyrosine Kinase-1 and Soluble Endoglin Secretion, Decrease Hypertension, and Rescue Endothelial Dysfunction. Hypertension 2017, 69, 457–468. [Google Scholar] [CrossRef]
- Binder, N.K.; de Alwis, N.; Beard, S.; Kadife, E.; Harper, A.; Kaitu’u-Lino, T.J.; Brownfoot, F.C.; Hannan, N.J. Sulfasalazine for the treatment of preeclampsia in a nitric oxide synthase antagonist mouse model. Placenta 2023, 132, 20–26. [Google Scholar] [CrossRef]
- de Alwis, N.; Binder, N.K.; Beard, S.; Mangwiro, Y.T.; Kadife, E.; Cuffe, J.S.; Keenan, E.; Fato, B.R.; Kaitu’u-Lino, T.J.; Brownfoot, F.C.; et al. The L-NAME mouse model of preeclampsia and impact to long-term maternal cardiovascular health. Life Sci. Alliance 2022, 5, e202201517. [Google Scholar] [CrossRef]
- Singh, J.; Ahmed, A.; Girardi, G. Role of complement component C1q in the onset of preeclampsia in mice. Hypertension 2011, 58, 716–724. [Google Scholar] [CrossRef]
- Alexander, B.T.; Kassab, S.E.; Miller, M.T.; Abram, S.R.; Reckelhoff, J.F.; Bennett, W.A.; Granger, J.P. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension 2001, 37, 1191–1195. [Google Scholar] [CrossRef]
- Davisson, R.L.; Hoffmann, D.S.; Butz, G.M.; Aldape, G.; Schlager, G.; Merrill, D.C.; Sethi, S.; Weiss, R.M.; Bates, J.N. Discovery of a spontaneous genetic mouse model of preeclampsia. Hypertension 2002, 39, 337–342. [Google Scholar] [CrossRef]
- Adams, D.M.; Beckers, K.F.; Flanagan, J.P.; Gomes, V.C.L.; Liu, C.C.; Sones, J.L. Reversal of maternal obesity attenuates hypoxia and improves placental development in the preeclamptic-like BPH/5 mouse model. Biocell 2023, 47, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Beckers, K.F.; Schulz, C.J.; Flanagan, J.P.; Adams, D.M.; Gomes, V.C.L.; Liu, C.C.; Childers, G.W.; Sones, J.L. Sex-specific effects of maternal weight loss on offspring cardiometabolic outcomes in the obese preeclamptic-like mouse model, BPH/5. Physiol. Rep. 2022, 10, e15444. [Google Scholar] [CrossRef]
- Mahany, E.B.; Han, X.; Borges, B.C.; da Silveira Cruz-Machado, S.; Allen, S.J.; Garcia-Galiano, D.; Hoenerhoff, M.J.; Bellefontaine, N.H.; Elias, C.F. Obesity and High-Fat Diet Induce Distinct Changes in Placental Gene Expression and Pregnancy Outcome. Endocrinology 2018, 159, 1718–1733. [Google Scholar] [CrossRef] [PubMed]
- Kolade, O.O.; O’Moore-Sullivan, T.M.; Stowasser, M.; Coombes, J.S.; Fassett, R.G.; Marwick, T.H.; Sharman, J.E. Arterial stiffness, central blood pressure and body size in health and disease. Int. J. Obes. 2012, 36, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Parvanova, A.; Reseghetti, E.; Abbate, M.; Ruggenenti, P. Mechanisms and treatment of obesity-related hypertension-Part 1: Mechanisms. Clin. Kidney J. 2024, 17, sfad282. [Google Scholar] [CrossRef]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef]
- Rust, O.A.; Bofill, J.A.; Zappe, D.H.; Hall, J.E.; Burnett, J.C., Jr.; Martin, J.N., Jr. The origin of endothelin-1 in patients with severe preeclampsia. Obstet. Gynecol. 1997, 89, 754–757. [Google Scholar] [CrossRef]
- Lu, Y.P.; Hasan, A.A.; Zeng, S.; Hocher, B. Plasma ET-1 Concentrations Are Elevated in Pregnant Women with Hypertension -Meta-Analysis of Clinical Studies. Kidney Blood Press. Res. 2017, 42, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, F.; Constantino, L.; Machado, R.; Petronilho, F.; Dal-Pizzol, F. Plasma nitric oxide, endothelin-1, arginase and superoxide dismutase in pre-eclamptic women. J. Obstet. Gynaecol. Res. 2008, 34, 957–963. [Google Scholar] [CrossRef]
- Weil, B.R.; Westby, C.M.; Van Guilder, G.P.; Greiner, J.J.; Stauffer, B.L.; DeSouza, C.A. Enhanced endothelin-1 system activity with overweight and obesity. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H689–H695. [Google Scholar] [CrossRef] [PubMed]
- Nacci, C.; Leo, V.; De Benedictis, L.; Carratù, M.R.; Bartolomeo, N.; Altomare, M.; Giordano, P.; Faienza, M.F.; Montagnani, M. Elevated endothelin-1 (ET-1) levels may contribute to hypoadiponectinemia in childhood obesity. J. Clin. Endocrinol. Metab. 2013, 98, E683–E693. [Google Scholar] [CrossRef] [PubMed]
- Maynard, S.; Min, J.Y.; Merchan, J.; Lim, K.H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt-1) may contribute to endothelial dysfunction, hypertension, and proteinuria in pre-eclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef]
- Maynard, S.E.; Karumanchi, S.A. Angiogenic factors and preeclampsia. Semin. Nephrol. 2011, 31, 33–46. [Google Scholar] [CrossRef]
- Nagamatsu, T.; Fujii, T.; Kusumi, M.; Zou, L.; Yamashita, T.; Osuga, Y.; Momoeda, M.; Kozuma, S.; Taketani, Y. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: An implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology 2004, 145, 4838–4845. [Google Scholar] [CrossRef]
- Holme, A.M.; Roland, M.C.; Henriksen, T.; Michelsen, T.M. In vivo uteroplacental release of placental growth factor and soluble Fms-like tyrosine kinase-1 in normal and preeclamptic pregnancies. Am. J. Obstet. Gynecol. 2016, 215, 782.e781–782.e789. [Google Scholar] [CrossRef]
- Faupel-Badger, J.M.; Staff, A.C.; Thadhani, R.; Powe, C.E.; Potischman, N.; Hoover, R.N.; Troisi, R. Maternal angiogenic profile in pregnancies that remain normotensive. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 158, 189–193. [Google Scholar] [CrossRef]
- Spradley, F.T.; Palei, A.C.; Granger, J.P. Obese melanocortin-4 receptor-deficient rats exhibit augmented angiogenic balance and vasorelaxation during pregnancy. Physiol. Rep. 2013, 1, e00081. [Google Scholar] [CrossRef]
- Herse, F.; Fain, J.N.; Janke, J.; Engeli, S.; Kuhn, C.; Frey, N.; Weich, H.A.; Bergmann, A.; Kappert, K.; Karumanchi, S.A.; et al. Adipose tissue-derived soluble fms-like tyrosine kinase 1 is an obesity-relevant endogenous paracrine adipokine. Hypertension 2011, 58, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Higgins, L.; Greenwood, S.L.; Wareing, M.; Sibley, C.P.; Mills, T.A. Obesity and the placenta: A consideration of nutrient exchange mechanisms in relation to aberrant fetal growth. Placenta 2011, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binder, N.K.; de Alwis, N.; Fato, B.R.; Beard, S.; Mangwiro, Y.T.M.; Kadife, E.; Brownfoot, F.; Hannan, N.J. Investigating the Impact of Maternal Obesity on Disease Severity in a Mouse Model of Preeclampsia. Nutrients 2025, 17, 1586. https://doi.org/10.3390/nu17091586
Binder NK, de Alwis N, Fato BR, Beard S, Mangwiro YTM, Kadife E, Brownfoot F, Hannan NJ. Investigating the Impact of Maternal Obesity on Disease Severity in a Mouse Model of Preeclampsia. Nutrients. 2025; 17(9):1586. https://doi.org/10.3390/nu17091586
Chicago/Turabian StyleBinder, Natalie K., Natasha de Alwis, Bianca R. Fato, Sally Beard, Yeukai T. M. Mangwiro, Elif Kadife, Fiona Brownfoot, and Natalie J. Hannan. 2025. "Investigating the Impact of Maternal Obesity on Disease Severity in a Mouse Model of Preeclampsia" Nutrients 17, no. 9: 1586. https://doi.org/10.3390/nu17091586
APA StyleBinder, N. K., de Alwis, N., Fato, B. R., Beard, S., Mangwiro, Y. T. M., Kadife, E., Brownfoot, F., & Hannan, N. J. (2025). Investigating the Impact of Maternal Obesity on Disease Severity in a Mouse Model of Preeclampsia. Nutrients, 17(9), 1586. https://doi.org/10.3390/nu17091586





